Abstract
The blood pressure response to exercise is exaggerated in type 2 diabetes mellitus (T2DM). However, the underlying mechanisms remain unclear. It is hypothesized that one mechanism mediating the potentiated cardiovascular response in T2DM is the sensitization of chemically sensitive afferent neurons by activation of metaboreceptors. To test this hypothesis, we examined transient receptor potential cation channel subfamily V member 1 (TRPV1)-induced cardiovascular responses in vivo and muscle afferent discharge ex vivo in T2DM rats. Additionally, TRPV1 and protein kinase C (PKC) protein levels in dorsal root ganglia (DRG) subserving skeletal muscle were assessed. For 14-16 weeks, Sprague-Dawley rats were given either a normal diet (control) or high fat diet in combination with a low dose (35 and 25 mg/kg) of streptozotocin (T2DM). Administration of capsaicin, TRPV1 agonist, in hindlimb evoked significantly greater increases in mean arterial pressure and renal sympathetic neve activity in decerebrated T2DM than control. In a muscle-nerve preparation, the discharge to capsaicin exposure in group IV afferents isolated from T2DM was likewise significantly augmented at a magnitude that was proportional to glucose concentration. Moreover, the discharge to capsaicin was potentiated by acute exposure of group IV afferents to a high glucose environment. T2DM showed significantly increased phospholyrated-TRPV1 and −PKCα levels in DRG neurons as compared with control. These findings suggest that group IV muscle afferents are sensitized by PKC-induced TRPV1 overactivity in early-stage T2DM with hyperglycemia and, thereby, may contribute to the potentiated circulatory response to TRPV1 activation in the disease.
Keywords: blood pressure, sympathetic nerve activity, skeletal muscle afferents, sensory neurons, TRPV1, type 2 diabetes mellitus
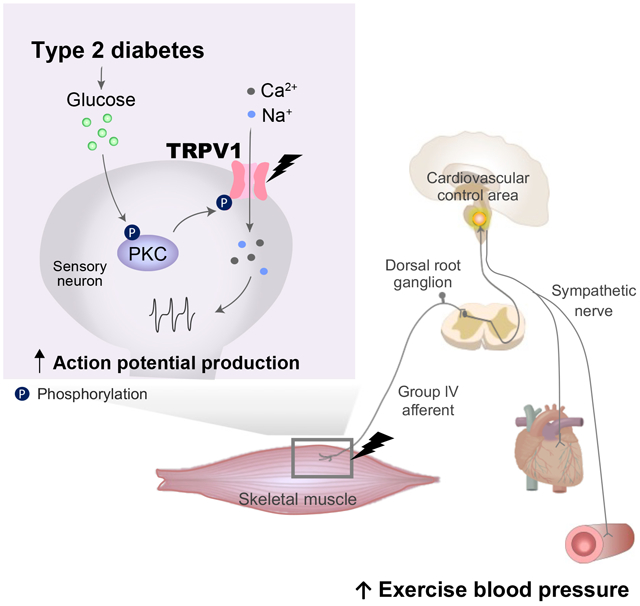
Graphical Abstract
INTRODUCTION
Physical activity can play a crucial role in improving cardiovascular health in type 2 diabetes mellitus (T2DM)1-3. However, the blood pressure (BP) response to exercise is exaggerated in T2DM4-9. Since such excessive BP rises can lead to adverse cardiac events and/or stroke during or immediately after a bout of exercise10, 11, identifying the potential mechanism that mediates this abnormal response is physiologically and clinically relevant. A recent study from our laboratory demonstrated that the exercise pressor reflex (EPR), an important neural mechanism originating in working skeletal muscle controlling cardiovascular function during physical activity12, 13, is abnormally augmented in T2DM rats14. The EPR is, in part, mediated by the muscle metaboreflex which is predominantly activated via chemically sensitive muscle afferents stimulated by the metabolites produced during muscle contraction12, 15. Importantly, previous work8 has demonstrated abnormally heightened metaboreflex function in T2DM patients. However, the mechanism underlying the potentiated responsiveness of this relex in this disease remains unclear.
Group IV afferents innervating skeletal muscle are predominantly chemically sensitive and primarily transmit information about the metabolic state of exercising muscles (i.e., the afferent arm of the muscle metaboreflex)8. Cutaneous innervation also consists, in part, of group IV fibers and spontaneous hyperactivity induced by neuronal damage is abnormally enhanced in group IV fiber terminals of skin in diabetic rats16, 17. In fact, a previous study has shown that a relationship exists between the degree of spontaneous hyperactivity and thermal hypersensitivity (mainly induced by activation of group IV fibers) in cutaneous sensory neurons18. Thus, it is logical to suggest that the responsiveness of group IV afferents in muscle may also be enhanced in T2DM. Moreover, alterations in the sensitivity of skeletal muscle group IV afferents could possibly be responsible for the heightened cardiovascular response in this desease. However, to date, the reactivity of group IV muscle afferents in T2DM remain unkonwn.
Hyperglycemia, a pathological characteristic of T2DM, leads to insulin secretion as well as the accumulation of advanced glycation end products (AGE)19 and the inflammatory cytokine high-mobility group box-protein 1 (HMGB1)20. High glucose21, insulin22, AGE19 and HMGB120, 22, 23 are known to activate protein kinase C (PKC) in sensory neurons. PKC, in turn, activates the transient receptor potential cation channel subfamily V member 1 (TRPV1) metaboreceptor in pheripheral sensory neurons24. Importantly, a recent study reported that TRPV1-evoked Ca2+ responses in dorsal root ganglia (DRG) mediated by increases in skin HMGB1 were PKC-dependently enhanced in type 1 diabetic hyperglycemia25. TRPV1 is a metaboreceptor widely expressed in group IV skeletal muscle afferents26. Thus, it is logical to further suggest that alterations in group IV skeletal muscle sensory neurons in T2DM may result from abnormalities in the PKC/TRPV1 pathway in the DRG that subserve the metaboreflex.
In this study, therefore, it was hypothesized that cardiovascular and sympathetic responses to TRPV1 activation and/or group IV muscle afferent discharge to capsaicin exposure are enhanced by augmentations in the activity of the PKC/TRPV1 pathway in T2DM. A combination of a high fat diet (HFD) and a low dose of streptozocin (STZ) has been shown to effectively generate a rat model of diabetes that mimics the metabolic characteristics of common T2DM in humans unlike that manifest in the genetically engineered Zucker diabetic rat and db/db mouse27, 28. Moreover, we previously demonstrated that the EPR was abnormally exaggerated in HFD/STZ diabetic rats14. Therefore, we utilized the HFD/STZ model in the current study. We investigated in both healthy controls and HFD/STZ-induced T2DM rats 1) the BP and sympathetic responses to the administration of a TRPV1 agonist in the hindlimb in vivo, 2) the action potential responses to administration of a TRPV1 agonist in group IV muscle afferents ex vivo, 3) the protein expression levels of the receptor for advanced glycation end products (RAGE), PKC and TRPV1 in primary sensory (DRG) neurons subserving skeletal muscle, and 4) the plasma levels of known triggers for PKC/TRPV1 activation (i.e. glucose, insulin, AGE and HMGB1)
MATERIALS AND METHODS
The authors declare that all supporting data are available with the article. A complete description of the methods used in this study is available in the online-only Data Supplement.
Type 2 Diabetic Model
All experiments were performed in male Sprague-Dawley rats (7-8 wk of age). To induce T2DM, rats were intraperitoneally injected with streptozotocin twice (35 mg/kg at first week and 25 mg/kg at second week) and fed a high-fat diet for 3-4 mo. In the control group, a benign vehicle (saline) was injected in lieu of streptozotocin, and the rats were fed a normal diet. All studies were performed in accordance with the US Department of Health and Human Services NIH Guide for the Care and Use of Laboratory Animals. The procedures outlined were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center.
Experimental Protocol
Experiment 1: Chemical reflex and skeletal muscle reflex testing in vivo.
Mean arterial pressure (MAP), heart rate (HR) and renal sympathetic nerve activity (RSNA) were continuously measured at rest and during distinctly separate stimulation trials. To determine the role of TRPV1 agonist on chemical reflex in vivo, we administered capsaicin (Sigma-Aldrich, 0.3 μg or 1.0 μg/100 μl) vs. saline as a vehicle control into the arterial supply of the right hindlimb29, 30. In a second distinct stimulation trial, the EPR was activated by statically contracting the triceps surae muscles of the right hindlimb for 30 s via electrical stimulation of isolated L4 and L5 ventral roots29-32.
Experiment 2: Single fiber recording ex vivo.
Using the extensor digitorum longus (EDL) muscle-nerve preparation, single-unit action potential responses to capsaicin administration were measured in group IV fibers33. The isolated EDL muscle-nerve preparation was kept under laminar superfusion with modified Krebs-Henseleit solution (110.9 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25.0 mM NaHCO3, and 20.0 mM glucose) for 4-7 hours. Frequency as well as the latency of the response to administration of capsaicin (Sigma-Aldrich, 1 μM, for 30 s) into the receptive field were also evaluated. Conduction velocity was measured by electrically stimulating the receptive field in EDL muscle.
In a subset of experiments, to investigate the impact of acute high glucose exposure on the capsaicin-evoked firing, the isolated EDL muscle-nerve preparation was kept under laminar superfusion with Krebs-Henseleit solution that included either 6 mM, 20 mM or 75 mM glucose for 7-10 hours.
Experiment 3: Western blotting, Blood collection and ELISA.
RAGE, total PKCα (t-PKCα), phosphorylated PKCα (p-PKCα), total TRPV1 (t-TRPV1), phosphorylated TRPV1 (p-TRPV1) and βactin protein levels in DRG (L3-L5) neurons were quantified by western blotting. Fasting blood glucose levels were assessed by glucometer measurements. Insulin, HMGB1 and AGE concentrations were determined in blood plasma obtained from the tail vein using ELISA. Insulin resistance index (HOMA-IR) was calculated according to the formula: fasting insulin (μU/L) × fasting glucose (nmol/L)/22.5.
Statistical Analysis
Data were analyzed using SPSS software. All data are expressed as mean ± SD. Data normality was assessed by utilizing the Shapiro-Wilk test. Data were analyzed using Student's unpaired t tests, Mann-Whitney U non-parametric test, two- and one-way ANOVA, post-hoc Fisher's Least Significant Difference test and Pearson’s correlation coefficient analysis. The level of statistical significance was defined as P<0.05.
Results
Morphometric characteristics and baseline hemodynamics
Morphometric characteristics and baseline hemodynamics for control and T2DM rats are summarized in Table 1. There was no significant difference in initial and end-point body weight, epididymal fat pad weight, heart weight-to-body weight ratios, heart weight-to-tibial length ratios and lung weight-to-body weight ratios between control and T2DM rats. Additionally, baseline MAP, HR and RSNA under 1% isoflurane anesthesia as well as after decerebration were not significantly different between groups.
Table 1.
Morphometric characteristics and baseline hemodynamics.
| Variables | CON | T2DM |
|---|---|---|
| Morphometric characteristics | ||
| Initial body weight, g | 193 ± 6 | 196 ± 6 |
| End-point body weight, g | 460 ± 51 | 473 ± 42 |
| Epididymal fat pad weight, g | 6.45 ± 1.30 | 9.07 ± 3.97 |
| Heart weight/body weight, mg/g | 2.68 ± 0.20 | 2.51 ± 0.19 |
| Heart weight/tibial length, mg/mm | 31.5 ± 2.6 | 28.4 ± 2.3 |
| Lung weight/body weight, mg/g | 5.0 ± 0.6 | 5.9 ± 1.3 |
| 1% isoflurane anesthesia | ||
| MAP, mmHg | 98 ± 20 | 96 ± 25 |
| HR, beats/min | 397 ± 40 | 365 ± 35 |
| RSNA, signal to noise ratio | 5.9 ± 3.9 | 6.2 ± 3.3 |
| After decerebration | ||
| MAP, mmHg | 89 ± 13 | 88 ± 19 |
| HR, beats/min | 463 ± 64 | 405 ± 48 |
| RSNA, signal to noise ratio | 9.9 ± 6.7 | 7.7 ± 4.7 |
Values are means ± SD. CON, control; HR, heart rate; MAP, mean arterial pressure; RSNA, renal sympathetic nerve activity; T2DM, type 2 diabetes mellitus. Body weight, CON: n = 17, T2DM: n = 17; Epididymal fat pad weight, CON: n = 7, T2DM: n = 7; heart and lung weight and tibial length, MAP, HR and RSNA, CON: n = 7, T2DM: n = 10.
Chemical Reflex Testing
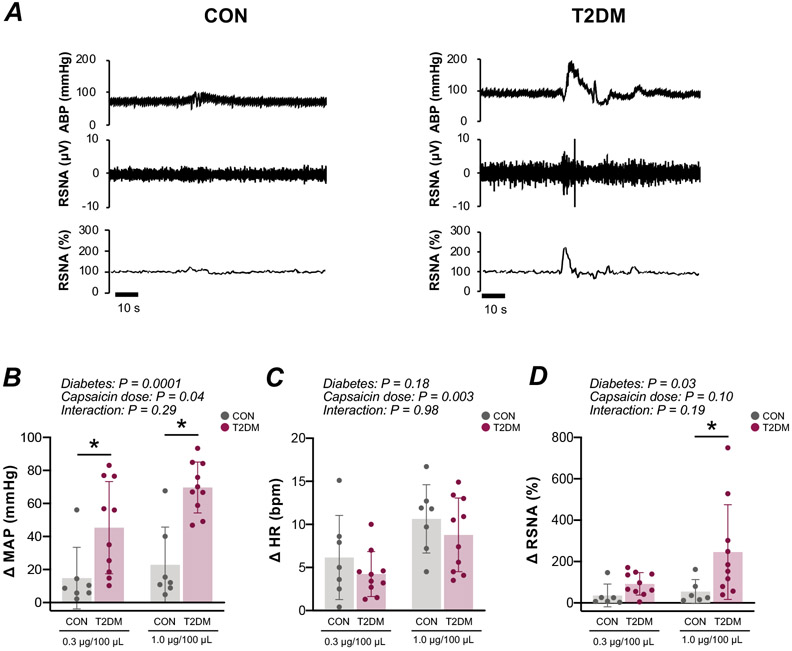
A representative example of the cardiovascular response to stimulation of chemically-sensitive skeletal muscle afferent fibers in vivo is illustrated in the raw recordings depicted in Fig. 1A. The MAP (diabetes effect: P<0.01; control vs. T2DM at 0.3 μg/100 μL and 1.0 μg/100 μL capsaicin: P<0.05) and RSNA (diabetes effect: P<0.05; control vs. T2DM at 1.0 μg/100 μL capsaicin: P<0.05) responses to the administration of capsaicin in the hindlimb of T2DM rats were augmented compared with control (Fig. 1B and D). The HR response was not significantly different between groups (Fig. 1C). The peak responses appeared within 10 seconds after intra-arterial bolus administration of capsaicin. The MAP and HR responses to injection of 1.0 μg/100 μL capsaicin were significantly exacerbated compared with injection of 0.3 μg/100 μL capsaicin (capsaicin dose effect: P<0.05; Fig. 1B and C). The RSNA responses to injection of 1.0 μg/100 μL capsaicin did not differ compared with 0.3 μg/100 μL capsaicin (Fig. 1D). Consistent with previous results29, 30, there was no significant differences in cardiovascular or sympathetic responses to saline administration (capsaicin vehicle control) between control and T2DM (MAP: 14 ± 5 vs. 16 ± 8 mmHg; HR: 3 ± 5 vs. 2 ± 1 bpm; RSNA: 9 ± 3 vs. 10 ± 2 % changes from baseline; n=3, respectively).
Figure 1. Cardiovascular and sympathetic responses to hindlimb intra-arterial capsaicin administration in male control (CON) and type 2 diabetes mellitus (T2DM) rats.
A, Representative raw recordings of individual arterial blood pressure (ABP) and renal sympathetic nerve activity (RSNA) responses to capsaicin. Peak changes in mean arterial pressure (MAP) [CON, n = 7; T2DM, n = 10; two-way ANOVA, diabetes: F=25.74, P<0.0001; capsaicin dose: F=4.51, P=0.04; interaction: F=1.14, P=0.29. Post-hoc Fisher's least Significant Difference (LSD), 0.3 and 1.0 μg/100 μL, P=0.008 and 0.0001, respectively.], heart rate (HR) [CON, n = 7; T2DM, n = 10; two-way ANOVA, diabetes: F=1.91, P=0.18; capsaicin dose: F=10.86, P=0.003; interaction: F=0.01, P=0.98] and RSNA [CON, n = 6; T2DM, n = 10; two-way ANOVA, diabetes: F=6.03, P=0.02; capsaicin dose: F=2.95, P=0.10; interaction: F=1.80, P=0.19. Post-hoc Fisher's least Significant Difference (LSD), 0.3 and 1.0 μg/100 μL, P=0.81 and 0.01, respectively.] in response to 0.3 and 1.0 μg/100 μL capsaicin are shown in panels B, C, and D. Plots show means±SD and individual data points. * P<0.05 compared with CON.
Single Fiber Nerve Recordings
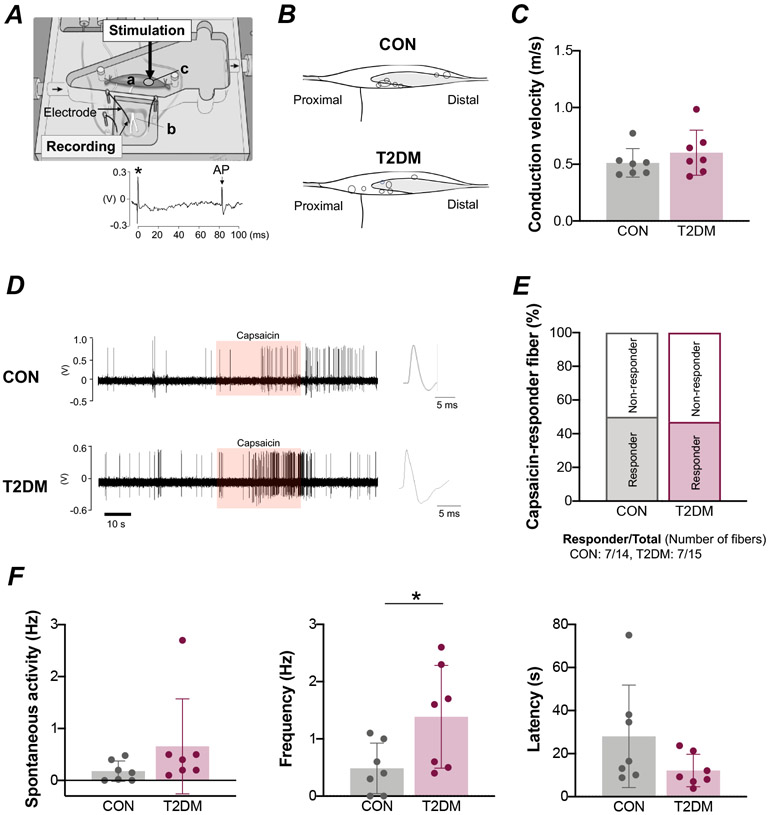
A schematic diagram illustrating the single-fiber experimental setup is shown in Figure 2A. The position of the group IV muscle afferent receptive fields assessed in this study are shown in Figure. 2B. Fiber conduction velocities were 0.51 ± 0.13 m/s (a range of 0.41–0.77 m/s) in control rats and 0.60 ± 0.20 m/s (a range of 0.39–0.98 m/s) in T2DM animals, which was not statistically different (Fig. 2C). An original recording demonstrating the action potential responses to capsaicin are shown in figure 2D. The percentage of group IV fibers demonstrated to be responsive to capsaicin was 50% (responder/total number of fibers: 7/14) in the control group, which was not significantly different from the 47% determined for the T2DM group (responder/total number of fibers: 7/15, Figure. 2E). Spontaneous neural activity was likewise not significantly different between groups (Fig. 2F). Using a capsaicin dose of 0.1 μM to activate the afferent’s receptive field, the frequency of response in group IV fibers was significantly greater in diabetic rats than in control animals (P<0.05; Fig. 2F). The response latency to capsaicin stimulation did not differ between groups (Fig. 2F).
Figure 2. Response of group IV muscle afferents to a capsaicin stimulus in male control (CON) and type 2 diabetes mellitus (T2DM) rats.
A, Schematic diagram illustrating the experimental setup. An isolated extensor digitorum longus (EDL) muscle (a)–peroneal nerve (b) preparation was placed in the test chamber. An electrical or capsaicin stimulus was applied to the receptive field of the nerve (c). The bottom figure shows a recording of an evoked action potential (AP) in a single-muscle fiber. The asterisk indicates electrical stimulus artifact. B, Each spot in the illustration represents the receptive field of a group IV muscle afferent. Shading illustrates the tendinous area. C, Conduction velocity in group IV muscle afferents [CON, n = 7; T2DM, n = 7; Mann-Whitney U-test, P=0.38]. D, Representative raw recording of individual group IV fiber activity in response to capsaicin administration (orange shading). E, Percentage of group IV fibers that responded to capsaicin. F, Spontaneous activity in group IV muscle afferents [CON, n = 7; T2DM, n = 7; Mann-Whitney U-test, P=0.07]. Frequency [CON, n = 7; T2DM, n = 7; Student’s t-test, P=0.04] and latency [CON, n = 7; T2DM, n = 7; Student’s t-test, P=0.13] in the response to capsaicin administration in group IV muscle afferents. Values are means ± SD. * P < 0.05 compared with CON.
Protein Expression
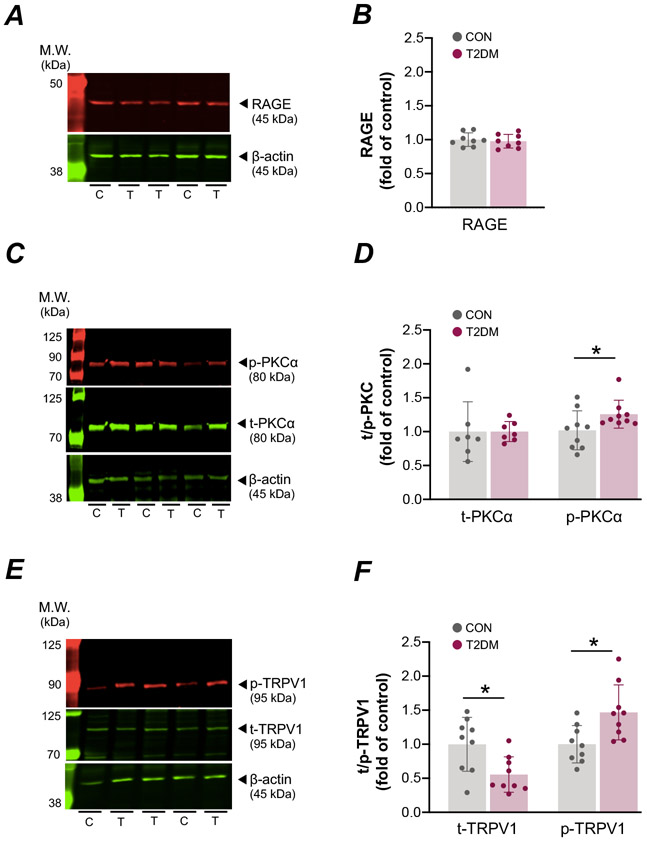
RAGE protein levels in DRG were not different between control and T2DM (Fig. 3A and B). The level of pPKCα protein was significantly higher in T2DM animals as compared to control rats (P<0.05; Fig. 3C and D). As compared to control, the pTRPV1 (S800) protein was significantly higher in T2DM animals, whereas tTRPV1 protein levels were significantly lower in T2DM (P<0.05; Fig. 3E and F).
Figure 3. RAGE, PKCα and TRPV1 protein levels in the dorsal root ganglia (DRG) of male control (CON) and type 2 diabetes mellitus (T2DM) rats.
Representative western blots (A) and quantified expression (B) of the receptor for advanced glycation end products (RAGE) in DRG tissue [CON, n = 8; T2DM, n = 8; Student’s t-test, P=0.61]. Representative western blots (C and E) and quantified expression (D and F) of total and phosphorylated PKCα [CON, n = 7; T2DM, n = 9; Student’s t-test, P=0.91 and P = 0.005] and TRPV1 [CON, n = 9; T2DM, n = 9; Student’s t-test, P=0.01 and P = 0.01], respectivley. Values are means ± SD, * P < 0.05 compared with CON. C, control; T, T2DM.
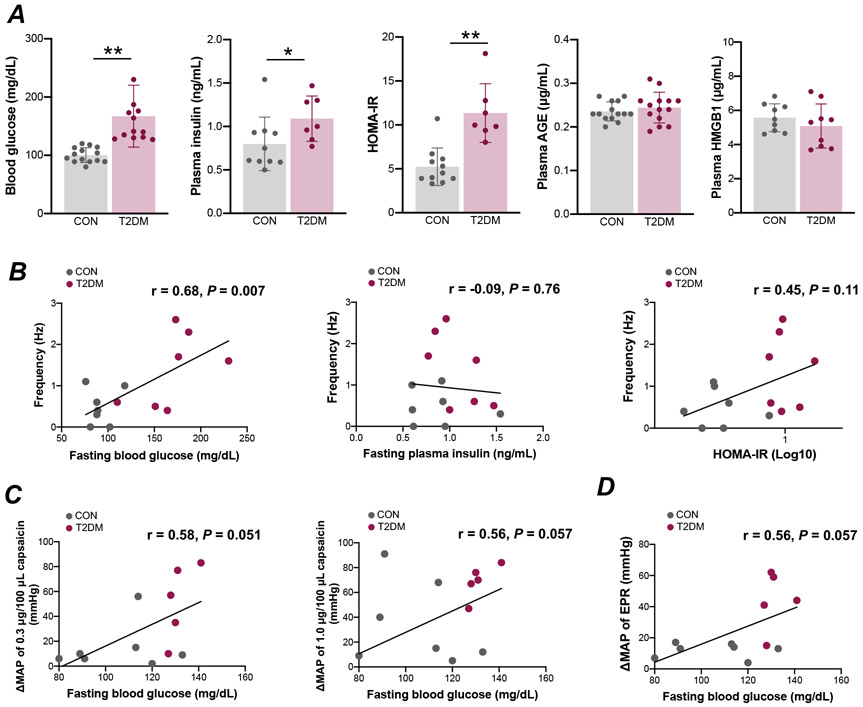
Correlation Between Blood Parameters and Nerve and Blood pressure Responses
T2DM animals exhibited hyperglycemia (Fig. 4A; P<0.01), hyperinsulinemia (Fig. 4A; P<0.05) and insulin resistance (Fig. 4A; P<0.01) after overnight fasting. Plasma AGE and HMGB1 levels did not differ between groups (Fig. 4A). A significant positive correlation was found between fasting blood glucose and the response to capsaicin in group IV fibers in vitro (Fig. 4B; r=0.68, P<0.01). There were no significant correlations between the response to capsaicin and either plasma insulin (Fig. 4B; r=−0.09, P=0.76) or HOMA-IR (Fig. 4 B; r=0.45, P=0.11). Furthermore, there was a positive, but not statistically sigfinicant, relationship between fasting blood glucose and changes in the MAP response to 0.3 μg/100 μL capsaicin (Fig. 4 C; r=0.58, P=0.051) and 1.0 μg/100 μL capsaicin administration (Fig. 4C; r=0.56, P=0.057) as well as activation of the EPR (Fig. 4D; r=0.56, P=0.057) in vivo.
Figure 4. Correlation between capsaicin-induced responses and blood parameters in male control (CON) and type 2 diabetes mellitus (T2DM) rats.
Fasting blood glucose [CON, n = 14; T2DM, n = 12; Student’s t-test, P=0.0001], fasting plasma insulin [CON, n = 11; T2DM, n = 7; Mann-Whitney U-test, P=0.006], homeostatic model assessment-insulin resistance (HOMA-IR) [CON, n = 11; T2DM, n = 7; Mann-Whitney U-test, P=0.00004], plasma advanced glycation end products (AGE) [CON, n = 14; T2DM, n = 14; Student’s t-test, P=0.45] and high-mobility group box-protein 1 (HMGB1) [CON, n = 9; T2DM, n = 9; Student’s t-test, P=0.35] are shown in A. Values are means ± SD. * P < 0.05 compared with CON, ** P < 0.01 compared with CON. Correlation between group IV fiber responses to capsaicin in vitro and fasting blood glucose [n = 12; Pearson’s correlation, r=0.68, P=0.007] (B), fasting plasma insulin [n = 14; Pearson’s correlation, r=−0.09, P=0.76] (B) and homeostatic model assessment-insulin resistance (HOMA-IR) [n = 14; Pearson’s correlation, r=0.45, P=0.11] (B) from control and type 2 diabetes mellitus (T2DM) rats. Correlation between fasting blood glucose and changes in mean arterial pressure (MAP) in response to administration of 0.3 μg/100 μL capsaicin [n = 12; Pearson’s correlation, r=0.58, P=0.051] (C) and 1.0 μg/100 μL capsaicin [n = 12; Pearson’s correlation, r=0.56, P=0.057] (C) as well as activation of the exercise pressor reflex (EPR) [n = 12; Pearson’s correlation, r=0.56, P=0.057] (D), in vivo from control and T2DM rats.
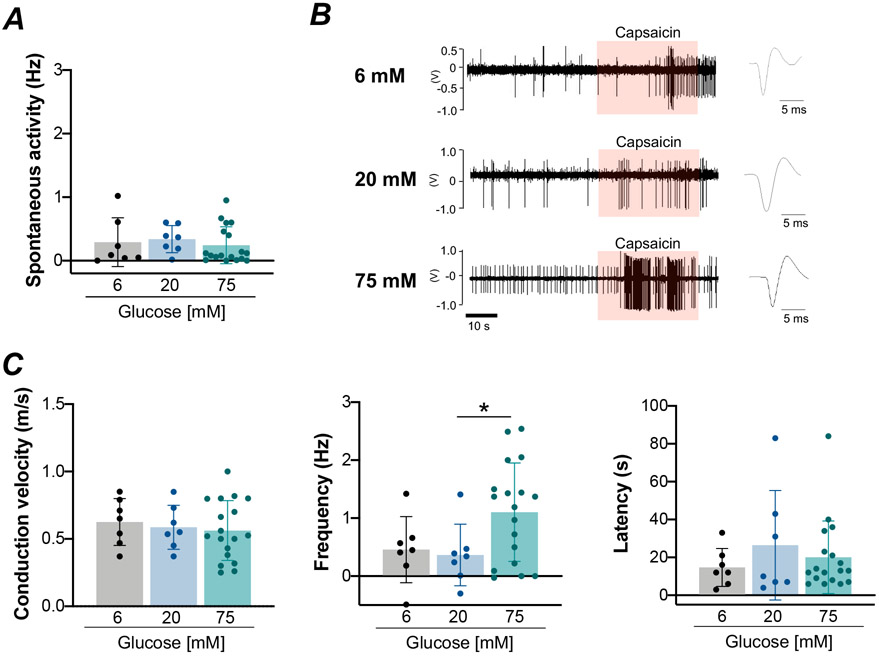
Impact of acute hyperglycemic exposure on TRPV1-mediated discharge in group IV fibers
In group IV muscle fibers from animals with normal fasting glucose levels (90 ± 16 mg/dL, a range of 59–122 mg/dL, n=32), conduction velocities did not differ between 6, 20 and 75 mM glucose-exposure (Fig. 5A). Acute high-glucose exposure with 75 mM glucose significantly augmented capsaicin-mediated nerve firing compared to 20 mM glucose exposure in group IV fibers (ANOVA: P<0.05; 20 mM vs. 75 mM glucose: P<0.05; 6 mM vs. 75 mM glucose: P=0.06; Fig. 5B and C). There were no significant differences in spontaneous activity (P=0.56; Fig. 5C) and latency (P=0.84; Fig. 5C) between glucose conditions.
Figure 5. Response of group IV muscle afferents to a capsaicin stimulus after acute high-glucose exposure in normal male rats.
A, Conduction velocity in group IV muscle afferents [6 mM glucose, n = 7; 20 mM glucose, n = 7; 75 mM glucose, n = 18; one-way ANOVA, F=0.25, P=0.78]. B, Representative raw recording of individual group IV fiber activity in response to capsaicin administration (orange shading) at 6 mM, 20 mM and 75 mM glucose exposure. C, Spontaneous activity in group IV muscle afferents [6 mM glucose, n = 7; 20 mM glucose, n = 7; 75 mM glucose, n = 18; Kruskal-Wallis one-way ANOVA, P=0.56]. Group IV muscle afferent response frequency [6 mM glucose, n = 7; 20 mM glucose, n = 7; 75 mM glucose, n = 18; one-way ANOVA, F=3.46, P=0.045; Post-hoc Fisher's least Significant Difference (LSD), 6 mM vs. 75 mM glucose, P=0.06; 20 mM vs. 75 mM glucose, P=0.03] and latency [6 mM glucose, n = 7; 20 mM glucose, n = 7; 75 mM glucose, n = 18; Kruskal-Wallis one-way ANOVA, P=0.84] in response to capsaicin administration. Values are means ± SD. * P<0.05 compared with 20 mM glucose.
DISCUSSION
The major novel findings from this investigation were (1) in vivo, the BP and sympathetic responses to hindlimb administration of a TRPV1 agonist were abnormally potentiated in T2DM rats, (2) ex vivo, the frequency of TRPV1-induced action potentials were markedly increased in skeletal muscle group IV fibers isolated from T2DM animals, (3) the expression of phosphorylated TRPV1 and PKCα in DRG were enhanced in diabetic animals, (4) the discharge response to capsaicin administration was significantly associated with fasting blood glucose, and (5) TRPV1-induced action potentials were significantly potentiated by acute high-glucose exposure. These data provide the first evidence suggesting that, in early stage T2DM, the function/activation of TRPV1 is increased in skeletal muscle afferents. These alterations are associated with hyperglycemia and likely play a role in mediating the enhanced BP response characteristic of T2DM.
TRPV1-Evoked Cardiovascular Response in Type 2 Diabetic Rats
Recent studies from our group14 and others34 demonstrated that the cardiovascular and sympathetic responses to activation of the EPR are exaggerated in T2DM rats. Moreover, Holwerda et al8 demonstrated that BP and muscle SNA responses were heightened during post exercise muscle ischemia (i.e. metaboreflex response) in T2DM patients. Herein, our data supported that an intra-arterial injection of capsaicin, a TRPV1 agonist, into the hindlimb induced augmented increases in BP and RSNA in T2DM rats (Fig. 1B and D). It should be noted that there remains some question among scientists in the field as to whether the TRPV1 receptor significantly contributes to expression of the muscle metaboreflex component of the EPR. Several lines of evidence in both mice and rats support the contention that the receptor does play an important role. For example, in TRPV1-null mice, systolic BP, diastolic BP and MAP responses to intravenous infusion of capsaicin are lower compared with wild-type mice, suggesting that TRPV1 regulates systemic BP35. Importantly, a recent study36 using a novel TRPV1 null mouse model to directly study the EPR demonstrated that the metaboreflex is, in part, mediated by activation of TRPV1 in skeletal muscle afferents consistent with a large number of previous studies in healthy37 and hypertensive rats29, with some exceptions 38. With regard to those exceptions, Ducrocq and colleagues38 recently demonstrated that blocking TRPV1 did not reduce expression of the EPR in healthy rats. Although the reasons for the discrepancy among studies is not readily clear, it could be due to a number of factors including, but not limited to, the the breed of rat studied, differences in techniques and protocols utilized, or health status of the animals investigated. Indeed, we have previously reported that blocking TRPV1 receptors in healthy Wistar-Kyoto rats does not appear to significantly attenuate EPR activity whereas it has a marked mitigating effect on the expression of the EPR in spontaneously hypertensive animals29. Keeping this in mind, the finding of the current study that the TRPV1-evoked cardiovascular response was exaggerated in T2DM animals does suggests it could be a contributory factor to the excessive BP response mediated by metabolite (e.g. protons39 and acid40) production in this disease.
TRPV1-Induced Sensitization of Group IV Fibers in T2DM
In the group IV muscle afferents isolated from T2DM rats, we showed that capsaicin-induced firing of action potentials was enhanced compared with normal rats (Fig. 2F). This data suggests that the neuronal function evoked by TRPV1 stimulation is abnormally augmented in the group IV afferents of diabetic animals. TRPV1 was also expressed in about 49% of muscle afferent neurons traced from skeletal muscle26. This proportion is higher than the P2X3 (13 %) metaboreceptor26, whereas it is similar to acid-sensing ion channel (ASIC) receptors (50%)41. Consistent with previous studies26, 42, the augmented action potential response to capsaicin was present in about 50% of the group IV afferents tested in both control and T2DM rats (Fig. 2E). Since the cardiovascular response evoked by TRPV1 activation is engaged upon stimulation of group IV afferents in skeletal muscle12, 36, 37, these data open the possibility that TRPV1 hyper-responsiveness in group IV muscle afferents in T2DM could, at least in part, mediate the exaggerated increase in the BP response to TRPV1 activaton by the metabolites produced during exercise.
Mechanism for TRPV1-mediated Sensitization of Sensory Afferents in Type 2 Diabetes
TRPV1 sensitization, which leads to an increase in Ca2+ influx, has been attributed to increased phosphorylation, particularly at the S800 PKC-dependent, phosphorylation site24. In this study, both phosphorylated-PKC (Fig. 3C and D) and −TRPV1 at the S800 site (Fig. 3E and F) were increased in DRG from T2DM rats. As shown previously in a T1DM rat model24, 43, our data suggest that TRPV1 activation was increased in T2DM, and this overactivation was possibly mediated via PKC signaling. Since TRPV1 is a key receptor in the induction of afferent action potential firing24, TRPV1 overactivity in T2DM may be a contributory factor to the sensitization of muscle afferents in this disease. Interestingly, total TRPV1 levels were decreased in DRG neurons in T2DM rats consistent with an earlier investigation43. Despite such an overall decrease in TRPV1 expression, this previous study demonstrated neuronal functional responses to TRPV1 stimulation were increased through a PKC-dependent mechanism43. Consistent with this previous report, our data suggested that the differences in group IV afferent responsiveness between control and T2DM was not attributable to alterations in total TRPV1 abundance but were more likely due to sensitization of the TRPV1 receptor itself.
Trigger for TRPV1 Sensitization in Type 2 Diabetic Animals
In T1DM rats, we have previously shown that muscle afferents were sensitized, and plasma HMGB1 and PKC activation in DRG were increased44. A recent study demonstrated that high glucose causes sensitization of sensory neurons through the HMGB1/RAGE/PKC/TRPV1 pathway in T1DM25. However, in our current study, neither AGE, HMGB1, nor RAGE were different between controls and T2DM rats (Fig. 4A). Blood glucose (T2DM: 163±53 mg/dL) was mildly increased compared to that previously reported for T1DM animals (> 270 mg/dL)25. Moreover, activation of RAGE by AGE results after accumulation of AGE over a considerable period of time (months to years)19 not reached in the current study. Indeed, the diabetic animal model used in this study is one of early-stage T2DM rather than an established, long-term form of the disease. Thus, unlike T1DM25, TRPV1 sensitization in mildly-hyperglycemic, early-stage T2DM rats, as were used in the current study, might not occur as a result of RAGE/PKC signaling but rather a completely different pathway.
Peripheral hyperinsulinemia is a pathophysiological characteristic of T2DM. Previous work45 has elucidated that insulin sensitizes TRPV1. Plasma insulin increased in the diabetic rat model used in the current study (Fig. 4A). However, there was no significant correlations among the response to capsaicin and plasma insulin (Fig. 4B). On the other hand, a positive correlation was found between fasting blood glucose and the group IV fiber response to the TRPV1 agonist capsaicin in normal and T2DM rats (Fig. 4B). In addition, changes in the MAP response to activation of TRPV1 or the EPR in vivo positively correlated with fasting blood glucose levels in normal and T2DM rats, although statistical significance was not reached (Fig. 4C and D). To further investigate whether the presence of a high glucose environment independently potentiates TRPV1-induced neuronal responsiveness in group IV fibers, we measured TRPV1-induced action potentials in response to high-glucose exposure in the ex vivo muscle-nerve preparation isolated from normal rats (Fig. 5). Data from these studies suggests that the enchanced TRPV1-evoked neuronal response in T2DM is, at least in part, mediated by hyperglycemia in this disease. Our data are consistent with results from a recent study by Bestall et al25, which demonstrated that exposure of DRG neurons to high-glucose conditions for 24 hours significantly increased phosphorylation of TRPV1 (S800 site). Moreover, high glucose induced phosphorylation of PKC in DRG neurons in vitro21. Thus, in mildly-hyperglycemic, early-stage T2DM, glucose may contribute to neuronal sensitization via PKC/TRPV1.
Limitations
Limitations to this investigation are acknowledged. First, only male rats were examined and results may not be applicable to female rats. Male rats were used in this study for ease of comparison to the majority of previous reports that likewise used male rats only. Clearly, future studies in female rats are needed and warranted. Second, the effects of the HFD itself on the cardiovascular responses evoked was not verified. However, a pre-collicular decerebration was performed in the current study which removed the hypothalamus. As such, the abnormal cardiovascular responses observed were likely not affected by known HFD-induced BP increases via opioid receptors in the hypothalamus46. Moreover, since visceral fat and body weight did not differ among groups, it is unlikely that adiposity played a major role in the TRPV1 sensitization noted in the study. Third, the cardiovascular response to EPR activation as well as the quantification of group IV fiber discharge during TRPV1 blockade in vivo were not examined in the current investigation. Such studies will be important to definitely demonstrate the TRPV1 receptor mediates, at least in part, the abnormal cardiovascular response to exercise in T2DM. This is especially appreciated given that although a number of studies29, 36, 37, 47, 48 have previously shown an important role for TRPV1 in the expression of the metaboreflex component of the EPR, a recent study38 suggests that the TRPV1 may not play a role in evoking the skeletal muscle reflex in normal healthy rats. Moreover, since the chemical used in this study to activate group IV afferents was capsaicin, an exogenous substance, additional studies are warranted to verify the response of TRPV1 to endogenously produced metabolites (proton39, acid40 and heat49). Future investigation using the recently developed TRPV1 null mouse model36 for the study of the EPR may prove useful in addressing these needs. Lastly, since involvement of the glucose-TRPV1 pathway was only indirectly examined in the current investigation, additional studies are warranted in the future to verify its role in the sensitization of muscle afferents.
In conclusion, the findings of the present study suggest the cardiovascular and sympathetic responses to activation of the TRPV1 channel are abnormally heightened in early-stage T2DM resulting, at least in part, from a hyperglycemia-induced increase in group IV skeletal muscle afferent fiber sensitivity. This sensitization is possibly associated with activation of the PKC/TRPV1 pathway.
PERSPECTIVES
Previous studies have provided evidence of excessive BP responses to physical activity in patients with well established T2DM4-9. More recently, a study in non–diabetic older adults has further demonstrated an association between insulin resistance and an exaggerated pressor response to exercise, particularly under ischemic conditions50. Since an excessive rise in BP in response to physical activity is known to be an independent risk factor for the development of adverse cardiovascular events 10, 11, dissection of the mechanisms underlying the abnormal cardiovascular responses to exercise in individuals with T2DM or insulin resistance may lead to the development of new therapies aimed at improving long term health outcomes. The results of the current study suggest that the TRPV1 receptor in skeletal muscle afferents could be associated with the exaggerated BP and sympathetic nerve responses to metaboreflex activation in T2DM. Moreover, previous studies suggest that TRPV1-blockade in T2DM may improve blood glucose control and insulin resistance51. Thus, the receptor may also be a target for treating hyperglycemia in diabetes via pharmacological therapy. Since these possibilities are based on findings from animal studies, extensive research in the human diebetic population will be required to demonstrate that targeted TRPV1 therapy is clinically safe and effective.
Supplementary Material
Novelty and Significance.
What is NEW?
Cardiovascular and sympathetic responses to TRPV1 stimulation are exaggerated in type 2 diabetic rats.
Neural functional responses to activation of TRPV1 are potentiated in group IV muscle afferents of type 2 diabetic rats.
The expression of phosphorylated-TRPV1 and PKCα protein are increased in sensory neurons of type 2 diabetic rats.
What is relevant?
The study findings implicate a role for TRPV1-induced neuronal sensitization in mediating the abnormally high blood pressure response to exercise in type 2 diabetes mellitus.
Summary
In early-stage type 2 diabetes with hyperglycemia, the function/activation of TRPV1 was enhanced in group IV muscle afferent fibers. These peripheral changes may contribute importantly to the potentiated circulatory response to exercise in this disease.
ACKNOWLEDGEMENTS
We thank Martha Romero and Julius Lamar, Jr. for their expert technical assistance.
SOURCES OF FUNDING
This research was supported by grants from the Southwestern School of Health Professions Interdisciplinary Research Grant Program (to M.M.), the Lawson & Rogers Lacy Research Fund in Cardiovascular Disease (to J.H.M.) the National Institutes of Health Heart, Lung and Blood Institute (R01HL-133179 to S.A.S. and W.V.; R01HL-151632 to M. M.), the UT Southwestern O’Brien Kidney Research Core Center (P30DK079328 to W.V.) and the JSPS KAKENHI (JP17K01769 to N.H.).
Abbreviations:
- AGE
advanced glycation end products
- BP
blood pressure
- EPR
exercise pressor reflex
- DRG
dorsal root ganglion
- EDL
extensor digitorum longus
- ELISA
enzyme-linked immunosorbent assay
- HMGB1
high mobility group box 1
- HOMA-IR
homeostatic model assessment for insulin resistance
- MAP
mean arterial pressure
- PKC
protein kinase C
- RAGE
receptor for advanced glycation end products
- RSNA
renal sympathetic nerve activity
- SNA
sympathetic nerve activity
- T2DM
type 2 diabetes mellitus
- TRPV1
transient receptor potential vanilloid 1
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Marwick TH, Hordern MD, Miller T, Chyun DA, Bertoni AG, Blumenthal RS, Philippides G, Rocchini A. Exercise training for type 2 diabetes mellitus: Impact on cardiovascular risk: A scientific statement from the american heart association. Circulation. 2009;119:3244–3262 [DOI] [PubMed] [Google Scholar]
- 2.Wing RR, Epstein LH, Paternostro-Bayles M, Kriska A, Nowalk MP, Gooding W. Exercise in a behavioural weight control programme for obese patients with type 2 (non-insulin-dependent) diabetes. Diabetologia. 1988;31:902–909 [DOI] [PubMed] [Google Scholar]
- 3.Winnick JJ, Sherman WM, Habash DL, Stout MB, Failla ML, Belury MA, Schuster DP. Short-term aerobic exercise training in obese humans with type 2 diabetes mellitus improves whole-body insulin sensitivity through gains in peripheral, not hepatic insulin sensitivity. The Journal of Clinical Endocrinology & Metabolism. 2008;93:771–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brett SE, Ritter JM, Chowienczyk PJ. Diastolic blood pressure changes during exercise positively correlate with serum cholesterol and insulin resistance. Circulation. 2000;101:611–615 [DOI] [PubMed] [Google Scholar]
- 5.Huot M, Arsenault BJ, Gaudreault V, Poirier P, Perusse L, Tremblay A, Bouchard C, Despres JP, Rheaume C. Insulin resistance, low cardiorespiratory fitness, and increased exercise blood pressure: Contribution of abdominal obesity. Hypertension. 2011;58:1036–1042 [DOI] [PubMed] [Google Scholar]
- 6.Papavasileiou MV, Thomopoulos C, Antoniou I, Papadimitriou G, Seferou M, Makris TK. Impaired glucose metabolism and the exaggerated blood pressure response to exercise treadmill testing in normotensive patients. J Clin Hypertens (Greenwich). 2009;11:627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott JA, Coombes JS, Prins JB, Leano RL, Marwick TH, Sharman JE. Patients with type 2 diabetes have exaggerated brachial and central exercise blood pressure: Relation to left ventricular relative wall thickness. Am J Hypertens. 2008;21:715–721 [DOI] [PubMed] [Google Scholar]
- 8.Holwerda SW, Restaino RM, Manrique C, Lastra G, Fisher JP, Fadel PJ. Augmented pressor and sympathetic responses to skeletal muscle metaboreflex activation in type 2 diabetes patients. Am J Physiol Heart Circ Physiol. 2016;310:H300–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrofsky JS, Stewart B, Patterson C, Cole M, Al Malty A, Lee S. Cardiovascular responses and endurance during isometric exercise in patients with type 2 diabetes compared to control subjects. Med Sci Monit. 2005;11:CR470–477 [PubMed] [Google Scholar]
- 10.Hoberg E, Schuler G, Kunze B, Obermoser AL, Hauer K, Mautner HP, Schlierf G, Kubler W. Silent myocardial ischemia as a potential link between lack of premonitoring symptoms and increased risk of cardiac arrest during physical stress. Am J Cardiol. 1990;65:583–589 [DOI] [PubMed] [Google Scholar]
- 11.Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of myocardial infarction onset study investigators. N Engl J Med. 1993;329:1677–1683 [DOI] [PubMed] [Google Scholar]
- 12.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. The Journal of physiology. 1972;224:173–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: Its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–242 [DOI] [PubMed] [Google Scholar]
- 14.Kim H-K, Hotta N, Ishizawa R, Iwamoto GA, Vongpatanasin W, Mitchell JH, Smith SA, Mizuno M. Exaggerated pressor and sympathetic responses to stimulation of the mesencephalic locomotor region and exercise pressor reflex in type 2 diabetic rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2019;317:R270–R279 [DOI] [PubMed] [Google Scholar]
- 15.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups iii and iv afferents in cats. J Appl Physiol. 1983;55:105–112 [DOI] [PubMed] [Google Scholar]
- 16.Burchiel KJ, Russell LC, Lee RP, Sima AA. Spontaneous activity of primary afferent neurons in diabetic bb/wistar rats. A possible mechanism of chronic diabetic neuropathic pain. Diabetes. 1985;34:1210–1213 [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Levine JD. Hyper-responsivity in a subset of c-fiber nociceptors in a model of painful diabetic neuropathy in the rat. Neuroscience. 2001;102:185–192 [DOI] [PubMed] [Google Scholar]
- 18.Bedi SS, Yang Q, Crook RJ, Du J, Wu Z, Fishman HM, Grill RJ, Carlton SM, Walters ET. Chronic spontaneous activity generated in the somata of primary nociceptors is associated with pain-related behavior after spinal cord injury. J Neurosci. 2010;30:14870–14882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. The Korean journal of physiology & pharmacology : official journal of the Korean Physiological Society and the Korean Society of Pharmacology. 2014;18:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maeda T, Ozaki M, Kobayashi Y, Kiguchi N, Kishioka S. Hmgb1 as a potential therapeutic target for neuropathic pain. Journal of pharmacological sciences. 2013;123:301–305 [DOI] [PubMed] [Google Scholar]
- 21.Chattopadhyay M, Mata M, Fink DJ. Continuous delta-opioid receptor activation reduces neuronal voltage-gated sodium channel (nav1.7) levels through activation of protein kinase c in painful diabetic neuropathy. J Neurosci. 2008;28:6652–6658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meents JE, Neeb L, Reuter U. Trpv1 in migraine pathophysiology. Trends Mol Med. 2010;16:153–159 [DOI] [PubMed] [Google Scholar]
- 23.Zochodne DW. Mechanisms of diabetic neuron damage: Molecular pathways. Handbook of clinical neurology. 2014;126:379–399 [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Joseph J, Ro JY, Chung MK. Modality-specific mechanisms of protein kinase c-induced hypersensitivity of trpv1: S800 is a polymodal sensitization site. Pain. 2015;156:931–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bestall SM, Hulse RP, Blackley Z, Swift M, Ved N, Paton K, Beazley-Long N, Bates DO, Donaldson LF. Sensory neuronal sensitisation occurs through hmgb-1-rage and trpv1 in high-glucose conditions. Journal of cell science. 2018;131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin DS, Kim EH, Song KY, Hong HJ, Kong MH, Hwang SJ. Neurochemical characterization of the trpv1-positive nociceptive primary afferents innervating skeletal muscles in the rats. J Korean Neurosurg Soc. 2008;43:97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52:313–320 [DOI] [PubMed] [Google Scholar]
- 28.Magalhães DA, Kume WT, Correia FS, Queiroz TS, Allebrandt Neto EW, Santos MPD, Kawashita NH, França SA. High-fat diet and streptozotocin in the induction of type 2 diabetes mellitus: A new proposal. An Acad Bras Cienc. 2019;91:e20180314. [DOI] [PubMed] [Google Scholar]
- 29.Mizuno M, Murphy MN, Mitchell JH, Smith SA. Antagonism of the trpv1 receptor partially corrects muscle metaboreflex overactivity in spontaneously hypertensive rats. J Physiol. 2011;589:6191–6204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith SA, Williams MA, Mitchell JH, Mammen PP, Garry MG. The capsaicin-sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation. 2005;111:2056–2065 [DOI] [PubMed] [Google Scholar]
- 31.Mizuno M, Mitchell JH, Crawford S, Huang CL, Maalouf N, Hu MC, Moe OW, Smith SA, Vongpatanasin W. High dietary phosphate intake induces hypertension and augments exercise pressor reflex function in rats. Am J Physiol Regul Integr Comp Physiol. 2016;311:R39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. The Journal of physiology. 2001;537:961–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taguchi T, Sato J, Mizumura K. Augmented mechanical response of muscle thin-fiber sensory receptors recorded from rat muscle-nerve preparations in vitro after eccentric contraction. Journal of neurophysiology. 2005;94:2822–2831 [DOI] [PubMed] [Google Scholar]
- 34.Grotle AK, Crawford CK, Huo Y, Ybarbo KM, Harrison ML, Graham J, Stanhope KL, Havel PJ, Fadel PJ, Stone AJ. Exaggerated cardiovascular responses to muscle contraction and tendon stretch in ucd type-2 diabetes mellitus rats. American journal of physiology. Heart and circulatory physiology. 2019;317:H479–h486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phan TX, Ton HT, Gulyás H, Pórszász R, Tóth A, Russo R, Kay MW, Sahibzada N, Ahern GP. Trpv1 expressed throughout the arterial circulation enables inflammatory vasoconstriction. The Journal of physiology. 2020;598:5639–5659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Q, Garry MG. A murine model of the exercise pressor reflex. The Journal of physiology. 2020; 598 (15): 3155–3171. [DOI] [PubMed] [Google Scholar]
- 37.Smith SA, Leal AK, Williams MA, Murphy MN, Mitchell JH, Garry MG. The trpv1 receptor is a mediator of the exercise pressor reflex in rats. The Journal of physiology. 2010;588:1179–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ducrocq GP, Estrada JA, Kim JS, Kaufman MP. Blocking the transient receptor potential vanilloid-1 does not reduce the exercise pressor reflex in healthy rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2019;317:R576–r587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caterina MJ, Julius D. The vanilloid receptor: A molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517 [DOI] [PubMed] [Google Scholar]
- 40.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543 [DOI] [PubMed] [Google Scholar]
- 41.Molliver DC, Immke DC, Fierro L, Paré M, Rice FL, McCleskey EW. Asic3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain. 2005;1:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hotta N, Kubo A, Mizumura K. Effect of protons on the mechanical response of rat muscle nociceptive fibers and neurons in vitro. Neuroscience research. 2015;92:46–52 [DOI] [PubMed] [Google Scholar]
- 43.Hong S, Wiley JW. Early painful diabetic neuropathy is associated with differential changes in the expression and function of vanilloid receptor 1. J Biol Chem. 2005;280:618–627 [DOI] [PubMed] [Google Scholar]
- 44.Ishizawa R, Kim H-K, Hotta N, Iwamoto GA, Vongpatanasin W, Mitchell JH, Smith SA, Mizuno M. Skeletal muscle reflex-induced dysregulation of sympathetic nerve activity and sensitization of muscle afferents in type 1 diabetic rats. Hypertension. 2020; 75: 1072–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Buren JJ, Bhat S, Rotello R, Pauza ME, Premkumar LS. Sensitization and translocation of trpv1 by insulin and igf-i. Mol Pain. 2005;1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnes MJ, Lapanowski K, Conley A, Rafols JA, Jen KLC, Dunbar JC. High fat feeding is associated with increased blood pressure, sympathetic nerve activity and hypothalamic mu opioid receptors. Brain Research Bulletin. 2003;61:511–519 [DOI] [PubMed] [Google Scholar]
- 47.Wang HJ, Li YL, Gao L, Zucker IH, Wang W. Alteration in skeletal muscle afferents in rats with chronic heart failure. J Physiol. 2010;588:5033–5047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xing J, Lu J, Li J. Contribution of nerve growth factor to augmented trpv1 responses of muscle sensory neurons by femoral artery occlusion. American journal of physiology. Heart and circulatory physiology. 2009;296:H1380–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824 [DOI] [PubMed] [Google Scholar]
- 50.Hotta N, Hori A, Okamura Y, Baba R, Watanabe H, Sugawara J, Vongpatanasin W, Wang J, Kim HK, Ishizawa R, Iwamoto GA, Mitchell JH, Smith SA, Mizuno M. Insulin resistance is associated with an exaggerated blood pressure response to ischemic rhythmic handgrip exercise in non-diabetic older adults. J Appl Physiol (1985). 2020; 129: 144–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gram DX, Holst JJ, Szallasi A. Trpv1: A potential therapeutic target in type 2 diabetes and comorbidities? Trends Mol Med. 2017;23:1002–1013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.