Abstract
The effect of age on autonomic nervous system was assessed at rest and while standing using systolic blood pressure (SBP) and diastolic blood pressure (DBP), heart rate, and power spectral analysis of the time duration between 2 consecutive R waves of an electrocardiogram (RR) interval variability, as well as on plasma norepinephrine and epinephrine levels in mild to moderate hypertensive patients (DBP, 90–110 mm Hg). Patients younger than 60 years (n=57) and older than 60 years (n=32), were evaluated after a 3‐ to 4‐week placebo period. Plasma catecholamines were measured in the supine position at rest and after 10 minutes of standing. Power spectral analysis of the RR interval variability was performed in each condition using the high‐frequency (HF) band (0.15–0.4 Hz) as an index of parasympathetic activity and the low‐frequency (LF) band (0.05–0.15 Hz) and LF‐HF ratio to estimate sympathetic activity. The total power was calculated as the sum of LF and HF power. supine SBP was significantly higher in older patients (P<.05). SBP and DBP increased significantly only in younger patients during standing (P<.05), while the changes were smaller and nonsignificantly lower in older patients. HR was similar in both groups at rest and increased similarly during standing. Norepinephrine and epinephrine levels were similar at rest and increased similarly in both groups of patients during standing. At rest, lower LF and HF components were observed in older patients. The LF component increased less and the HF component decreased less in older patients during standing. A lower sympathetic and parasympathetic basal cardiac tone was observed at rest in older hypertensive patients. Moreover, reduced hemodynamic and sympathetic responses to standing as assessed by SBP, DBP, and the LF component of HR variability were observed in older hypertensives in the presence of a normal catecholamine response. These observations could reflect a decreased sensitivity of cardiac β‐adrenoceptors with aging.
Both age and sex influence autonomic control of the cardiovascular system. 1 , 2 Epidemiologic studies have reported a relationship between aging and increasing blood pressure. 3 , 4 , 5 The physiologic changes in the cardiovascular system and in its autonomic regulatory mechanisms could explain the rise of blood pressure with age. Several studies have reported a decrease in baroreceptor sensitivity with aging in both normotensive and hypertensive persons. 6 , 7 , 8 It has been postulated that a reduced baroreflex sensitivity could result from anatomic changes in baroreceptors or from decreased arterial wall compliance secondary to aging, as well as from increased sympathetic nerve activity. 6 , 9 Heart rate (HR) variability, analyzed by spectral powers, is now a generally accepted marker of autonomic function in hypertensive patients, specifically for cardiac autonomic tone, displaying individually reproducible circadian variations. We used the time duration between 2 consecutive R waves of an electrocardiogram (RR) interval for the analysis. Several studies have used the evaluation of the beat‐to‐beat variability of RR intervals to assess the alterations in autonomic functions and their influence on the risk of cardiovascular disease and death. 10 A progressive reduction in beat‐to‐beat HR variability has been reported with age. 10 , 11 Moreover, attenuation in the activity of both sympathetic and parasympathetic components has also been observed with age. 12 , 13 There is little information in the literature about the effect of aging on cardiac autonomic activity assessed by HR variability in hypertensive patients, however.
The aim of this study was to evaluate autonomic nervous system (ANS) activity at rest and after standing using hemodynamic and autonomic parameters such as systolic blood pressure (SBP), diastolic blood pressure (DBP), HR, plasma norepinephrine (NE) and epinephrine (E) levels, as well as spectral power analysis of RR interval variability in young (younger than 60 years) and older (older than 60 years) patients with mild to moderate untreated hypertension and a sitting DBP between 90 and 110 mm Hg.
METHODS
Participants
The effects of age on ANS basal activity and reactivity to standing were studied in 65 men and 24 women by assessing SBP, DBP, HR, plasma NE and E, and RR interval variability in the frequency domain. Participants were selected in the present study on the basis of specific inclusion and exclusion criteria. Healthy patients with mild to moderate hypertension aged between 18 and 74 years inclusively were studied. The hypertensive patients who participated in the present protocol were recruited for various other studies. Previously treated patients stopped their current medication and undertook, along with previously untreated patients, a period of placebo treatment for 3 to 4 weeks. At the end of the placebo period, patients were required to have a clinic sitting DBP level >90 mm Hg and <110 mm Hg to be eligible for study enrollment. Secondary causes of hypertension were excluded by history, physical examination, and laboratory test results. Patients with known cardiac events in the past 12 months, insulin dependent diabetes, a history of drug or alcohol abuse, neurologic or psychiatric illnesses, or liver diseases were excluded. All participants gave informed written consent approved by the ethic committees of both hospitals. This study was performed under Drugs Directorate Guidelines for the Conduct of Clinical Investigations of Canada and according to the Declaration of Helsinki.
Study Design
After completing the placebo period, patients who qualified for study inclusion were evaluated in the clinical research units of either Sacré‐Coeur Hospital or of Hôtel‐Dieu Hospital or of the Institut de Recherches Cliniques de Montreal in the morning at 8 am± 1 hour. The ANS was evaluated by the measurement of plasma catecholamine levels and analysis of RR interval variability. Hemodynamic parameters were noninvasively monitored during resting in the supine position for 20 minutes and during 10 minutes of active standing, using a Holter monitoring device (Holter 6600, read with Burdick version 3.0; Deerfield, WI). Blood samples for the determination of plasma catecholamines levels were obtained at rest and after 10 minutes of active standing. The measurements were made using a high performance liquid chromatographic technique. The measurements of hormones were carried out blinded by a third‐party technician.
Biochemistry
Catecholamines. Twenty minutes after positioning an intravenous line in the antecubital vein with a 20‐gauge needle, blood samples were taken after 20 minutes of rest and after 10 minutes of standing. Venous blood was drawn into prechilled tubes containing sodium heparin 143 USP/10 mL for catecholamines and tubes containing ethylenediaminetetraacetic acid (K3) 15% (0.057 mL, 0.34 M) for drug levels. The samples were vortexed and centrifuged immediately at 4°C for 20 minutes at 3000 rpm. Plasma samples (2 mL) for catecholamine assay were stored at −80°C in tubes containing 40 µL of a preservative solution composed of ethylenediaminetetraacetic acid (0.950 g) and glutathione (0.600 g) in 10 mL of water adjusted to pH 7.0. All plasma samples were stored at −80°C until assayed. For the determination of catecholamines by high‐performance liquid chromatography, a modification of the method of Remie and Zaagsma 14 as described by Hjemdahl, 12 was used as previously reported by de Champlain and colleagues 4 The detection limit was 10 pg/mL for NE and E. The recovery in plasma was of 98% for NE and 95% for E, and the interassay variability was 4% for NE and 6% for E.
Autonomic and Hemodynamic Parameters. Beat‐to‐beat recording of HR was conducted with 3‐lead electrocardiography based on the X, Y, and Z signals from a modified Frank lead system, digitally recorded (Burdick, ALTIR DISC 6632R, Deerfield, WI) in the supine and upright positions. The electrocardiographic signal was digitalized at 500 samples/s/channel. For optimal spectral analysis reproducibility, the HR recording had to meet a number of conditions: ideally, the signal needed to be regular, without artifacts and important arrhythmias, and of sufficiently long duration. Usually, validated reliable software R‐wave detection and an accurate fiducial marker of each R‐wave ensure that only high quality data were submitted for spectral analysis. Segments of 256 seconds for each condition (rest, standing) were chosen for normalized power spectral analysis: during the last 10 minutes in the supine position and during the period of 10 minutes active standing. Data were analyzed using a computer program based on a fast Fourier transform algorithm. High‐frequency (HF) power (0.15–0.4 Hz) was used as an index of parasympathetic activity, and low‐frequency (LF) power (0.05–0.15 Hz) as well as the LF‐HF ratio were used as indices of sympathetic activity.
Sitting Clinic Office Blood Pressure and HR. SBP and DBP levels were measured in the sitting position, according to the World Health Organization guidelines, with a mercury sphygmomanometer (3 measurements in the sitting position at 2‐minute intervals after 5 minutes resting, followed by 1 measurement in the standing position after at least 2 minutes of equilibration).
Statistics
Results are expressed as mean ± SD. Nonpaired Student's t test was used for the analysis of the effects of age on clinic blood pressure reading, HR, catecholamine levels, and spectral analysis of RR interval variability as well as for the differences between values observed at the same times following stimulation by active standing. Statistical analyses were performed with the Statistica (edition 2000) program (StatSoft, Tulsa, OK). A P value <.05 was used for statistical significance of comparisons between the 2 groups.
RESULTS
Demographics
Fifty‐seven persons aged 18 to 60 years and 32 persons older than 60 years with mild to moderate hypertension were studied in the present protocol (Table I). There were significant differences between the 2 groups of patients with respect to duration of hypertension and weight, which were respectively longer and lower in older patients. Moreover, the clinic sitting SBP was significantly higher in older patients (P<.05), but the clinic DBP and HR did not differ between the 2 groups.
Table I.
Population Demographics
| Younger Than 60 y | Older Than 60 y | |
|---|---|---|
| No. of patients (male/female) | 57 (42/15) | 32 (23/9) |
| Age, y | 51.7±6.4 | 65.4±4.0a |
| Duration of hypertension, mo | 93.5±65.9 | 139.1±109.3a |
| Clinic sitting SBP, mm Hg | 145.4±13.1 | 155.0±12.4a |
| Clinic sitting DBP, mm Hg | 99.9±9.5 | 99.3±5.4 |
| Clinic sitting heart rate, bpm | 73.7±9.1 | 71.9±9.7 |
| Weight, kg | 85.8±16.4 | 78.9±18.4a |
| a P<.05 between groups. Abbreviations: bpm, beats per minute; DBP, diastolic blood pressure; SBP, systolic blood pressure. | ||
Blood Pressure and HR During Standing
At rest in the supine position, SBP was significantly higher in older patients (P>.05), whereas DBP levels were identical in both groups (Figure 1). Standing increased SBP and SBP levels to a slight degree in the younger patients; changes in pressure were smaller in older patients (P<.05). No significant differences in HR were observed at rest or after stimulation during active standing in either group.
Figure 1.
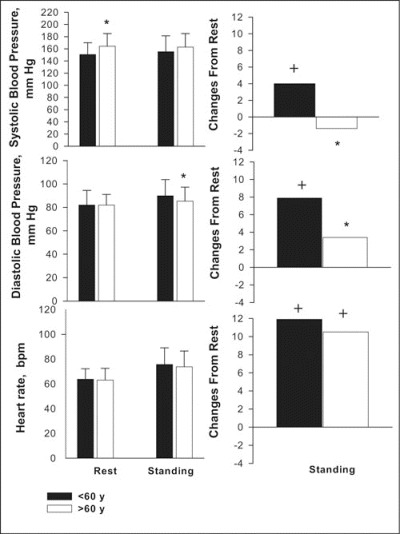
Changes in systolic and diastolic blood pressure levels (mm Hg) and HR in hypertensive patients younger than 60 years and older than 60 years at the end of the period of placebo treatment (younger than 60 years, n=57; older than 60 years, n=32), respectively, after 20 minutes of rest in the supine position and 10 minutes of standing stimulation. * indicates significance (P<.05) between groups; +, significance (P<.05) of changes from rest.
NE and E Levels During Standing
NE plasma levels tended to be higher at rest and during active standing in older hypertensive patients, but those differences were not significant (Figure 2). The significant increases in NE levels from rest to standing did not differ between groups. E plasma levels were slightly but significantly lower in older patients at rest; however, the significant increases in E levels during active standing were not significantly different between groups.
Figure 2.
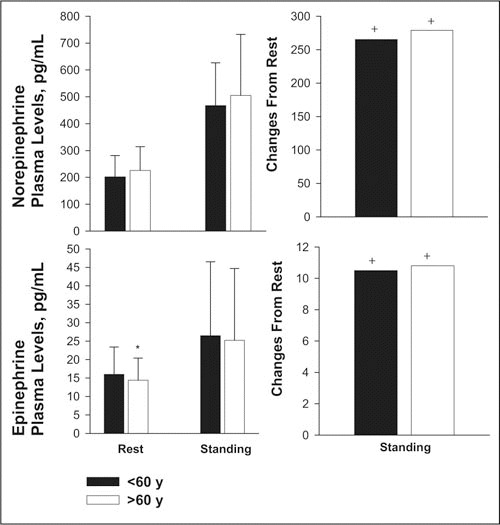
Changes in norepinephrine and epinephrine plasma levels (pg/mL) in hypertensive patients younger than 60 years and older than 60 years at the end of the period of placebo treatment (younger than 60 years, n= 57; older than 60 years, n=32,) respectively, after 20 minutes of rest in the supine position and 10 minutes of standing stimulation. * indicates significance (P<.05) between groups; +, significance (P<.05) of changes from rest.
The LF and HF Components and the LF‐HF Ratio of Power Spectra of RR Variability
The LF component was found to be significantly lower in older compared with younger hypertensive patients after 20 minutes of rest in the supine position and during active standing. The increases in the LF component from the supine position to active standing were also significantly greater in younger hypertensive patients (P>.05; Figure 3 and Figure 4).
Figure 3.
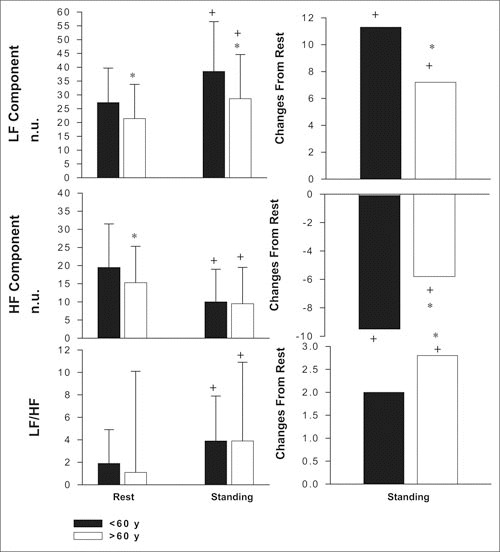
Changes in low‐frequency (LF), high‐frequency (HF), and LF‐HF ratio of power spectrum in hypertensive patients younger than 60 years (n=57) and older than 60 years (n=32) at the end of the period of placebo treatment, respectively, after 20 minutes of rest in the supine position and 10 minutes of standing stimulation. Values are given as means ± SD.* indicates significance (P<.05) between groups; +, significance (P<.05) of changes from rest; n.u., normalized units.
Figure 4.
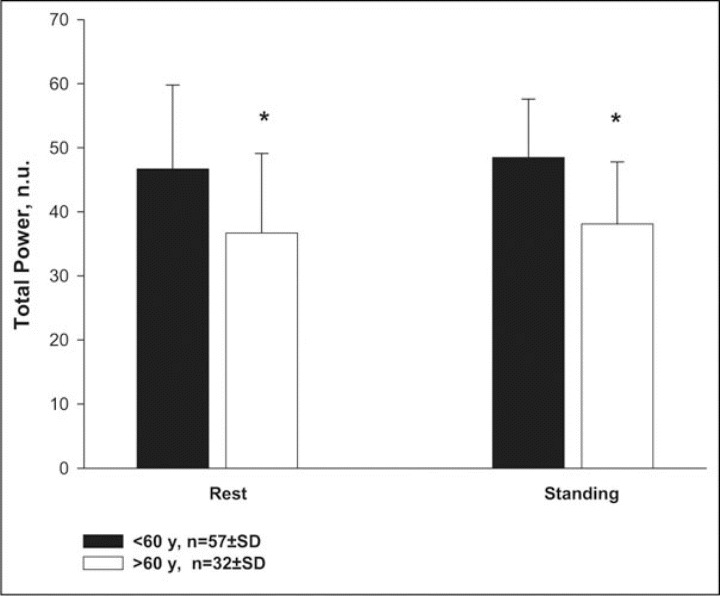
Total power of heart rate variability in hypertensive patients younger than 60 years (n=57) and older than 60 years (n=32) at the end of the period of placebo treatment, respectively, after 20 minutes of rest in the supine position and 10 minutes of standing stimulation. Values are given as means ± SD.* indicates significance (P<.05) between groups; n.u., normailized units.
The HF component was significantly lower at rest in the supine position in older hypertensive patients, but there were no differences between groups during active standing. The decrease in the HF component from rest to active standing, however, was significantly smaller in older patients (P<.05). Regardless of age, no significant difference in the ratio of LF‐HF components was observed during rest or active standing. This ratio increased significantly more in the older group during standing.
Total power was significantly higher (P<.05) in younger hypertensive patients during rest in the supine position and after active standing.
DISCUSSION
The findings reported in the present study showed significant differences in autonomic responses between younger (younger than 60 years) and older (older than 60 years) hypertensive patients. Older patients with untreated hypertension were found to have significantly higher SBP, whereas no differences were observed between groups for DBP and HR. Following active standing, the HR increased similarly in both groups, but the increases in SBP and DBP were attenuated in the older group. The autonomic control of blood pressure appears to decline with aging. Indeed, a decrease in cardiac sympathetic and parasympathetic components was observed in the older population. Decreased baroreflex sensitivity, sympathetic dysfunctions, 15 increased arterial stiffness, and a decline in arterial compliance 16 were postulated to be responsible for hemodynamic and autonomic changes. Our results are in line with the observations by Franklin and colleagues 9 in a population‐based study in which SBP was reported to increase progressively with age, but DBP rose until 60 years of age, then began to decline.
In our study, basal plasma NE and E values after standing were not significantly different between groups. Conflicting results have been reported in the literature regarding catecholamine plasma levels in older hypertensive patients. In some studies, plasma NE levels have been observed to increase with advancing age in both hypertensive and normotensive persons, 6 , 17 only in normotensive persons, 18 only in hypertensive patients, 2 or in neither group. 19 , 20 The increase in NE plasma levels with advancing age may reflect increased peripheral sympathetic activity as a consequence of the decreased peripheral responsiveness to adrenergic stimuli 21 or with an age‐related decrease in NE clearance. 22 , 23 An elevation in plasma epinephrine has also been reported in older hypertensive compared with normotensive persons, suggesting increased sympathoadrenal activity in this group. 24 Earlier investigations showed that total plasma NE spillover was greater in older healthy adults compared with young controls. 25 In contrast, the regional cardiac sympathetic responses were reported to be either similar or attenuated in older adults compared with young controls. 14 In other studies, however, as in the present study, a lack of increase in NE plasma levels with age in hypertensive patients was observed. 26
Only a few age‐related observations in patients with untreated hypertension regarding power spectral analysis of the RR interval have previously been reported. In contrast with our observation of catecholamines levels, we found that both LF and HF components (RR variability) under basal resting conditions were significantly higher in younger hypertensive patients at rest, suggesting higher sympathetic and parasympathetic tone in that population. Moreover, the changes in the LF and HF components from rest to standing were greater in younger patients after postural stimulation. The present data thus suggest a higher autonomic activity and reactivity in the younger hypertensive population during postural changes.
It has previously been reported in normotensive, healthy persons that the LF component declined linearly until the age of 62 years and then stabilized, whereas the HF component declined only between the ages of 9 to 30 years. 27 Also, in the study by Lipsitz and associates, 28 younger patients demonstrated a higher total power and LF power, but the HF component was not affected by age. Our results confirm previous observations by Yamasaki and colleagues, 29 who found that the LF and HF components declined gradually with aging in normotensive participants. Age‐related changes in autonomic function have also been reported by Finley and coworkers, 8 who found that LF and HF power as well as the LF‐HF ratio decreased in the supine position with age. Korkushko and associates 30 also reported that LF and HF power increased progressively during the first 3 decades of life, then declined thereafter; the HF component decreases more slowly than LF power. In healthy individuals, the response to orthostatic stimulation was found to be affected by age because the total power and LF power were greater in younger participants, while the HF component remained unchanged during aging. 31 The RR interval at rest and during passive tilt demonstrated a significant age dependency, with both components significantly decreasing with age. 32 In older hypertensive patients (older than 60 years), LF and HF power were reported to be significantly lower than in middle‐aged patients, 33 as in the present study. Similar findings were also presented by Yo and coworkers, 34 who reported a decline in total power in older hypertensive patients.
It was postulated that in healthy conditions, a reduction of LF power in individuals with advanced age could decrease vulnerability to coronary artery accidents, sudden death and arrhythmias, and acute cardiac events. 35 , 36 It has also been suggested that the changes in HR variability secondary to aging are associated with a new equilibrium between sympathetic and parasympathetic activities in the elderly. 33 , 37 , 38 , 39 , 40 , 41
CONCLUSIONS
Our findings suggest an attenuation of the ANS in the regulation of cardiac function in older essential hypertensive patients. Although the exact mechanism of this attenuation is not completely known, it is postulated that the effect of aging might by related to the parallel age‐related reduction in sympathetic vasomotor responsiveness 42 and reduced respiratory sinus arrhythmia. 26 In addition, our finding of a lower LF component of spectral power in older hypertensives, in the presence of a normal catecholamine response, suggests that those changes may be secondary to the age‐related decrease of cardiac β‐receptor sensibility. Indeed, our study suggests that β‐blockers may not represent ideal antihypertensive therapy in the older population of hypertensive patients. 33
Acknowledgments and disclosures:
The authors wish to express their deep gratitude to the research nursing staff of Sacré‐Coeur (J. Fouquette) and Hôtel‐Dieu Hospitals (Centre Hospitalier Universitaire de Montréal) (N. Denault, M. Bouchard) for their invaluable medical assistance and to J. LeGuerrier for her excellent technical assistance in the analysis of plasma catecholamines. Dr de Champlain is the holder of a J. C. Edwards career investigatorship in cardiovascular research. This study was supported in part by grants from the Canadian Institutes for Health Research and by the Cardiovascular Network from the Fond de Recherche en Santé du Québec.
References
- 1. Brecht HM, Schoeppe W. Relation of plasma noradrenaline to blood pressure, age, sex and sodium balance in patients with stable essential hypertension and in normotensive subjects. Clin Sci Mol Med Suppl. 1978;4:81s–83s. [DOI] [PubMed] [Google Scholar]
- 2. Chemla D, Hebert JL, Coirault C, et al. Total arterial compliance estimated by stroke volume‐to‐aortic pulse pressure ratio in humans. Am J Physiol. 1998;274:H500–H505. [DOI] [PubMed] [Google Scholar]
- 3. Cleophas TJ, Van Marum R. Age‐related decline in autonomic control of blood pressure: implications for the pharmacological management of hypertension in the elderly. Drugs Aging. 2003;20(5):313–319. [DOI] [PubMed] [Google Scholar]
- 4. De Champlain J, Karas M, Nguyen P, et al. Different effects of nifedipine and amlodipine on circulating catecholamine levels in essential hypertensive patients. J Hypertens. 1998;16:1357–1369. [PubMed] [Google Scholar]
- 5. Esler M, Skews H, Leonard P. Age‐dependence of noradrenaline kinetics in normal subjects. Clin Sci (Lond). 1981;60:217–219. [DOI] [PubMed] [Google Scholar]
- 6. Esler MD, Turner AG, Kaye DM. Aging effects on human sympathetic neuronal function. Am J Physiol. 1995;268(1, pt 2):R278–R285. [DOI] [PubMed] [Google Scholar]
- 7. Ferrari AU, Radaelli A, Centola M. Invited review: aging and the cardiovascular system. J Appl Physiol. 2003;95(6):2591–2597. [DOI] [PubMed] [Google Scholar]
- 8. Finley JP, Nugent ST, Hellenbrant W. Heart rate variability in children. Spectral analysis of developmental changes between 5 and 24 years. Can J Physiol Pharmacol. 1987;65:2048–2052. [DOI] [PubMed] [Google Scholar]
- 9. Franklin SS, Gustin W, Wong ND, et al. Hemodynamic patterns of age related changes in blood pressure. Circulation. 1997;96:308–315. [DOI] [PubMed] [Google Scholar]
- 10. Goldstein DS. Arterial baroreflex sensitivity, plasma catecholamines, and pressor responsiveness in essential hypertension. Circulation. 1983;68:234–240. [DOI] [PubMed] [Google Scholar]
- 11. Hellman JB, Stacy RW. Variation of respiratory sinus arrhythmia with age. J Appl Physiol. 1976;41(5, pt 1):734–738. [DOI] [PubMed] [Google Scholar]
- 12. Hjemdahl P. Plasma catecholamines as markers for sympathoadrenal activity in human primary hypertension. Pharmacol Toxicol. 1988;63(suppl 1):27–31. [DOI] [PubMed] [Google Scholar]
- 13. Hogikyan RV, Supiano MA. Arterial alpha‐adrenergic responsiveness is decreased and SNS activity is increased in older humans. Am J Physiol. 1994;266(5, pt 1):E717–E724. [DOI] [PubMed] [Google Scholar]
- 14. Remie R, Zaagsma J. A new technique for the study of vascular presynaptic receptors in freely moving rats. Am J Physiol. 1980;251(2, pt 2):463–467. [DOI] [PubMed] [Google Scholar]
- 15. Laitinen T, Hartikainen J, Vanninen E, et al. Age and gender dependency of baroreflex sensitivity in healthy subjects. J Appl Physiol. 1998;84(2):576–583. [DOI] [PubMed] [Google Scholar]
- 16. Lakatta EG. Catecholamines and cardiovascular function in aging. Endocrinol Metab Clin North Am. 1987;16(4):877–891. [PubMed] [Google Scholar]
- 17. Masaoka S, Lev‐Ran A, Hill LR, et al. Heart rate variability in diabetes: relationship to age and duration of the disease. Diabetes Care. 1985;8(1):64–68. [DOI] [PubMed] [Google Scholar]
- 18. Messerli FH, Frohlich ED, Suarez DZ, et al. Borderline hypertension: Relationship between age, hemodynamics and circulating catecholamines. Circulation. 1981;64:760–764. [DOI] [PubMed] [Google Scholar]
- 19. Messerli FH. The age factor in hypertension. Hosp Pract (Off Ed). 1986;21(1):103–105, 109–112. [DOI] [PubMed] [Google Scholar]
- 20. Odemuyiwa O, Farrel TG, Malick M, et al. Influence of age on the relation between heart rate variability, left ventricular ejection fraction, frequency of ventricular extrasystoles, and sudden death after myocardial infarction. Br Heart J. 1992;67(5):387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Malley K, Docherty JR, Kelly JG. Adrenoceptor status and cardiovascular function in aging. J Hypertens Suppl. 1988;6(1):S59–S62. [PubMed] [Google Scholar]
- 22. Pagani M, Lombardi F, Guzzetti S, et al. Power spectral density of heart rate variability as an index of sympathovagal interaction in normal and hypertensive subjects. J Hypertens Suppl. 1984;2(3):S383–S385. [PubMed] [Google Scholar]
- 23. Piccirillo G, Fimognari FL, Viola E, et al. Age‐adjusted normal confidence intervals for heart rate variability in healthy subjects during head‐up tilt. Int J Cardiol. 1995;50:117–124. [DOI] [PubMed] [Google Scholar]
- 24. Piccirillo G, Bucca C, Bauco C, et al. Power spectral analysis of heart rate in subjects over a hundered years old. Int J Cardiol. 1998;63(1):53–61. [DOI] [PubMed] [Google Scholar]
- 25. Ryan SM, Goldberger AL, Pincus SM, et al. Gender‐and age‐related differences in heart rate dynamics: are women more complex than men? J Am Coll Cardiol. 1994;24(7):1700–1707. [DOI] [PubMed] [Google Scholar]
- 26. Shannon DC, Carley DW, Benson H. Aging of modulation of heart rate. Am J Physiol. 1987;253(22):H874–H877. [DOI] [PubMed] [Google Scholar]
- 27. Smith WM. Epidemiology of hypertension. Med Clin North Am. 1977;61:467–486. [DOI] [PubMed] [Google Scholar]
- 28. Lipsitz LA, Mietus J, Moody GB. Spectral characteristics of heart rate variability before and during postural tilt: relation to aging and risk of syncope. Circulation. 1990;81:1803–1810. [DOI] [PubMed] [Google Scholar]
- 29. Yamasaki Y, Kodama M, Matsuhisa M. Diurnal heart rate variability in healthy subjects: effects of aging and sex difference. Am J Physiol. 1996;271:H303–H310. [DOI] [PubMed] [Google Scholar]
- 30. Korkushko OV, Shatilo VB, Plachinda YUI. Autonomic control of cardiac chronotropic function in man as a function of age: Assessment by power spectral analysis of heart rate variability. J Auton Nerv Syst. 1991;32:191–198. [DOI] [PubMed] [Google Scholar]
- 31. Tuck ML, Stern N, Sowers JR. Enhanced 24‐hour norepinephrine and renin secretion in young patient with essential hypertension:relation with circadian pattern of arterial blood pressure. Am J Cardiol. 1985;55(1):112–115. [DOI] [PubMed] [Google Scholar]
- 32. Tsuji H, Larson MG, Venditti FJ Jr, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94(11):2850–2855. [DOI] [PubMed] [Google Scholar]
- 33. Lindholm LH, Carlberg B, Samuelsson O. Should ß‐blockers remain first choice in the treatment of primary hypertension? A meta‐analysis. Lancet. 2005;366:1545–1553. [DOI] [PubMed] [Google Scholar]
- 34. Yo Y, Nagano M, Nagano N, et al. Effects of age and hypertension on autonomic nervous regulation during passive head‐up tilt. Hypertension. 1994;23(suppl 1):I82–I86. [DOI] [PubMed] [Google Scholar]
- 35. Sowers JR, Zawada EF. Hypertension in the aged. In: Zawada ET Jr, Sica DA, eds. Geriatric Nephrology and Urology. Littleton, MA: PSG Publishing Co.;1985:265–281. [Google Scholar]
- 36. Stern N, Beahm E, McGinty D, et al. Dissociation of 24‐hour catecholamine levels from blood pressure in older men. Hypertension. 1985;7(6, pt 1):1023–1029. [DOI] [PubMed] [Google Scholar]
- 37. Veith RC, Featherstone JA, Linares OA, et al. Age differences in plasma norepinephrine kinetics in humans. J Gerontol. 1986;41:319–324. [DOI] [PubMed] [Google Scholar]
- 38. Kohara K, Igase M, Maguchi M, et al. Autonomic nervous function in essential hypertension in the elderly. Evaluation by power spectral analysis of heart rate variability. Am J Hypertens. 1996;9(11):1084–1089. [DOI] [PubMed] [Google Scholar]
- 39. Yamada Y, Miyaijima E, Tochikubo O, et al. Age‐related changes in muscle sympathetic nerve activity in essential hypertension. Hypertension. 1989;13(6, pt 2):870–877. [DOI] [PubMed] [Google Scholar]
- 40. Khan N, McAlister FA. Re‐examining the efficacy of β‐blockers for the treatment of hypertension: a meta‐analysis. CMAJ. 2006;174(12):1737–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Izzo JL Jr. Hypertension in the elderly: a pathophysiologic approach to therapy. J Am Geriatr Soc. 1982;30:352–359. [DOI] [PubMed] [Google Scholar]
- 42. Uchino BN, Holt‐Lunstad J, Boor LE, et al. Aging and cardiovascular reactivity to stress: longitudinal evidence for changes in stress reactivity. Psychol Aging. 2005;20(1):134–143. [DOI] [PubMed] [Google Scholar]


