Abstract
Despite advances in the prevention and treatment of hypertension over the past decade, hypertension remains an important public health challenge. Recent efforts to reduce the prevalence of hypertension have focused on nonpharmacologic means, specifically diet. An increased intake of minerals such as potassium, magnesium, and calcium by dietary means has been shown in some but not all studies to reduce blood pressure in patients with hypertension. This review will discuss the roles of potassium, magnesium, and calcium in the prevention and treatment of essential hypertension with specific emphasis on clinical trial evidence, mechanism of action, and recommendations for dietary intake of these minerals. A high intake of these minerals through increased consumption of fruits and vegetables may improve blood pressure levels and reduce coronary heart disease and stroke.
J Clin Hypertens (Greenwich). 2008;10(7 suppl 2):2–11.
Hypertension remains the leading cause of cardiovascular disease (CVD), affecting approximately 1 billion individuals worldwide. 1 Although more than 72 million Americans, or nearly 1 in 3 adults, are estimated to have hypertension, blood pressure (BP) control is achieved in only 35% 2 ; recent surveys estimate that a higher percentage of patients have BP levels reduced to <140/90 mm Hg. Nearly 90% of adults will probably develop hypertension, especially systolic elevations, by age 65. 3 Hypertension is associated with an increased risk of mortality and morbidity from stroke, coronary heart disease, heart failure, and end‐stage renal disease. A major focus of research remains the poor control rates in hypertensive individuals. Evidence suggests that goals set by the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) are attainable in a high percentage of cases. 4 , 5 Poor BP control is even more of a challenge in patients with diabetes and chronic kidney disease due to the lower recommended BP goals. 6 Hypertension remains the most common reason for visits to physicians' offices and the primary reason for prescription drug use.
DIET IN THE PREVENTION AND TREATMENT OF HYPERTENSION
Several epidemiologic studies suggest that diet plays an important role in determining BP. Dietary therapies known to lower BP include weight loss, reduced sodium intake, increased potassium intake, and a diet rich in fruits and vegetables. 7 , 8 , 9 The land‐mark Dietary Approaches to Stop Hypertension (DASH) trial, 10 , 11 in which patients received all of their food preprepared, demonstrated that modification of diet significantly lowered BP in patients with stage 1 hypertension and high‐normal BP. the DASH diet, which emphasizes fruits, vegetables, and low‐fat dairy products, also lowers BP in persons with isolated systolic hypertension. 12 recognizing the importance of diet, several guidelines have been developed by both national and international organizations incorporating recommendations for increased nutrient intake for hypertension prevention. 13 , 14 , 15 , 16 , 17 these guidelines will be discussed in detail.
DASH EATING PLAN
The DASH study 10 was a controlled feeding study of 11 weeks' duration designed to assess the effects of modifying whole diets on BP. the DASH trial demonstrated that a diet rich in fruit and vegetables and low‐fat dairy products with reduced saturated and total fat can result in a clinically significant reduction in BP compared with the typical american diet. 18 the reduction in BP began within 2 weeks of feeding and was maintained for the following 6 weeks. among normotensive individuals, this diet reduced systolic BP and diastolic BP by 3.5 and 2.1 mm hg, respectively (figure 1). a subgroup analysis of the DASH trial also found that the BP‐lowering effects were more pronounced in hypertensive (11.4 and 5.5 mm hg, respectively) and black patients. 19 although potassium intake was increased by 1447 to 2776 mg/d through increased consumption of fruit and vegetables, reductions in BP cannot be attributed to potassium alone, as the diet was also rich in calcium, magnesium, and other nutrients.
Figure 1.
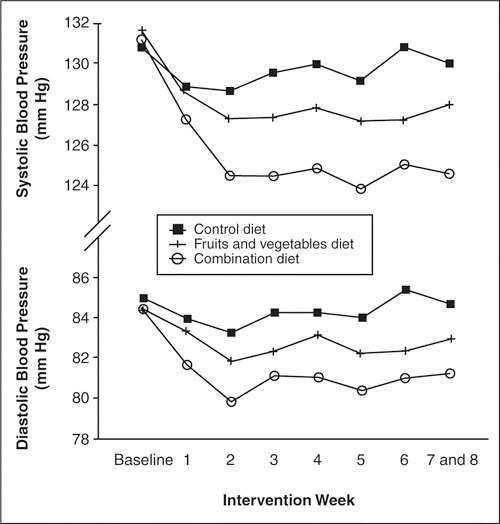
Mean systolic and diastolic blood pressure at baseline and during each intervention week, according to diet, for 379 patients with complete sets of weekly blood pressure measurements. Reprinted with permission from Appel et al. 10
In a subgroup analysis of patients with isolated systolic hypertension, 12 the DASH diet was found to be as effective as initial treatment of isolated systolic hypertension. Svetkey and coworkers 3 further demonstrated that in patients with stage 1 hypertension, a marked reduced sodium intake in combination with the DASH diet improved BP control still further (figure 2). among DASH‐Sodium trial participants, sustained reductions in BP were observed over a 1‐year period despite a gradual increase in sodium intake. 20
Figure 2.
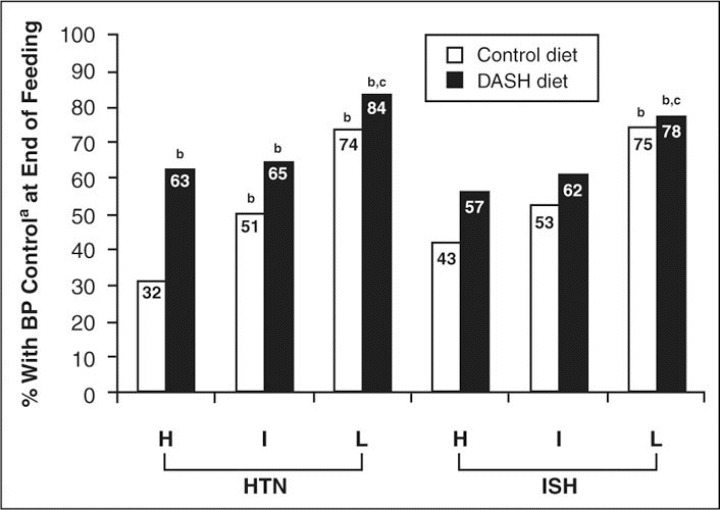
End of feeding blood pressure (BP) control rates (%) by diet and sodium level for Dietary Approaches to Stop Hypertension (DASH)‐ Sodium trial participants who had hypertension (HTN) at baseline (systolic BP >140 mm Hg or diastolic BP >90 mm Hg) and in those who had isolated systolic HTN (ISH) at baseline (systolic BP >140 mm Hg and diastolic BP <90 mm Hg). H indicates higher sodium level (142 mmol/d); I, intermediate sodium level (107 mmol/d); L, lower sodium level (65 mmol/d); aBP control was defined as systolic BP <140 mm Hg and diastolic BP <90 mm Hg. bP<.01 compared with control diet at higher sodium level; cP<.05 compared with DASH diet at higher sodium level. Reprinted with permission from Svetkey et al. 3
POTASSIUM IN THE PREVENTION OF HYPERTENSION
Cardioprotective effects of a relatively high potassium intake have been hypothesized as a basis for low CVD rates in populations consuming primitive diets and in vegetarians in industrialized countries. 21 in isolated societies consuming diets high in fruits and vegetables, hypertension affects only 1% of the population, whereas in industrialized countries that consume diets high in processed foods and large amounts of dietary sodium, 1 in 3 persons have hypertension. 22 In primitive diets, the daily intake of potassium is higher, while that of sodium is lower than in the modern diet. The rates of intake for primitive cultures range from 20 to 40 mmol/d for sodium and from 150 to 290 mmol/d for potassium. In comparison, the daily rates of intake of potassium and sodium for members of industrialized societies consuming large amounts of processed foods are 80 to 250 mmol/d for sodium and 30 to 70 mmol/d for potassium. In the modern Western diet, therefore, the potassium‐to‐sodium intake ratio on a molar basis is usually <0.4, whereas in primitive cultures the intake ratio is >3 and closer to 10. 7
Evidence of Benefits of Potassium on BP
A consistent body of evidence from observational studies 8 , 23 , 24 and clinical trials 25 , 26 , 27 , 28 indicates that high levels of potassium are associated with lower BP. One population study in St Lucia suggested that an increase of only 20 to 30 mmol/d (742–1173 mg/d) of potassium in the diet could result in a 2‐ to 3‐mm Hg reduction of BP in a population. 29 The Yanomamo Indians in Brazil, who consume very little sodium and follow mostly a vegetarian diet, are also known for having low average BP and no hypertension. Of course, they are extremely active, with little obesity. As part of the INTERSALT study, this population was found to have a very low urinary sodium excretion (0.9 mmol/d), a mean systolic BP of 94.5 mm Hg, and a mean diastolic BP of 61.4 mm Hg. 30 The INTERSALT study 31 also provided evidence that potassium intake (as measured by 24‐hour urinary potassium excretion) is an important determinant of population BP, independent of that of sodium. While most intervention studies have focused on high levels of potassium intake, observational studies indicate that even increasing potassium by 750 to 1000 mg/d can lower BP by 2 or 3 mm Hg. 8 , 23 , 24 While this may appear to be a small change for an individual, this degree of BP lowering may translate into an important cardiovascular benefit in terms of reducing stroke and other CVD events in a population. 32 , 33
Several meta‐analyses 25 , 26 , 27 , 28 report a significant reduction in BP with potassium supplementation (Table I). An earlier meta‐analysis by Cappuccio and MacGregor 25 of 19 clinical trials examining the effect of potassium supplementation on BP found that oral potassium supplements significantly lowered both systolic BP and diastolic BP (5.9 mm Hg and 3.4 mm Hg, respectively). Another meta‐analysis of 33 randomized controlled trials (RCTs)26 also documented that potassium supplementation significantly lowered BP. The BP‐lowering effect was more pronounced in blacks compared with whites and those consuming a diet high in sodium chloride. As with the previous meta‐analyses, the BP response was greater in hypertensive than normotensive patients (3.5/2.5 mm Hg vs 0.97/0.34 mm Hg, respectively), which was of borderline statistical significance. A meta‐analysis of of 40 sodium trials and 27 potasisum trials by Geleijnse et al. 27 also indicated that a reduced intake of sodium and increased intake of potassium are important contributors to the prevention of hypertension. However, a more recent meta‐analysis of 5 RCTs, conducted by Dickinson and associates, 28 reported that potassium supplementation resulted in statistically nonsignificant reductions in both systolic BP (3.9 mm Hg) and diastolic BP (1.5 mm Hg). It should be remembered that some studies with potassium supplementation were of short duration and included small numbers of participants.
Table I.
Summary of Meta‐Analyses of Potassium Trials
| Meta‐Analysis | No. of Trials | Intervention | Average Duration | Mean BP Lowering (SBP/DBP), mm Hg | 95% CI |
|---|---|---|---|---|---|
| Cappucio, 1991 25 | 19 | 100 mmol/d diet; 48–120 mmol/d KCl; 66 mmol/d K Glu +Cit | 39 d | 5.9/3.4 | −6.6 to –5.2 |
| −4.0 to 2.8 | |||||
| Whelton, 1997 26 | 33 | 100–200 mmol/d diet; 60–120 mmol/d Kcl; 120 mmol/d K Cit + Bicarb | 5 wk | 3.1/2.0 | −1.9 to –4.3 |
| −0.5 to –3.4 | |||||
| Geleijnse, 2003 27 | 27 | 44 mmol (1.7 g) (form not given) | >2 wk | 2.4/1.6 | −3.8 to –1.0 |
| −2.7 to –0.5 | |||||
| Dickinson, 2006 28 | 5 | >100 mmol/d diet; 48–120 mmol/d Kcl; 120 mmol/d K Cit + Bicarb | >8 wk | 11.2/5.0 | −25.2 to 2.7 |
| −12.5 to 2.4 | |||||
| Abbreviations: BP, blood pressure; CI, confidence interval; DBP, diastolic blood pressure; SBP, systolic blood pressure. Forms of K+ include Kcl, citrate (Cit), gluconate (Glu), and bicarbonate (Bicarb). One Meq or mmol of K+ equals 39.09 mg. | |||||
All of these meta‐analyses do reveal a dose‐response relationship between BP lowering and potassium intake. Significant BP lowering with supplemental doses of potassium in the range of 1900 to 4700 mg/d (49–122 mmol/d) has been reported to result in BP lowering of approximately 2 to 6 mm hg for diastolic BP and 2 to 4 mm hg for systolic BP. the high variability between results reflects the variability observed in different studies. in addition, the effect of potassium on BP is influenced by pretreatment BP level; age; race; sex; comorbid conditions; intake of sodium, magnesium, calcium, or other ions; diet; exercise; weight; type of potassium used; concomitant medications; and duration of use. a summary of the findings of all meta‐analyses on the effects of potassium on BP to date is given in Figure 3.
Figure 3.
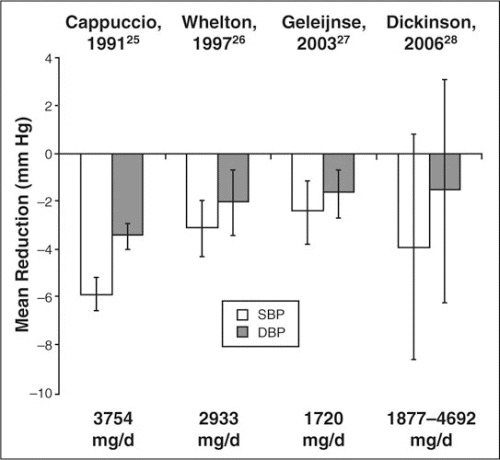
Overview of meta‐analyses of studies investigating the blood pressure‐lowering effects of potassium. 25–28 SBP indicates systolic blood pressure; DBP, diastolic blood pressure.
Although conflicting results regarding the effects of potassium supplementation on BP have been reported in clinical studies, 34 , 35 more recent trials have demonstrated results consistent with that of the meta‐analysis by Whelton and colleagues 26 (Table II). Gu and coworkers 36 found that moderate potassium supplementation (60 mmol KCl) taken for 12 weeks resulted in a reduction in systolic BP, but not diastolic BP, in a Chinese population. Similarly, Kawano and colleagues 37 documented that a 4‐week potassium supplementation period (during which 64 mmol/d of potassium was given as slow‐release KCl) resulted in small but significant reductions in office, home, and 24‐hour BP in Japanese men and women. Braschi and colleagues 38 further examined the effect of low‐dose potassium supplementation on BP and found that 24 mmol/d of slow‐release KCl administered for 6 weeks resulted in significant reductions in mean arterial pressure and diastolic BP in healthy volunteers.
Table III.
Major Dietary Sources of Potassium
| Food | Serving Size | Potassium, mg a | Sodium, mg b |
|---|---|---|---|
| Apricots | 3 medium | 281 | 1 |
| Apricots (dried) | 8 halves | 490 | 13 |
| Asparagus | 6 spears | 278 | 2 |
| Avocado | 1/2 medium | 604 | 4 |
| Banana | 1 medium | 569 | 1 |
| Beans (white, cooked) | 1/2 cup | 416 | 7 |
| Beans (green) | 1 cup | 189 | 5 |
| Broccoli | 1 stalk | 267 | 10 |
| Cantaloupe | 1/4 medium | 251 | 12 |
| Carrots | 2 small | 341 | 47 |
| Dates | 10 medium | 648 | 1 |
| Grapefruit | 1/2 medium | 135 | 1 |
| Mushrooms | 4 large | 414 | 15 |
| Orange | 1 medium | 311 | 2 |
| Orange juice | 1 cup | 496 | 3 |
| Peach | 1 medium | 202 | 1 |
| Peanuts (plain) | 2 1/2 oz | 740 | 2 |
| Potato | 1 medium | 504 | 4 |
| Prunes (dried) | 8 large | 940 | 11 |
| Raisins | 1/4 medium | 271 | 10 |
| Spinach | 1/2 cup | 291 | 45 |
| Squash (acorn) | 1/2 baked | 749 | 2 |
| Sunflower seeds | 3 1/2 oz | 920 | 30 |
| Sweet potato | 1 small | 367 | 15 |
| Tomato | 1 small | 244 | 3 |
| Watermelon | 1 slice (6 1/2 in) | 600 | 6 |
| a1000 mg=25.6 mmol. b1000 mg=44 mmol. Reprinted with permission from Moser. 47 | |||
Mechanisms by Which Potassium Lowers BP
The homeostasis of sodium and potassium plays an important role in endothelium‐dependent vasodilatation. 39 Sodium retention decreases the synthesis of nitric oxide, an arteriolar vasodilator elaborated by endothelial cells, and increases the plasma level of asymmetric dimethyl‐L‐arginine, an endogenous inhibitor of nitric oxide production. 40 a diet rich in potassium as well as increases in serum potassium, even within the physiologic range, cause endothelium‐dependent vasodilatation by hyperpolarizing the endothelial cell through stimulation of the sodium pump and opening potassium channels. 41 In addition, other proposed mechanisms by which potassium can influence BP include natriuresis, modulation of baroreceptor sensitivity, reduced vasoconstrictive sensitivity to norepinephrine and angiotensin II, increased serum and urinary kallikrein, increased sodium/potassium AT Pase activity, alteration in DNA synthesis, and proliferation in vascular smooth muscle and sympathetic nervous system cells. 42 , 43
Dietary Guidelines for Potassium
Maintaining an adequate intake of dietary potassium (>90 mmol [3500 mg]/d) has been recommended for the primary prevention of hypertension by the JNC 7.13 The Institute of Medicine has recommended a sodium intake <65 mmol/d (3.8 g/d) and an increase in potassium to 120 mmol/d. 44 In 2006, the American Heart Association issued new guidelines suggesting an increase in potassium intake to 120 mmol/d (4.7 g/d), which is the level provided in the DASH diets in which all food is supplied. 14 The Canadian Hypertension Society recommends that the daily dietary intake of potassium should be ≥60 mmol, since this intake has been associated with a reduced risk of stroke‐related mortality. 45 , 46 The most recent European Society of Hypertension guidelines also support an increased potassium intake based on the DASH diet. 18 In addition, the 2003 World Health Organization/International Society of Hypertension statement recommends a diet high in fruits and vegetables, a reduction of dietary sodium intake, and increased dietary potassium intake for reducing the incidence of hypertension. 16 Some sources of high‐potassium, low‐sodium foods are listed in Table III. 47
Table II.
Other Clinical Trial Evidence of the Effect of Potassium on Blood Pressure
| Study | No. of Patients | Intervention | Average Duration | Mean BP Lowering (SBP/DBP), mm Hg | P Value |
|---|---|---|---|---|---|
| Appel, 1997 10 | 459 | DASH diet | 8 wk | 3.5/2.1 No HTN | <.001 |
| 11.4/5.5 HTN | .003 | ||||
| <.001 | |||||
| Sacks, 2001 11 | 412 | IM Na diet + control diet/DASH diet; low Na diet + control diet/DASH diet | 30 d | 2.1/1.1 | <.05 |
| 1.3/0.6 | <.001 | ||||
| 4.6/2.4 | <.001 | ||||
| 1.7/1.0 | <.001 | ||||
| Gu, 2001 36 | 150 | 60 mmol/d KCl | 12 wk | 5.0/0.63 | <.001 |
| NS | |||||
| Kawano, 1998 37 | 64 mmol/d KCl (slow‐release) | 4 wk | 2.7/1.4 | <.05 | |
| Braschi, 2003 38 | 59 | 24 mmol/d KCl/d (slow‐release) | 6 wk | ↓MAP 7.01; 7.60/6.46 | <.001 |
| Abbreviations: BP, blood pressure; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; HTN, hypertension; IM, intermediate; MAP, mean arterial pressure; SBP, systolic blood pressure. | |||||
EFFECT OF MAGNESIUM ON BP
Epidemiologic, observational, and clinical trial data indicate that a diet high in magnesium (at least 500–1000 mg/d) may lower BP, but the results are inconsistent. 48 , 49 In most epidemiologic studies, an inverse relationship has been shown between dietary magnesium intake and BP. 42 , 50 However, data from clinical studies have been less convincing, and the therapeutic value of magnesium in the prevention and treatment of essential hypertension remains unclear. 49
Magnesium supplementation for patients with hypertension has been tested in intervention trials, but there is no clear evidence of benefit. 51 In a study by Sacks and associates, 52 magnesium was given in combination with potassium and calcium to 96 patients, but no significant effect was observed at 6 months. In the DASH trial, 10 there was an increase in urinary excretion of magnesium in study participants on the combination diet (low‐fat dairy products and fruit and vegetables), which was consistent with an increase in dietary intake of this nutrient. However, it is not clear whether the effect of the combination diet in reducing BP was related to increased magnesium intake for either the hypertensive or normotensive patients.
Meta‐analyses of magnesium supplementation have also revealed conflicting results. A review of 29 studies of magnesium was inconclusive due to flaws in methodology but suggested that a negative association of BP with magnesium was not present. 53 In contrast, a meta‐analysis of 20 RCTs (with a median intake of 15.4 mmol/d of magnesium) revealed a dose‐dependent BP reduction with magnesium supplementation. 48 However, adequately powered trials with sufficiently high doses of magnesium supplements need to be performed to confirm this relationship. A more recent meta‐analysis of 105 trials randomizing 6805 participants with at least 8 weeks of follow‐up found no evidence that magnesium supplements had any important effect on BP. 28 On the basis of the available data, guideline committees have not specifically advocated an increased magnesium intake for the treatment of elevated BP.
Mechanism by Which Magnesium Might Lower BP
Magnesium may lower BP by acting like a natural calcium channel blocker. Specifically, magnesium competes with sodium for binding sites on vascular smooth muscle cells, increases prostaglandin E, binds to potassium in a cooperative manner, and induces endothelial‐dependent vasodilation and BP reduction. 48 , 54 , 55
Magnesium is also an essential cofactor for the delta‐6‐desaturase enzyme, which is the rate‐limiting step for the conversion of linoleic acid to gamma‐linolenic acid. 56 Gamma‐linolenic acid in turn elongates to form dihomo‐gamma‐linoleic acid, the precursor for prostaglandin E1, both a vasodilator and platelet inhibitor. 57 Low magnesium states lead to insufficient amounts of prostaglandin E1, causing vasoconstriction and increased BP. 56
In addition to BP, magnesium regulates intracellular calcium, sodium, potassium, and pH as well as left ventricular mass, insulin sensitivity, and arterial compliance. 49 , 50 , 54 Research involving new imaging techniques such as P nuclear magnetic resonance and magnesium‐specific ion‐selective electrodes, which measure intracellular and extracellular free concentrations of magnesium, will further enhance our understanding of the role of magnesium in hypertension. 50 Magnesium is more effective in reducing BP when administered in a natural form as a combination of magnesium, potassium, and calcium than when given alone. 43
Recommendations for Magnesium Intake
In view of the ill‐defined role of magnesium in hypertension, magnesium supplementation at present is recommended in hypertensive patients receiving diuretic therapy who have resistant or secondary hypertension or who have frank magnesium deficiency. 49 Magnesium in hypertension is still utilized in the treatment of preeclampsia and eclampsia, but some experts have recently questioned its benefits. Despite conflicting evidence, a magnesium‐rich diet should be encouraged, particularly in communities predisposed to the development of hypertension.
CALCIUM IN THE PREVENTION OF HYPERTENSION
Population studies indicate that high intakes of calcium from the diet are linked with low BP, whereas clinical trials using calcium supplements to lower BP have been less compelling. 58 , 59 A high dietary intake of calcium has been associated with both a decrease in BP and the risk of developing hypertension. In two studies, individuals receiving >800 mg/d of calcium compared with 400 mg/d achieved a 23% reduction in risk of developing hypertension. 60 , 61 Ascherio and coworkers 23 also demonstrated in more than 30,000 normotensive male health professionals aged 40 to 75 years that men consuming <250 mg/d of calcium had a 50% greater chance of developing hypertension than those who consumed ≥400 mg/d. In a prospective cohort of 28,886 US women older than 45 years, 62 dietary intake of calcium was inversely associated with risk of hypertension; however, no change in BP was observed with calcium supplementation. Sacks and colleagues 52 reported no significant effect on BP in 94 patients randomly assigned to receive placebo or combination therapy with either calcium and magnesium or calcium and potassium for 6 months.
Calcium supplementation has not been shown to prevent an increase in BP or hypertension. In the Trial of Hypertension Prevention (TOHP), 35 445 normotensive patients were randomly assigned to receive either placebo or 1 g of calcium daily for 6 months. There was no difference in BP between the groups. In addition, TOHP‐1 tested the separate effects of micronutrients on BP in hypertensive patients with a diastolic BP of 80 to 89 mm Hg. Increases in dietary calcium and magnesium had only a small effect on BP, whereas restriction of sodium intake and weight reduction resulted in significant decreases in BP. 35
Two meta‐analyses of randomized trials reported a modest reduction in BP with calcium supplementation, but neither recommended calcium supplementation. 63 , 64 Another meta‐analysis of the effects of calcium supplements on BP reported a reduction in systolic BP and diastolic BP of 2.1 mm Hg and 1.1 mm Hg, respectively, in hypertensive patients. 65 In this meta‐analysis, calcium‐containing foods were found to be more effective in lowering BP than was calcium supplementation. A more recent meta‐analysis of 13 RCTs with between 8 and 15 weeks' follow‐up showed that participants receiving calcium supplementation had a statistically significant reduction in systolic BP but not diastolic BP when compared with a control group. 28 Due to the heterogeneity between trials, the evidence relating calcium supplementation and BP reduction is weak. As with other studies with various minerals and BP, many studies have been short in duration and have had few participants. More double‐blind placebo‐controlled trials of longer duration and better quality are needed to more accurately assess the effect of calcium supplementation on BP and cardiovascular outcomes.
Mechanisms by Which Calcium Might Lower BP
Resnick 66 has offered 2 possible mechanisms for the various responses to calcium supplementation, discussed in detail below. A reduction in calcium in the diet may cause calcium depletion from all membrane storage sites, resulting in less stability of the vascular smooth muscle cell membrane. 67 When present in optimal concentrations, calcium stabilizes vascular cell membranes, inhibits its own entry into cells, and reduces vasoconstriction. 42 Calcium works in combination with other ions such as sodium, potassium, and magnesium to provide an ionic balance to the vascular membrane, vasodilatation, and resulting reduced BP.
Recommendations for Calcium Intake
There is presently no evidence to recommend the use of calcium supplementation for cardiovascular protection other than an adequate dietary intake. 21 For normotensive persons, calcium supplementation above the recommended daily dietary intake is not recommended as a means of preventing an increase in BP. In addition, calcium supplementation above the recommended daily dietary intake is not recommended as a treatment for hypertension.
RESNICK'S IONIC HYPOTHESIS
The ionic hypothesis of hypertension and other metabolic disorders by Resnick 66 is characterized by the following: 1) increased intracellular free calcium and reduced intracellular free magnesium determine the amount of vasoconstriction or vasodilatation 66 ; 2) elevated glucose and low‐density lipoprotein cholesterol values increase intracellular calcium and/or lower intracellular magnesium in vascular smooth muscle cells 68 ; 3) hypertension, insulin resistance, and type II diabetes mellitus are associated with increased intracellular calcium and decreased intracellular magnesium, which all respond to weight loss 69 ; 4) weight loss also decreases intracellular calcium levels 70 ; 5) dietary calcium‐suppressible hormones like parathyroid hormone (PTH) and 1,25 vitamin D are vasoactive and promote calcium uptake in vascular smooth muscle cells and cardiac muscle 71 ; 6) the higher the PTH concentration, the greater the decrease in BP, and the greater the reduction in PTH and 1,25 vitamin D, the greater the BP reduction 72 ; 7) individuals with salt‐sensitive and calcium‐sensitive hypertension have elevated intracellular calcium PTH and 1,25 vitamin D, but low intracellular magnesium 73 ; 8) dietary calcium reverses abnormal calcium indices and lowers BP 74 ; 9) dietary potassium reduces urinary calcium excretion and 1,25 vitamin D plasma levels 75 ; and 10) magnesium intake reduces tissue calcium accumulation. 76
In summary, the overall effect of diet on BP is determined by the net contribution of various nutrients on cytosolic free minerals such as potassium, calcium, and magnesium. Steady‐state mineral concentrations are determined by both the direct ionic effects on glucose or calcium and the ionic effects on hormones (PTH, 1,25 vitamin D). 72 A discussion of some additional clinical effects of various minerals on BP was recently reported in The Journal of Clinical Hypertension. 77
CONCLUSIONS
Americans consume double the sodium and about half the potassium that is recommended by current guidelines. The average US dietary intake of potassium is 45 mEq/d with a potassium‐to‐sodium ratio of <1:2. 78 This is less than the recommended intake of 4700 mg/d (121 mEq/d) of potassium, with a potassium/sodium ratio of >5:1. Low potassium intake in the United States is considered a contributor to the prevalence of hypertension. 27 Therefore, if Americans were able to increase their potassium intake alone, the number of adults with known hypertension might decrease. As data from TOHP, 35 PREMIER, 79 and the Trial of Preventing Hypertension (TROPHY) 80 indicate, lifestyle changes such as weight loss, salt restriction, increased exercise, and dietary changes may prevent the progression to hypertension. The results of the DASH studies further indicate that a diet rich in fruits and vegetables is important for the prevention of hypertension and major public health problems such as coronary heart disease and stroke. An increase in potassium with a decrease in sodium is probably the most important dietary choice (after weight loss) that should be implemented to reduce CVD. Unfortunately, the population that could most benefit from increased potassium, magnesium, and calcium in the diet may have the least access to these more expensive food types (fruits and vegetables).
Disclosure:
Editorial support was funded by Unilever's Promise Institute for Heart Health Nutrition and the authors received an honorarium, funded by Unilever's Promise Institute for Heart Health Nutrition, for time and effort spent preparing this article.
References
- 1. Israili ZH, Hernandez‐Hernandez R, Valasco M. The future of antihypertensive treatment. Am J Ther. 2007;14:121–134. [DOI] [PubMed] [Google Scholar]
- 2. Rosamund W, et al. >Heart disease and stroke—2008 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Circulation. 2008;17(4):e25. [DOI] [PubMed] [Google Scholar]
- 3. Svetkey LP, Simons‐Morton DG, Proschan MA, et al. Effect of the dietary approaches to stop hypertension diet and reduced sodium intake on blood pressure control. J Clin Hypertens (Greenwich). 2004;6:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cushman WC, Basile J. Achieving blood pressure goals: why aren't we? J Clin Hypertens (Greenwich). 2006;8:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hajjar I, Kotchen JM, Kotchen TA. Hypertension: trends in prevalence, incidence, and control. Annu Rev Public Health. 2006;27:465–490. [DOI] [PubMed] [Google Scholar]
- 6. Carter BL. Implementing the new guidelines for hypertension: JNC7, ADA, WHO‐ISH. J Manag Care Pharm. 2004;10(5 suppl A):S18–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Young DB, Lin H, McCabe RD. Potassium's cardiovascular protective mechanisms. Am J Physiol. 1995;268(4, pt 2):R825–R837. [DOI] [PubMed] [Google Scholar]
- 8. INTERSALT Cooperative Research Group . INTERSALT: an international study of electrolyte excretion and blood pressure. Results for 24 hr urinary sodium and potassium excretion. BMJ. 1988;297:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elford J, Phillips A, Thomson AG, et al. Migration and geographic variations in blood pressure in Britain. BMJ. 1990;300:291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Appel LJ, Moore TH, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. Research Group . N Engl J Med. 1997;336:1117–1124. [DOI] [PubMed] [Google Scholar]
- 11. Sacks FM, Svetkey LP, Vollmer WM, et al; DASH‐Sodium Collaborative Research Group . Effects on blood pressure or reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 12. Moore TJ, Conlin PR, Ard J, et al. DASH (Dietary Approaches to Stop Hypertension) diet is effective treament for stage 1 isolated systolic hypertension. Hypertension. 2001;38:155–158. [DOI] [PubMed] [Google Scholar]
- 13. The Seventh Report of the Joint National Committeee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. [DOI] [PubMed] [Google Scholar]
- 14. Appel LJ, Brands MW, Daniels SR, et al. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47:296–308. [DOI] [PubMed] [Google Scholar]
- 15. Guidelines Committee . 2003 European Society of Hypertension‐European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–1063. [DOI] [PubMed] [Google Scholar]
- 16. Whitworth JA; World Health Organization, International Society of Hypertension Writing Group . 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21(11):1983–1992. [DOI] [PubMed] [Google Scholar]
- 17. Williams B, Poulter NR, Brown MJ, et al. British Hypertension Society guidelines for hypertension management, 2004 (BHS‐IV): Summary. BMJ. 2004;328:634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vollmer WM, Sacks FM, Svetkey LP. New insights into the effects on blood pressure of diets low in salt and high in fruits and vegetables and low‐fat dairy products. Curr Control Trials Cardiovasc Med. 2001;2:71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Svetkey LP, Simons‐Morton D, Vollmer WM, et al. Effects of Dietary Patterns on Blood Pressure‐Subgroup Analysis of the Dietary Approaches to Stop Hypertension (DASH) randomized clinical trial. Arch Intern Med. 1999;159:285–293. [DOI] [PubMed] [Google Scholar]
- 20. Ard JD, Coffman CJ, Lin PH, et al. One‐year follow‐up study of blood pressure and dietary patterns in Dietary Approaches to Stop Hypertension (DASH)‐sodium participants. Am J Hypertens. 2004;17:1156–1162. [DOI] [PubMed] [Google Scholar]
- 21. Srinath Reddy K, Katan MB. Diet, nutrition, and the prevention of hypertension and cardiovascular diseases. Public Health Nutr. 2004;7(1A):167–186. [DOI] [PubMed] [Google Scholar]
- 22. Adrogue HJ, Madias NE. Sodium and potassium in the pathogenesis of hypertension. N Engl J Med. 2007;356:1966–1978. [DOI] [PubMed] [Google Scholar]
- 23. Ascherio A, Rimm EB, Giovannucci EL, et al. A prospective study of nutritional factors and hypertension among US men. Circulation. 1992;86(5):1475–1484. [DOI] [PubMed] [Google Scholar]
- 24. Tunstall‐Pedoe H. Does dietary potassium lower blood pressure and protect against coronary heart disease and death. Findings from the Scottish Heart Health Study. Semin Nephrol. 1999;19(5):500–502. [PubMed] [Google Scholar]
- 25. Cappuccio FP, MacGregor GA. Does potassium supplementation lower blood pressure? A meta‐analysis of published trials. J Hypertens. 1991;9(5):465–473. [DOI] [PubMed] [Google Scholar]
- 26. Whelton PK, He J, Cutler JA, et al. Effects of oral potassium on blood pressure: meta‐analysis of randomized controlled clinical trials. JAMA. 1997;277:1624–1632. [DOI] [PubMed] [Google Scholar]
- 27. Geleijnse JM, Kok FJ, Grobbee DE. Blood pressure response to changes in sodium and potassium intake: a metaregression analysis of randomised trials. J Hum Hypertens. 2003;17:471–480. [DOI] [PubMed] [Google Scholar]
- 28. Dickinson HO, Nicolson DJ, Campbell F, et al. Potassium supplementation for the management of primary hypertension in adults. Cochrane Database Syst Rev. 2006;3:CD004641. [DOI] [PubMed] [Google Scholar]
- 29. Khaw KT, Rose G. Population study of blood pressure and associated factors in St Lucia, West Indies. Int J Epidemiol. 1982;11(4):372–377. [DOI] [PubMed] [Google Scholar]
- 30. Mancilha‐Carvalho JJ, Souza e Silva NA. The Yanomami Indians in the INTERSALT study. 2003. Ar Qbras Cardiol. 2003;80(3):295–300. [DOI] [PubMed] [Google Scholar]
- 31. Dyer AR, Elliott P, Shipley M. Urinary electrolyte excretion in 24 hours and blood pressure in the INTER SALT Study. II. Estimates of electrolyte‐blood pressure associations corrected for regression dilution bias. The INTERSALT Cooperative Research Group . Am J Epidemiol. 1994;139(9):940–951. [DOI] [PubMed] [Google Scholar]
- 32. Khaw KT, Barrett‐Connor E. Dietary potassium and stroke associated mortality. N Engl J Med. 1987;316:235–240. [DOI] [PubMed] [Google Scholar]
- 33. Ascherio A, Rimm EB, Hernan MA, et al. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men. Circulation. 1998;98:1198–1204. [DOI] [PubMed] [Google Scholar]
- 34. Grimm RH Jr, Neaton JD, Elmer PJ, et al. The influence of oral potassium chloride on blood pressure in hypertensive men. N Engl J Med. 1990;322:569–574. [DOI] [PubMed] [Google Scholar]
- 35. The Trials of Hypertension Prevention Collaborative Research Group . The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. JAMA. 1992;267:1213–1220. [DOI] [PubMed] [Google Scholar]
- 36. Gu D, He J, Wu X, et al. Effect of potassium supplementation on blood pressure in Chinese: a randomized, placebo‐controlled trial. J Hypertens. 2001;19:1325–1331. [DOI] [PubMed] [Google Scholar]
- 37. Kawano Y, Minami J, Takishita S, et al. Effects of potassium supplementation on office, home, and 24‐h blood pressure in patients with essential hypertension. Am J Hypertens. 1998;11(10):1141–1146. [DOI] [PubMed] [Google Scholar]
- 38. Braschi A, Naismith DJ, Braschi A. The effect of low‐dose potassium supplementation on blood pressure in apparently healthy volunteers. Br J Nutr. 2003;90(1):53–60. [DOI] [PubMed] [Google Scholar]
- 39. Panza JA, Quyyumi AA, Brush JE Jr, et al. Abnormal endothelium‐dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323(1):22–27. [DOI] [PubMed] [Google Scholar]
- 40. Fujiwara N, Osanai T, Kamada T, et al. Study on the relationship between plasma nitrite and nitrate level and salt sensitivity in human hypertension: modulation of nitric oxide synthesis by salt intake. Circulation. 2000;101(8):856–861. [DOI] [PubMed] [Google Scholar]
- 41. Haddy FJ, Vanhoutte PM, Feletou M. Role of potassium in regulating blood flow and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290(3):R546–R552. [DOI] [PubMed] [Google Scholar]
- 42. Undurti DN. Nutritional factors in the pathobiology of human essential hypertension. Nutrition. 2001;17:337–346. [DOI] [PubMed] [Google Scholar]
- 43. Preuss HG. Diet, genetics and hypertension. J Am Coll Nutr. 1997;16:296–305. [DOI] [PubMed] [Google Scholar]
- 44. Food and Nutrition Board, Institute of Medicine, National Academies of Science . Dietary Reference Intakes (DRIs): Recommended Intakes for Individuals, Elements. http://www.iom.edu/Object.File/Master/21/372/0.pdf. Accessed June 9, 2008.
- 45. Burgess E, Lewanczuk R, Bolli P, et al. Lifestyle modifications to prevent and control hypertension. 6 Recommendations on potassium, magnesium and calcium. Canadian Hypertension Society, Canadian Coalition for High Blood Pressure Prevention and Control, Laboratory Centre for Disease Control at Health Canada, Heart and Stroke Foundation of Canada . CMAJ. 1999;160(9):S35–S45. [PMC free article] [PubMed] [Google Scholar]
- 46. Khan NA, Hemmelgam B, Padwal R, et al. The 2007 Canadian Hypertension Education Program recommendations for the management of hypertension: part 2: therapy. Can J Cardiol. 2007;23:539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moser M. Clinical Management of Hypertension. 8th ed. Caddo, OK: Professional Communications, Inc.; 2008. [Google Scholar]
- 48. Jee SH, Miller ER 3rd, Guallar E, et al. The effect of magnesium supplementation on blood pressure: a meta‐analysis of randomized clinical trials. Am J Hypertens. 2002;15:691–696. [DOI] [PubMed] [Google Scholar]
- 49. Touyz RM. Role of magnesium in the pathogenesis of hypertension. Mol Aspects Med. 2003;24:107–136. [DOI] [PubMed] [Google Scholar]
- 50. Resnick LM. Magnesium in the pathophysiology and treatment of hypertension and diabetes mellitus: where are we in 1997? Am J Hypertens. 1997;10:368–370. [DOI] [PubMed] [Google Scholar]
- 51. Myers VH, Champagne CM. Nutritional effects on blood pressure. Curr Opin Lipidol. 2007;18:20–24. [DOI] [PubMed] [Google Scholar]
- 52. Sacks FM, Brown LE, Appel L, et al. Combinations of potassium, calcium, and magnesium supplements in hypertension. Hypertension. 1995;26(6, pt 1):950–956. [DOI] [PubMed] [Google Scholar]
- 53. Mizushima S, Cuppauccio FP, Nichols R, et al. Dietary magnesium intake and blood pressure: a qualitative overview of the observational studies. J Hum Hypertens. 1998;12:447–453. [DOI] [PubMed] [Google Scholar]
- 54. Bo S, Pisu E. Role of dietary magnesium in cardiovascular disease prevention, insulin sensitivity and diabetes. Curr Opin Lipidol. 2008;19:50–56. [DOI] [PubMed] [Google Scholar]
- 55. Uzui H, Lee JD. Role of magnesium in hypertension therapy. Clin Calcium. 2005;15:117–122. [PubMed] [Google Scholar]
- 56. Das UN. Essential fatty acids: biology and their clinical implications. Asia Pacific J Pharmacol. 1991;6:317. [Google Scholar]
- 57. Das UN. Nutrients, essential fatty acids and prostaglandins interact to augment immune responses and prevent genetic damage and cancer. Nutrition. 1989;5:106–110. [PubMed] [Google Scholar]
- 58. Suter PM, Sierro C, Vetter W. Nutritional factors in the control of blood pressure and hypertension. Nutr Clin Care. 2002;5:9–19. [DOI] [PubMed] [Google Scholar]
- 59. Vaskonen T. Dietary minerals and modification of cardiovascular risk factors. J Nutr Biochem. 2003;14:492–506. [DOI] [PubMed] [Google Scholar]
- 60. McCarron DA, Reusser ME. Nonpharmacologic therapy in hypertension: from single components to overall dietary management. Prog Cardiovasc Dis. 1999;41:451–460. [DOI] [PubMed] [Google Scholar]
- 61. Witteman JC, Willett WC, Stampfer MJ, et al. A prospective study of nutritional factors and hypertension among US women. Circulation. 1989;80(5):1320–1327. [DOI] [PubMed] [Google Scholar]
- 62. Wang L, Manson JE, Buring JE, et al. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle‐aged and older women. Hypertension. 2008;51:1073–1079. [DOI] [PubMed] [Google Scholar]
- 63. Bucher HC, Cook RJ, Guyatt GH, et al. Effects of dietary calcium supplementation on blood pressure. A meta‐analysis of randomized controlled trials. JAMA. 1996;275:1016–1022. [DOI] [PubMed] [Google Scholar]
- 64. Allender PS, Cutler JA, Follmann D, et al. Dietary calcium and blood pressure: a meta‐analysis of randomized clinical trials. Ann Intern Med. 1996;124:825–831. [DOI] [PubMed] [Google Scholar]
- 65. Griffith LE, Guyatt GH, Cook RJ, et al. The influence of dietary and nondietary calcium supplementation on blood pressure: an updated meta‐analysis of randomized clinical trials. Am J Hypertens. 1999;12:84–92. [DOI] [PubMed] [Google Scholar]
- 66. Resnick LM. Cellular ions in hypertension, insulin resistance, obesity, and diabetes: a unifying theme. J Am Soc Nephrol. 1992;3:S78–S85. [DOI] [PubMed] [Google Scholar]
- 67. Resnick LM. Calcium metabolism in hypertension and allied metabolic disorders. Diabetes Care. 1991;14:505–520. [DOI] [PubMed] [Google Scholar]
- 68. Resnick LM, Barbagallo M, Gupta RK, et al. Ionic basis of hypertension in diabetes mellitus: role of hyperglycemia. Am J Hypertens. 1993;6:413–417. [DOI] [PubMed] [Google Scholar]
- 69. Resnick LM, Gupta RK, Bhargava KK, et al. Cellular ions in hypertension, diabetes and obesity: a nuclear magnetic resonance spectroscopic study. Hypertension. 1991;17:951–957. [DOI] [PubMed] [Google Scholar]
- 70. Jacobs DB, Sowers JR, Hmeidan A, et al. Effects of weight reduction on cellular cation metabolism and vascular resistance. Hypertension. 1993;21:308–314. [DOI] [PubMed] [Google Scholar]
- 71. Resnick LM. Calcium‐regulating hormones and human hypertension. In: Crass MF, Avioli LV, eds. Calcium‐Regulating Hormones and Cardiovascular Function. Boca Raton, FL: CRC Press; 1995:295–320. [Google Scholar]
- 72. Resnick LM, Oparil S, Chait A, et al. Factors affecting blood pressure responses to diet: the Vanguard Study. Am J Hypertens. 2000;13:956–965. [DOI] [PubMed] [Google Scholar]
- 73. Resnick L, Müller F, Laragh J. Calcium regulating hormones in essential hypertension: relation to plasma rennin activity and sodium metabolism. Ann Intern Med. 1986;105:649–654. [DOI] [PubMed] [Google Scholar]
- 74. Lijnen P, Petrov V. Dietary calcium, blood pressure and cell membrane cation transport systems in males. J Hypertens. 1995;13:875–882. [DOI] [PubMed] [Google Scholar]
- 75. Lawton WJ, Fitz AE, Anderson EA, et al. Effect of dietary potassium on blood pressure, renal function, muscle sympathetic nerve activity, and forearm vascdular resistance and flow in normotensive and borderline hypertensive humans. Circulation. 1990;81:173–184. [DOI] [PubMed] [Google Scholar]
- 76. Zhang A, Cheng TP, Altura BM. Magnesium regulates intracellular free ionized calcium concentration and cell geometry in vascular smooth muscle cells. Biochim Biophys Acta. 1992;1134:25–29. [DOI] [PubMed] [Google Scholar]
- 77. Moser M, Franklin SS, Handler J. The nonpharmacologic treatment of hypertension: how effective is it? An update. J Clin Hypertens (Greenwich). 2007;9(3):209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Karppanen H, Karppanen P, Mervaala E. Why and how to implement sodium, potassium, calcium, and magnesium changes in food items and diets? J Hum Hypertens. 2005;19(S3):S10–S19. [DOI] [PubMed] [Google Scholar]
- 79. Elmer PJ, Obarzanck E, Vollmer WM, et al. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18‐month results of a randomized trial. Ann Intern Med. 2006;144:485–495. [DOI] [PubMed] [Google Scholar]
- 80. Julius S, Nesbitt SD, Egan RM, et al; TROPHY Study Group . Trial of preventing hypertension: Design and 2‐year progress report. Hypertension. 2004;44:146–151. [DOI] [PubMed] [Google Scholar]


