Abstract
The objective was to estimate the prevalence of plasma aldosterone concentration:plasma renin activity ratio >30 ng/dL:ng/mL/h in patients with resistant hypertension and to describe the computed tomography findings of adrenal glands in those with elevated ratios. In a cross‐sectional design, 492 patients were enrolled. All patients with plasma aldosterone concentration:plasma renin activity ratio ≥30 ng/dL:ng/mL/h (n=77) underwent abdominal computed tomography. Patients with an adrenal image of possible aldosterone‐producing adenoma underwent a saline‐loading test. The prevalence of elevated plasma aldosterone concentration:plasma renin activity ratio was 15.7% (95% confidence interval, 12.6–19.2). Twelve patients showed adrenal abnormalities on computed tomography. The level of renin was low in 50% of the sample. Results indicate a low prevalence of aldosterone‐producing adenoma. Our evidence points out the importance of confirming the hypothesis that essential hypertension, low‐renin hypertension, and idiopathic hyperaldosteronism could be the same disease, but at different neurohormonal stages, and aldosterone‐producing adenoma may be yet another disease.
Historically, primary aldosteronism (PAL) was thought to be an uncommon cause of hypertension. Aldosterone‐producing adenoma (APA) or bilateral adrenal hyperplasia accounted for a similarly small proportion (ie, <2% of all hypertension). 1 Since hiramatsu and colleagues 2 described the utility of plasma aldosterone concentration (PAC):plasma renin activity (PRA) ratio (ARR) in screening hypertensive patients for PAL, many studies demonstrated a high prevalence; Calhoun and associates 3 found a PAL prevalence of 20% in patients with resistant hypertension (RH).
In recent years, increasing evidence indicates that aldosterone affects the heart, vasculature, and kidney and that it can promote vascular remodeling, collagen formation, and endothelial dysfunction. 4
Thus, the investigation of PAL in patients with RH, defined as office blood pressure (BP) >140/90 mm Hg despite the use of ≥3 antihypertensive medications at pharmacologically effective doses, is clinically important for improving the management of these patients. The biochemical diagnosis of PAL is made by an ARR >30 and is confirmed by an aldosterone suppression test. Unfortunately, in RH, it is difficult to discontinue antihypertensive drugs to measure PAC and PRA, 5 and there is risk when performing an aldosterone suppression test with saline load or fludrocortisone, 6 mainly in patients with left ventricular hypertrophy (LVH), 7 a common problem in RH. The best way to investigate systematic PAL with accuracy and low risk in patients who have RH is not consistently defined. Dietary salt loading, which is a less dangerous test, has not yet been compared with fludrocortisones, the gold standard.
The second step in PAL is to diagnose APA, the major surgically curable subtype. The best way to make this diagnosis has not been established, because the accuracy of computed tomography (CT) is contested and adrenal vein sampling (considered the gold standard) requires a technical expertise available only in few centers and at high cost.
The objective of this study was to screen consecutive patients with RH to estimate the prevalence of ARRs >30 and to describe the CT findings in adrenal glands of those with elevated ARRs.
METHODS
Participants and Baseline Procedures
In a cross‐sectional design, 492 consecutive patients with RH were enrolled for collection of blood samples to measure ARRs. All patients were on a stable treatment regimen of at least 3 antihypertensive medications at optimal doses, and they were compliant with medicines and diet. Other causes of secondary hypertension were excluded. Obstructive sleep apnea was not evaluated.
Blood samples were obtained under standardized conditions in the morning after at least 30 minutes of resting in sitting position. All patients were advised to consume a liberal salt diet (>220 mmol of sodium by Brazilian patterns) and stopped taking β‐blockers, spironolactone, amiloride, and triamterene for 2 weeks. All other antihypertensive drugs were continued.
Verapamil slow‐release formulation could be prescribed to any patient who had discontinued β‐blockers. PAC and PRA were measured by radioimmunoassay (PAC by Coat‐a‐Count Aldosterone kit [DPC, Los Angeles, CA] and PRA by GammaCoat Plasma Renin Activity [DiaSorin, Stillwater, MN]). A measurement of serum potassium by indirect potentiometry (Roche Diagnostics, Indianapolis, IN) on the same day was performed, and if this level was <3.5 mEq/dL, the patient was scheduled to repeat blood sampling after potassium correction. All patients with ARRs =≥30 (PAC expressed in ng/100 mL, PRA in ng/mL/h) were diagnosed as suggestive of PAL and were invited to undergo abdominal CT. CT was performed in a spiral dual slice facility (Siemens, Erlange, DE). Axial 5‐mm (2 mm of increment) slices with fine cuts of 2 to 3 mm through the adrenal glands before and after administration of nonionic contrast were obtained. Patients with abdominal CT findings of possible APA had a confirmatory test with a saline‐loading test (SLT) as described by Mulatero and coworkers 6 and with suspension of spirolactone use.
All participants gave written informed consent and the local ethics committee previously approved the study protocol. The characteristics of this cohort, as well as the baseline procedures and the diagnostic definitions, were described elsewhere. 8 In brief, all hypertensive patients who fulfilled the criteria for RH (office BP >140/90 mm Hg using at least 3 antihypertensive drugs at full doses, always including a diuretic) were submitted to a standard protocol that included a thorough clinical examination, laboratory evaluation, 12‐lead electrocardiography, 24‐hour ambulatory blood pressure monitoring, and 2‐dimensional echocardiography. Compliance with antihypertensive treatment was evaluated in the first interview by a validated standard questionnaire. 9 Demographic and anthropometric characteristics, as well as cardiovascular risk factors and target organ damage information, were recorded. BP was measured twice by a trained physician, with patients in a seated position, using an Omron HEM 907 XL (Vernon Hills, IL) and a suitably sized cuff. Ambulatory blood pressure monitoring was performed using Mobil O Graph equipment (version 12, Cardios, São Paulo, Brazil), approved by the British Society of Hypertension. A reading was taken every 10 minutes throughout the day and every 20 minutes at night. Parameters evaluated included mean 24‐hour, daytime, and nighttime systolic BP (SBP), diastolic BP (DBP), pulse pressure, and nocturnal reduction of SBP/DBP.
Statistical Analysis
The prevalence and respective 95% confidence interval (CI) of elevated ARR were estimated.
Clinical and sociodemographic characteristics were analyzed through means, standard deviations, and proportions. The statistical significance of the differences between the groups with and without elevated ARRs was assessed using the chi‐squared test for proportions and the unpaired Student t‐ or Kruskal‐Wallis test for means.
RESULTS
All 492 patients fulfilled the inclusion criteria and were analyzed. There was a predominance of women in the population (72%); the prevalence of diabetes, LVH, and nephropathy was, respectively, 38.2%, 81.6%, and 38.5%.
The Figure provides a flow diagram of the investigation. The prevalence of elevated ARR was 77 of 492 (15.7%; 95% CI, 12.6–19.2). Twelve of 76 patients (15.8%) who had CT exhibited adrenal abnormalities. Only 1 among the 5 patients with a unilateral nodule image compatible with adenoma did not demonstrate suppression of aldosterone secretion after the saline infusion test.
Figure.
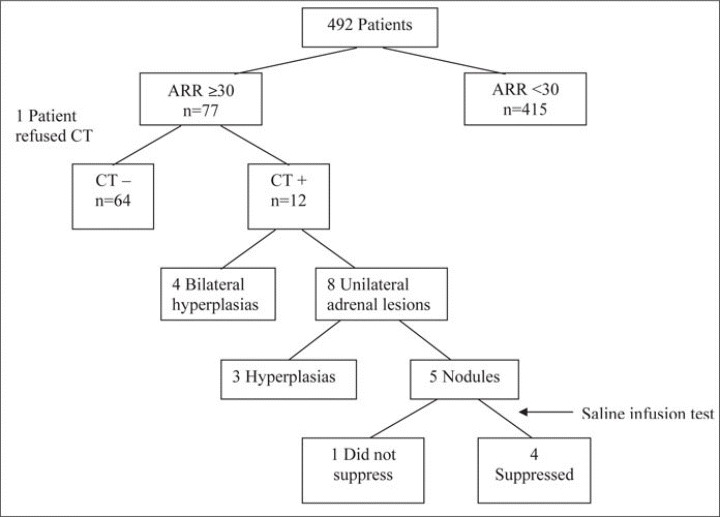
Flow diagram of primary aldosteronism investigation process. ARR indicates aldosterone/renin ratio.
Table I describes patients demographic and clinical characteristics according to ARR. BP was higher in patients with an elevated ARR than in patients with ARRs <30.
Table I.
Demographic and Clinical Characteristics of Patients According to Aldosterone/Renin Ratio (ARR)
| Characteristic | ARR ≥30 | ARR <30 | P Value |
|---|---|---|---|
| Sex, No. (M/F) | 19/58 | 119/296 | .47 |
| Age, y | 60.7 (10.7) | 60.6 (11.4) | .97 |
| BMI, kg/m2 | 29.7 (5.8) | 29.6 (6.2) | .86 |
| 24‐h SBP, mm Hg | 139.6 (17.6) | 134.7 (17.7) | .03 |
| 24‐h DBP, mm Hg | 80.3 (10.1) | 76.0 (12.3) | .004 |
| 24‐h PP, mm Hg | 59.3 (13.6) | 58.7 (12.3) | .71 |
| Dippers, % (No.) | 49.3 (38) | 54.2 (225) | .32 |
| No. of drugs | 4.0 (0.96) | 3.8 (1.0) | .14 |
| Potassium, mmol/L | 4.1 (0.59) | 4.2 (0.55) | .22 |
| Creatinine, mmol/L | 81.3 (30.5) | 99.7 (209.3) | .44 |
| Creatinine, mg/dL | 0.92 (0.35) | 1.12(2.36) | .44 |
| Aldosterone, ng/dL | 14.2 (6.3) | 11.4 (7.4) | .002 |
| Renin activity, ng/mL/h | 0.21 (0.16) | 6.9 (9.8) | .0001a |
| ARR | 129.2 (161.8) | 6.5 (7.5) | .0001a |
| Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; PP, pulse pressure; SBP, systolic blood pressure. Values are mean (SD) unless otherwise indicated. aKruskal‐Wallis test. | |||
Renin was low in 50% of the sample, with median of 1.9 ng/mL/h (0.65–6.30 as first and third quartiles). None of the patients with a high ARR had a renin value >1.0 ng/mL/h, whereas 77.6% of the patients with a low ARR had a renin value >1.0 ng/mL/h. The aldosterone level distribution is given in Table II. Only one adrenal abnormality (a nodule) on CT occurred in patients with an aldosterone level <9 ng/dL.
Table II.
Aldosterone Level According to Aldosterone/Renin Ratio (ARR)
| Aldosterone Level | ARR ≥30 | ARR <30 | Total |
|---|---|---|---|
| <9 ng/L | 22.1 (17) | 44.8 (186) | 41.3 (203) |
| 9–15 ng/L | 41.5 (32) | 28.7 (119) | 30.7 (151) |
| >15 ng/L | 36.4 (28) | 26.5 (110) | 28.0 (138) |
| Values are % (No.). | |||
DISCUSSION
This study was designed to investigate prevalence of ARRs >30 in patients with RH. The prevalence of 15.7% was similar to that found by other authors in patients without RH using the same cutoff of an ARR >30. 7 , 10 , 11
The figures in our study could be underestimated because the patients stopped taking β‐blockers, decreasing the possibility of false‐positive results, but continued taking angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, and diuretic drugs that could increase false‐negative results. However, some recent studies demonstrated a low interference of antihypertensive drugs in the ARR results, 12 and Nishizaka and coworkers 13 validated this ratio for screening for PAL in RH with patients continuing all antihypertensive drugs except spironolactone.
The patients of our two groups (ARR <30 or ARR >30) did not have any difference in age, body mass index, renal function, or serum potassium concentration. The 24‐hour SBP and DBP values were higher in the group with ARRs >30 than in the group with ARRs <30. This result demonstrates the difficulty of controlling BP in patients with elevated ARRs.
All patients in the elevated ARR group had low PRA, and the difference between the two groups was statistically significant. This point is quite important, because although the mean PAC is higher in the group with ARRs >30 than in the group with ARRs <30, the ratio was much more dependent on the denominator (PRA). The aldosterone level distribution shows that levels <9 ng/dL are more common in the group with ARRs <30, and the one patient with an adrenal nodule on CT scan with PAC <9 ng/dL demonstrates aldosterone suppression on the SLT. This result is compatible with findings in the literature 6 and points to the idea that the best screening test for PAL is a high ARR level with PAC >9 ng/dL.
CT revealed 12 adrenal abnormalities, and among these were 5 nodules compatibles with adenoma. However, only 1 nodule was not suppressed by the SLT in a patient with both ARR and PAC elevated. The prevalence of nodules by CT in this work was 6.6%, which is compatible with the figures presented at a recent review of adrenal incidentaloma. 14
Our study found only one probable APA, an adrenal unilateral nodule of 1.5 × 1.1 cm on CT that was not suppressed on the SLT. This prevalence of 1.3% is very low, mainly because it was found in a cohort of patients with RH in which the probability of PAL should be much higher, although, as far as we know, no one else has studied this issue in a cohort of patients with RH.
The SLT was performed after and not before CT because of the high prevalence of LVH and renal damage in this cohort of patients with RH. This strategy ensured that only patients with the potential benefit of surgical cure were submitted to a high‐risk procedure.
We decided not to perform adrenal vein sampling, based on important evidence that the biochemical lateralization in patients without hypokalemia is not accurate. The results from Greenslopes Hospital in Australia 15 showed that 53% of the patients operated on had their hypertension cured and 62% had it biochemically cured. Some patients who had hypertension that was biochemically cured demonstrated persistent hypertension, and others without a biochemical cure had hypertension cured. These figures point to a random result in terms of curing hypertension when a decision was based on adrenal vein sampling without anatomic image demonstration. One study showed that a better accuracy of adrenal vein sampling vs CT was retrospective, wherein 79% of the hypertensive patients investigated for PAL had hypokalemia. 16
Greenslopes and Princess Alexandra Hospitals investigated PAL in all hypertensive patients independent of BP level, performing a suppression test in all patients with ARRs >30 and lateralizing all those patients who had a diagnosis of PAL confirmed by this criterion. 7 The cost of this routine is unaffordable in many health care systems. 17 Moreover, the advantages of this strategy may be questioned, because the Greenslopes results showed that only 53% of any kind of hypertension was controlled. This level of control can be achieved with usual antihypertensive drugs, at lower cost and risk. Even in RH, Chapman and associates 18 showed that a regimen with spironolactone can be effective in controlling BP.
The low prevalence of PAL found in this and in Hood and colleagues' study 10 raises a question about the risks and benefits of screening for this condition. The ARR is much more dependent on PRA level, and evidence supports the hypothesis that essential hypertension, low‐renin hypertension, and idiopathic hyperaldosteronism could be the same disease, but at different neurohormonal stages. 19 , 20 Therefore, in patients with RH, the treatment should be individualized with a measure of PRA, and in those with low PRA levels, an aldosterone antagonist should be commenced to control BP and restore the renin level.
Disclosures:
The study was funded by a research grant from Petrobras‐Brazilian Petroleum Company and FINEP ‐Financiadora Nacional de Estudos e Projetos contract number 0104070700. Dr Nogueira and Dr Bloch have research grants from the Brazilian National Research Council‐CNPq.
References
- 1. Stewart PM. Mineralocorticoid hypertension. Lancet. 1999;353:1341–1347. [DOI] [PubMed] [Google Scholar]
- 2. Hiramatsu K, Yamada T, Jukimura Y, et al. A screening test to identify aldosterone‐producing adenoma by measuring plasma renin activity. Arch Intern Med. 1981;141:1589–1594. [PubMed] [Google Scholar]
- 3. Calhoun DA, Nishizaka MK, Zaman MA, et al. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40:892–896. [DOI] [PubMed] [Google Scholar]
- 4. Epstein M. Aldosterone blockade: an emerging strategy for abrogating progressive renal disease. Am J Med. 2006;119:912–919. [DOI] [PubMed] [Google Scholar]
- 5. Mulatero P, Dluhy RG, Giachetti G, et al. Diagnosis of primary aldosteronism: from screening to subtype differentiation. Trends Endocrinol Metab. 2005;16:114–119. [DOI] [PubMed] [Google Scholar]
- 6. Mulatero P, Milan A, Fallo F, et al. Comparison of confirmatory tests for diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 2006;91:2618–2623. [DOI] [PubMed] [Google Scholar]
- 7. Stowasser M, Gordon RD, Gunasekera TG, et al. High rate of detection of primary aldosteronism, including surgically treatable forms, after “non‐selective” screening of hypertensive patients. J Hypertens. 2003;21:2149–2157. [DOI] [PubMed] [Google Scholar]
- 8. Salles G, Leocadio S, Bloch K, et al. Combined QT interval and voltage criteria improve left ventricular hypertrophy detection in resistant hypertension. Hypertension. 2005;46:1207–1212. [DOI] [PubMed] [Google Scholar]
- 9. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care. 1986;24:67–74. [DOI] [PubMed] [Google Scholar]
- 10. Hood S, Cannon J, Foo R, et al. Prevalence of primary hyperaldosteronism assessed by aldosterone/renin ratio and spironolactone testing. Clin Med. 2005;5:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lim PO, Rodgers P, Cardale K, et al. Potentially high prevalence of primary aldosteronism in a primary‐care population. Lancet. 1999;353:40. [DOI] [PubMed] [Google Scholar]
- 12. Schwartz GL, Turner ST. Screening for primary aldosteronism in essential hypertension: diagnostic accuracy of the ratio of plasma aldosterone concentration to plasma renin activity. Clin Chem. 2005;51:386–394. [DOI] [PubMed] [Google Scholar]
- 13. Nishizaka MK, Pratt‐Ubunama M, Zaman MA, et al. Validity of plasma aldosterone‐to‐renin activity ratio in African American and white subjects with resistant hypertension. Am J Hypertens. 2005;18:805–812. [DOI] [PubMed] [Google Scholar]
- 14. Young WF Jr. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:601–610. [DOI] [PubMed] [Google Scholar]
- 15. Rutherford JC, Taylor WL, Stowasser M, et al. Succcess of surgery in primary aldosteronism judged by residual autonomous aldosterone production. World J Surg. 1998;22:1243–1245. [DOI] [PubMed] [Google Scholar]
- 16. Nwariaku FE, Miller BS, Auchus R, et al. Primary hyperaldosteronism: effect of adrenal vein sampling on surgical outcome. Arch Surg. 2006;141:497–502. [DOI] [PubMed] [Google Scholar]
- 17. Kaplan NM. The current epidemic of primary aldosteronism: cause and consequences. J Hypertens. 2004;22:863–869. [DOI] [PubMed] [Google Scholar]
- 18. Chapman N, Dobson J, Wilson S; Anglo‐Scandinavian Cardiac Outcomes Trials Investigators . Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49:839–845. [DOI] [PubMed] [Google Scholar]
- 19. Lim PO, Struthers AD, MacDonald TM. The neurohormonal natural history of essential hypertension: towards primary or tertiary aldosteronism? J Hypertens. 2002;20:11–15. [DOI] [PubMed] [Google Scholar]
- 20. Padfield PL. Prevalence and role of raised aldosterone to renin ratio in the diagnosis of primary aldosteronism: a debate on the scientific logic of the use of the ratio in practice. Clin Endocrinol (Oxf). 2003;59:422–426. [DOI] [PubMed] [Google Scholar]


