Abstract
Ambulatory 24‐hour pulse pressure predicts progression of albuminuria in persons with diabetes mellitus. The authors assessed whether nocturnal blood pressure (BP) patterns added predictive information and examined the multivariate‐adjusted association of nocturnal BP patterns with progression of urine albumin excretion during follow‐up in a multiethnic cohort of older people (n=957) with type 2 diabetes mellitus who were free of macroalbuminuria. Albuminuria was assessed by spot urine measurement of albumin‐to‐creatinine ratio at baseline and annually for 3 years. Participants were categorized according to their sleep/wake systolic BP ratio as dippers (ratio ≤0.9; n=295), nondippers (flat nocturnal pattern, ratio >0.9 to 1; n=475), and nocturnal BP risers (ratio >1; n=187). The proportion exhibiting progression of albuminuria in dippers, nondippers, and risers was 17.6%, 22.9%, and 27.3%, respectively (P for linear trend = .01). A nocturnal BP rise was independently associated with progression of albuminuria (hazard ratio, 1.68; 95% confidence interval [CI], 1.09–2.60; P=.02), whereas office pulse pressure was not. When ambulatory 24‐hour pulse pressure was added to the model, the nocturnal BP rise remained an independent predictor of progression of albuminuria (hazard ratio, 1.58; 95% CI, 1.02–2.45; P=.04). Nocturnal nondipping (without BP increase) was not an independent predictor. In conclusion, nocturnal BP rise on ambulatory monitoring is superior to office BP to predict worsening of albuminuria in elderly individuals with type 2 diabetes and adds to the information provided by 24‐hour pulse pressure.
Albuminuria is an independent predictor of cardiovascular morbidity and mortality in people with and without diabetes mellitus—an association that extends to urine albumin levels below the currently accepted, arbitrary cutoff point for microalbuminuria. 1 , 2 , 3 , 4 , 5 , 6 Moreover, an increase in urinary albumin excretion is associated with higher cardiovascular morbidity and mortality, 7 and a decrease in albuminuria by drug therapy is associated with better cardiovascular and renal outcomes. 8 Albuminuria is prevalent in older and middle‐aged people with type 2 diabetes mellitus in whom it is often present when diabetes is diagnosed 9 , 10 , 11 and in whom cardiovascular and renal complication rates are the highest. 12 , 13 , 14 Thus, it is particularly relevant to identify predictors of worsening albuminuria in older people with diabetes mellitus.
In persons with diabetes mellitus, ambulatory blood pressure monitoring (ABPM) has been shown to predict progression of albuminuria better than office blood pressure (BP). 15 , 16 , 17 , 18 Several ABPM variables appear to be of value to predict worsening of albuminuria. Pulse pressure is of particular interest in the elderly because it is a measure of increased arterial stiffness prevalent in that age group. We previously reported that 24‐hour pulse pressure is the most informative ABPM variable, outperforming office pulse pressure and ambulatory systolic BP (SBP) and diastolic BP for predicting progression of albuminuria in elderly patients with diabetes. 18 In a subsequent study, we confirmed those findings after longer follow‐up and also found that a lack of nocturnal decrease in BP (nondipping, as compared with the normal dipping pattern) did not add to the prediction of albuminuria progression provided by pulse pressure. 19 In contrast, a study in patients with type 1 diabetes mellitus reported that a nondipping pattern was associated with progression from normoalbuminuria to microalbuminuria. 17 Their analyses did not adjust for clinical characteristics, office BP, or other ambulatory BP measurements. It is possible that after considering glycemic control and office BP, a nondipping nocturnal pattern (measured at an additional cost) might not have improved the prediction of progression to microalbuminuria. In addition, a nocturnal elevation in BP has been shown to be associated with higher cardiovascular risk after adjusting for 24‐hour pulse pressure and other characteristics. 20 This raises the question as to whether a categorization of nocturnal patterns that distinguishes patients with a nighttime increase (using 3 categories: dippers, nondippers, and risers) might be more informative than the usual dichotomous classification (dippers vs nondippers). Therefore, we investigated whether nocturnal BP patterns, using an assessment that discriminates between a flat pattern and a nocturnal rise, improves the prediction of worsening of albuminuria in persons with diabetes.
METHODS
Data Collection
We studied participants enrolled in the multisite Informatics for Diabetes Education and Telemedicine (IDEATel) study, 21 which has been described in detail elsewhere. IDEATel evaluates telemedicine as a means of managing the care of older Medicare beneficiaries with diabetes who reside in medically underserved areas of New York State. Inclusion criteria in IDEATel were: age 55 years or older; being a current Medicare beneficiary; having diabetes as defined by a physician's diagnosis and being on treatment with diet, an oral hypoglycemic agent, or insulin; residence in a federally designated medically underserved area in New York State; and fluency in either English or Spanish. All IDEATel participants signed informed consent, and the protocol was approved by the institutional review boards at all participating institutions.
This study examined the relationship between data obtained at the baseline IDEATel examination, conducted during the years 2000 and 2001, and the urinary albumin content, measured by spot urine albumin‐to‐creatinine ratio, at 3 consecutive annual follow‐up visits. Prescription drug use was ascertained by interviewer‐administered questionnaire and confirmed through review of medication bottles. Medication use was successfully ascertained in 99.5% of the participants at the baseline visit. For analytic purposes, antihypertensive medications were categorized into 4 classes: angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), β‐blockers, calcium channel blockers, and diuretics (the use of other types of antihypertensive drugs was rare). Height, weight, and seated BP were measured, blood and spot urine samples were collected, and 24‐hour ABPM was performed.
Laboratory Measures
Urine albumin level was measured using the immunoprecipitin method (Diasorin, Stillwater, MN) from a random spot sample. Values <5.7 mg/dL were assigned a value of 4.0 mg/dL. Urine creatinine level was measured using the picric acid colorimetric method. Both analyses were performed using a Roche/Hitachi 717 automated analyzer (Roche Diagnostics, Indianapolis, IN). Hemoglobin A1c was analyzed by boronate affinity chromatography with the Primus CLC 385 (Primus, Kansas City, MO). Total cholesterol, triglyceride, and high‐density lipoprotein cholesterol levels were measured using enzymatic colorimetric methods (Vitros; Johnson & Johnson, New Brunswick, NJ). Biochemical analyses were performed at Penn Medical Laboratory (currently MedStar, Inc.) in Washington, DC.
Albumin‐to‐Creatinine Ratio
Albumin‐to‐creatinine ratio (ACR) (milligrams of albumin per grams of creatinine) was calculated from a morning spot urine sample. Urine albumin excretion was categorized into normoalbuminuria (ACR <17 in men and <25 in women), microalbuminuria (ACR 17–250 in men and 25–355 in women), and macroalbuminuria (ACR >250 in men and >355 in women); these thresholds are designed to identify people with urinary albumin excretion rates >30 mg/24 h and >300 mg/24 h, respectively. 22 Participants who had macroalbuminuria at baseline were excluded from this study.
Progression of albuminuria was defined as an increase in ACR to a level in a higher category, provided that it did not revert to a lower category in a subsequent measurement (ie, the worsening had to be persistent). In patients with normoalbuminuria at baseline, progression was defined as microalbuminuria or macroalbuminuria at follow‐up. In patients with microalbuminuria at baseline, progression was defined as macroalbuminuria at follow‐up. In participants with microalbuminuria at baseline, improvement in albuminuria was defined as having a measurement within a lower albuminuria category at a follow‐up visit, provided that it did not revert to a higher category in a subsequent measurement. The group with improvement in albuminuria was identified for descriptive purposes only. In all statistical analyses, this group was combined with patients without progression, to preserve statistical power. Thus, our analyses compared participants with progression of albuminuria with those without progression.
Resting BP Measurement
Resting BP was measured at the baseline and follow‐up visits by trained and certified research personnel using the Dinamap Monitor Pro 100 (Critikon, Tampa, FL) automated oscillometric device. Three measurements were obtained at 1‐minute intervals in a seated position after 5 minutes of rest in a quiet room, using a standardized protocol. 23 The average of the second and third measurements was recorded as the resting BP.
Office pulse pressure was defined as the difference between systolic and diastolic resting BP.
Ambulatory BP Monitoring
ABPM was performed at the IDEATel baseline visit using a Spacelabs 90207 oscillometric monitor (SpaceLabs, Redmond, WA) following a published protocol. 24 BP was recorded every 20 minutes for a 24‐hour period with the machine programmed to deflate in 8‐mm Hg bleed steps. Sleep and wake intervals were defined based on diary entries and confirmed by a telephone interview on the morning when monitoring ended. A minimum of 6 valid wake readings and 4 valid sleep readings were required for the computation of wake and sleep averages. A reading was accepted as valid if it was nonartifactual and within physiologic range. Our group has used this approach over the years because 6 daytime readings have been estimated to provide a reliable estimate of daytime mean BP values, and the nighttime BP is less variable than daytime BP. 25 Of note, our method is less stringent than the usual requirement of at least one valid reading per hour over the 24‐hour period 26 and may thus result in increased noise‐to‐signal ratio. Ambulatory 24‐hour pulse pressure was defined as the mean difference between all systolic and diastolic BP readings. Nocturnal dipping was defined as a ratio of mean sleep to mean wake SBP of ≤0.90 (a decrease in sleep SBP of at least 10% relative to wake SBP). Nondipping or flat nocturnal pattern was defined as a ratio >0.9 to 1.0, and a nocturnal rise was defined as a ratio >1.0. 27
Statistical Analysis
Variables that were positively skewed, including ACR, were log‐transformed for the analyses to better approximate a normal distribution. Comparisons of baseline characteristics according to category of progression of albuminuria were made using chi‐square or Fisher exact tests (when any expected cell frequency was <5) for categoric variables, Student t for continuous variables approximating a normal distribution, and the Mann‐Whitney U test for continuous variables that were not normally distributed.
The main goal of the Cox proportional hazards models was to test the independent association of a nocturnal rise in BP with progression of albumin excretion after adjustment for other baseline covariates. Office and ambulatory 24‐hour pulse pressure were selected as BP covariates for these models because we previously found them to be strong predictors of progression of albuminuria in this population. 18 , 19 We also adjusted for the following covariates, assessed at baseline, selected because of their biologically plausible association with albuminuria: age, sex, IDEATel randomization group, body mass index, baseline urinary albumin‐to‐creatinine ratio (log‐transformed), hemoglobin A1c, triglycerides, high‐density lipoprotein cholesterol, years since diagnosed with diabetes, number of antihypertensive medications, use of ACE inhibitors, and active smoking. Office pulse pressure and nocturnal BP patterns were the BP variables entered in the first model; ambulatory 24‐hour pulse pressure and nocturnal patterns were entered in the second model. Correctness of the proportional hazards assumption was verified using the Harrell and Lee modification of the Schoenfeld goodness of fit test. 28 Collinearity between BP variables was assessed by calculating the tolerance for each of them in the final model. None of the BP variables exhibited tolerance values <0.20 (ie, there was no indication of excessive collinearity). 29 All predictor variables were considered fixed at baseline (ie, none was treated as time‐dependent). The significance of interaction terms was assessed in fully adjusted models using the likelihood ratio test. Statistical analyses were performed using SPSS version 13.0 (SPSS, Chicago, IL) and SAS version 9.0 (SAS Institute Inc, Cary, NC).
RESULTS
Sampling and follow‐up in this study are summarized in Figure 1. There were 1180 IDEATel participants with complete baseline data. Of those, 223 were excluded from the analysis because they had macroalbuminuria at baseline. The mean follow‐up in the remaining 957 participants was 30.4±9 months. Among them, 212 exhibited worsening of their albuminuria. Of the 301 participants who had microalbuminuria at baseline, 94 exhibited improvement of albuminuria during follow‐up. Those participants exhibiting improvement were similar to those without a significant change in microalbuminuria during follow‐up, except that the former tended to be younger and to have a lower albumin‐to‐creatinine ratio at baseline.
Figure 1.
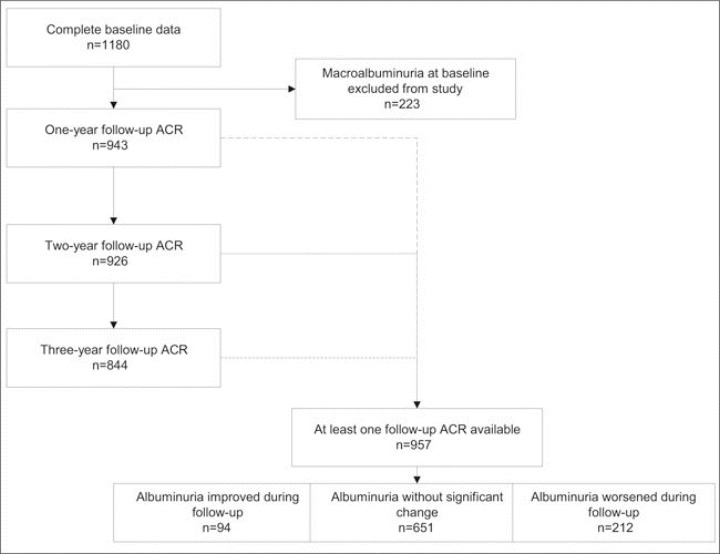
Sampling for this study. ACR indicates albumin‐to‐creatinine ratio.
Participants exhibiting progression of albuminuria were more likely to be older, to be Hispanic, to smoke (Table I), and to have higher mean levels of albumin excretion, hemoglobin A1c and 24‐hour systolic and pulse pressure, and lower body mass index. This group was also more likely to exhibit a nocturnal rise in SBP.
Table I.
Selected Baseline Characteristics of 957 Participants Categorized by Progression of Albuminuria During Follow‐Upa
| Characteristic | No Progression (n=745) | Progression (n=212) | P Value |
|---|---|---|---|
| Age, y | 70.53 | 71.83 | .009 |
| Female, % | 63 | 59 | .29 |
| Race/ethnicity, % | .036 | ||
| White | 50 | 42 | |
| Hispanic | 37 | 44 | |
| African American | 13 | 14 | |
| Randomized to telemedicine, % | 51 | 44 | .10 |
| Body mass index, kg/m2 | 31.6±6.3 | 30.4±5.9 | .01 |
| Waist circumference, cm | 106±15 | 106±14 | .37 |
| Office systolic blood pressure, mm Hg | 139.4±21.8 | 139.4±22.2 | .99 |
| Office diastolic blood pressure, mm Hg | 70.7±10.9 | 69.9±17.8 | .41 |
| Office pulse pressure, mm Hg | 68.7±17.0 | 69.4±17.8 | .61 |
| Use of ACE inhibitors/ARBs, % | 61 | 63 | .47 |
| ACR (log‐transformed), mg/g | 1.4±0.5 | 1.5±0.5 | .01 |
| Duration of diabetes, y | 10±9 | 12±9 | .005 |
| Hemoglobin A1c | 7.3±1.4 | 7.6±1.6 | .001 |
| HDL cholesterol, mg/dL | 47.9±13.9 | 45.9±12.6 | .06 |
| Triglycerides, mg/dL | 167.4±98.5 | 175.7±107.3 | .32 |
| Currently smoking, % | 6 | 11 | .017 |
| 24‐h systolic blood pressure, mm Hg | 131.4±13.5 | 133.7±14.9 | .049 |
| 24‐h diastolic blood pressure, mm Hg | 69.1±8.2 | 68.3±8.7 | .22 |
| 24‐h pulse pressure, mm Hg | 62.3±10.9 | 65.4±12.1 | .001 |
| Nocturnal blood pressure pattern | .011b | ||
| Dipping | 33 | 25 | |
| Flat (nondipping) | 49 | 51 | |
| Rising | 18 | 24 | |
| Abbreviations: ACE, angiotensin‐converting enzyme; ACR, albumin‐to‐creatinine ratio; ARBs, angiotensin receptor blockers; HDL, high‐density lipoprotein. aFrom the Informatics for Diabetes Education and Telemedicine (IDEATel) study. 21 Values are expressed as mean ± SD unless otherwise indicated. b P for linear trend. | |||
The percentages of patients who exhibited progression of albuminuria among the normal dippers, nondippers, and risers were 17.6%, 22.9%, and 27.3%, respectively (Table II; P for trend = .011). As previously reported, 19 a 24‐hour pulse pressure ≥65 mm Hg was also associated with worsening of albuminuria (P=.001). Figure 2 depicts the proportions of participants with progression of albuminuria for each category of nocturnal BP pattern, stratified by 24‐hour pulse pressure. These data suggested that nocturnal BP patterns may be most informative in the subgroup of participants with elevated 24‐hour pulse pressure. Thus, tests for a possible interaction between nocturnal patterns (both as categoric and continuous variables) and 24‐hour pulse pressure (as a continuous variable) were performed in the Cox models.
Table II.
Percentage of Patients Exhibiting Progression of Albuminuria at Follow‐Up, Within Blood Pressure Categories (n=957)a
| Blood Pressure Variable | Progression of Albuminuria, % | P Value |
|---|---|---|
| Office blood pressureb | .27 | |
| Controlled (n=342) | 23.4 | |
| Uncontrolled (n=615) | 21.5 | |
| 24‐h blood pressurec | .43 | |
| Controlled (n=139) | 23.0 | |
| Uncontrolled (n=818) | 22.0 | |
| Nocturnal pattern | .011d | |
| Dipping (n=295) | 17.6 | |
| Flat (n=475) | 22.9 | |
| Rising (n=187) | 27.3 | |
| 24‐h pulse pressure | .001 | |
| <65 mm Hg (n=570) | 18.6 | |
| ≥65 mm Hg (n=387) | 27.4 | |
| aFrom the Informatics for Diabetes Education and Telemedicine (IDEATel) study. 21 bUncontrolled office blood pressure was defined as systolic blood pressure >130 mm Hg or diastolic blood pressure >80 mm Hg. cUncontrolled 24‐hour blood pressure was defined as mean systolic blood pressure >120 mm Hg or mean diastolic blood pressure >70 mm Hg. d P for trend. | ||
Figure 2.
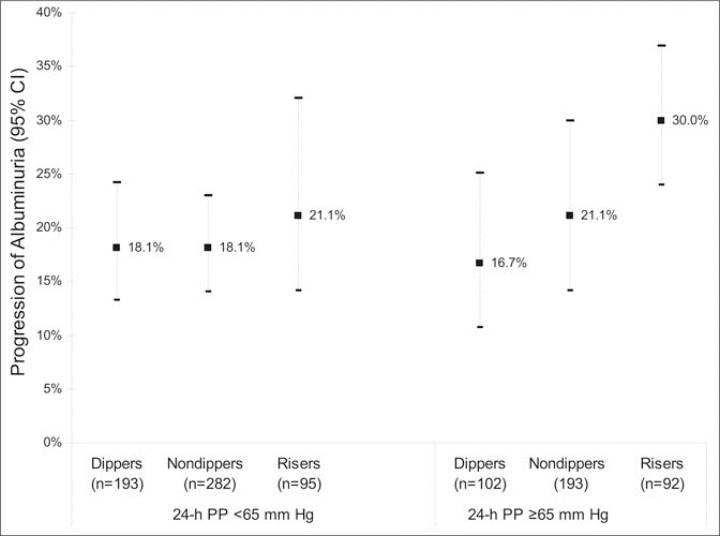
Percentages of patients (95% confidence interval [CI]) with progression of albuminuria according to category of nocturnal blood pressure pattern and stratified by 24‐hour pulse pressure (PP). The P for trend across categories of nocturnal blood pressure was .007 when 24‐hour PP was ≥65 mm Hg and .62 when 24‐hour PP was <65 mm Hg.
Cox proportional hazards models were used to test whether nocturnal BP patterns were independently predictive of the risk of progression of albuminuria. In the first model (Table III), which included office pulse pressure, nocturnal BP rise was an independent predictor of progression of albuminuria; the adjusted hazards ratio was 1.68 (95% confidence interval, 1.09 to 2.60; P=.019). Office pulse pressure was not an independent predictor in this model. The inclusion of 24‐hour mean SBP in the model did not change the results substantially, nor did adjustment for the use of diuretics. Other variables independently associated with progression of albuminuria were age, hemoglobin A1c, number of antihypertensive medications, and active smoking. Of note, BMI was not independently associated with progression of albuminuria. When the night‐to‐day SBP ratio was included in the multivariate model instead of the categoric nocturnal SBP variables, an independent log‐linear association with the progression of albuminuria was observed (P=.048 for the log10‐transformed night‐to‐day SBP ratio). In the second Cox model, which included ambulatory 24‐hour pulse pressure, nocturnal BP rise remained an independent predictor of progression of albuminuria; the hazard ratio was 1.58 (95% confidence interval, 1.02 to 2.5; P=.04). Hemoglobin A1c, 24‐hour pulse pressure, smoking, and the number of antihypertensive medications were also independently significant. There was no evidence that 24‐hour pulse pressure modified the multivariate‐adjusted association between nocturnal BP elevation and progression of albuminuria (P=.38 for the interaction term [night‐today SBP ratio × 24‐hour pulse pressure]).
Table III.
Results of Proportional Hazards Model for Progression of Albuminuria in 957 Participants Without Macroalbuminuria at Baseline
| Model 1a | Model 2b | |||
|---|---|---|---|---|
| Variable | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value |
| Age, y | 1.03 (1.0–1.05) | .048 | 1.02 (0.99–1.05) | .14 |
| Female sex | 0.83 (0.61–1.15) | .27 | 0.79 (0.57–1.09) | .14 |
| Randomization group (telemedicine) | 0.76 (0.56–1.04) | .08 | 0.77 (0.57–1.04) | .09 |
| Body mass index, kg/m2 | 0.98 (0.95–1.01) | .14 | 0.98 (0.95–1.01) | .16 |
| Baseline ACR (log‐transformed), mg/g | 1.25 (0.88–1.76) | .21 | 1.15 (0.82–1.61) | .43 |
| Hemoglobin A1c, % | 1.11 (1.01–1.22) | .035 | 1.12 (1.02–1.23) | .02 |
| HDL cholesterol, mg/dL | 0.99 (0.98–1.00) | .08 | 0.99 (0.98–1.00) | .11 |
| Triglycerides, mg/dL | 1.00 (0.99–1.00) | .43 | 1.00 (0.99–1.00) | .41 |
| Duration of diabetes, y | 1.01 (0.99–1.03) | .21 | 1.01 (0.99–1.03) | .26 |
| Use of ACE inhibitors or ARBs | 1.01 (0.73–1.57) | .73 | 1.03 (0.71–1.51) | .87 |
| Antihypertensive medications, No. | 1.19 (1.02–1.41) | .03 | 1.22 (1.04–1.44) | .02 |
| Currently smoking | 2.05 (1.25–3.35) | .004 | 2.09 (1.23–3.4) | .003 |
| Nocturnal blood pressure pattern | ||||
| Dipping | (reference) | — | (reference) | — |
| Flat | 1.37 (0.94–2.00) | .11 | 1.35 (0.92–1.97) | .13 |
| Rising | 1.68 (1.09–2.60) | .02 | 1.58 (1.02–2.45) | .04 |
| Office pulse pressure, 10 mm Hg | 1.01 (0.92–1.10) | .82 | — | — |
| 24‐h pulse pressure, 10 mm Hg | — | — | 1.19 (1.04–1.36) | .014 |
| Abbreviations: ACR, albumin‐to‐creatinine ratio; ACE, angiotensin‐converting enzyme; ARBs, angiotensin receptor blockers; CI, confidence interval; HDL, high‐density lipoprotein. aModel 1: selected characteristics, nocturnal blood pressure patterns, and office pulse pressure. bModel 2: selected characteristics, nocturnal blood pressure patterns, and 24‐hour pulse pressure. From the Informatics for Diabetes Education and Telemedicine (IDEATel) study. 21 All covariates are listed in the Table. | ||||
DISCUSSION
Our main finding was that a nocturnal rise in BP was associated with progression of albuminuria after adjusting for several covariates, including 24‐hour ambulatory pulse pressure. We believe this finding is clinically relevant because progression of albuminuria is associated with greater cardiovascular risk 7 , 8 and that progression may be slowed and in some cases even reversed with appropriate antihypertensive treatment. The role of nocturnal hypertension as a predictor of albuminuria has been described in people with type 1 diabetes. 17 In a previous study of progression of albuminuria in type 2 diabetes, however, we failed to identify nocturnal BP patterns as an independent predictor. 19 We believe that this was probably due to the fact that we used a dichotomous classification of the nocturnal BP pattern, dividing the sample into persons with a normal nocturnal BP fall (dippers) and persons without that decrease (nondippers). In doing so, we grouped together 2 subgroups with different risks: patients with a flat nocturnal pattern (a decline of <10% relative to daytime BP) and patients with a nocturnal rise. In the current study we separated the “risers” from the “nondippers.” This analytic framework resulted in an observed independent association between nocturnal BP elevation and progression of albuminuria. It has also been observed by others that collapsing several categories into one, or categorizing a continuous variable, may result in loss of information. 30 , 31
Our results have practical implications for clinicians when they perform risk stratification of their patients in regards to progression of albuminuria. In addition, our findings raise the possibility that a similar phenomenon may have occurred in studies assessing the value of ABPM to predict cardiovascular events. Two studies that examined nocturnal patterns as dipping/no dipping did not find an association with cardiovascular risk, 32 , 33 whereas a study that assessed the nocturnal rise did report a significant association. 20 In an analysis nested within the Systolic Hypertension in Europe Trial, Staessen and colleagues 20 found that the night‐today BP ratio added significantly to 24‐hour SBP to predict major cardiovascular events.
Several approaches to antihypertensive drug treatment, including the addition of a diuretic, the use of extended‐release formulations, and nightly dosing, have been shown to lower nocturnal BP in nondippers, bringing their circadian cycles closer to a normal pattern, 34 , 35 , 36 , 37 , 38 , 39 and to reduce the severity of proteinuria in some patients. 38 Unfortunately, our observational design, with its nonrandomized antihypertensive treatment assignment, precluded an unbiased assessment of the role different medication classes, formulations, and dosing schedules may play in the association between nocturnal BP and the progression of albuminuria in this population. 40 For example, it is apparent that the number of antihypertensive medications was associated with greater risk of progression of albuminuria in this study because patients with more severe hypertension were prescribed more drugs, and not due to a deleterious effect of the drugs themselves. For the same reason, ACE inhibitors and ARBs did not appear to have a protective effect against albuminuria in our analyses.
It remains to be determined whether interventions that lower nocturnal BP will decrease cardiovascular risk in people with diabetes, particularly after the 24‐hour pulse pressure has already been lowered. This hypothesis should be assessed by properly designed randomized clinical trials, which should include follow‐up with serial ABPM studies.
This study has several limitations. First, we measured albumin urinary excretion using a single spot urine sample. Whereas a 24‐hour urine collection, or 3 measurements instead of 1, may provide a more accurate measurement of renal albumin excretion, assessment of albuminuria in a spot urine sample has been accepted as valid and may be the only feasible alternative in large studies. 41 , 42 , 43 , 44 Moreover, there is no reason to expect that misclassification of albuminuria caused by our measurement procedure would be differential with respect to the predictors. Second, our sample was composed of older patients, with long‐standing diabetes, prevalent end‐organ damage, and advanced cardiovascular stiffness at the time of the evaluation. Thus, our findings may not generalize to younger patients, and particularly to patients with type 1 diabetes. Third, like all observational studies, ours is subject to the risk of residual confounding due to poorly measured or unmeasured confounders. For example, certain predictors of progression of albuminuria, such as chronic excessive use of nonsteroidal anti‐inflammatory agents and high protein diets, were not measured in this sample. Finally, the IDEATel baseline examination did not include an assessment of renal function, such as a serum creatinine measurement. Patients with advanced renal failure were excluded from enrollment, but we do not know whether the addition of a serum creatinine measurement would have substantially changed our results.
The strengths of this study include a longitudinal design and a large sample that was well characterized, elderly, and multiethnic and had adequate representation of women. Detailed information regarding pertinent covariates, including a medication inventory and comprehensive laboratory and anthropometric measurements, was available. ABPM was performed using a well‐validated methodology. 45
In conclusion, a nocturnal rise in SBP recorded by 24‐hour ambulatory monitoring was associated with higher risk of progression of albuminuria in this cohort of elderly people with type 2 diabetes. This association was statistically significant after adjustment for 24‐hour ambulatory pulse pressure and other baseline covariates.
Disclosure:
This research was supported by Cooperative Agreement 95‐C‐90998 from the Centers for Medicare and Medicaid Services.
References
- 1. MacLeod JM, Lutale J, Marshall SM. Albumin excretion and vascular deaths in NIDDM. Diabetologia. 1995;38:610–616. [DOI] [PubMed] [Google Scholar]
- 2. Rossing P, Hougaard P, Borch‐Johnsen K, et al. Predictors of mortality in insulin dependent diabetes: 10 year observational follow up study. BMJ. 1996;313:779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ljungman S, Wikstrand J, Hartford M, et al. Urinary albumin excretion‐a predictor of risk of cardiovascular disease. A prospective 10‐year follow‐up of middle‐aged non‐diabetic normal and hypertensive men. Am J Hypertens. 1996;9:770–778. [DOI] [PubMed] [Google Scholar]
- 4. Donnelly R, Yeung JM, Manning G. Microalbuminuria: a common, independent cardiovascular risk factor, especially but not exclusively in type 2 diabetes. J Hypertens Suppl. 2003;21(suppl 1):S7–S12. [PubMed] [Google Scholar]
- 5. Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. [DOI] [PubMed] [Google Scholar]
- 6. Wachtell K, Ibsen H, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Ann Intern Med. 2003;139:901–906. [DOI] [PubMed] [Google Scholar]
- 7. Spoelstra‐de Man AM, Brouwer CB, Stehouwer CD, et al. Rapid progression of albumin excretion is an independent predictor of cardiovascular mortality in patients with type 2 diabetes and microalbuminuria. Diabetes Care. 2001;24:2097–2101. [DOI] [PubMed] [Google Scholar]
- 8. Shahinfar S, Dickson TZ, Ahmed T, et al. Losartan in patients with type 2 diabetes and proteinuria: observations from the RENAAL Study. Kidney Int Suppl. 2002;82:64–67. [DOI] [PubMed] [Google Scholar]
- 9. Fabre J, Balant LP, Dayer PG, et al. The kidney in maturity onset diabetes mellitus: a clinical study of 510 patients. Kidney Int. 1982;21:730–738. [DOI] [PubMed] [Google Scholar]
- 10. Klein R, Klein BE, Moss SE. Prevalence of microalbuminuria in older‐onset diabetes. Diabetes Care. 1993;16:1325–1330. [DOI] [PubMed] [Google Scholar]
- 11. Olivarius Nde F, Andreasen AH, Keiding N, et al. Epidemiology of renal involvement in newly‐diagnosed middle‐aged and elderly diabetic patients. Cross‐sectional data from the population‐based study “Diabetes Care in General Practice,” Denmark. Diabetologia. 1993;36:1007–1016. [DOI] [PubMed] [Google Scholar]
- 12. Fillenbaum GG, Pieper CF, Cohen HJ, et al. Comorbidity of five chronic health conditions in elderly community residents: determinants and impact on mortality. J Gerontol A Biol Sci Med Sci. 2000;55:M84–M89. [DOI] [PubMed] [Google Scholar]
- 13. Garcia MJ, McNamara PM, Gordon T, et al. Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow‐up study. Diabetes. 1974;23:105–111. [DOI] [PubMed] [Google Scholar]
- 14. Lee CD, Folsom AR, Pankow JS, et al. Cardiovascular events in diabetic and nondiabetic adults with or without history of myocardial infarction. Circulation. 2004;109:855–860. [DOI] [PubMed] [Google Scholar]
- 15. Nielsen S, Schmitz A, Poulsen PL, et al. Albuminuria and 24‐h ambulatory blood pressure in normoalbuminuric and microalbuminuric NIDDM patients. A longitudinal study. Diabetes Care. 1995;18:1434–1441. [DOI] [PubMed] [Google Scholar]
- 16. Poulsen PL, Hansen KW, Mogensen CE. Ambulatory blood pressure in the transition from normo‐ to microalbuminuria. A longitudinal study in IDDM patients. Diabetes. 1994;43:1248–1253. [DOI] [PubMed] [Google Scholar]
- 17. Lurbe E, Redon J, Kesani A, et al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med. 2002;347:797–805. [DOI] [PubMed] [Google Scholar]
- 18. Palmas W, Moran A, Pickering T, et al. Ambulatory pulse pressure and progression of urinary albumin excretion in older patients with type 2 diabetes mellitus. Hypertension. 2006;48:301–308. [DOI] [PubMed] [Google Scholar]
- 19. Palmas W, Pickering T, Eimicke J, et al. Value of ambulatory arterial stiffness index and 24‐hour pulse pressure to predict progression of albuminuria in elderly people with diabetes mellitus. Am J Hypertens. 2007;20:493–500. [DOI] [PubMed] [Google Scholar]
- 20. Staessen JA, Thijs L, O'Brien ET, et al. Ambulatory pulse pressure as predictor of outcome in older patients with systolic hypertension. Am J Hypertens. 2002;15:835–843. [DOI] [PubMed] [Google Scholar]
- 21. Shea S, Starren J, Weinstock RS, et al. Columbia University's Informatics for Diabetes Education and Telemedicine (IDEATel) Project: rationale and design. J Am Med Inform Assoc. 2002;9:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 suppl 1):S1–S266. [PubMed] [Google Scholar]
- 23. Bild DE, Bluemke DA, Burke GL, et al. Multi‐ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 24. Pickering TG. Ambulatory Blood Pressure. Redmond, WA: Spacelabs Medical; 1994. [Google Scholar]
- 25. Llabre MM, Ironson GH, Spitzer SB, et al. How many blood pressure measurements are enough? An application of generalizability theory to the study of blood pressure reliability. Psychophysiology. 1988;25:97–106. [DOI] [PubMed] [Google Scholar]
- 26. Di Iorio A, Marini E, Lupinetti M, et al. Blood pressure rhythm and prevalence of vascular events in hypertensive subjects. Age Ageing. 1999;28:23–28. [DOI] [PubMed] [Google Scholar]
- 27. Roman MJ, Pickering TG, Schwartz JE, et al. Is the absence of a normal nocturnal fall in blood pressure (nondipping) associated with cardiovascular target organ damage? J Hypertens. 1997;15:969–978. [DOI] [PubMed] [Google Scholar]
- 28. Kleinbaum DG, Klein M. Survival Analysis. 2nd ed. New York, NY: Springer; 2005. [Google Scholar]
- 29. Hedblad B, Ogren M, Engstrom G, et al. Heterogeneity of cardiovascular risk among smokers is related to degree of carbon monoxide exposure. Atherosclerosis. 2005;179:177–183. [DOI] [PubMed] [Google Scholar]
- 30. Streiner DL. Breaking up is hard to do: the heartbreak of dichotomizing continuous data. Can J Psychiatry. 2002;47:262–266. [DOI] [PubMed] [Google Scholar]
- 31. MacCallum RC, Zhang S, Preacher KJ, et al. On the practice of dichotomization of quantitative variables. Psychol Methods. 2002;7:19–40. [DOI] [PubMed] [Google Scholar]
- 32. Khattar RS, Swales JD, Banfield A, et al. Prediction of coronary and cerebrovascular morbidity and mortality by direct continuous ambulatory blood pressure monitoring in essential hypertension. Circulation. 1999;100:1071–1076. [DOI] [PubMed] [Google Scholar]
- 33. Nakano S, Ito T, Furuya K, et al. Ambulatory blood pressure level rather than dipper/nondipper status predicts vascular events in type 2 diabetic subjects. Hypertens Res. 2004;27:647–656. [DOI] [PubMed] [Google Scholar]
- 34. Hermida RC, Calvo C, Ayala DE, et al. Administration time‐dependent effects of valsartan on ambulatory blood pressure in elderly hypertensive subjects. Chronobiol Int. 2005;22:755–776. [DOI] [PubMed] [Google Scholar]
- 35. Kario K, Schwartz JE, Pickering TG. Changes of nocturnal blood pressure dipping status in hypertensives by nighttime dosing of alpha‐adrenergic blocker, doxazosin: results from the HALT study. Hypertension. 2000;35:787–794. [DOI] [PubMed] [Google Scholar]
- 36. Qiu YG, Zhu JH, Tao QM, et al. Captopril administered at night restores the diurnal blood pressure rhythm in adequately controlled, nondipping hypertensives. Cardiovasc Drugs Ther. 2005;19:189–195. [DOI] [PubMed] [Google Scholar]
- 37. White WB, Mehrotra DV, Black HR, et al. Effects of controlled‐onset extended‐release verapamil on nocturnal blood pressure (dippers versus nondippers). COER‐Verapamil Study Group . Am J Cardiol. 1997;80:469–474. [DOI] [PubMed] [Google Scholar]
- 38. Uzu T, Harada T, Namba T, et al. Thiazide diuretics enhance nocturnal blood pressure fall and reduce proteinuria in immunoglobulin A nephropathy treated with angiotensin II modulators. J Hypertens. 2005;23:861–865. [DOI] [PubMed] [Google Scholar]
- 39. Uzu T, Kimura G. Diuretics shift circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1999;100:1635–1638. [DOI] [PubMed] [Google Scholar]
- 40. Psaty BM, Koepsell TD, Lin D, et al. Assessment and control for confounding by indication in observational studies. J Am Geriatr Soc. 1999;47:749–754. [DOI] [PubMed] [Google Scholar]
- 41. Nelson RG, Knowler WC, Pettitt DJ, et al. Assessment of risk of overt nephropathy in diabetic patients from albumin excretion in untimed urine specimens. Arch Intern Med. 1991;151:1761–1765. [PubMed] [Google Scholar]
- 42. Gatling W, Knight C, Mullee MA, et al. Microalbuminuria in diabetes: a population study of the prevalence and an assessment of three screening tests. Diabet Med. 1988;5:343–347. [DOI] [PubMed] [Google Scholar]
- 43. Hutchison AS, O'Reilly DS, MacCuish AC. Albumin excretion rate, albumin concentration, and albumin/creatinine ratio compared for screening diabetics for slight albuminuria. Clin Chem. 1988;34:2019–2021. [PubMed] [Google Scholar]
- 44. Chaiken RL, Khawaja R, Bard M, et al. Utility of untimed urinary albumin measurements in assessing albuminuria in black NIDDM subjects. Diabetes Care. 1997;20:709–713. [DOI] [PubMed] [Google Scholar]
- 45. Pang TC, Brown MA. Accuracy of ambulatory blood pressure monitors in routine clinical practice. Am J Hypertens. 2006;19:801–809. [DOI] [PubMed] [Google Scholar]


