Abstract
The aim of this study was to estimate the cost‐effectiveness of renin‐angiotensin‐aldosterone system blockers in patients with diabetic nephropathy. A cost‐effectiveness analysis was performed based on a meta‐analysis of studies investigating the effect of angiotensin‐converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) as part of a treatment regimen on the incidence of end‐stage renal disease (ESRD) in patients with diabetic nephropathy. The primary outcome was the cost to prevent 1 patient from developing ESRD. Cost analysis was performed from a third‐party payer perspective in 2006 US dollars. As part of a treatment regimen, ARBs significantly reduced the incidence of ESRD and doubling of serum creatinine concentration (P<.05) but not total mortality. The cost to prevent 1 patient from developing ESRD was $31,729 (95% confidence interval, $19,443–$85,442; P<.01), $189,190 (P=.13) and $51,585 (P=.068) for patients receiving ARBs, ACE inhibitors, or either of them, respectively. This study demonstrates that blocking the RAAS, which delays the progression to ESRD, appears to be cost‐effective. The current analysis favors ARBs in terms of cost‐effectiveness.
Diabetic nephropathy is the leading cause of end‐stage renal disease (ESRD), accounting for about 30% of new cases in the European Union 1 and more than 40% in the United States. 2 The incidence of ESRD is increasing, and by 2004 some 1,783,000 persons worldwide were estimated to be on treatment for ESRD. 3 Because ESRD is associated with a substantial clinical and economic burden that significantly impacts health care systems, expenditures on ESRD will increase and facilities that deliver health care for ESRD may be overburdened. In the United States, ESRD costs are projected to rise from $18.5 billion in 2004 (6.7% of the total Medicare budget) to about $28.3 billion in 2010. 2 , 4
In Greece, there has also been an increase in the frequency of both diabetes and ESRD. 5 , 6 The incidence of ESRD has doubled over the past decade and was estimated at 195 new patients per million of the general population in 2004, one of the highest rates in Europe. 1 In 2003, the total economic burden of renal replacement therapy (RRT) in the Greek social insurance system accounted for about 1.03% of social insurance expenditures. 7
The pathogenesis of diabetic nephropathy is related to chronic hyperglycemia, and the mechanisms by which the latter leads to ESRD include hypertension; complex interactions of growth factors, angiotensin II, and endothelin; hemodynamic alterations in the renal microcirculation; and structural changes in the glomerulus. 8 Agents that block the renin‐angiotensin‐aldosterone system (RAAS) have been shown to interfere with many of the above factors and may delay the progression to ESRD. 8 , 9 Pharmacologic blockade of the RAAS is related to additional costs for the health care system; these need to be balanced against treatment costs for ESRD. Economic evaluations of angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) based on single clinical trials have recently been performed, 7 , 10 , 11 , 12 , 13 suggesting that treatment of patients with diabetic nephropathy with these agents results in substantial cost savings. The overall cost benefit of treatment with RAAS blockers, based on a systematic review of the effects of these drugs on ESRD incidence in diabetic nephropathy, has not been estimated, however. Moreover, because of the huge structural differences in health care systems, the costs associated with ESRD cannot be extrapolated a priori and unconditionally to all countries; separate cost‐effectiveness analyses are necessary. 14 This is particularly important in Greece, which has a distinct public health care system, a complex hybrid of “Beveridge” and “Bismark” types, with fragmented funding and delivery and unreasonably high expenditures. 15 , 16
The primary aim of the current study was to estimate the cost‐effectiveness of RAAS‐blocking agents in patients with diabetic nephropathy in Greece, based on a meta‐analysis of the relevant studies. Secondary objectives include estimating the cost savings per patient receiving RAAS blocker and investigating the applicability of the results to US findings.
METHODS
A cost‐effectiveness analysis was performed based on a systematic review and meta‐analysis of randomized controlled trials investigating the effect of ACE inhibitor‐ or ARB‐based treatment regimens compared with treatment programs that did not include an RAAS inhibitor on the incidence of ESRD in patients with diabetic nephropathy. The number needed to treat (NNT) to prevent 1 patient from developing ESRD was estimated. The primary outcome of the analysis was the cost to prevent 1 patient from developing ESRD and the secondary outcome was the cost savings per patient receiving an RAAS blocker.
Systematic Review and Meta‐Analysis
A systematic literature search of MEDLINE/PubMed and EMBASE databases was performed to identify English‐language original articles on the effects of RAAS blockers in diabetic nephropathy in humans published from 1977 (when ACE inhibitors were approved by the US Food and Drug Administration for clinical use in humans) through December 31, 2006. Medical subject heading terms and search words used were “angiotensin‐converting enzyme inhibitors,”“captopril,”“enalapril,”“cilazapril,”“enalaprilat,”“fosinopril,”“lisinopril,”“perindopril,”“ramipril,”“saralasin,”“angiotensin receptor antagonists,”“angiotensin receptor blockers,”“losartan,”“irbesartan,”“valsartan,”“olmesartan,”“candesartan,”“eprosartan,” and “telmisartan” combined with “diabetes” or “diabetic nephropathy.” Reference lists of identified articles, including previous relevant meta‐analyses and narrative reviews 9 , 17 , 18 , 19 were also evaluated for additional relevant studies and information.
Inclusion criteria for the meta‐analysis were as follows: eligible studies had to be randomized controlled trials in adults and examine the effect of an ACE inhibitor‐ or ARB‐based treatment regimen compared with regimens that did not include these medications on the incidence of ESRD in patients with diabetic nephropathy. The studies also had to have a minimum follow‐up of 1 year and had to have been published as full‐length articles in peer‐reviewed English‐language journals. Studies in both types of diabetes mellitus and all stages of diabetic nephropathy were included.
The first 2 authors assessed each identified trial independently. They extracted data on the characteristics of the participants, interventions, comparisons, and outcomes (ESRD, doubling of serum creatinine concentration, all‐cause mortality). Standard criteria to assess the quality of the trials were used (allocation concealment, intent‐to‐treat analysis, percentage of loss to follow‐up, blinding). Differences in data between the 2 authors were resolved by consensus.
The treatment effects were summarized as relative risks and risk differences (means with 95% confidence intervals), using the DerSimonian and Laird random effects model to pool the data. The overall risk difference of ESRD was estimated just for the trials that had reported ≥1 patients developing ESRD, to calculate the NNT. The assumption of heterogeneity of treatment effects between studies was tested with chi‐square tests, and P values <.05 indicate heterogeneity across combined studies. 20 Analyses were performed using SPSS 13.0 (SPSS Inc, Chicago, IL), Review Manager 4.2 for Windows (Wintertree Software Inc, The Cochrane Collaboration, Oxford, England), and EasyMA 2001 Software (software for meta‐analysis of clinical trials, Department of Clinical Pharmacology, Lyon, France).
Cost‐Effectiveness Analyses
Cost‐effectiveness analysis was performed from the perspective of the Greek social security system, which covers 100% of all direct costs for the management of ESRD. The cost of pharmaceutical treatment was estimated based on the price reported in the last edition of the Greek National Formulary (updated in 2006) 21 multiplied by the mean days of therapy by daily dose in each study. The daily doses and the duration of the treatment were estimated from the included studies. A weighted average cost of treatment was calculated for each drug class. The lifetime ESRD direct cost was calculated using the cost of RRT in Greece as estimated in a recent study by our group 7 and the expected remaining lifetimes of the ESRD patients. 1 All costs were discounted at a rate of 3% per year and are reported in 2006 Euros (€) [1€ = $1.34; 2007 values].
Total mortality and cardiovascular morbidity and mortality were not included in the model because there were no significant differences between the treatment groups in 2 of the major studies (the Reduction of Endpoints in NIDDM With the Angiotensin II Antagonist Losartan [RENAAL] study 22 and Irbesartan in Diabetic Nephropathy Trial [IDNT] 23 ); thus, these parameters did not affect the direct medical costs. Since there were no significant differences in the incidence of side effects (with the exception of cough with ACE inhibitors) and use of nonstudy medications between treatment groups in both studies, 22 , 23 it was assumed that there was also no difference in the cost related to side effects and nonstudy medications between the groups. 7 , 11 Doubling of serum creatinine (DSC) concentration was not included in the model because it did not affect the direct medical costs. 7 , 11 The costs associated with the patient's follow‐up (eg, cost of clinical visits and monitoring of urine protein excretion, serum creatinine, and potassium) were not included because this monitoring should be performed routinely in all individuals with diabetes and renal disease 24 ; therefore, there should again be no difference between the groups with regard to these costs. 7 , 11
Sensitivity analyses tested the effect of modifying the input parameters on the economic end points. To examine the applicability of the findings to the US setting, the epidemiologic data, prices, and charges were substituted with the respective levels of the US setting. 2 , 25
RESULTS
Meta‐Analysis of Studies With RAAS Blockers in Diabetic Nephropathy
Of the 1028 originally identified articles, 1004 were excluded because of nonrandomized design, lack of diabetic nephropathy patients, no evaluation of renal outcomes, follow‐up <1 year, or duplicate publication. Of the remaining 24 studies, 20 (7269 patients) compared an ACE inhibitor‐based regimen with treatment that did not include an ACE inhibitor and 4 (3329 patients) compared ARB treatment with non‐ARB therapy, with a weighted mean follow‐up of 41.62 months (Table I).
Table I.
Included Studies and Patient Characteristics
| Trial | Year | Level of Albuminuria | Type of Diabetes | HTN at Baseline | Reported ESRD Cases | Intervention | Follow‐Up, mo | N | T+ | T− |
|---|---|---|---|---|---|---|---|---|---|---|
| ACE Inhibitor‐Based Treatment Compared With Therapy Without an ACE Inhibitor | ||||||||||
| Parving et al 26 | 1989 | Macro‐ | 1 | ‐ | Yes | Captopril 25–100 mg/d | 12 | 32 | 15 | 17 |
| Bauer et al 27 | 1992 | Macro‐ | Mixed | Yes | ‐ | Enalapril 5–40 mg/d | 18 | 33 | 18 | 15 |
| Chase et al 28 | 1993 | Micro‐ | 1 | ‐ | ‐ | Captopril 100 mg/d | 24 | 15 | 9 | 6 |
| Lewis et al 29 | 1993 | Micro‐ | 1 | Yes | Yes | Captopril 75 mg/d | 36 | 409 | 207 | 202 |
| Phillips et al 30 | 1993 | Mixed | 1 | Yes | ‐ | Cilazapril 2.5–5 mg/d | 24 | 25 | 14 | 11 |
| Ravid et al 31 | 1993 | Micro‐ | 2 | ‐ | ‐ | Enalapril 10 mg/d | 60 | 94 | 49 | 45 |
| Bakris et al 32 | 1994 | Micro‐ | 1 | ‐ | ‐ | Lisinopril 78 mg/d | 18 | 15 | 8 | 7 |
| Capek et al 33 | 1994 | Micro‐ | 2 | Yes | ‐ | Captopril 37.5 mg/d | 12 | 15 | 9 | 6 |
| Sano et al 34 | 1994 | Micro‐ | 2 | Yes | ‐ | Enalapril 5 mg/d | 48 | 62 | 31 | 31 |
| Laffel et al 35 | 1995 | Micro‐ | 1 | ‐ | ‐ | Captopril 100 mg/d | 24 | 143 | 70 | 73 |
| Maschio et al 36 | 1996 | Macro‐ | 2 | Yes | ‐ | Benazepril 10 mg/d | 36 | 21 | 6 | 15 |
| Crepaldi et al 37 | 1998 | Micro‐ | 1 | ‐ | ‐ | Lisinopril 10–20 mg/d | 36 | 66 | 32 | 34 |
| Garg et al 38 | 1998 | Micro‐ | 1 | ‐ | ‐ | Ramipril 5 mg/d | 12 | 11 | 7 | 4 |
| Nankervis et al 39 | 1998 | Micro‐ | Mixed | Yes | ‐ | Perindopril 4 mg/d | 36 | 31 | 17 | 14 |
| Cordonnier et al 40 | 1999 | Macro‐ | 2 | Yes | ‐ | Perindopril 4 mg/d | 24 | 19 | 9 | 10 |
| Mathiesen et al 41 | 1999 | Macro‐ | 1 | ‐ | ‐ | Captopril 100 mg/d | 48 | 40 | 19 | 21 |
| Micro‐HOPE 42 | 2000 | Micro‐ | Mixed | Yes | Yes | Ramipril 10 mg/d | 54 | 1140 | 553 | 587 |
| Bojestig et al 43 | 2001 | Micro‐ | 1 | ‐ | ‐ | Ramipril 1.25–5 mg/d | 24 | 55 | 37 | 18 |
| Katayama et al 44 | 2002 | Micro‐ | 1 | Yes | ‐ | Captopril 37.5 mg/d, imidapril 5 mg/d | 18 | 131 | 104 | 27 |
| DIABHYCAR 45 | 2004 | Mixed | 2 | ‐ | Yes | Ramipril 1.25 mg/d | 48 | 4912 | 2443 | 2469 |
| ARB‐Based Treatment Compared With Therapy Without an ARB | ||||||||||
| Brenner et al 22 | 2001 | Macro‐ | 2 | Yes | Yes | Losartan 50–100 mg/d | 40.8 | 1513 | 751 | 762 |
| Lewis et al 23 | 2001 | Macro‐ | 2 | Yes | Yes | Irbesartan 75–300 mg/d | 30 | 1148 | 579 | 569 |
| Parving et al 46 | 2001 | Micro‐ | 2 | Yes | ‐ | Irbesartan 150–300 mg/d | 24 | 590 | 389 | 201 |
| Muirhead et al 47 | 1999 | Micro‐ | 2 | Yes | ‐ | Valsartan 80–160 mg/d | 13 | 90 | 54 | 24 |
| Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; ESRD, end‐stage renal disease; HTN, hypertension; N, number of patients participating in each trial; T+, number of patients receiving active treatment; T−, number of patients receiving placebo or no treatment. | ||||||||||
Eight of the trials with ACE inhibitors reported weak evidence of a reduced risk of ESRD and DSC concentration, but 3 trials with ARBs showed a significantly reduced risk of ESRD and DSC concentration. The overall treatment effect was in favor of the RAAS blockade when compared with non‐RAAS treatment in reducing the risk of ESRD (10,024 patients; relative risk, 0.77; 95% confidence interval [CI], 0.67–0.89; P=.0004) and DSC concentration (10,005 patients; relative risk, 0.75; 95% CI, 0.63–0.90; P=.002) (Figure 1). Statistically significant reductions in total mortality were not found in the 20 trials evaluating ACE inhibitors (relative risk, 0.91; 95% CI, 0.71–1.17; P=.48) or in the 4 trials with ARBs (relative risk, 0.99; 95% CI, 0.85–1.17; P=.95).
Figure 1.
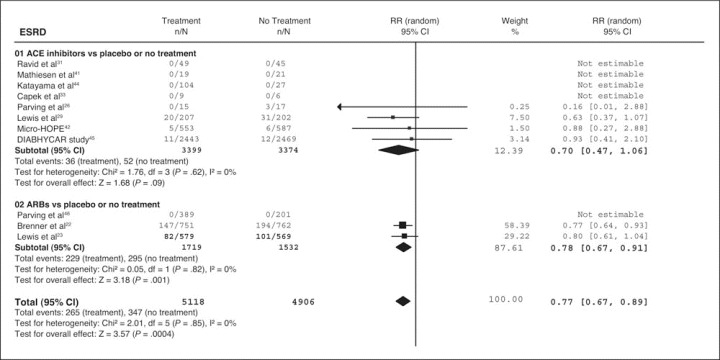
Effect of angiotensin‐converting enzyme (ACE) inhibitor‐based or angiotensin receptor blocker (ARB)‐based therapy compared with regimens without renin‐angiotensin‐aldosterone system (RAAS) inhibitors on renal outcomes (end‐stage renal disease [ESRD] and doubling of serum creatinine concentration). RR indicates relative risk; CI, confidence interval.
The risk difference of ESRD was statistically significant only for the RENAAL study 23 and for the overall effect of ARBs (RENAAL study and IDNT) (Figure 2). 22 , 23 The risk difference was not statistically significant in trials with ACE inhibitors, which reported progression to ESRD, 26 , 29 , 42 , 45 or for the overall effect of both ACE inhibitors and ARBs, because these analyses were dominated by the Microalbuminuria Cardiovascular Renal Outcomes—Heart Outcomes Prevention Evaluation (MICRO‐HOPE) 42 and the Noninsulin‐Dependent Diabetes, Hypertension, Microalbuminuria, Proteinuria, Cardiovascular Events, and Ramipril (DIABHYCAR) studies, 45 which contributed 23.38% and 23.86%, respectively, to the weight of the summary estimate.
Figure 2.
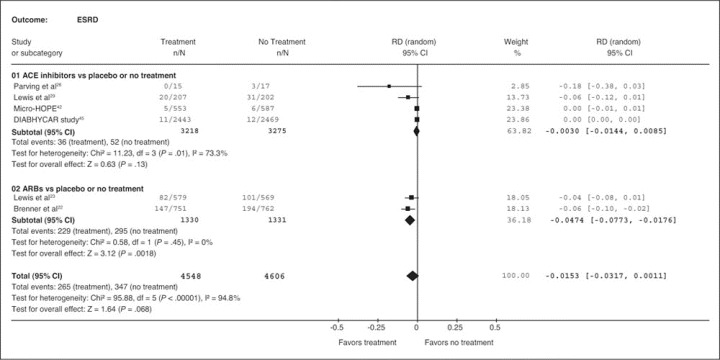
Risk difference of angiotensin‐converting enzyme (ACE) inhibitor‐based or angiotensin receptor blocker (ARB)‐based treatment compared with regimens without renin‐angiotensin‐aldosterone system (RAAS) blockade on incidence of end‐stage renal disease (ESRD). RD indicates risk difference; CI, confidence interval.
The NNT to prevent 1 patient from developing ESRD was 21 (95% CI, 12.94–56.82), which means that only 21 patients with diabetic nephropathy needed to receive ARBs for 3 years (weighted mean follow‐up) to prevent 1 patient from developing ESRD. The mean NNT to prevent 1 patient from developing ESRD was 333 for patients receiving ACE inhibitors (P=.61) and 65 for patients receiving any of the agents that block the RAAS (P=.068); these results were not statistically significant (upper 95% confidence limit could not be estimated because the corresponding CI of the risk differences included zero.)
Cost‐Effectiveness Analyses
The weighted mean lifetime direct cost of ESRD from the perspective of the public insurance system organizations was estimated at $195,692 (€146,039) in Greece and $265,374 in the United States for a 65‐year‐old diabetic patient (mean age of the studies' populations) (Table II). The mean weighted acquisition cost per patient per year for ACE inhibitors, ARBs, and the average of both in Greece is $144.92, $763.50, and $291.39, respectively, and in the United States is $355.15, $1090.16, and $529.18, respectively.
Table II.
The Weighted Mean Lifetime Direct Cost of End‐Stage Renal Disease (Discounted at 3%, in US $)
| First Year Cost, $ | Average Cost PPPY, $ | Expected Remaining Lifetime, y | Established Therapy, % | Lifetime Cost, $ | |
|---|---|---|---|---|---|
| Greecea | |||||
| Dialysis | 46,796.34 | 42,585.89 | 4.40 | 81.80 | 191,588.36 |
| Renal transplantation | 36,026.07 | 21,720.55 | 9.20 | 18.20 | 214,134.62 |
| ESRD | 195,691.78 | ||||
| United Statesb | |||||
| Dialysis | 72,000.00 | 68,108.53 | 3.70 | 71.11 | 255,893.04 |
| Renal transplantation | 108,000.00 | 19,858.38 | 10.10 | 28.89 | 288,711.23 |
| ESRD | 265,374.22 | ||||
| Abbreviations: ESRD, end‐stage renal disease; PPPY, per patient per year. aData for Greece are available from European Renal Association‐European Dialysis and Transplant Association Annual Report 2004 1 and a relative study by Stafylas and colleagues. 7 bData for the United States are available from US Renal Data System Annual Data Report 2006. 2 | |||||
The cost to prevent 1 patient from developing ESRD was therefore $31,729 (95% CI, $19,443–$85,442) for the patients receiving ARBs, and the net cost savings per patient over 3 years of treatment was $7770 (95% CI, $1940–$13,631) in Greece. The net cost savings per patient receiving ARBs was also statistically significant in the United States (Table III). For patients receiving ACE inhibitors, the cost to prevent 1 patient from developing ESRD was $189,190 in Greece, but this strategy was not cost‐saving in the United States. For patients receiving ACE inhibitors or ARBs, the net cost savings per patient was more than $2000 in both settings, but these results did not reach statistical significance (P>.05 for overall effect) and there was heterogeneity among combined trials (P<.05 for heterogeneity).
Table III.
The Cost to Prevent 1 Patient From Developing End‐Stage Renal Disease and the Net Cost Savings per Patient (Discounted at 3%, in US $)
| NNT | P Value for Overall Effect a | Mean Treatment Cost, $ | Cost to Prevent 1 ESRD, $ | Net Cost Savings per Patient,b$ | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |||
| Greece | ||||||||
| ACE inhibitors | 333 | (69‐NAc) | .13 | 567.58 | 189,190 | (39,412‐NAc) | 19.50 | (2251‐NAc) |
| ARBs | 21 | (13–57) | .0018 | 1503.74 | 31,729 | (19,443–85,442) | 7770.76 | (1940–13,631) |
| ACE inhibitors or ARBs | 65 | (32‐NAc) | .068 | 789.24 | 51,585 | (24,900‐NAc) | 2204.82 | (5413, NAc) |
| United States | ||||||||
| ACE inhibitors | 333 | 69‐NAc) | .13 | 1379.60 | 459,860 | (95,799‐NAc) | −583.46 | (2442‐NAc) |
| ARBs | 21 | (13–57) | .0018 | 1999.87 | 42,197 | (25,858–113,633) | 10,577.10 | (2671–18,524) |
| ACE inhibitors or ARBs | 65 | (32‐NAc) | .068 | 1526.46 | 99,770 | (48,160‐NAc) | 2533.73 | (6885‐NAc) |
| Abbreviations: ACE, angiotensin‐converting enzyme; ARBs, angiotensin receptor blockers; CI, confidence interval; ESRD, end‐stage renal disease; NA, not applicable; NNT, number needed to treat. a P value for overall effect <.05 indicates statistical significance. P value for heterogeneity more than .05 was just for the trials comparing ARBs vs placebo or no treatment indicating homogeneity across combined studies. bNet cost savings per patient receiving ACE inhibitors for a mean duration of 4 years, receiving ARBs for a mean duration of 3 years, ACE inhibitors or ARBs for a mean duration of 3.7 years. cThe upper 95% confidence limit cannot be estimated because the lower 95% limit of the risk difference includes zero. | ||||||||
Results of sensitivity analyses consistently indicated the benefits of ARBs. The modification of inclusion criteria in the meta‐analysis (inclusion of trials reporting zero incidence of ESRD) did not qualitatively change the results, but the risk difference of ARB‐based regimens was not statistically significant anymore (P=.64). Modification of the economic parameters failed to change the outcome of the analysis with respect to cost‐effectiveness. The increase in drug acquisition cost and the inclusion of the cost of clinical and laboratory monitoring of the patients with diabetic nephropathy reduced the net cost savings per patient, but the results still favored ARBs. If the analysis was performed from a broader social perspective, the net cost savings would be even greater because of the substantially higher total (direct and indirect) costs of ESRD. The applicability of the conclusions to the US setting supports the robustness of the current study.
DISCUSSION
The present study demonstrated that treatment of patients with diabetic nephropathy with agents that block the RAAS as part of the treatment regimen is cost‐effective, resulting in a 23% reduction of the incidence of ESRD and in net cost savings for the insurance system organizations. The findings appear to favor ARBs; the results are statistically significant (P<.05) only for this drug class, with net cost savings per patient of more than $7770 compared with $20 for ACE inhibitors (P=.61) and $2205 for ACE inhibitors or ARBs (P=.068) in Greece. The cost to prevent 1 patient from developing ESRD was $51,585 for the patients receiving RAAS‐blocking agents ($31,729 for ARBs compared with $189,190 for ACE inhibitors).
In the United States, the main findings were also applicable, but treatment with ACE inhibitors did not result in cost savings. The net cost savings per patient receiving ARBs were about $10,577 (P<.01). For patients receiving ACE inhibitors or ARBs, the net cost savings per patient was more than $2000, but these results did not reach statistical significance (P>.05 for overall effect) and there was heterogeneity among combined trials (P<.05 for heterogeneity).
The weighted mean lifetime direct cost of ESRD from the perspective of the insurance system organizations was estimated at $195,692 (€146,039) in Greece and $265,374 in the United States for a 65‐year‐old diabetic patient (mean age of the study population). The lower cost of ESRD in Greece is due to lower physician fees and salaries of personnel in the Greek National Health Service, the lower charges for blood tests, and the fact that the cost of RRT embraces a different range of services.
In the current meta‐analysis, RAAS blockers were found to be renoprotective, with about a 23% reduction in risk of ESRD and 25% reduction in risk of DSC. In contrast, the included studies have not shown a reduction in all‐cause mortality. ARBs significantly reduced the risk of ESRD and DSC (22% and 21%, respectively). The point estimates of effect of all outcomes favored ACE inhibitors compared with ARBs. There was, however, considerable imprecision surrounding these summary estimates because of low event rates and because of heterogeneity in trial results.
The findings of the present meta‐analysis are similar to results of a previous one, 9 despite the differences in the inclusion criteria and the inclusion of a more recent trial 45 in our meta‐analysis. There were no trials comparing ACE inhibitors with ARBs in the included trials. A regression analysis of treatment effects of the 2 drug classes by Strippoli and colleagues 9 using active treatment as the explanatory variable showed no significant difference between these 2 agents for the risk of any outcome, but the trials had important differences in study design. 22 , 23 , 42 These results are in accordance with those of a study by Lacourciere and associates 48 and the recently published DETAI L study, 49 supporting that ACE inhibitors and ARBs provide similar renoprotection in patients with diabetic nephropathy.
The results of the current study are in accordance with the economic evaluations of irbesartan in the treatment of diabetic nephropathy (net cost savings per patient, $2778 at 3 years and $16,026 at 10 years of treatment) which used models simulating the progression to ESRD based on the results of IDNT. 12 , 23 The findings are also in accordance with the results of the RENAAL study economic evaluations based on an estimation of the mean number of ESRD days saved per patient during the trial. 7 , 11 , 22 The net cost savings per patient over 3.5 years were $3522 in the US setting 11 and $2232 in the Greek setting. 7 In our study, the net cost savings per patient were higher than the above mainly because of the substantial increase in the cost of ESRD and secondly because the calculations of the current study are based on the average weighted lifetime cost of RRT .
As the renoprotective properties of ARBs are considered a class effect, it is possible that agents other than those tested to date will have a similar economic impact when used in patients with diabetic nephropathy. 7 Since large clinical trials with hard renal outcomes in patients with diabetic nephropathy are not available for the rest of ARBs, however, the findings from the above economic analyses should be generalized with some caution to the whole group. Other antihypertensive compounds (ie, nondihydropyridine calcium channel blockers and aldosterone receptor antagonists) have also been shown in some studies to have renoprotective effects possibly beyond blood pressure reduction, 19 but large‐scale outcome trials are needed to confirm these findings before economic evaluations are performed. It should be noted that in all of the cited trials, a diuretic was used with the RAAS inhibitors in a large number of cases.
Limitations
A major limitation of this study was the perspective of the cost analysis. Although economic evaluations should ideally be conducted from a societal perspective, 50 this analysis was conducted from the perspective of the Greek social security system, which covers almost 100% of the population. 16 The estimated cost of ESRD is a good approximation of the cost to the social security system under current reimbursement policies and it permits the generalization and comparability of results across studies. It should be noted that the results of this analysis are particularly relevant to the cost‐containment efforts initiated through the recent reform of the Greek National Health Service. 16 If we needed to perform this study from the societal perspective, this method would have underestimated the real cost of ESRD and consequently the net cost savings per patient, given that the costs do not include patient out‐of‐pocket costs or productivity losses. The costs of the clinical and laboratorial monitoring before the development of ESRD were also not included in the analysis, because these were performed routinely in all patients with diabetes and nephropathy. 24 The acquisition cost of nonstudy medications was not included in the study because there was just a small but not significantly greater use in the non‐RAAS treated groups 11 , 22 , 23 , 42 , 45 ; this would obviously augment the cost‐effectiveness ratio. Other limitations were the small number of included trials and the indirect comparison between ACE inhibitors and ARBs by using other agents as a common comparator; there were no trials directly comparing the 2 agents and reporting incidence of ESRD. Another possible limitation was the heterogeneity of the included studies, mainly concerning the type of diabetes and the level of albuminuria. Therefore, the results favoring ARBs should be interpreted with caution.
CONCLUSIONS
Treatment of patients with diabetic nephropathy with RAAS‐blocking agents is a cost‐effective strategy that resulted in a reduction in the incidence of ESRD and in net cost savings for the insurance system of more than $2000 per patient over 3 years of treatment. Thus, this study adds data from an idiosyncratic setting to the existing evidence that ARBs and ACE inhibitors should be used as initial antihypertensive therapy in patients with diabetic nephropathy. In most cases, other medications are necessary to reduce BP to goal levels. The relative risk reduction of ESRD and the net cost savings were statistically significant for ARBs but not for ACE inhibitors. The current analysis favored the use of ARBs, which resulted in substantially more net cost savings than ACE inhibitors. An economic evaluation of an adequately powered comparative trial of ACE inhibitors compared with ARBs with renal and all‐cause mortality as primary outcomes would be informative about the incremental cost‐effectiveness of the 2 agents.
References
- 1. Registry ERA‐EDTA. European Renal Association‐European Dialysis and Transplant Association Registry 2004 Annual Report. Amsterdam, the Netherlands: Academic Medical Center, Department of Medical Informatics; 2006. [Google Scholar]
- 2. US Renal Data System. USRDS 2006 Annual Data Report: Atlas of End‐Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2006. [Google Scholar]
- 3. Grassmann A, Gioberge S, Moeller S, et al. ESRD patients in 2004: global overview of patient numbers, treatment modalities and associated trends. Nephrol Dial Transplant. 2005;20(12):2587–2593. [DOI] [PubMed] [Google Scholar]
- 4. Xue JL, Ma JZ, Louis TA, et al. Forecast of the number of patients with end‐stage renal disease in the United States to the year 2010. J Am Soc Nephrol. 2001;12(12):2753–2758. [DOI] [PubMed] [Google Scholar]
- 5. Athyros VG, Bouloukos VI, Pehlivanidis AN, et al. The prevalence of the metabolic syndrome in Greece: The MetS‐Greece Multicentre Study. Diabetes Obes Metab. 2005;7(4):397–405. [DOI] [PubMed] [Google Scholar]
- 6. Ioannidis G, Papadaki O, Tsakiris D. Statistical and epidemiological data of the renal replacement therapy in Greece 1986–2000. Report of the Hellenic Renal Registry. Hellenic Nephrol. 2002;14(4):525–548. [Google Scholar]
- 7. Stafylas PC, Sarafidis PA, Lasaridis AN, et al. Cost‐effectiveness of losartan in diabetic nephropathy: a Greek perspective. J Nephrol. In press. [PubMed] [Google Scholar]
- 8. Powers A. Diabetes mellitus. In: Braunwalds E, Fauci A, Kasper D, et al, eds. Harrison's Principles of Internal Medicine. 15th ed. New York, NY: McGraw‐Hill; 2001: 2109–2137. [Google Scholar]
- 9. Strippoli GF, Craig M, Deeks JJ, et al. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. BMJ. 2004;329(7470):828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodby RA, Firth LM, Lewis EJ. An economic analysis of captopril in the treatment of diabetic nephropathy. The Collaborative Study Group . Diabetes Care. 1996;19(10):1051–1061. [DOI] [PubMed] [Google Scholar]
- 11. Herman WH, Shahinfar S, Carides GW, et al. Losartan reduces the costs associated with diabetic end‐stage renal disease: the RENAAL study economic evaluation. Diabetes Care. 2003;26(3):683–687. [DOI] [PubMed] [Google Scholar]
- 12. Rodby RA, Chiou CF, Borenstein J, et al. The cost‐effectiveness of irbesartan in the treatment of hypertensive patients with type 2 diabetic nephropathy. Clin Ther. 2003;25(7):2102–2119. [DOI] [PubMed] [Google Scholar]
- 13. Palmer AJ, Tucker DM, Valentine WJ, et al. Cost‐effectiveness of irbesartan in diabetic nephropathy: a systematic review of published studies. Nephrol Dial Transplant. 2005;20(6):1103–1109. [DOI] [PubMed] [Google Scholar]
- 14. Boulenger S, Nixon J, Drummond M, et al. Can economic evaluations be made more transferable? Eur J Health Econ. 2005;6(4):334–346. [DOI] [PubMed] [Google Scholar]
- 15. Mossialos E, Allin S, Davaki K. Analysing the Greek health system: a tale of fragmentation and inertia. Health Econ. 2005;14(suppl 1):S151–S168. [DOI] [PubMed] [Google Scholar]
- 16. Stafilas PC, Sarafidis PA, Lasaridis AN, et al. An economic evaluation of the 2003 European Society of Hypertension‐European Society of Cardiology guidelines for the management of mild‐to‐moderate hypertension in Greece. Am J Hypertens. 2005;18(9, pt 1):1233–1240. [DOI] [PubMed] [Google Scholar]
- 17. Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin‐converting enzyme inhibition: a patient‐level meta‐analysis. Ann Intern Med. 2003;139(4):244–252. [DOI] [PubMed] [Google Scholar]
- 18. Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin‐angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta‐analysis. Lancet. 2005;366(9502):2026–2033. [DOI] [PubMed] [Google Scholar]
- 19. Sarafidis PA, Khosla N, Bakris GL. Antihypertensive therapy in the presence of proteinuria. Am J Kidney Dis. 2007;49(1):12–26. [DOI] [PubMed] [Google Scholar]
- 20. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Intervention 4.2.6. The Cochrane Library, 4th ed. Chichester, UK: John Wiley & Sons, Ltd; 2006. [Google Scholar]
- 21. National Organization for Medicines . National Formulary 2003. Athens: National Organization for Medicines; 2003. [Google Scholar]
- 22. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. [DOI] [PubMed] [Google Scholar]
- 23. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. [DOI] [PubMed] [Google Scholar]
- 24. American Diabetes Association . Standards of medical care in diabetes—2006. Diabetes Care. 2006;29(suppl 1):S4–S42. [PubMed] [Google Scholar]
- 25. Fleming T, ed. Red Book: Pharmacy's Fundamental Reference. Montvale, NJ: Thomson PDR; 2004: 177–619. [Google Scholar]
- 26. Parving HH, Hommel E, Damkjaer NM, et al. Effect of captopril on blood pressure and kidney function in normotensive insulin dependent diabetics with nephropathy. BMJ. 1989;299(6698):533–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bauer JH, Reams GP, Hewett J, et al. A randomized, double‐blind, placebo‐controlled trial to evaluate the effect of enalapril in patients with clinical diabetic nephropathy. Am J Kidney Dis. 1992;20(5):443–457. [DOI] [PubMed] [Google Scholar]
- 28. Chase HP, Garg SK, Harris S, et al. Angiotensin‐converting enzyme inhibitor treatment for young normotensive diabetic subjects: a two‐year trial. Ann Ophthalmol. 1993;25(8):284–289. [PubMed] [Google Scholar]
- 29. Lewis EJ, Hunsicker LG, Bain RP, et al. The Collaborative Study Group . The effect of angiotensin‐converting enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329(20):1456–1462. [DOI] [PubMed] [Google Scholar]
- 30. Phillips PJ, Phillipou G, Bowen KM, et al. Diabetic microalbuminuria and cilazapril. Am J Med. 1993;94: 58S–60S. [PubMed] [Google Scholar]
- 31. Ravid M, Savin H, Jutrin I, et al. Long‐term stabilizing effect of angiotensin‐converting enzyme inhibition on plasma creatinine and on proteinuria in normotensive type II diabetic patients. Ann Intern Med. 1993;118(8):577–581. [DOI] [PubMed] [Google Scholar]
- 32. Bakris GL, Slataper R, Vicknair N, et al. ACE inhibitor mediated reductions in renal size and microalbuminuria in normotensive, diabetic subjects. J Diabetes Complications. 1994;8(1):2‐6. [DOI] [PubMed] [Google Scholar]
- 33. Capek M, Schnack C, Ludvik B, et al. Effects of captopril treatment versus placebo on renal function in type 2 diabetic patients with microalbuminuria: a long‐term study. Clin Investig. 1994;72(12):961–966. [DOI] [PubMed] [Google Scholar]
- 34. Sano T, Kawamura T, Matsumae H, et al. Effects of long‐term enalapril treatment on persistent microalbuminuria in well‐controlled hypertensive and normotensive NIDDM patients. Diabetes Care. 1994;17(5):420–424. [DOI] [PubMed] [Google Scholar]
- 35. Laffel LM, McGill JB, Gans DJ. The beneficial effect of angiotensin‐converting enzyme inhibition with captopril on diabetic nephropathy in normotensive IDDM patients with microalbuminuria. North American Microalbuminuria Study Group . Am J Med. 1995;99(5):497–504. [DOI] [PubMed] [Google Scholar]
- 36. Maschio G, Alberti D, Janin G, et al. Effect of the angiotensin‐converting‐enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin‐Converting‐Enzyme Inhibition in Progressive Renal Insufficiency Study Group . N Engl J Med. 1996;334(15):939–945. [DOI] [PubMed] [Google Scholar]
- 37. Crepaldi G, Carta Q, Deferrari G, et al. Effects of lisinopril and nifedipine on the progression to overt albuminuria in IDDM patients with incipient nephropathy and normal blood pressure. The Italian Microalbuminuria Study Group in IDDM . Diabetes Care. 1998;21(1):104–110. [DOI] [PubMed] [Google Scholar]
- 38. Garg SK, Chase HP, Jackson WE, et al. Renal and retinal changes after treatment with ramipril and pentoxifyline in subjects with IDDM. Ann Ophthalmol. 1998;30: 33–37. [Google Scholar]
- 39. Nankervis A, Nicholls K, Kilmartin G, et al. Effects of perindopril on renal histomorphometry in diabetic subjects with microalbuminuria: a 3‐year placebo‐controlled biopsy study. Metabolism. 1998;47(12 suppl 1):12–15. [DOI] [PubMed] [Google Scholar]
- 40. Cordonnier DJ, Pinel N, Barro C, et al. Expansion of cortical interstitium is limited by converting enzyme inhibition in type 2 diabetic patients with glomerulosclerosis. The Diabiopsies Group . J Am Soc Nephrol. 1999;10(6):1253–1263. [DOI] [PubMed] [Google Scholar]
- 41. Mathiesen ER, Hommel E, Hansen HP, et al. Randomised controlled trial of long term efficacy of captopril on preservation of kidney function in normotensive patients with insulin dependent diabetes and microalbuminuria. BMJ. 1999;319(7201):24–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heart Outcomes Prevention Investigation Study Investigators . Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO‐HOPE substudy. Lancet. 2000;22;355(9200):253–259. [PubMed] [Google Scholar]
- 43. Bojestig M, Karlberg BE, Lindstrom T, et al. Reduction of ACE activity is insufficient to decrease microalbuminuria in normotensive patients with type 1 diabetes. Diabetes Care. 2001;24(5):919–924. [DOI] [PubMed] [Google Scholar]
- 44. Katayama S, Kikkawa R, Isogai S, et al. Effect of captopril or imidapril on the progression of diabetic nephropathy in Japanese with type 1 diabetes mellitus: a randomized controlled study (JAPAN‐IDDM). Diabetes Res Clin Pract. 2002;55(2):113–121. [DOI] [PubMed] [Google Scholar]
- 45. Marre M, Lievre M, Chatellier G, et al. Effects of low dose ramipril on cardiovascular and renal outcomes in patients with type 2 diabetes and raised excretion of urinary albumin: randomised, double blind, placebo controlled trial (the DIABHYCAR study). BMJ. 2004;328(7438):495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parving HH, Lehnert H, Brochner‐Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870–878. [DOI] [PubMed] [Google Scholar]
- 47. Muirhead N, Feagan BF, Mahon J, et al. The effects of valsartan and captopril on reducing microalbuminuria in patients with type 2 diabetes mellitus: a placebo‐controlled trial. Curr Ter Res. 1999;60: 650–660. [Google Scholar]
- 48. Lacourciere Y, Belanger A, Godin C, et al. Long‐term comparison of losartan and enalapril on kidney function in hypertensive type 2 diabetics with early nephropathy. Kidney Int. 2000;58(2):762–769. [DOI] [PubMed] [Google Scholar]
- 49. Barnett AH, Bain SC, Bouter P, et al. Angiotensin‐receptor blockade versus converting‐enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351(19):1952–1961. [DOI] [PubMed] [Google Scholar]
- 50. Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party . BMJ. 1996;313(7052):275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]


