Abstract
Blacks have the highest rates of hypertension and cardiovascular disease, with earlier onset, greater severity, and more target organ damage including coronary disease, heart failure, stroke, and end‐stage renal disease. A major reason is the greater prevalence of other cardiovascular disease risk factors, particularly obesity, inactivity, and diabetes mellitus, along with socioeconomic differences, adherence, and achievement of goals. This review focuses on the burden of cardiovascular disease in blacks. Therapeutic lifestyle changes and pharmacologic interventions to decrease clinical events in this high‐risk group are described. Intensive blood pressure control is a primary means of “stopping the clock” in the progression of cardiovascular disease and renal disease. Thiazide diuretics remain primary first‐step agents, especially for uncomplicated hypertension; calcium channel blockers are also efficacious. However, renin‐angiotensin system modulators may also be beneficial, especially with a diuretic, considering the high prevalence in this group of patients of compelling indications for use of such agents.
Cardiovascular disease (CVD) is the number one cause of mortality worldwide, responsible for nearly 17 million deaths yearly. Hypertension, an established major risk factor for CVD, affects approximately 31% of American adults 1 and an estimated 40% of black adults in the United States, which is one of the highest prevalence rates in the world. 2 Blacks are at greater risk for hypertension, with an early age at onset and greater severity than white Americans, 3 , 4 and they are more likely to develop and die of serious microvascular and macrovascular complications. 5 Detection and control of high blood pressure (BP) are therefore critical to reducing the risk of CVD in this population.
Although hypertension awareness and control rates may be improving among blacks, as suggested by recent analyses of trends between 1999 and 2004 from the National Health and Nutrition Examination Survey (NHANES), 3 there is also evidence of a nonsignificant rise in overall hypertension prevalence during this same period. 3 The persistently high prevalence of hypertension, the increasing frequency of obesity, the decrease in physical activity, and the aging of the population suggest that CVD will continue to be a major cause of death and disability. This report focuses on the burden of CVD specifically related to hypertension in black individuals and describes evidence related to therapeutic lifestyle changes and pharmacologic interventions that may decrease the risk of clinical consequences. Early and intensive control of hypertension involving lifestyle changes and pharmacologic interventions is needed to prevent or stop the progression of CVD events in this high‐risk group. As monotherapy, thiazide diuretics remain the primary first‐step antihypertensive agent, particularly for uncomplicated hypertension; long‐acting calcium channel blockers (CCBs) are also efficacious antihypertensive agents in blacks. Although agents that act on the renin‐angiotensin system (RAS) have sometimes been avoided in blacks, their use may be beneficial when included in combination therapy with a diuretic.
THE HYPERTENSION‐RELATED MORBIDITY AND MORTALITY IN THE BLACK POPULATION
Black individuals with hypertension are more likely to die of associated complications than whites (Figure 1), 6 and the rates of nonfatal stroke, fatal stroke, and death of heart disease are increased by 1.3, 1.8, and 1.5 times, respectively. 2 Physiologic factors that have been observed in association with hypertension among blacks may account to some extent for the higher risk of morbidity and mortality among them compared with their white counterparts. 7 For example, an excess prevalence of salt sensitivity, lower plasma renin activity, and lower levels of endothelium‐derived vasodilators such as nitric oxide have been documented in black Americans as well as black individuals worldwide. 8 , 9 A study that matched black and white patients with treated hypertension for the same daytime BP found that blacks tend to have significantly higher nocturnal BP than whites. This difference could contribute to the higher rates of target organ damage and mortality among hypertensive patients in this group.
Figure.
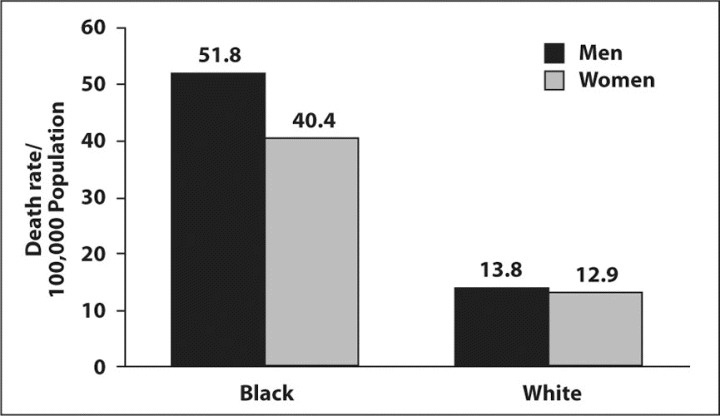
Differences in deaths from hypertension between black and white Americans. Reprinted with permission from Ferdinand. 6
Nevertheless, the excess risk of hypertensive diseases and mortality in blacks in the United States may be due to adverse diet and other environmental factors. As in the general US population, blacks have reduced their intake of dietary saturated fat, 10 but overall consumption of fat remains high (as it does in all Americans). In addition, NHANES data indicate that the prevalence of overweight and obesity (body mass index [BMI] ≥25 kg/m2) is increasing remarkably in all Americans, with a prevalence of approximately 66% in 2003–2004. 2 Disparities in weight according to race were observed for NHANES 2001–2002 for women but not men; approximately 42% of black women are obese (BMI ≥30 kg/m2), compared with 19% of white women and 26% of Hispanic women. 2 There is also evidence of excess sodium and decreased potassium intake among black patients. Despite evidence that a sedentary lifestyle increases CVD risk, trends observed among schoolchildren suggest that physical activity is decreasing in the American population, with 23% of all children not engaging in physical activity during leisure time. 11 Moreover, data suggest that non‐Hispanic black children are less likely than non‐Hispanic white children to participate in organized or leisure time physical activity. 11 Among black girls, physical activity appears to decline with each year through adolescence at a greater rate than among whites. Furthermore, it is distressing that only 24% of black adults report regular physical activity. 2 Therefore, to halt the growing rates of CVD in all Americans and specifically black Americans, efforts should be made starting with school‐aged children to encourage increased physical activity; avoidance of high‐calorie foods; and emphasis on diets rich in fresh fruits, vegetables, whole grains, and low‐saturated fat protein.
In addition to possible physiologic and environmental factors, the greater prevalence of CVD in black individuals also results from higher rates of comorbid cardiovascular risk factors, such as diabetes mellitus and dyslipidemia. Blacks in the United States have a disproportionate burden of diabetes (11% vs 5.2% for non‐Hispanic whites) and end‐stage renal disease (ESRD) (≈4.8 per 1000 vs ≈1.1 per 1000 in whites). 12 , 13 These data confirm that this population group is almost twice as likely to develop diabetes as age‐matched non‐Hispanic whites 14 ; among adults with diabetes, death from heart disease is 2 to 4 times higher than among adults without diabetes. 2 The primary etiologies of ESRD are usually hypertension and diabetes, and patients with ESRD most often die of cardiovascular causes. 2 Recent data suggest that blacks have 16 times the rate of ESRD as whites of a similar age. 13 Hypercholesterolemia, particularly in combination with hypertension, is also an important modifiable risk factor that requires clinical attention. A recent examination of 2001–2002 NHANES data in adults aged 20 years and older showed that combined hypertension and hypercholesterolemia was more common in blacks (22%) than in non‐Hispanic whites (19%) or Hispanics (9.8%). 15 Unfortunately, rates for treatment and control were relatively low for all groups.
Hypertension is a potent risk factor, leading to excessive CVD morbidity and mortality. Rates of hypertension and hypertension‐related morbidity and mortality are affected by appropriate treatment to goal levels and adherence with prescribed medication. It has been estimated that BP control is achieved in 52.4% of non‐Hispanic blacks with treated hypertension, compared with 68.2% of non‐Hispanic whites with treated hypertension. 3 Although some studies suggest that the relatively lower control rate in blacks is related to differential access to health care, 16 economic barriers and access to care are not the only contributors to racial differences in BP control. A study involving veterans with hypertension found some persistent racial differences in BP control despite equal access to health care and after taking into account numerous other potential mediating factors. 17 Of note, and similar to other studies, the study reported that blacks in the United States had poorer rates of medication adherence. 17
THE IMPORTANCE OF INTENSIVE BP CONTROL TO DRIVE RISK REDUCTION
The relationship between hypertension and CVD is continuous and consistent. 18 Beginning at a BP level of 115/75 mm Hg, the risk of a CVD event doubles with each increment of 20/10 mm Hg. 19 To reduce the risk of hypertension‐related CVD, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) 18 recommends achieving a BP target of <140/90 mm Hg in patients without comorbidities, or <130/80 mm Hg in individuals with diabetes mellitus or chronic kidney disease. The JNC 7 treatment guidelines emphasize the need for healthy lifestyle modifications in all hypertension categories, with the addition of one or more medications, as required, to achieve BP goals. 18 According to JNC 7, combination therapy may be considered when BP is >20/10 mm Hg above the desired goal.
Because blacks tend to experience more severe hypertension at younger ages, the International Society on Hypertension in Blacks (ISHIB) Working Group has stated that early identification of high‐normal BP (130–139/85–89 mm Hg) and intensive treatment are especially important in blacks. 4 In contrast to JNC 7, ISHIB states that first‐step combination therapy should be considered in black patients when their systolic BP is ≥15 mm Hg or diastolic BP is ≥10 mm Hg above target level to prevent target organ damage and other complications (ie, >155/100 mm Hg in patients without comorbidities). 4 Future recommendations from ISHIB may potentially confirm the recommendation of the benefits of combination therapy.
Lifestyle Modifications
Therapeutic lifestyle modifications (Table) are an important means for reducing risk in all individuals. Findings from large‐scale international clinical trials, including the Dietary Approaches to Stop Hypertension (DASH) study, 20 demonstrate that reduction of sodium intake and consumption of more fruits, vegetables, and low‐fat foods lowers BP, especially in blacks. More recent findings suggest that sodium reduction may not only lower BP but also may prevent CVD. 21 An analysis of data from 2415 prehypertensive adults (approximately 18%–20% blacks) randomized to reduced sodium intake or usual care in the Trials of Hypertension Prevention (TOHP) I and II 21 showed that CVD risk was significantly lowered in the intervention group compared with the control group (adjusted relative risk, 0.7; P=.018). Results were similar when analyzed for interaction by race, confirming the benefit of a low‐sodium diet for CVD risk reduction in blacks. 21 Physical activity and weight loss are also crucial lifestyle modifications. Data from the Atherosclerosis Risk in Communities study 22 (N=14,575), which included a substantial proportion of black individuals (n=3885), suggest that physical activity, whether at work, during leisure time, or through sports, reduces the risk of ischemic stroke. Although it is difficult to replicate in practice the results observed in clinical trials, wider acceptance and implementation of appropriate nonpharmacologic therapies would greatly enhance efforts to decrease the prevalence and severity of both hypertension and CVD. To halt the ongoing rise in CVD, therapeutic lifestyle changes remain the bedrock of primary prevention.
Table.
Therapeutic Lifestyle Interventions in Hypertension
| Modification | Recommendation |
|---|---|
| Reduce weight | Maintain normal body weight (BMI, 18.5–24.9 kg/m2) |
| Adopt DASH eating plan | Consume a diet rich in fruits, vegetables, and low‐fat dairy products with a reduced content of saturated and total fat |
| Reduce dietary sodium | Reduce dietary sodium intake to no more than 2.4 g sodium or 6 g sodium chloride |
| Increase physical activity | Engage in regular aerobic physical activity, such as brisk walking, at least 30 minutes per day, most days of the week |
| Modify alcohol consumption | Limit alcohol consumption to no more than 2 drinks per day in most men and no more than 1 drink per day in most women |
| Abbreviations: BMI, body mass index; DASH, Dietary Approaches to Stop Hypertension. Adapted from Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. 18 | |
Pharmacologic Therapy
Even with appropriate use of lifestyle modifications, most patients with hypertension will require pharmacologic therapy to control their BP. Lowering BP with medication has been shown to decrease the risk of cardiac events, stroke, and heart failure and to slow progression of atherosclerosis in a wide range of patients. 23 As monotherapy, thiazide diuretics remain the primary first‐step agents in hypertension, especially uncomplicated hypertension. In addition, combination therapy with an RAS blocker and a diuretic to achieve BP goals promptly is particularly important in black Americans, considering the high rates of chronic kidney disease and type 2 diabetes in this population. Studies in 1982 had demonstrated less efficacy with the angiotensin‐converting enzyme (ACE) inhibitor captopril even in doses up to 450 mg/d as monotherapy in blacks when compared with other agents such as hydrochlorothiazide. 24 Because of the perception that lower plasma renin activity in this population already reflects suppression of the RAS, some physicians have tended to avoid RAS‐blocking agents in this group. Some other studies have reported, however, that RAS blockers are useful in blacks when used in higher doses and especially in combination with a diuretic or long‐acting CCB. 6 Diuretics and dietary sodium reduction activate the RAS and are often necessary to diminish race‐related differences in antihypertensive efficacy.
Clinical Trial Evidence
The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) 25 confirmed the efficacy of treatment with a long‐acting CCB, thiazide diuretic, or ACE inhibitor in preventing cardiovascular events in 42,448 high‐risk men and women (of whom 15,133 [35%] were black) aged 55 years and older who had hypertension and at least one other CVD risk factor. There was no significant difference among the 3 antihypertensive therapy groups in the primary outcome of combined nonfatal myocardial infarction or fatal coronary heart disease in the cohort overall or among black patients. However, systolic BP was significantly higher in the lisinopril group (+4 mm Hg) compared with chlorthalidone. Compared with chlorthalidone, black patients receiving lisinopril had a 19% higher risk of all CVD events, a 40% higher risk of stroke, and a 32% greater risk of heart failure. 26 The higher incidence of stroke among black patients in the ACE inhibitor group may have been the result of using a combination of ACE inhibitors and β‐blockers as first‐ and second‐step treatments, as this combination is a relatively ineffective antihypertensive regimen in this population. Because of the study design, neither a diuretic nor CCB could be added to the ACE inhibitor.
The Trial of Preventing Hypertension (TROPHY) 27 was designed to assess whether treatment with an angiotensin II receptor blocker (ARB) would prevent the development and progression to stage 1 hypertension in 772 patients, of whom 79 (10%) were black. All patients had prehypertension (systolic BP, 120–139 mm Hg and/or diastolic BP 80–89 mm Hg) at baseline. Results after 2 years showed that treatment with candesartan led to a relative risk reduction of 66% (P<.001) for the development of stage 1 hypertension compared with placebo. Subgroup analysis showed that the hazard ratios for the development of new‐onset hypertension were 0.55 (0.44–0.67) in whites and 0.74 (0.42–1.32) in blacks. 27 The provocative results of TROPHY suggest that early pharmacologic intervention may be effective in delaying the progression of hypertension in patients with prehypertension. Some experts however, have questioned the design and conclusions of this study.
Although variations may exist in the response of black patients to ACE inhibitors or ARBs, the addition of a diuretic or long‐acting CCB to an ACE inhibitor or ARB regimen essentially removes any differences in response. In a small study of blacks (N=88) with hypertension treated with an ARB (valsartan 160 mg/d) adding hydrochlorothiazide to their regimen was more effective in incrementally lowering BP than doubling the valsartan dose or adding an ACE inhibitor (benazepril). 28 Another study demonstrated the antihypertensive efficacy of combining hydrochlorothiazide with losartan in 440 black American adults with mild to moderate hypertension. Combination therapy with an ARB and a diuretic has been shown to lead to greater BP reductions than ARB monotherapy in blacks, without compromising tolerability. 29 In addition, in a study of 482 black patients with stage 1 or stage 2 hypertension, combination therapy with an ARB (valsartan) and a diuretic (hydrochlorothiazide) was as effective as monotherapy with high‐dose amlodipine in reducing BP and was associated with a more favorable tolerability profile. 30 The better tolerability profile that typically results from combination therapy compared with higher‐dose monotherapy is an important consideration given that tolerability influences adherence and hence BP control.
Therefore, while overall, multiple trials have confirmed that ACE inhibitors and ARBs (usually given with a thiazide diuretic) are effective in preventing or attenuating diabetes, kidney disease, coronary heart disease, and heart failure in patients with hypertension, combination therapy that includes a thiazide diuretic and/or a long‐acting CCB may be especially effective in black patients. And although patients in different racial groups have been included in a number of completed and ongoing studies of RAS inhibition in the prevention of hypertension complications, more inclusive research is needed. This would give clinicians even more confidence in using RAS blockers again, usually in combination, to help halt the progression of CVD and renal disease in the nonwhite population.
NEW‐ONSET DIABETES
Hypertension itself without any treatment increases the risk of developing diabetes by 2.5‐fold. 18 Diabetes in hypertension is associated with substantial morbidity and mortality, and prevention is an important concern, particularly among blacks, who are at greater risk for both conditions. This fact may be taken into consideration when selecting an antihypertensive agent. Certain classes of antihypertensive agents, namely the thiazide diuretics and more particularly β‐blockers, may have an adverse impact on the metabolic profile and increase glucose intolerance, whereas CCBs appear to be neutral overall. 18 In contrast, ACE inhibitors and ARBs may have favorable effects on insulin sensitivity, and studies have suggested that these agents reduce the risk of new‐onset diabetes compared with other antihypertensive agents in patients with hypertension or CVD. 31 , 32 The 23% reduction in new‐onset diabetes observed with valsartan compared with a CCB‐based treatment regimen in the Valsartan Antihypertensive Long‐Term Use Evaluation (VALUE) study 32 (11.5% compared with 14.5% of new‐onset diabetes) is an important consideration when treating high‐risk black patients. The mechanisms responsible for the potential beneficial effects of RAS‐blocking agents on glucose tolerance have yet to be established; nevertheless, it may be reasonable to treat hypertension with agents that present less risk of new‐onset type 2 diabetes. Data from the Diabetes Reduction Approaches With Ramipril and Rosiglitazone Medications (DREAM) study, however, reported that treatment with ramipril (an ACE inhibitor) had no significant effect on diabetes incidence in patients at low risk for CVD. 33 Despite the negative findings of DREAM, a large meta‐analysis of 22 clinical trials involving 143,153 participants with hypertension, heart failure, or multiple CVD risk factors found that the rate of new‐onset diabetes was lowest with ARBs and ACE inhibitors and highest with β‐blockers and diuretics. 34 Unfortunately, the proportion of black patients in these studies was not analyzed.
It is of interest to note that in the blinded controlled ALLHAT study, CHD outcome was not adversely affected by the differences in new‐onset diabetes between the ACE inhibitor‐based and the diuretic‐based treatment groups (8% and 11.5% new‐onset diabetes, respectively).
RENAL DISEASE
The African American Study of Kidney Disease and Hypertension (AASK) 35 was conducted in black patients (N=1094) with hypertensive nephropathy to determine the effect of different antihypertensive regimens on renal disease progression. Compared with an amlodipine‐based regimen, a ramipril‐based regimen was associated with a significant decrease in progression of chronic kidney disease, especially in patients with high levels of proteinuria. 35 Moreover, AASK showed equivalent BP reductions among ramipril‐based therapies that included loop diuretics and other agents as needed and the CCB‐ and β‐blocker‐based therapies. 35 None of the treatment regimens had a significant effect on cardiovascular events, possibly because of the limited power of the study. 36 The Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) 37 study assessed the effects of losartan (an ARB)‐based treatment on renal and CVD outcomes in 1513 patients with type 2 diabetes and nephropathy, of whom 230 (15%) were black. Losartan‐based therapy significantly reduced signs of renal disease progression by 16% (P=.02) compared with a regimen that did not include an ACE inhibitor or an ARB and was generally well tolerated; unfortunately, subgroup analysis of black patients was not provided. 37 In the recently reported Diovan Reduction of Proteinuria (DROP) 38 study, 391 patients (110 blacks) with hypertension, type 2 diabetes, and albuminuria were randomized to valsartan 160 mg, 320 mg, or 640 mg (plus other medication) for 30 weeks. By study end, twice as many patients returned to normoalbuminuria with valsartan 640 mg than with 160 mg (24% vs 12%; P<.01). The high‐dose ARB was well tolerated. 38 Preliminary data suggest that blacks required high valsartan dosages (640 mg/d) to reach similar BP reduction and control rates compared with nonblack matched controls. 39 Therefore, regardless of race or ethnicity, it appears that the use of an RAS‐blocking agent as one component of therapy in hypertensive patients with significant renal disease is indicated.
CVD OUTCOMES
The Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) study 40 evaluated the benefits of an ARB‐based compared with an atenolol‐based treatment regimen in older patients (N=9193) with essential hypertension and left ventricular hypertrophy. CVD morbidity and mortality were significantly reduced by 13% (P=.021) with the losartan‐based regimen. 40 Most of the benefits were noted with reduction of strokes. More than 70% of patients were also receiving a diuretic. However, an analysis of the black subgroup (6%; n=533) showed that the hazard ratio for the primary composite end point (fatal and nonfatal MI and CVD mortality) favored the β‐blocker atenolol in these patients (hazard ratio, 1.666; P=.033), whereas it favored the ARB losartan in whites (hazard ratio, 0.829; P=.003). 41 BP reductions in black patients were similar in both treatment groups, and the regression of electrocardiographic left ventricular hypertrophy was greater with losartan, making the difference in CVD mortality outcome difficult to explain. 41 However, these results should be interpreted with caution because black patients accounted for a relatively small proportion of the entire trial cohort, and this subgroup experienced a small number (75) of overall events. 41
In general, interpretation of subgroup analyses using small cohorts from large randomized trials is problematic.
The Valsartan Antihypertensive Long‐Term Use Evaluation (VALUE) 32 was designed to test the hypothesis that at similar levels of BP control, ARB treatment based on valsartan would lead to greater reductions in CVD morbidity and mortality than an amlodipine‐based regimen in high‐risk patients with hypertension. The primary outcome did not differ between the 2 treatment groups; however, BP reductions were greater with amlodipine, especially early in the trial. As noted, the incidence of new‐onset diabetes was significantly (P<.0001) lower in the valsartan group, but overall CVD events were lower in the CCB group; it has been assumed that the greater reduction in BP might have accounted for the difference in outcome. 32 Although only 4% (639 of 15,245) of the VALUE patient population was black, the VALUE findings underscore the importance of prompt BP control in hypertensive patients and suggest that clinicians should use any means necessary to lower BP effectively. 32
ONGOING TRIALS
A number of studies are currently under way that may help to further elucidate the role of RAS inhibition in preventing CVD, renal, and metabolic morbidity and mortality. 42 , 43 Ongoing and future clinical trials may provide additional evidence regarding the value of RAS blockade in high‐risk and black patients.
CONCLUSIONS
Blacks have a disproportionately greater burden of morbidity and mortality from cardiovascular and renal causes. Hypertension, which is highly prevalent in blacks, is a key target for risk reduction. Therapeutic lifestyle modifications effectively reduce BP in this population; however, most patients will also require pharmacologic intervention to achieve BP goals. Because of the high frequency of diabetes and kidney disease in black patients with hypertension, they may benefit from treatment with agents that block the RAS; however, most often the addition of a thiazidetype diuretic or long‐acting CCB will be required for adequate BP reduction. The ISHIB guidelines state that 2‐drug therapy should be considered as a first‐step in blacks when systolic BP is ≥15 mm Hg or diastolic BP is ≥10 mm Hg above target level, regardless of the presence or absence of comorbid conditions (155/100 or 145/90 mm Hg depending on comorbidities). Clinical trials demonstrate the efficacy of antihypertensive therapy with RAS inhibitors as part of a treatment regimen in lowering BP and reducing CVD risk. Several ongoing studies should further clarify the role of these agents in the prevention of cardiac, metabolic, and renal disease in high‐risk patients. The primary means of “stopping the clock” in the progression of CVD and renal disease includes efforts toward prevention, early detection, and control of hypertension and other modifiable risk factors. More effective use of therapeutic lifestyle and pharmacologic interventions can substantially reduce CVD morbidity and mortality in black individuals to a greater degree than has been accomplished to date.
References
- 1. Fields LE, Burt VL, Cutler JA, et al. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398–404. [DOI] [PubMed] [Google Scholar]
- 2. American Heart Association . Heart Disease and Stroke Statistics: 2007 Update. Dallas, TX: American Heart Association; 2007. [Google Scholar]
- 3. Ong KL, Cheung BMY, Man YB, et al. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. [DOI] [PubMed] [Google Scholar]
- 4. Douglas JG, Bakris GL, Epstein M, et al. Management of high blood pressure in African Americans: consensus statement of the Hypertension in African Americans Working Group of the International Society on Hypertension in Blacks. Arch Intern Med. 2003;163:525–541. [DOI] [PubMed] [Google Scholar]
- 5. Ferdinand KC, Saunders E. Hypertension‐related morbidity and mortality in African Americans: why we need to do better. J Clin Hypertens (Greenwich). 2006;8(suppl 1):21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferdinand KC. Recommendations for the management of special populations: racial and ethnic populations. Am J Hypertens. 2003;16:50s–54s. [DOI] [PubMed] [Google Scholar]
- 7. Smith SC Jr, Clark LT, Cooper RS, et al. Discovering the full spectrum of cardiovascular disease: Minority Health Summit 2003: report of the Obesity, Metabolic Syndrome, and Hypertension Writing Group. Circulation. 2005;111:e134–e139. [DOI] [PubMed] [Google Scholar]
- 8. Nesbitt SD. Hypertension in black patients: special issues and considerations. Curr Hypertens Rep. 2005;7:244–248. [DOI] [PubMed] [Google Scholar]
- 9. Vita JA. Nitric oxide and vascular reactivity in African American patients with hypertension. J Card Fail. 2003;9:s199–s204. [DOI] [PubMed] [Google Scholar]
- 10. Popkin BM, Siega‐Riz AM, Haines PS, et al. Where's the fat? trends in U.S. diets 1965‐1996. Prev Med. 2001;32:245–254. [DOI] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention. Physical activity levels among children aged 9–13 years: United States, 2002. MMWR Morb Mortal Wkly Rep. 2003;52:785–788. [PubMed] [Google Scholar]
- 12. Tarver‐Carr ME, Powe NR, Eberhardt MS, et al. Excess risk of chronic kidney disease among African‐American versus white subjects in the United States: a population‐based study of potential explanatory factors. J Am Soc Nephrol. 2002;13:2363–2370. [DOI] [PubMed] [Google Scholar]
- 13. US Renal Data System. USRDS 2006 Annual Data Report: Atlas of End‐Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2006. [Google Scholar]
- 14. National Diabetes Education Program. The Diabetes Epidemic Among African Americans Fact Sheet. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health; 2005. [Google Scholar]
- 15. Wong ND, Lopez V, Tang S, et al. Prevalence, treatment, and control of combined hypertension and hypercholesterolemia in the United States. Am J Cardiol. 2006;98:204–208. [DOI] [PubMed] [Google Scholar]
- 16. Ferdinand KC. Hypertension in minority populations. J Clin Hypertens (Greenwich). 2006;8:365–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bosworth HB, Dudley T, Olsen MK, et al. Racial differences in blood pressure control: potential explanatory factors. Am J Med. 2006;119(1):70.e9–70.e15. [DOI] [PubMed] [Google Scholar]
- 18. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 19. Prospective Studies Collaboration . Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 20. Svetkey LP, Simons‐Morton D, Vollmer WM, et al; DASH Research Group . Effects of dietary patterns on blood pressure: subgroup analysis of the Dietary Approaches to Stop Hypertension (DASH) randomized clinical trial. Arch Intern Med. 1999;159:285–293. [DOI] [PubMed] [Google Scholar]
- 21. Cook NR, Cutler JA, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow‐up of the trials of hypertension prevention (TOHP). BMJ. 2007;334(7599):885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Evenson KR, Rosamond WD, Cai J, et al; Atherosclerosis Risk in Communities (ARIC) Study Investigators. Physical activity and ischemic stroke risk: the Atherosclerosis Risk in Communities Study. Stroke. 1999;30:1333–1339. [DOI] [PubMed] [Google Scholar]
- 23. Nissen SE, Tuzcu EM, Libby P, et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA. 2004;292:2217–2226. [DOI] [PubMed] [Google Scholar]
- 24. Moser M, Lunn J. Responses to captopril and hydrochlorothiazide in black patients with hypertension. Clin Pharmacol Ther. 1982;32(3):307–312. [DOI] [PubMed] [Google Scholar]
- 25. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid‐Lowering Treatment to prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 26. Wright JT Jr, Dunn JK, Cutler JA, et al; ALLHAT Collaborative Research Group . Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA. 2005;293:1595–1608. [DOI] [PubMed] [Google Scholar]
- 27. Julius S, Nesbitt SD, Egan BM, et al; Trial of Preventing Hypertension (TROPHY) Study Investigators. Feasibility of treating prehypertension with an angiotensin‐receptor blocker. N Engl J Med. 2006;354:1685–1697. [DOI] [PubMed] [Google Scholar]
- 28. Weir MR, Smith DHG, Neutel JM, et al. Valsartan alone or with a diuretic or ACE inhibitor as treatment for African American hypertensives: relation to salt intake. Am J Hypertens. 2001;14:665–671. [DOI] [PubMed] [Google Scholar]
- 29. Flack JM, Saunders E, Gradman A. Antihypertensive efficacy and safety of losartan alone and in combination with hydrochlorothiazide in adult African Americans with mild to moderate hypertension. Clin Ther. 2001;23:1193–1208. [DOI] [PubMed] [Google Scholar]
- 30. Weir MR, Ferdinand KC, Flack JM, et al; AADVANCE investigators . A noninferiority comparison of valsartan/hydrochlorothiazide combination versus amlodipine in black hypertensives. Hypertension. 2005;46:508–513. [DOI] [PubMed] [Google Scholar]
- 31. Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM‐Overall programme. Lancet. 2003;362:759–766. [DOI] [PubMed] [Google Scholar]
- 32. Julius S, Kjeldsen SE, Weber M, et al; VALUE trial group . Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. [DOI] [PubMed] [Google Scholar]
- 33. The DREAM trial investigators . Effect of ramipril on the incidence of diabetes. N Engl J Med. 2006;355:1551–1562. [DOI] [PubMed] [Google Scholar]
- 34. Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta‐analysis. Lancet. 2007;369:201–207. [DOI] [PubMed] [Google Scholar]
- 35. Agodoa LY, Appel L, Bakris GL, et al. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA. 2001;285:2719–2728. [DOI] [PubMed] [Google Scholar]
- 36. Norris K, Bourgoigne J, Gassman J, et al; AASK Study Group . Cardiovascular outcomes in the African American Study of Kidney Disease and hypertension (AASK) trial. Am J Kidney Dis. 2006;48:739–751. [DOI] [PubMed] [Google Scholar]
- 37. Brenner BM, Cooper ME, De Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. [DOI] [PubMed] [Google Scholar]
- 38. Hollenberg NK, Parving HH, Viberti G, et al. Albuminuria response to very high‐dose valsartan in type 2 diabetes mellitus. J Hypertens. 2007;25:1921–1926. [DOI] [PubMed] [Google Scholar]
- 39. Weir MR, Hollenberg NK, Daley WL, et al. Dose‐related antihypertensive effects of valsartan therapy in African American patients with type 2 diabetes: the Diovan Reduction of Proteinuria (DROP) study. J Clin Hypertens (Greenwich). 2007;9(suppl A):A186. Abstract p‐446 Mp‐4. [Google Scholar]
- 40. Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- 41. Julius S, Alderman MH, Beevers G, et al. Cardiovascular risk reduction in hypertensive black patients with left ventricular hypertrophy: the LIFE study. J Am Coll Cardiol. 2004;43:1047–1055. [DOI] [PubMed] [Google Scholar]
- 42. The ONTARGET/TRANSCEND Investigators . Rationale, design, and baseline characteristics of 2 large, simple, randomized trials evaluating telmisartan, ramipril, and their combination in high‐risk patients: the Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial/Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND) trials. Am Heart J. 2004;148:52–61. [DOI] [PubMed] [Google Scholar]
- 43. McMurray JJ, Califf R, Holman R, et al. Cardiologists should care about glucose: most people with CV disease or risk factors have diabetes or significant glycaemic abnormalities. Results of screening over 39,000 subjects for NAVIGATOR . Eur Heart J. 2004;25(suppl):239 [Abstract 1406]. [Google Scholar]


