Abstract
The efficacy and safety of the angiotensin receptor blocker olmesartan medoxomil (OLM) was assessed in 550 elderly Japanese hypertensive patients who were followed for 24 weeks in daily clinical practice. Patients were given OLM alone or in combination with other antihypertensive drugs at the discretion of the investigators. After 24 weeks of treatment, systolic and diastolic blood pressure (BP) significantly decreased from baseline (P<.0001). When patients were classified as either young‐old (65–74 years) or older‐old (75 years and older), with either isolated systolic hypertension (ISH) or systolic‐diastolic hypertension (SDH), the reduction of diastolic BP in ISH patients was significantly smaller than that in SDH patients (5.0 vs 15.2 mm Hg; P<.0001), indicating that OLM did not cause excessive reduction of diastolic BP in ISH patients. Treatment was well tolerated in all groups. In conclusion, the medication was safe and effective in reducing BP levels in ISH patients aged 75 years and older, as well as in other elderly hypertensive patients.
Hypertension in the elderly is characterized by elevated systolic blood pressure (SBP) and normal diastolic blood pressure (DBP), a condition known as isolated systolic hypertension (ISH). This phenomenon in elderly individuals is caused by the progressive increase in arterial stiffness accompanying advanced age, resulting in decreased elasticity of blood vessels and widened pulse pressure (PP), the difference between SBP and DBP. 1 , 2 ISH is especially prevalent in individuals aged 75 years and older.
Elevated SBP and PP are considered risk factors for cardiovascular diseases such as stroke, ischemic heart disease, and heart failure. 3 , 4 , 5 , 6 , 7 The Systolic Hypertension in the Elderly Program (SHEP) Study 8 and Systolic Hypertension in Europe (Syst‐Eur) Trial 9 demonstrated that the risk of cardiovascular events is reduced by lowering SBP and PP with antihypertensive drugs, specifically a diuretic‐based regimen in the SHEP study and a calcium channel blocker (CCB)‐based treatment program in the Syst‐Eur trial. Thus, management of hypertension in elderly patients with elevated SBP and PP is recommended. 10 , 11 , 12
In some elderly hypertensive patients with ISH, there are concerns that pharmacologic antihypertensive therapy to reduce SBP might lower DBP excessively as an unwanted adverse drug reaction (ADR). Findings from the SHEP study demonstrated a trend toward a slight increase in cardiovascular events in patients in whom DBP was reduced excessively to levels <55 to 60 mm Hg, although in small numbers of patients with DBP levels of 45 to 50 mm Hg, this effect was not seen. 13 Therefore, it is considered desirable to reduce SBP without excessively reducing DBP in patients with ISH. In ISH patients treated with agents such as diuretics, this trend toward slightly elevated risk of cardiovascular events is usually not seen because SBP is reduced to a greater extent than DBP (ie, PP is decreased).
Angiotensin II type 1 (AT1) receptor blockers (ARBs) are among the 6 classes of antihypertensive drugs recommended as possible initial therapy for hypertension. 14 , 15 Olmesartan medoxomil (OLM) is an ARB that exhibits high AT1 receptor selectivity. 16 , 17 , 18 , 19 , 20 , 21 OLM monotherapy or in combination with CCBs, diuretics, or angiotensin‐converting enzyme (ACE) inhibitors can be safely used in both young‐old (aged 65–74 years) and older‐old (aged 75 years and older) patients. 22 Some data suggest that ARBs are not as effective as CCBs in elderly patients. 23
Few reports have focused specifically on the efficacy and safety of ARBs in older‐old patients with ISH. In the present study, we performed a post hoc analysis of previous data to assess the efficacy and safety of OLM as monotherapy or in combination with other antihypertensive agents in young‐old and older‐old patients with ISH as well as in those with systolic‐diastolic hypertension (SDH).
METHODS
Study Design and Participants
The present study followed an open, prospective cohort design. The study protocol conformed to Japanese pharmaceutical affairs law and was approved by the In‐house Ethical Committee of Sankyo (now Daiichi Sankyo) and the Ministry of Health, Labor and Welfare of Japan. This study was carried out in medical institutions registered according to Good Post‐Marketing Surveillance Practice in Japan.
Participants were OLM‐naïve hypertensive patients aged 65 years and older. Physicians from several medical institutions were asked to select and register patients at the Registration Center within 14 days of examining them. The registration period lasted for 6 months from July to December 2004.
Drug Administration
Based on indications and doses described in the Japanese package insert, patients were given OLM (mainly 5 or 10 mg/d) either alone, in combination with other drugs, or by switching from other antihypertensive medications. If BP remained uncontrolled, the dose of OLM could be increased at the discretion of the investigators. Use of concomitant therapy was not restricted. Treatment was given for a standard observation period of 24 weeks.
Main Outcome Measures and Data Analysis
Efficacy and safety were the main outcome measures. BP, clinical laboratory test results, and ADRs were recorded. Patients were classified according to age as young‐old (aged 65–74 years) or older‐old (aged 75 years and older) at enrollment and to BP level just before starting OLM therapy, with no wash‐out period. Those with SBP/DBP ≥140/≥90 mm Hg were defined as having SDH, whereas individuals whose levels were ≥140/<90 mm Hg were defined as having ISH.
Efficacy of treatment with the ARB was assessed in all patients who completed the study except in those with poor OLM compliance based on patients' interviews. Safety was assessed in terms of laboratory parameters and ADRs.
Statistical Analysis
Baseline characteristics were compared between ISH and SDH patients using the t test for continuous variables and Fisher exact test for categorical variables. The time course of changes in BP was analyzed by Dunnett test for comparison against baseline level. BP and PP at 24 weeks were compared with baseline levels by paired t test. Changes of BP and PP from baseline levels were compared between ISH and SDH patients by t test. The incidence rate of ADRs was analyzed by Fisher exact test. The 2‐tailed test was used for the analysis and P values <5% were defined as significant. Continuous variables were expressed as mean ± SD, and categorical variables were expressed as rates (%). ADRs were classified based on the preferred term from the Medical Dictionary for Regulatory Activities. Statistical analyses were performed using SAS System Release 8.2 (SAS Institute Inc, Cary, NC). This study was performed as a post hoc analysis of a previous study conducted in 646 patients. 22
RESULTS
Study Population and Patient Disposition
A total of 646 patients treated at 102 medical institutions in Japan were enrolled during the registration period. Of these patients, 12 did not subsequently revisit their hospital and hence were lost to follow‐up. In addition, 80 patients who subsequently were found not to meet the criteria for ISH or SDH were removed from this analysis. Safety was evaluated in 554 patients. Of these, 282 patients were young‐old (SDH, n=141; ISH, n=141) and 272 older‐old (SDH, n=110; ISH, n=162). A total of 4 patients who violated the inclusion criteria (2 patients who were not enrolled within 14 days and 2 with poor drug compliance) were excluded from the efficacy assessment; therefore, efficacy was assessed in 550 patients. Of these, 280 patients were young‐old (SDH, n=140; ISH, n=140) and 270 older‐old (SDH, n=109; ISH, n=161).
Baseline Characteristics
The baseline characteristics of patients in whom efficacy was evaluated are summarized in Table I. Mean age was 74.8 years (range, 65–95 years). Most patients (≥98%) were outpatients. All patients were diagnosed as having essential hypertension, except for one in the young‐old ISH group who was diagnosed as having arteriosclerosis. Baseline SBP/DBP in SDH patients either newly diagnosed (n=251) or already on therapy (n=299) was significantly higher than in ISH patients in both age groups (P≤.0001). Baseline PP in ISH was significantly higher than in SDH patients irrespective of age group (P<.0001).
Table I.
Baseline Characteristics of the Study Population by Age and Type of Hypertension
| Characteristic | Young‐Old (Age, 65–74 Y) | Older‐Old (Age, 75 Y and Older) | ||
|---|---|---|---|---|
| SDH (n=140) | ISH (n=140) | SDH (n=109) | ISH (n=161) | |
| Age, y | 69.6±2.8 | 70.3±2.7a | 78.9±3.5 | 80.4±4.3a |
| Women | 82 (58.6) | 101 (72.1)a | 81 (74.3) | 117 (72.7) |
| BMI, kg/m2 | 24.1±4.2 | 23.5±2.8 | 22.9±3.7 | 22.8±3.4 |
| SBP, mm Hg | 166.8±15.5 | 160.0±11.8b | 168.1±14.7 | 161.5±11.6b |
| DBP, mm Hg | 96.2±7.5 | 79.5±7.1b | 94.4±5.7 | 78.8±7.5b |
| PP, mm Hg | 70.6±14.2 | 80.5±14.0b | 73.6±13.8 | 82.7±12.1b |
| Pulse rate, beats per minute | 73.9±11.4 | 71.9±9.1 | 75.0±11.4 | 72.5±10.5 |
| Previous antihypertensive medication | ||||
| None | 82 (58.6) | 55 (39.3) | 61 (56.0) | 53 (32.9) |
| Diuretic | 5 (3.6) | 12 (8.6) | 4 (3.7) | 18 (11.2) |
| α‐blocker | 3 (2.1) | 5 (3.6) | 0 (0) | 8 (5.0) |
| β‐blocker | 8 (5.7) | 7 (5.0) | 7 (6.4) | 11 (6.8) |
| CCB | 33 (23.6) | 66 (47.1) | 37 (33.9) | 83 (51.6) |
| ACE‐inhibitor | 12 (8.6) | 13 (9.3) | 4 (3.7) | 14 (8.7) |
| ARB | 18 (12.9) | 15 (10.7) | 10 (9.2) | 32 (19.9) |
| Others | 0 (0) | 0 (0) | 0 (0) | 1 (0.6) |
| Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CCB, calcium channel blocker; DBP, diastolic blood pressure; ISH, isolated systolic hypertension; PP, pulse pressure; SBP, systolic blood pressure; SDH, systolic‐diastolic hypertension. Values are mean ± SD or No. (%). a P<.05. b P<.0001 (t test for continuous variables, Fisher exact test for categorical variables compared with SDH). | ||||
The most frequently observed comorbid condition at baseline in either group was hyperlipidemia (33%–49%). Diabetes mellitus was the second most frequently observed complication in the young‐old group (approximately 20%), whereas heart disease was the second most frequent complication in older‐old patients (approximately 23%).
Most patients (72.4%) were taking other medications as well, including other antihypertensive drugs such as CCBs (30.9%), diuretics (6.5%), and β‐blockers (4.7%); lipid‐lowering drugs such as statins (26.2%); and antidiabetic agents (9.5%) (Table I).
Drug Dose
The average initial/maximum daily doses of OLM were 9.7±3.2/12.5±4.9 mg in the young‐old SDH group, 10.1±4.2/12.9±6.5 mg in the young‐old ISH group, 6.8±3.4/10.8±5.1 mg in the older‐old SDH group, and 6.6±3.0/11.6±5.8 mg in the older‐old ISH group, respectively (Table II). The most frequently used initial daily dose of OLM was 10 mg in ≥70% of young‐old patients and 5 mg in ≥70% of older‐old patients. During the study period, the most frequently used maximum daily dose was 10 mg, followed by 5 mg and 20 mg (Table II). About 76% of young‐old SDH patients received OLM monotherapy at baseline, and 67% of them were still on OLM monotherapy after 24 weeks. About 47% of older‐old ISH patients received OLM monotherapy at baseline, and 44% of them were still on OLM monotherapy after 24 weeks. The most frequently used concomitant antihypertensive drug at baseline was a CCB in ≥20% of young‐old and older‐old patients, followed by a diuretic (≥3%) and a β‐blocker (≥3%); after 24 weeks, the most common was a CCB in ≥26%, followed by a diuretic (≥6%) and a β‐blocker (≥3%) (Table II).
Table II.
Antihypertensive and Other Medication Taken by Study Participants
| Young‐Old | Older‐Old | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SDH | ISH | SDH | ISH | |||||||||
| Weeks | 0 (n=l40) | 12 (n=126) | 24 (n=122) | 0 (n=l40) | 12 (n=134) | 24 (n=130) | 0 (n=109) | 12 (n=95) | 24 (n=87) | 0 (n=161) | 12 (n=153) | 24 (n=l42) |
| OLM dose, mg | 9.7±3.2 | 11.9±4.9 | 12.3±4.9 | 10.1±4.2 | 12.1±5.9 | 12.6±6.6 | 6.8±3.4 | 10.3±4.5 | 10.7±4.9 | 6.6±3.0 | 10.6±5.2 | 11.4±5.7 |
| 5 mg | 24 (17.1) | 13 (10.3) | 9 (7.4) | 22 (15.7) | 9 (6.7) | 10 (7.7) | 78 (71.6) | 22 (23.2) | 19 (21.8) | 117 (72.7) | 45 (29.4) | 38 (26.8) |
| 10 mg | 108 (77.1) | 82 (65.1) | 80 (65.6) | 107 (76.4) | 99 (73.9) | 89 (68.5) | 27 (24.8) | 59 (62.1) | 52 (59.8) | 40 (24.8) | 77 (50.3) | 65 (45.8) |
| 20 mg | 8 (5.7) | 31 (24.6) | 33 (27.0) | 10 (7.1) | 23 (17.2) | 27 (20.8) | 4 (3.7) | 14 (14.7) | 16 (18.4) | 4 (2.5) | 31 (20.3) | 39 (27.5) |
| 40 mg | 0 (0) | 0 (0) | 0 (0) | 1 (0.7) | 3 (2.2) | 4 (3.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Concomitant drugs | ||||||||||||
| Antihypertensive | 34 (24.3) | 39 (31.0) | 40 (32.8) | 55 (39.3) | 55 (41.0) | 58 (44.6) | 36 (33.0) | 39 (41.1) | 37 (42.5) | 86 (53.4) | 83 (54.2) | 79 (55.6) |
| Diuretic | 5 (3.6) | 9 (7.1) | 8 (6.6) | 8 (5.7) | 7 (5.2) | 9 (6.9) | 5 (4.6) | 6 (6.3) | 7 (8.0) | 18 (11.2) | 14 (9.2) | 14 (9.9) |
| α‐blocker | 2 (1.4) | 2 (1.6) | 2 (1.6) | 4 (2.9) | 2 (1.5) | 3 (2.3) | 0 (0) | 1 (1.1) | 3 (3.4) | 5 (3.1) | 5 (3.3) | 3 (2.1) |
| β‐blocker | 5 (3.6) | 6 (4.8) | 5 (4.1) | 5 (3.6) | 4 (3.0) | 4 (3.1) | 7 (6.4) | 8 (8.4) | 9 (10.3) | 9 (5.6) | 9 (5.9) | 9 (6.3) |
| CCB | 28 (20.0) | 30 (23.8) | 32 (26.2) | 46 (32.9) | 49 (36.6) | 49 (37.7) | 30 (27.5) | 30 (31.6) | 31 (35.6) | 66 (41.0) | 67 (43.8) | 64 (45.1) |
| ACE inhibitor | 2 (1.4) | 2 (1.6) | 2 (1.6) | 4 (2.9) | 4 (3.0) | 5 (3.8) | 0 (0) | 0 (0) | 0 (0) | 6 (3.7) | 5 (3.3) | 4 (2.8) |
| ARB | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.6) | 1 (0.7) | 1 (0.7) |
| Potassium‐sparing diuretic | 1 (0.7) | 1 (0.8) | 0 (0) | 1 (0.7) | 1 (0.7) | 1 (0.8) | 1 (0.9) | 1 (1.1) | 0 (0) | 4 (2.5) | 4 (2.6) | 4 (2.8) |
| Lipid‐lowering drug | 34 (24.3) | 30 (23.8) | 31 (25.4) | 48 (34.3) | 49 (36.6) | 49 (37.7) | 25 (22.9) | 25 (26.3) | 23 (26.4) | 37 (23.0) | 40 (26.1) | 40 (28.2) |
| Antidiabetic drug | 14 (10.0) | 12 (9.5) | 12 (9.8) | 16 (11.4) | 16 (11.9) | 15 (11.5) | 6 (5.5) | 7 (7.4) | 7 (8.0) | 16 (9.9) | 16 (10.5) | 16 (11.3) |
| Other | 51 (36.4) | 48 (38.1) | 50 (41.0) | 72 (51.4) | 70 (52.2) | 69 (53.1) | 63 (57.8) | 56 (58.9) | 50 (57.5) | 98 (60.9) | 98 (64.1) | 89 (62.7) |
| Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; ISH, isolated systolic hypertension; OLM, olmesartan medoxomil; SDH, systolic‐diastolic hypertension. Values are mean ± SD or No. (%). | ||||||||||||
Efficacy
The time course of changes in BP is shown in Figure 1. SBP and DBP were significantly reduced starting from 2 weeks of treatment (P<.0001) and BP‐lowering effects were maintained from 4 weeks to the end of the observation period at 24 weeks.
Figure 1.
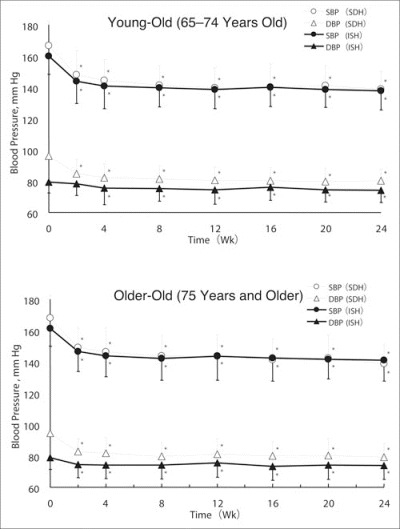
The time course of changes in blood pressure over the 24‐week course of treatment for the 4 study groups. DBP indicates diastolic blood pressure; ISH, isolated systolic hypertension; SBP, systolic blood pressure; SDH, systolic‐diastolic hypertension. *P<.0001 vs baseline.
Changes in SBP/DBP and PP at 24 weeks compared with baseline are shown in Figure 2. SBP/DBP in ISH patients was reduced by −22.5/−5.2 and −20.6/−4.8 mm Hg in young‐old and older‐old, respectively. DBP reductions were significantly less in ISH than in SDH patients in both age groups (P<.0001). DBP reductions in all ISH patients (5.0±9.1 mm Hg) also were significantly less than in all SDH patients (15.2±10.1 mm Hg; P<.0001). After 24 weeks of treatment, reductions in PP were significantly (P<.001) smaller in SDH patients than in ISH patients categorized as young‐old; the trend was the same but the difference did not achieve statistical significance in older‐old patients.
Figure 2.
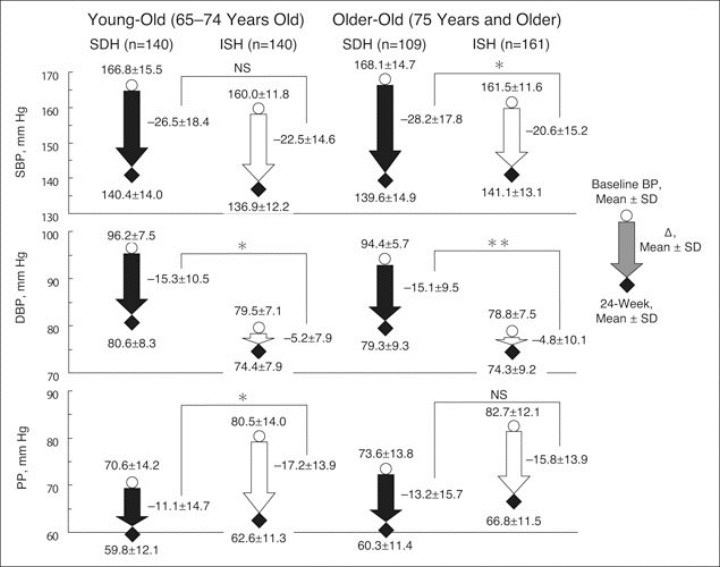
Changes in systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse pressure (PP) at 24 weeks compared with baseline for the 4 study groups. ISH indicates isolated systolic hypertension; SDH, systolic‐diastolic hypertension. *P<.001 SDH vs ISH; **P<.0001 SDH vs ISH.
Safety
The number of patients who had ADRs was 8 (5.67%) in the young‐old SDH group, 4 (2.84%) in the young‐old ISH group, 6 (5.45%) in the older‐old SDH group, and 9 (5.56%) in the older‐old ISH group. No statistical difference was detected among the 4 groups (P=.6309). ADRs that might have been associated with excessive lowering of BP are summarized in Table III. All of these were mild, and the cumulative incidence rate and severity were not different in any of the groups. Clinical laboratory evaluations (serum blood urea nitrogen and creatinine) showed little change from baseline to the final visit regardless of group.
Table III.
Adverse Drug Reactions That Might Have Been Associated With Excessive Blood Pressure Reduction and Abnormality of Renal Function Test in Study Participants
| Young‐Old | Older‐Old | |||
|---|---|---|---|---|
| SDH (n=141) | ISH (n=141) | SDH (n=110) | ISH (n=162) | |
| Dizzinessa | 1 (0.7) | 1 (1.4) | 1 (0.9) | ‐ |
| Hypotension | 1 (0.7) | ‐ | ‐ | ‐ |
| Blood pressure decrease | 1 (0.7) | ‐ | ‐ | ‐ |
| BUN increase | 1 (0.7) | ‐ | ‐ | 1 (0.6) |
| Serum creatinine increase | ‐ | ‐ | ‐ | 1 (0.6) |
| Abbreviation: BUN, blood urea nitrogen. Values are No. (%). aNo significant difference was found among the 4 groups (P=.6309, Fisher exact test). | ||||
DISCUSSION
In the present study, OLM alone or in combination with other antihypertensive medications decreased BP in all 4 groups of elderly hypertensive patients and with low incidence rates of ADRs. After 24 weeks of treatment, ≥44% of patients were still on OLM monotherapy; the most frequently used concomitant antihypertensive drug was a CCB in ≥26%, followed by a diuretic (≥6%) and a β‐blocker (≥3%). SBP/DBP in ISH patients was reduced by −22.5/−5.2 and −20.6/−4.8 mm Hg in the young‐old and older‐old groups, respectively, indicating that the decrease was roughly the same regardless of age. Decreases in DBP in young‐old and older‐old patients with ISH were significantly smaller than those in young‐old and older‐old patients with SDH.
ISH was present in 43.3% and 52.6% of young‐old and older‐old patients, respectively. This is in accordance with the notion that vascular elasticity decreases with advancing age.
The most frequently used initial daily dose of OLM was 10 mg in ≥70% of young‐old and 5 mg in ≥70% of older‐old patients, suggesting that a more cautious approach was used in elderly hypertensive patients of more advanced age, as recommended. 10 , 11 , 12 The dose was increased in many cases during the treatment period; the daily dose was increased to ≥20 mg in approximately 30% of young‐old patients with ISH and SDH. Among older‐old patients, the dose was increased to 10 mg in many cases, with it being increased to ≥20 mg in 27.5% of ISH and 18.4% of SDH patients; a concomitant antihypertensive drug was used in 55.6% of ISH and 42.5% of SDH patients in the older‐old group, suggesting that ISH tend to be more refractory to therapy.
Incidence rates of ADRs were not significantly different among the 4 groups of patients and were not related to doses of OLM. In addition, timing of appearance and severity of ADRs varied considerably among patients. The incidence of ADRs that might have been associated with excessive BP lowering was investigated in detail and no difference in the rates was seen among the 4 groups. Thus, as with other medications such as CCBs and diuretics, OLM appears to be safe in ISH patients aged 75 years and older as well as in other elderly patients.
This study confirms the results of previous trials of ARBs as well as other medications in elderly European and US patients with ISH. In the Valsartan in Isolated Systolic Hypertension (Val‐Syst) study 24 conducted in 421 elderly patients with ISH (mean age, 69±6 years), DBP in the valsartan group (n=208) decreased by 6 mm Hg, whereas SBP decreased by 31 mm Hg. In a subanalysis of the Losartan Intervention For Endpoint Reduction (LIFE) in Hypertension study, 25 660 elderly patients with ISH (mean age, 70±6 years) who were treated with losartan experienced SBP and DBP reductions of 28 and 9 mm Hg, respectively. Furthermore, in the Study on Cognition and Prognosis in the Elderly (SCOPE), 26 a total of 1518 ISH patients aged 70 to 89 years achieved reductions of 22 and 6 mm Hg, respectively, following treatment with candesartan. Izzo and coworkers 27 reported that OLM 40 mg reduced SBP by 17.7 mm Hg in patients with ISH. In the pilot study of the Hypertension in the Very Elderly Trial (HYVET‐Pilot), use of an ACE inhibitor and diuretic reduced stroke events and mortality in patients older than 80 years. 28
Some limitations of the present study should be considered. The design was to represent the “real world” of clinical practice; consequently, patients were not blinded to treatment and no placebo comparison was used. Therefore, the contribution of placebo‐like effects over time is unknown. The results of the present study were qualitatively and quantitatively similar, however, to a previous study 27 and to a post hoc analysis of our previous study conducted in 6261 patients.
CONCLUSIONS
OLM, usually prescribed in combination with other antihypertensive agents, was safe and effective at lowering BP levels in elderly ISH patients aged 75 years and older, as well as in other elderly hypertensive patients.
Acknowledgments and disclosures:
The authors express their gratitude to the many doctors who cooperated in this survey of OLM in elderly hypertensive patients and provided many valuable data. We are thankful to Suzanne Oparil, MD, Vascular Biology and Hypertension Program, University of Alabama at Birmingham, for her comments and suggestions in the preparation of the manuscript. This study was supported for funding, data collection, and statistical analysis by Sankyo (now Daiichi Sankyo). Ikuo Saito, MD and Toshio Kushiro, MD, provided intellectual advice about the study concept and design as well as scientific interpretation of the results. Koji Hirata, Yuki Sato, Fumiaki Kobayashi, Kei Sagawa, Katsutoshi Hiramatsu, and Masahiro Komiya are employees of Daiichi Sankyo Co, Ltd.
References
- 1. O'Rourke M. Mechanical principles in arterial disease. Hypertension. 1995;26:2–9. [DOI] [PubMed] [Google Scholar]
- 2. London GM, Guerin AP. Influence of arterial pulse and reflected waves on blood pressure and cardiac function. Am Heart J. 1999;138:220–224. [DOI] [PubMed] [Google Scholar]
- 3. National High Blood Pressure Education Program Working Group . National High Blood Pressure Education Program Working Group Report on Hypertension in the Elderly. Hypertension. 1994;23:275–285. [PubMed] [Google Scholar]
- 4. Madhavan S, Ooi WL, Cohen H, et al. Relation of pulse pressure and blood pressure reduction to the incidence of myocardial infarction. Hypertension. 1994;23:395–401. [DOI] [PubMed] [Google Scholar]
- 5. Kannel WB. Historic perspectives on the relative contributions of diastolic and systolic blood pressure elevation to cardiovascular risk profile. Am Heart J. 1999;138:205–210. [DOI] [PubMed] [Google Scholar]
- 6. Domanski M, Mitchell G, Pfeffer M, et al. Pulse pressure and cardiovascular disease‐related mortality: follow‐up study of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA. 2002;287:2677–2683. [DOI] [PubMed] [Google Scholar]
- 7. Staessen JA, Li Y, Thijs L, et al. Blood pressure reduction and cardiovascular prevention: an update including the 2003–2004 secondary prevention trials. Hypertens Res. 2005;28:385–407. [DOI] [PubMed] [Google Scholar]
- 8. SHEP Cooperative Research Group . Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 9. Staessen JA, Fagard R, Thijs L, et al. Randomised double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators . Lancet. 1997;350:757–764. [DOI] [PubMed] [Google Scholar]
- 10. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 11. Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 12. Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH2004). Hypertens Res. 2006;29:(suppl):S1–S105. [DOI] [PubMed] [Google Scholar]
- 13. Somes GW, Pahor M, Shorr RI, et al. The role of DBP when treating isolated systolic hypertension. Arch Intern Med. 1999;159:2004–2009. [DOI] [PubMed] [Google Scholar]
- 14. Mori H, Ukai H, Yamamoto H, et al. Current status of antihypertensive prescription and associated blood pressure control in Japan. Hypertens Res. 2006;29:143–151. [DOI] [PubMed] [Google Scholar]
- 15. Murai K, Obara T, Ohkubo T, et al; J‐HOME Study Group . Current usage of diuretics among hypertensive patients in Japan: the Japan Home versus Office Blood Pressure Measurement Evaluation (J‐HOME) study. Hypertens Res. 2006;29:857–863. [DOI] [PubMed] [Google Scholar]
- 16. Koike H, Konse T, Sada T, et al. Olmesartan medoxomil, a novel potent angiotensin II blocker. Annu Rep Sankyo Lab. 2003;55:1–99. [Google Scholar]
- 17. Oparil S. Comparative antihypertensive efficacy of olmesartan: comparison with other angiotensin II receptor antagonists. J Hum Hypertens. 2002;16(suppl 2):S17–S23. [DOI] [PubMed] [Google Scholar]
- 18. Brunner HR, Laeis P. Clinical efficacy of olmesartan medoxomil. J Hypertens Suppl. 2003;21:S43–S46. [DOI] [PubMed] [Google Scholar]
- 19. Brunner HR. Clinical efficacy and tolerability of olmesartan. Clin Ther. 2004;26(suppl A):A28–A32. [DOI] [PubMed] [Google Scholar]
- 20. Stumpe KO. Olmesartan compared with other angiotensin II receptor antagonists: head‐to‐head trials. Clin Ther. 2004;26(suppl A):A33–A37. [DOI] [PubMed] [Google Scholar]
- 21. Giles TD, Oparil S, Silfani TN, et al. Comparison of increasing doses of olmesartan medoxomil, losartan potassium, and valsartan in patients with essential hypertension. J Clin Hypertens (Greenwich). 2007;9:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kushiro T, Saito I, Sato Y, et al. Results of special survey of Olmetec Tablets (olmesartan medoxomil) for the elderly [in Japanese]. J Clin Therap Med. 2006;22:699–714. [Google Scholar]
- 23. Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. [DOI] [PubMed] [Google Scholar]
- 24. Malacco E, Vari N, Capuano V, et al. A randomized, double‐blind, active‐controlled, parallel‐group comparison of valsartan and amlodipine in the treatment of isolated systolic hypertension in elderly patients: the Val‐Syst Study. Clin Ther. 2003;25:2765–2780. [DOI] [PubMed] [Google Scholar]
- 25. Kjeldsen SE, Dahlof B, Devereux RB, et al. Effects of losartan on cardiovascular morbidity and mortality in patients with isolated systolic hypertension and left ventricular hypertrophy: a Losartan Intervention for Endpoint Reduction (LIFE) substudy. JAMA. 2002;288:1491–1498. [DOI] [PubMed] [Google Scholar]
- 26. Papademetriou V, Farsang C, Elmfeldt D, et al. Stroke prevention with angiotensin II type 1 receptor blocker candesartan in elderly patients with isolated systolic hypertension: the Study on Cognition and Prognosis in the Elderly (SCOPE). J Am Coll Cardiol. 2004;44:1175–1180. [DOI] [PubMed] [Google Scholar]
- 27. Izzo JL Jr, Neutel JM, Silfani T, et al. Efficacy and safety of treating stage 2 systolic hypertension with olmesartan and olmesartan/HCTZ: results of an open‐label titration study. J Clin Hypertens (Greenwich). 2007;9:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bulpitt CJ, Beckett NS, Cooke J, et al. Results of the pilot study for the Hypertension in the Very Elderly Trial. J Hypertens. 2003;21:2409–2417. [DOI] [PubMed] [Google Scholar]


