There is an increasing prevalence of hypertension (HTN) and obesity in the world. According to the Third National Health and Nutrition Examination Survey (NHANES III), the prevalence of HTN increases progressively with increasing body mass index (BMI), from about 15% among people with a BMI <25 kg/m2 to approximately 40% among those with a BMI ≥30 kg/m2. 1 HTN commonly clusters with other cardiovascular risk factors (eg, insulin resistance [IR], central obesity, dyslipidemia, and dysglycemia) to form the cardiometabolic syndrome (CMS), which is associated with increased cardiovascular mortality. 2 A number of reports implicate aldosterone in the pathogenesis of CMS 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 ; however, definitive studies directly targeting mineralocorticoid activation to correct the syndrome have yet to be conducted.
The pathogenesis of CMS‐related HTN is complex and has not been fully elucidated. 3 However, there is accumulating evidence of interacting factors including IR; the renin‐angiotensin‐aldoster‐one system (RAAS); oxidative stress; sodium retention and volume expansion; increased sympathetic nervous system activity; and increased levels of free fatty acids, adipokines (except adiponectin), and inflammatory cytokines. Activation of systemic and local RAAS has been increasingly implicated in obesity‐associated HTN. 4 , 5 , 6 In this article, we will concentrate on the last mediator in the RAAS, which is aldosterone.
ALDOSTERONE AND IR
Compared with patients who have essential HTN, patients with primary hyperaldosteronism experience more cardiovascular events. 7 Plasma aldosterone levels are associated with CMS per se, independent of the association with its separate components. 8 In fact, primary hyperaldosteronism was linked to dysglycemia 4 decades ago. 9 Fallo and associates 10 reported that in primary hyperaldosteronism, there are higher blood glucose levels and a higher prevalence of CMS compared with essential HTN. In a subsequent study, they showed that IR as measured by homeostatic model assessment was higher and adiponectin levels were lower in primary hyperaldosteronism than in essential HTN. 11 It has also been shown that resection of aldosteroneproducing tumors improves IR and decreases blood insulin and glucose levels. 12 In murine brown fat tissue, aldosterone dose‐dependently decreased insulin‐induced glucose uptake by about 25% and increased mRNA of the proinflammatory adipokines. 13 Several other mechanisms of aldosterone‐induced IR have been suggested, including effects of hypokalemia on pancreatic β‐cell function, direct effects on insulin receptors/signaling, stimulation of hepatic gluconeogenesis, effects on sodium‐glucose transport, and fibrosis‐induced dysfunction in the insulin‐producing or insulin‐sensitive tissues. 14 On the other hand, it seems that human adipocytes produce a (yet to be identified) mineralocorticoid‐releasing factor, which stimulates adrenal aldosterone production via paracrine or endocrine mechanisms. 15
ALDOSTERONE AND HTN
In addition to classic effects on blood volume and sodium and potassium renal handling, aldosterone has been shown to be the mediator of several maladaptive changes in the nervous and cardiovascular systems that promote HTN and cardiovascular disease (Figure). Aldosterone, either directly or indirectly through an angiotensin II (Ang II) effect, impairs endothelium‐dependent relaxation. This is associated with oxidative stress in the vessel wall, resulting in decreased bioavailability of nitric oxide (NO). Further, treatment with spironolactone has been shown to improve NO bioavailability and endothelium‐dependent vasorelaxation. 5 , 16 , 17
Figure.
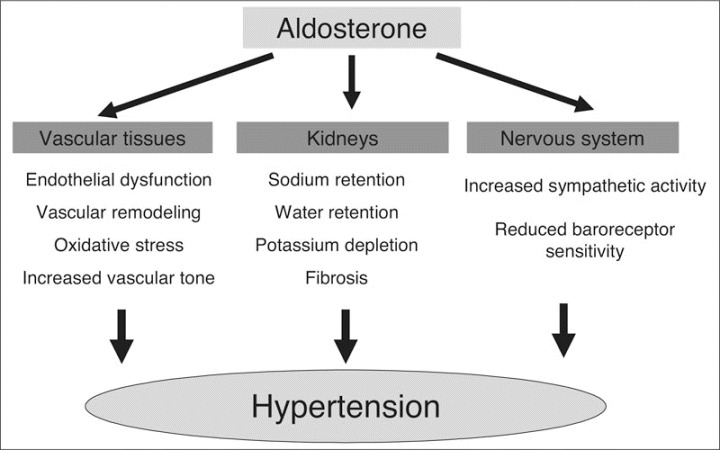
The hypertension‐promoting effects of aldosterone.
Aldosterone is involved in vascular injury and promotes collagen synthesis and fibrosis, leading to increased arterial stiffness.5, 16, 17 These maladaptive changes are augmented through cross talk between aldosterone and Ang II. Both Ang II and aldosterone stimulate vascular growth and remodeling. Along with the effects on other vascular beds, aldosterone has been implicated as a cause or contributor to pathologic hypertrophy of the glomerular mesangium and fibrosis of the renal interstitium. Also, aldosterone, acting via rapid nongenomic effects in vivo in humans, increases renal vascular resistance and thereby mediates arterial HTN if endothelial dysfunction is present. 18 Epstein and colleagues 19 conducted a multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group trial comparing the effects of concomitant eplerenone, an aldosterone antagonist, plus enalapril compared with enalapril plus placebo on albuminuria in 268 patients with type 2 diabetes mellitus. The use of eplerenone significantly reduced the urinary albumin:creatinine ratio independently of BP reduction. 19
Both cardiomyocytes and fibroblasts express mineralocorticoid receptor (MR) with a high affinity for both glucocorticoids and aldosterone. Aldosterone, like Ang II, can contribute to abnormal cardiac remodeling, including fibrosis and perivascular inflammation. 5 , 16 Further, we showed that a subpressor dose of spironolactone in Ren‐2 rats reduces cardiac fibrosis and remodeling as well as oxidative stress. These beneficial effects of MR blockade are mediated, in part, through inhibition of nicotinamide adenine dinucleotide phosphate‐oxidase. 20 Furthermore, it is important to note that aldosterone may exert its effects, in part, through stimulation of glucocorticoid receptors in tissues that express little 11β‐OH steroid dehydrogenase, such as the heart and vasculature. 16 , 20
In addition to vessels and the heart, Mrs have been found in the brain; aldosterone is also produced in the brain. 21 It is not clear, however, whether locally produced aldosterone exerts any significant physiologic or pathophysiologic effects. Experimental work has shown that activation of Mrs in the brain results in enhanced central sympathetic drive to the kidneys, heart, and vascular smooth muscle; increased release of vasopressin; and decreased baroreceptor sensitivity. Sodium intake is also increased by activation of Mrs in the brain, exacerbating HTN and associated end‐organ disease. 22 Much of the United States population consumes a high‐salt and high‐calorie diet, which likely facilitates the negative metabolic, cardiovascular, and renal effects of aldosterone. Accordingly, we will likely need to target both hygienic as well as pharmacologic approaches to reduce the detrimental effects of aldosterone.
CONCLUSIONS
The relationship between the RAAS and IR is complex. Aldosterone excess is involved in detrimental actions on glucose metabolism, HTN, and cardiovascular and renal disease. Collectively, these changes contribute to cardiovascular disease, as seen in CMS. While more research investigating the mechanism of interaction between aldosterone and IR is needed, it is increasingly apparent that therapeutic strategies targeting reductions in plasma aldosterone and/or blockade of Mrs should be considered not only for blood pressure lowering but also for improving other abnormalities that exist in CMS.
Disclosures:
This research was supported by the National Institutes of Health RO1‐HL073101 (JRS) and VA Merit 0018 (JRS).
References
- 1. Brown CD, Higgins M, Donato KA, et al. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000;8(9):605–619. [DOI] [PubMed] [Google Scholar]
- 2. Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease cardiovascular disease, and all causes in United States adults. Circulation. 2004;110(10):1245–1250. [DOI] [PubMed] [Google Scholar]
- 3. Stas SN, El‐Atat FA, Sowers JR. Pathogenesis of hypertension in diabetes. Rev Endocr Metab Disord. 2004;5(3):221–225. [DOI] [PubMed] [Google Scholar]
- 4. Sharma AM. Is there a rationale for angiotensin blockade in the management of obesity hypertension? Hypertension. 2004;44(1):12–19. [DOI] [PubMed] [Google Scholar]
- 5. Cooper SA, Whaley‐Connell A, Habibi J, et al. Renin‐angiotensin‐aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol. 2007;293(4):H2009–H2023. [DOI] [PubMed] [Google Scholar]
- 6. Manrique C, Lastra G, Whaley‐Connell A, et al. Hypertension and the cardiometabolic syndrome. J Clin Hypertens (Greenwich). 2005;7(8):471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Milliez P, Girerd X, Plouin PF, et al. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45(8):1243–1248. [DOI] [PubMed] [Google Scholar]
- 8. Bochud M, Nussberger J, Bovet P, et al. Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension. 2006;48(2):239–245. [DOI] [PubMed] [Google Scholar]
- 9. Conn JW. Hypertension, the potassium ion and impaired carbohydrate tolerance. N Engl J Med. 1965;273(21):1135–1143. [DOI] [PubMed] [Google Scholar]
- 10. Fallo F, Veglio F, Bertello C, et al. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab. 2006;91(2):454–459. [DOI] [PubMed] [Google Scholar]
- 11. Fallo F, Della Mea P, Sonino N, et al. Adiponectin and insulin sensitivity in primary aldosteronism. Am J Hypertens. 2007;20(8):855–861. [DOI] [PubMed] [Google Scholar]
- 12. Giacchetti G, Ronconi V, Turchi F, et al. Aldosterone as a key mediator of the cardiometabolic syndrome in primary aldosteronism: an observational study. J Hypertens. 2007;25(1):177–186. [DOI] [PubMed] [Google Scholar]
- 13. Kraus D, Jäger J, Meier B, et al. Aldosterone inhibits uncoupling protein‐1, induces insulin resistance, and stimulates proinflammatory adipokines in adipocytes. Horm Metab Res. 2005;37(7):455–459. [DOI] [PubMed] [Google Scholar]
- 14. Fallo F, Federspil G, Veglio F, et al. The metabolic syndrome in primary aldosteronism. Curr Hypertens Rep. 2007;9(2):106–111. [DOI] [PubMed] [Google Scholar]
- 15. Ehrhart‐Bornstein M, Arakelyan K, Krug AW, et al. Fat cells may be the obesity‐hypertension link: human adipogenic factors stimulate aldosterone secretion from adrenocortical cells. Endocr Res. 2004;30(4):865–870. [DOI] [PubMed] [Google Scholar]
- 16. McFarlane SI, Sowers JR. Aldosterone function in diabetes mellitus: effects on cardiovascular and renal disease. J Clin Endocrinol Metab. 2003;88(2):516–523. [DOI] [PubMed] [Google Scholar]
- 17. Schiffrin EL. Effects of aldosterone on the vasculature. Hypertension. 2006;47(3):312–318. [DOI] [PubMed] [Google Scholar]
- 18. Schmidt BM, Sammer U, Fleischmann I, et al. Rapid nongenomic effects of aldosterone on the renal vasculature in humans. Hypertension. 2006;47(4):650–655. [DOI] [PubMed] [Google Scholar]
- 19. Epstein M, Williams GH, Weinberger M, et al. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2006;1(5):940–951. [DOI] [PubMed] [Google Scholar]
- 20. Stas S, Whaley‐Connell A, Habibi J, et al. Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin‐angiotensin‐aldosterone system stimulation of reduced nicotinamide adenine dinucleotide phosphate oxidase and cardiac remodeling. Endocrinology. 2007;148(8):3773–3780. [DOI] [PubMed] [Google Scholar]
- 21. Sakai RR, McEwen BS, Fluharty SJ, et al. The amygdala: site of genomic and nongenomic arousal of aldosterone‐induced sodium intake. Kidney Int. 2000;57(4):1337–1345. [DOI] [PubMed] [Google Scholar]
- 22. Gomez‐Sanchez EP. Brain mineralocorticoid receptors: orchestrators of hypertension and end‐organ disease. Curr Opin Nephrol Hypertens. 2004;13(2):191–196. [DOI] [PubMed] [Google Scholar]


