Abstract
Patients with difficult to control hypertension typically require 2 or more agents to achieve goal blood pressure (BP) levels. Fixed‐dose combination therapies with lower doses generally are well tolerated and more effective than higher‐dose monotherapy. The authors performed prespecified and post hoc subgroup analyses of 2 double‐blind, randomized, placebo‐controlled trials that assessed the efficacy and safety of amlodipine and valsartan, alone and in combination, in patients with mild to moderate hypertension. Patients were randomized to amlodipine (study 1: 2.5 or 5 mg/d; study 2: 10 mg/d), valsartan (study 1: 40, 80, 160, or 320 mg/d; study 2: 160 or 320 mg/d), combination therapy across the same dose ranges, or placebo. Analyses were performed on changes from baseline in mean sitting systolic and diastolic BP and the occurrence of adverse events in specific subgroups of patients (ie, those with stage 2 hypertension [post hoc], the elderly [65 years or older], and blacks [both prespecified]). Amlodipine + valsartan combination therapy was associated with greater BP‐lowering effects in the subgroups compared with each respective monotherapy and placebo. These findings were consistent with the primary efficacy analysis results from the overall study populations. Combination regimens were generally well tolerated by all patient subgroups.
Hypertension is a highly prevalent condition, affecting nearly 1 in 3 adults ≥65 million persons) in the United States 1 , 2 , 3 , 4 and as many as 1 billion persons worldwide. 5 While improved awareness and control of hypertension during the past 30 years has resulted in substantial declines in mortality and morbidity associated with high blood pressure (BP), 5 prevalence rates have increased, 1 , 2 and an estimated 40% to 70% of patients in Europe and the United States do not achieve BP control (<140/90 mm Hg) despite the availability of numerous effective antihypertensive medications. 3 , 5 , 6 Hypertension is more prevalent in some patients, including the elderly and blacks. 3 The reasons why certain patient subgroups have a higher prevalence of hypertension are varied and complex; however, research suggests that arterial stiffness that occurs with aging may be an important underlying cause in the elderly. 7 The higher prevalence of hypertension among blacks likely results from multiple factors including a high‐salt diet and high prevalence rates of obesity and diabetes mellitus. 5 , 8
Data from the National Health and Nutrition Examination Survey (NHANES) 3 indicate not only a higher prevalence rate of hypertension but also lower control rates in treated patients 60 years or older compared with patients 40 to 59 years of age (50.0% vs 63.5%, respectively). Findings from NHANES also show that in blacks, hypertension prevalence rates for all age groups are significantly higher than in whites (13.3% vs 7.3% for 20‐ to 39‐year‐olds, 48.0% vs 27.9% for 40‐ to 59‐year‐olds, and 81.0% vs 65.4% for patients 60 years or older). 9 Although awareness (77.7% vs 70.4%) and treatment (68.2% vs 60.4%) rates are higher among blacks than whites, hypertension control rates in blacks are lower (48.9% vs 59.7%). 9
Poorly controlled BP is associated with increased risk of cardiovascular disease events including myocardial infarction, heart failure, stroke, and kidney disease, as well as the metabolic syndrome. 5 Because BP increases with age, older persons are at greater risk of developing hypertension and its associated risks of cardiovascular and renal disease. 5 The earlier onset of hypertension and the higher elevations in BP among blacks increase the rate of nonfatal stroke by 1.3 times, fatal stroke by 1.8 times, heart disease death by 1.5 times, and end‐stage renal disease by 4.2 times compared with whites. 4 , 10 There is a continuing disparity in mortality rates for heart, cerebrovascular, and renal diseases between blacks and whites in the United States. 11
More than two thirds of patients with stage 2 hypertension will require therapy with 2 or more drugs to achieve BP control, and those with lower BP goals (eg, patients with diabetes or renal disease) or higher elevations in BP may require 3 or more agents from different antihypertensive classes. 5 Combining agents from different antihypertensive classes targets multiple pathophysiologic mechanisms involved in BP elevations, which may lead to more effective BP lowering more promptly, at lower doses, and generally with fewer adverse events than with either component drug administered in high doses as monotherapy. 5 , 12 , 13 , 14 , 15 For all patients who require multiple‐drug therapy to control their BP, fixed‐dose combinations may improve adherence to therapy by simplifying regimens and lowering cost, important factors in the elderly. 5 , 16
Combination therapy with different classes of antihypertensive drugs—such as a diuretic plus a β‐blocker, calcium channel blocker, or a renin‐angiotensin system blocker, as well as combinations of a calcium channel blocker and a renin‐angiotensin system blocker—has been shown to have an additive BP‐lowering effect when compared with monotherapy. 5 , 14 Morbidity and mortality rates are reduced when these therapies have been used in hypertension treatment trials. The availability of a fixed‐dose combination of a calcium channel blocker and an angiotensin II receptor blocker in addition to other effective combinations might also provide clinicians with an additional therapeutic option for lowering BP effectively and safely. Two double‐blind, randomized, placebo‐controlled trials were conducted to evaluate multiple doses of amlodipine, a dihydropyridine calcium channel blocker, and valsartan, an angiotensin II receptor blocker, alone and in combination, for the treatment of uncomplicated mild to moderate hypertension. 17 One study was designed to evaluate the lower dose range and the other to evaluate the higher dose range of the combination. Subgroup analyses based on hypertension stage, age, and race for efficacy in each study and for safety in the combined study populations are reported.
PATIENTS AND METHODS
Two similarly designed, multicenter, double‐blind, randomized, multifactorial, placebo‐controlled, parallel group trials were conducted in patients 18 years or older with a mean sitting diastolic BP ≥95 mm Hg and <110 mm Hg. A brief overview of the methods follows, as the trials were reported elsewhere. 17
Both studies included a 2‐week washout period during which any antihypertensive medications being taken by eligible patients were discontinued. This was followed by a single‐blind, placebo run‐in period of 2 to 4 weeks, then an 8‐week, double‐blind, active‐treatment period. In study 1, patients were randomized to 1 of 15 treatment groups, including placebo; amlodipine in daily doses of 2.5 to 5 mg; valsartan in daily doses of 40, 80, 160, or 320 mg; and amlodipine and valsartan in combination. In study 2, patients were randomized to 1 of 6 treatment groups, including placebo, amlodipine 10 mg daily, valsartan in daily doses of 160 and 320 mg, and amlodipine and valsartan in combination.
A calibrated standard aneroid or mercury sphygmomanometer and cuff of appropriate size were used for BP measurement. BP measurements were taken at trough (ie, between 7 AM [23 hours post‐dose] and 10 AM [26 hours post‐dose]). The arm in which the highest sitting diastolic pressures were found at visit 1 was used for all subsequent readings throughout both studies. BP measurements were obtained by the same staff member for each patient, at the same time of day, using the same equipment, at every visit. BP was measured after the patient had been sitting for 5 minutes at the start of the single‐blind, placebo run‐in period, at randomization, and after weeks 1, 2, 4, 6, and 8 of the double‐blind, active‐treatment period. Systolic and diastolic BPs were measured 3 times at 1‐ to 2‐minute intervals, and the mean of the 3 measurements was recorded as the mean sitting BP for that visit. One standing BP (measured after the patient had been standing for 2 minutes) was taken at each visit.
Efficacy and Safety Variables in Subgroup Analyses
For the subgroup analyses, efficacy was assessed by measurement of sitting diastolic and systolic BPs. Safety was assessed by monitoring and recording all reported adverse events, as well as monitoring changes in clinical laboratory test results, physical examinations, and vital signs.
Definition of Subgroups
Subgroup analyses were performed based on the stage of hypertension, age, and race. Using the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) 5 guidelines issued in 2003, stage 2 hypertension was defined as a baseline systolic BP ≥160 mm Hg and/or diastolic BP ≥100 mm Hg; stage 1 hypertension was defined as a baseline systolic BP of 140 to 159 mm Hg and/or diastolic BP of 90 to 99 mm Hg. Using a standard research definition of elderly as 65 years or older, patients were divided into 2 age subgroups: 65 years or older and younger than 65 years. Racial subgroups analyzed included only blacks and whites because the numbers of patients in other racial groups were too small to make clinically meaningful comparisons. Race was determined by patient self‐identification.
Statistical Methods
Since the objective of the analyses was to determine whether the efficacy of amlodipine and valsartan were consistent in various subgroups to that observed in the overall patient population, descriptive statistics were used to summarize the change from baseline in BP by individual treatment group and by subgroup. The primary efficacy variable was mean sitting diastolic BP measured at the end point of each trial (ie, week 8, with last observation carried forward). The analysis population was the intent‐to‐treat population, defined as all randomized patients with at least 1 post‐baseline BP measurement. In both clinical studies, the descriptive statistics for change from baseline in mean sitting diastolic BP and mean sitting systolic BP were prespecified for race and age. Stage of hypertension analysis was determined post hoc. Since both trials were similar in design, duration, and entry criteria and used the same data collection methods, adverse event rates were pooled and summarized by subgroup and treatment group (ie, amlodipine, valsartan, amlodipine + valsartan, and placebo).
RESULTS
Patient Disposition and Baseline Characteristics
A total of 1911 patients were randomized in study 1 and 1250 patients were randomized in study 2. Approximately 91% (1738 of 1911) of patients randomized in study 1 and 93% (1167 of 1250) of those randomized in study 2 completed the 8‐week, double‐blind treatment period. The overall mean age was 54.4 years in study 1 and 56.9 years in study 2. Baseline mean sitting BPs were 152.8/99.3 mm Hg in study 1 and 156.7/99.1 mm Hg in study 2.
Stage 2 hypertension was identified in 53.8% (1029 of 1911) of patients in study 1 and 61.0% (763 of 1250) of patients in study 2. The proportions of black patients in study 1 and study 2 were 10.4% (199 of 1911) and 0.4% (5 of 1250), respectively. Patients 65 years or older comprised 18.2% (347 of 1911) of randomized patients in study 1 and 28.6% (358 of 1250) of randomized patients in study 2.
Pooled baseline and demographic variables for subgroup populations are shown in Table I. In the race subgroups, the mean age of white patients was greater than that of black patients (56.2 vs 49.5 years). Stage 2 patients were older than stage 1 patients (56.4 vs 54.1 years) and, as expected, had higher mean baseline BPs (160.8/101.0 vs 145.9/96.8 mm Hg). Higher systolic BPs were observed in patients 65 years or older (160.2 mm Hg) and white patients (155.5 mm Hg) compared with younger (152.7 mm Hg) and black patients (149.7 mm Hg). Within each subgroup, the baseline demographics were generally comparable across treatment groups.
Table I.
Pooled Baseline Patient Demographics and Blood Pressure (BP) Measurements for Studies 1 and 2 by Subgroup
| Variable | Age | Race | Hypertension Stage | |||
|---|---|---|---|---|---|---|
| Younger than 65 (n=2451) | 65 Years or Older (n=704) | White (n=2508) | Black (n=201) | Stage 1 (n=1355) | Stage 2 (n=1800) | |
| Age, mean, y | 50.9 | 71.1 | 56.2 | 49.5 | 54.1 | 56.4 |
| Age group, No. (%) | ||||||
| Younger than 65 years | 2451 (100) | ‐ | 1881 (75.0) | 192 (95.5) | 1099 (81.1) | 1352 (75.1) |
| 65 Years or older | ‐ | 704 (100) | 627 (25.0) | 9 (4.5) | 256 (18.9) | 448 (24.9) |
| Sex | ||||||
| Male, No. (%) | 1332 (54.3) | 317 (45.0) | 1337 (53.3) | 81 (40.3) | 689 (50.8) | 960 (53.3) |
| Female, No. (%) | 1119 (45.7) | 387 (55.0) | 1171 (46.7) | 120 (59.7) | 666 (49.2) | 840 (46.7) |
| Race, No. (%) | ||||||
| White | 1881 (76.7) | 627 (89.1) | 2508 (100) | ‐ | 1045 (77.1) | 1463 (81.3) |
| Black | 192 (7.8) | 9 (1.3) | ‐ | 201 (100) | 93 (6.9) | 108 (6.0) |
| Asian | 172 (7.0) | 29 (4.1) | ‐ | ‐ | 102 (7.5) | 99 (5.5) |
| Other | 206 (8.4) | 39 (5.5) | ‐ | ‐ | 115 (8.5) | 130 (7.2) |
| Mean sitting systolic BP, mm Hg (No.) | 152.7 (2451) | 160.2 (704) | 155.5 (2508) | 149.7 (201) | 145.9 (1355) | 160.8 (1800) |
| Mean sitting diastolic BP, mm Hg (No.) | 99.4 (2451) | 98.6 (704) | 99.1 (2508) | 100.0 (201) | 96.8 (1355) | 101.0 (1800) |
Changes in BP
Stage 2 Hypertension. Patients with stage 2 hypertension generally had greater reductions in mean sitting BPs than did patients with stage 1 hypertension in both study 1 (Table II) and study 2 (Figure 1 and Figure 2). In both stage 1 and stage 2 hypertension, all active therapies produced greater reductions in mean sitting BP compared with placebo. The majority of combination doses were associated with greater BP‐lowering effects than the respective monotherapies.
Table II.
Change From Baseline (SD) in Mean Sitting Systolic Blood Pressure/Mean Sitting Diastolic Blood Pressure (mm Hg) at the End of Study 1 in Patients With Stage 1 and Stage 2 Hypertension*
| Stage 1 Hypertension | |||||
|---|---|---|---|---|---|
| Amlodipine Dose, mg | Valsartan Dose, mg | ||||
| 0 | 40 | 80 | 160 | 320 | |
| 0 | −6.4 (10.4)/−8.4 (7.5) | −8.8 (11.8)/−9.1 (8.4) | −11.9 (11.8)/−9.8 (6.4) | −8.8 (14.2)/−11.2 (8.1) | −11.9 (11.6)/−12.6 (7.7) |
| 2.5 | −9.9 (11.4)/−8.9 (7.3) | −11.6 (9.0)/−11.2 (7.5) | −11.1 (10.2)/−12.5 (7.0) | −12.3 (11.3)/−13.0 (5.6) | −16.0 (8.7)/−14.1 (8.5) |
| 5 | −12.1 (10.1)/−10.6 (6.7) | −15.9 (9.4)/−14.7 (7.5) | −16.9 (13.1)/−13.9 (6.8) | −14.6 (15.0)/−14.2 (8.6) | −16.4 (9.7)/−15.0 (6.4) |
| Stage 2 Hypertension | |||||
| Amlodipine Dose, mg | Valsartan Dose, mg | ||||
| 0 | 40 | 80 | 160 | 320 | |
| 0 | −5.9 (15.2)/−4.7 (7.5) | −14.1 (16.7)/−10.4 (9.3) | −13.9 (16.7)/−9.3 (7.2) | −19.8 (13.1)/−10.6 (7.6) | −19.8 (14.0)/−13.6 (7.3) |
| 2.5 | −15.2 (15.2)/−9.3 (7.6) | −18.4 (11.9)/−10.1 (7.4) | −20.6 (15.0)/−13.6 (6.9) | −19.3 (14.5)/−13.0 (8.9) | −19.4 (14.7)/−13.7 (7.3) |
| 5 | −17.5 (13.0)/−11.6 (7.0) | −22.3 (12.0)/−14.2 (6.1) | −24.2 (13.1)/−14.6 (8.0) | −23.2 (11.6)/−13.9 (6.6) | −28.5 (10.7)/−16.5 (6.0) |
| *The number of patients in each treatment group ranged from 53 to 65 in the stage 1 hypertension subgroup and from 62 to 74 in the stage 2 hypertension subgroup. | |||||
Figure 1.
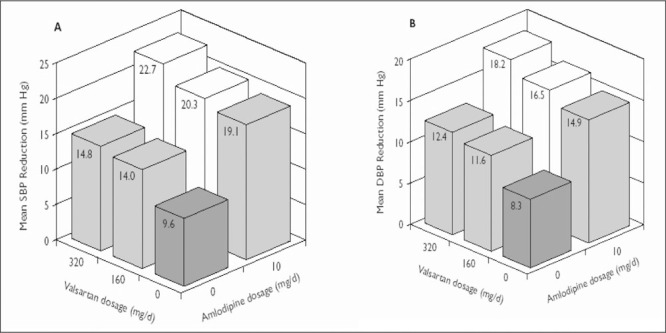
Change from baseline in (A) mean sitting systolic blood pressure (SBP) and (B) mean sitting diastolic blood pressure (DBP) at the end of study 2 in patients with stage 1 hypertension. The number of patients in each treatment group ranged from 69 to 90. The SD from the mean (systolic/diastolic, expressed in mm Hg) in each treatment group was as follows: placebo, 11.2/7.3; valsartan 160 mg, 12.5/8.4; valsartan 320 mg, 14.5/8.5; amlodipine 10 mg, 10.9/7.0; amlodipine 10 mg + valsartan 160 mg, 9.9/7.4; and amlodipine 10 mg + valsartan 320 mg, 11.7/7.1.
Figure 2.
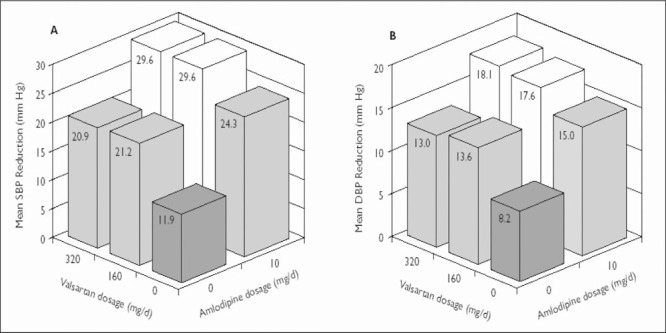
Change from baseline in (A) mean sitting systolic blood pressure (SBP) and (B) mean sitting diastolic blood pressure (DBP) at the end of study 2 in patients with stage 2 hypertension. The number of patients in each treatment group ranged from 117 to 140. The SD from the mean (systolic/diastolic, expressed in mm Hg) in each treatment group was as follows: placebo, 17.0/9.5; valsartan 160 mg, 13.0/8.7; valsartan 320 mg, 14.9/9.2; amlodipine 10 mg, 11.0/6.4; amlodipine 10 mg + valsartan 160 mg, 13.0/8.0; and amlodipine 10 mg + valsartan 320 mg, 13.8/8.6.
Maximum reductions in mean sitting BP were observed with the higher‐dose combination regimens in both stage 1 and stage 2 patients. In study 1, the reduction in mean sitting BP in patients with stage 2 hypertension was −28.5/−16.5 mm Hg with the combination of amlodipine 5 mg + valsartan 320 mg, compared with reductions of −24.2/−14.6 and −23.2/−13.9 mm Hg with combination doses of 5 mg + 80 mg and 5 mg + 160 mg, respectively (Table II). In study 2, the reductions in mean sitting BP in patients with stage 2 hypertension were similar with valsartan 160 mg and 320 mg. The BP response was also similar with the combinations of amlodipine 10 mg + valsartan 320 mg (−29.6/−18.1 mm Hg) and amlodipine 10 mg + valsartan 160 mg (−29.6/−17.6 mm Hg). By comparison, the reduction with placebo was −11.9/−8.2 mm Hg (Figure 2).
Elderly Patients. In both elderly (65 years or older) and younger (younger than 65 years) patients, all active therapies produced greater reductions in mean sitting BP compared with placebo (Table III, Figure 3, Figure 4). For the majority of the combination doses, there appeared to be a contribution to the overall efficacy from each of the respective monotherapy doses. An exception was the angiotensin II receptor blocker alone in doses of 80 mg in the 65 years or older patients, where the systolic BP response appeared to be similar to that of this dosage combined with the calcium channel blocker in doses of 5 mg. Similarly, diastolic BP effects were not different when valsartan 320 mg alone was compared with valsartan 320 mg + amlodipine 5 mg.
Table III.
Change From Baseline (SD) in Mean Sitting Systolic Blood Pressure/Mean Sitting Diastolic Blood Pressure (mm Hg) at the End of Study 1 in Elderly and Younger Patients*
| Elderly (65 Years or Older) Patients | |||||
|---|---|---|---|---|---|
| Amlodipine Dose, mg | Valsartan Dose, mg | ||||
| 0 | 40 | 80 | 160 | 320 | |
| 0 | −6.3 (13.8)/−7.0 (6.8) | −8.9 (17.9)/−9.4 (11.5) | −22.7 (9.3)/−12.7 (5.5) | −16.0 (13.7)/−13.1 (7.1) | −16.3 (14.7)/−17.1 (8.3) |
| 2.5 | −16.8 (14.1)/−12.2 (7.7) | −18.1 (10.7)/−13.3 (7.6) | −19.0 (13.4)/−14.6 (8.2) | −18.8 (18.3)/−13.5 (8.2) | −19.8 (14.0)/−14.7 (7.1) |
| 5 | −19.0 (12.8)/−13.8 (6.9) | −21.1 (11.0)/−16.9 (7.9) | −21.1 (12.5)/−15.7 (6.5) | −18.4 (16.5)/−14.7 (6.8) | −22.7 (13.2)/−14.9 (5.8) |
| Younger (Younger Than 65 Years) Patients | |||||
| Amlodipine Dose, mg | Valsartan Dose, mg | ||||
| 0 | 40 | 80 | 160 | 320 | |
| 0 | −6.1 (13.1)/−6.3 (7.8) | −12.6 (14.3)/−10.0 (8.3) | −11.6 (14.7)/−9.1 (6.9) | −14.1 (14.9)/−10.5 (7.9) | −16.3 (13.3)/−12.1 (6.9) |
| 2.5 | −12.2 (13.8)/−8.5 (7.3) | −14.7 (11.3)/−9.9 (7.3) | −15.8 (13.9)/−12.8 (6.6) | −15.4 (11.9)/−12.9 (7.4) | −17.3 (12.1)/−13.6 (8.0) |
| 5 | −13.8 (11.5)/−10.5 (6.8) | −19.3 (11.4)/−14.0 (6.4) | −20.6 (13.8)/−14.0 (7.6) | −19.6 (13.2)/−13.8 (7.7) | −22.3 (11.5)/−16.0 (6.3) |
| *The number of patients in each treatment group ranged from 15 to 32 in the elderly subgroup and from 96 to 108 in the younger subgroup. | |||||
Figure 3.
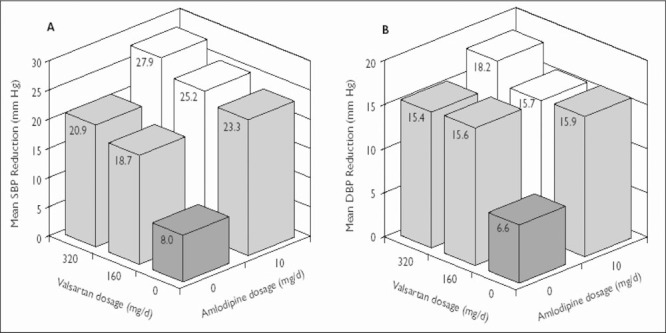
Change from baseline in (A) mean sitting systolic blood pressure (SBP) and (B) mean sitting diastolic blood pressure (DBP) (mm Hg) at the end of study 2 in elderly (65 years or older) patients. The number of patients in each treatment group ranged from 51 to 70. The SD from the mean (systolic/diastolic, expressed in mm Hg) in each treatment group was as follows: placebo, 15.2/8.8; valsartan 160 mg, 12.7/7.5; valsartan 320 mg, 16.8/10.2; amlodipine 10 mg, 11.2/6.6; amlodipine 10 mg + valsartan 160 mg, 12.7/8.0; and amlodipine 10 mg + valsartan 320 mg, 12.7/8.2.
Figure 4.
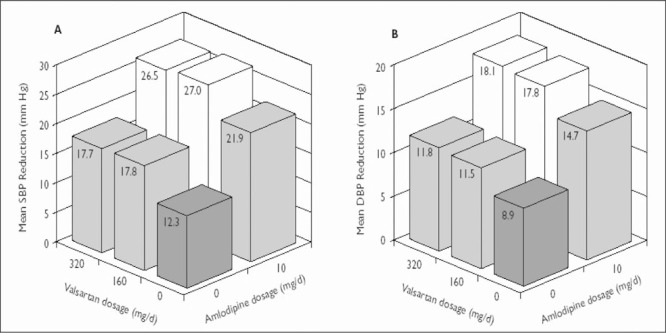
Change from baseline in (A) mean sitting systolic blood pressure (SBP) and (B) mean sitting diastolic blood pressure (DBP) (mm Hg) at the end of study 2 in younger (younger than 65 years) patients. The number of patients in each treatment group ranged from 138 to 155. The SD from the mean (systolic/diastolic, expressed in mm Hg) in each treatment group was as follows: placebo, 14.8/8.6; valsartan 160 mg, 13.5/8.8; valsartan 320 mg, 14.3/8.2; amlodipine 10 mg, 11.2/6.6; amlodipine 10 mg + valsartan 160 mg, 12.8/7.7; and amlodipine 10 mg + valsartan 320 mg, 13.8/8.0.
In elderly patients in study 1, reductions in mean sitting BP were −22.7/−14.9 mm Hg with amlodipine 5 mg + valsartan 320 mg and −6.3/−7.0 mm Hg with placebo (Table III) (difference of −16.4/−7.9 mm Hg). In study 2, reductions in mean sitting BP in the elderly were −25.2/−15.7 mm Hg and −27.9/−18.2 mm Hg for amlodipine + valsartan 10/160 mg and 10/320 mg, respectively, compared with −8.0/−6.6 mm Hg for placebo (Figure 3).
Black Patients. Because there were only 5 black patients in study 2, reductions in mean sitting BP from study 1 are reported. The number of black patients randomized to each dosage group ranged from 9 to 19 patients; interpretation of the treatment results is difficult because of the small number of patients. A smaller placebo response was observed in black patients than in white patients (Table IV). In both the black and white subpopulations, all active therapies produced greater reductions in mean sitting BP than placebo. Greater BP reductions were observed in each of the combination dose groups compared with the respective monotherapies in both subgroups. Mean sitting BP reductions in blacks with amlodipine + valsartan 5/160 mg and 5/320 mg were −19.6/−12.9 mm Hg and −17.5/−17.9 mm Hg, respectively, compared with +0.9/−1.2 mm Hg with placebo (Table IV).
Table IV.
Change From Baseline (SD) in Mean Sitting Systolic Blood Pressure/Mean Sitting Diastolic Blood Pressure (mm Hg) at the End of Study 1 in Black and White Patients*
| Black Patients | |||||
|---|---|---|---|---|---|
| Amlodipine Dose, mg | Valsartan Dose, mg | ||||
| 0 | 40 | 80 | 160 | 320 | |
| 0 | +0.9 (15.5)/–1.2 (8.0) | –8.6 (16.6)/–7.9 (10.2) | –5.3 (15.3)/–6.1 (9.4) | –4.6 (14.5)/–5.4 (11.0) | –9.3 (11.6)/–7.7 (5.9) |
| 2.5 | –6.9 (15.0)/–7.6 (5.0) | –16.5 (12.2)/–7.7 (6.5) | –7.5 (7.5)/–10.5 (5.3) | –19.3 (6.4)/–13.4 (9.4) | –14.9 (7.8)/–12.4 (6.3) |
| 5 | –10.1 (7.4)/–8.6 (4.1) | –14.0 (9.7)/–10.7 (6.7) | –19.7 (10.9)/–11.3 (9.3) | –19.6 (11.5)/–12.9 (5.7) | –17.5 (11.5)/–17.9 (6.5) |
| White Patients | |||||
| Amlodipine Dose, mg | Valsartan Dose, mg | ||||
| 0 | 40 | 80 | 160 | 320 | |
| 0 | –7.0 (12.6)/–6.9 (7.4) | –12.4 (15.1)/–9.8 (8.4) | –13.8 (14.2)/–9.9 (5.9) | –15.3 (15.0)/–11.4 (7.3) | –18.0 (13.9)/–14.4 (7.5) |
| 2.5 | –13.7 (13.6)/–9.3 (7.6) | –15.7 (10.9)/–10.7 (7.1) | –18.0 (14.1)/–13.5 (6.9) | –15.6 (14.3)/–12.4 (7.5) | –18.2 (13.1)/–13.8 (7.8) |
| 5 | –15.0 (11.2)/–11.2 (6.7) | –19.7 (11.4)/–14.2 (6.3) | –19.4 (13.3)/–14.3 (7.0) | –19.6 (14.3)/–14.2 (7.5) | –23.0 (11.8)/–15.5 (6.0) |
| *The number of patients in each treatment group ranged from 9 to 19 in blacks and from 95 to 106 in whites. | |||||
Safety and Tolerability
Safety and tolerability for the total pooled patient population from both studies were previously reported. 17 The current subgroup analyses indicate that amlodipine + valsartan combination therapy is well tolerated in the reported patient subgroups (Table V).
Table V.
Summary of Pooled Analyses of Adverse Events With Amlodipine and Valsartan, Alone and in Combination, in Different Patient Subgroups
| No. (%) of Adverse Events | |||||
|---|---|---|---|---|---|
| Amlodipine+Valsartan | Valsartan | Amlodipine | Placebo | Total | |
| Overall, No. | 1437 | 921 | 460 | 337 | 3155 |
| Total events | 634 (44.1) | 367 (39.8) | 210 (45.7) | 129 (38.3) | 1340 (42.5) |
| Stage 1 hypertension, No. | 613 | 400 | 202 | 140 | 1355 |
| Total events | 284 (46.3) | 172 (43.0) | 99 (49.0) | 57 (40.7) | 612 (45.2) |
| Stage 2 hypertension, No. | 824 | 521 | 258 | 197 | 1800 |
| Total events | 350 (42.5) | 195 (37.4) | 111 (43.0) | 72 (36.5) | 728 (40.4) |
| Black patients, No. | 98 | 61 | 29 | 13 | 201 |
| Total events | 53 (54.1) | 29 (47.5) | 15 (51.7) | 8 (61.5) | 105 (52.2) |
| White patients, No. | 1145 | 730 | 365 | 268 | 2508 |
| Total events | 475 (41.5) | 272 (37.3) | 159 (43.6) | 91 (34.0) | 997 (39.8) |
| Elderly (65 years or older) patients, No. | 323 | 205 | 95 | 81 | 704 |
| Total events | 121 (37.5) | 53 (25.9) | 34 (35.8) | 21 (25.9) | 229 (32.5) |
| Younger (younger than 65 years) patients, No. | 1114 | 716 | 365 | 256 | 2451 |
| Total events | 513 (46.1) | 314 (43.9) | 176 (48.2) | 108 (42.2) | 1111 (45.3) |
The overall incidence of adverse events was similar in patients with stage 2 hypertension and stage 1 hypertension (40.4% vs 45.2%). The incidence of adverse events in patients with stage 2 hypertension was similar with combination therapy (42.5%) to that observed with amlodipine monotherapy (43.0%) and higher than the incidence with valsartan monotherapy (37.4%) or placebo (36.5%). The most commonly occurring adverse events with combination therapy in stage 2 hypertension were peripheral edema (5.7%vs 2.0% for placebo), nasopharyngitis (4.2% vs 1.0%), and headache (3.8% vs 6.1%). The most commonly occurring adverse events with combination therapy in patients with stage 1 hypertension were headache (5.1% vs 5.7% for placebo), peripheral edema (4.9% vs 4.3%), and nasopharyngitis (4.4% vs 2.9%).
Elderly patients experienced fewer adverse events overall than did younger patients (32.5%vs 45.3%). This trend was observed for all treatment groups. The incidence of adverse events in the elderly with amlodipine + valsartan (37.5%) was similar to that observed with amlodipine monotherapy (35.8%) and lowest with valsartan monotherapy and placebo (both, 25.9%). The most commonly occurring adverse events with combination therapy in the elderly were peripheral edema (9.0% vs 2.5% for placebo), nasopharyngitis (3.1% vs 1.2%), and headache (2.8% vs 3.7%). In addition, the incidence of dizziness in the elderly was 1.9% with amlodipine + valsartan and 0.0% with placebo. Results were similar in younger patients; the most commonly occurring adverse events with combination therapy in patients younger than 65 years were headache (4.8% vs 6.6% for placebo), nasopharyngitis (4.7% vs 2.0%), and peripheral edema (4.3% vs 3.1% for placebo). In younger patients, 2.2% of those in the combination therapy group experienced dizziness, compared with 1.2% of patients in the placebo group. Adverse events associated with low BP, such as syncope, hypotension, orthostatic hypotension, postural dizziness, or lightheadedness, either did not occur or occurred at very low frequencies in both the elderly ≤0.3% of patients) and nonelderly ≤0.4% of patients).
The overall incidence of adverse events in the small number of black patients studied was greater than that observed in white patients (52.2% vs 39.8%). This trend was observed for all treatment groups. Among the most commonly occurring adverse events in black patients with combination therapy were nasopharyngitis (9.2% vs 7.7% for placebo), upper respiratory tract infection (7.1%vs 7.7%), headache (6.1% vs 7.7%), and peripheral edema (5.1% vs 15.4%). Among white patients, the most commonly occurring adverse events with combination therapy were peripheral edema (5.0% vs 2.6% for placebo), nasopharyngitis (4.0% vs 1.9%), headache (3.6% vs 6.0%), and upper respiratory tract infection (2.3% vs 1.1%).
DISCUSSION
The antihypertensive efficacy of dual therapy with amlodipine, a dihydropyridine calcium channel blocker, and valsartan, an angiotensin II receptor blocker, was evaluated in 2 placebo‐controlled, factorial design trials. In the overall study population of both studies, combination regimens were superior to both monotherapies and placebo in reducing mean sitting diastolic and systolic BPs, and a positive dose response was demonstrated. In study 1, reductions in mean sitting diastolic BP were statistically significantly greater with each combination of amlodipine 5 mg + valsartan 40 to 320 mg (range, 14.2–15.9 mm Hg) compared with the respective monotherapy doses of valsartan 40 to 320 mg (range, 9.7–13.4 mm Hg), amlodipine 5 mg (11.5 mm Hg), and placebo (6.8 mm Hg). In study 2, reductions in mean sitting diastolic BP were statistically significantly greater with amlodipine 10 mg + valsartan 160 mg and 320 mg (17.6 and 18.6 mm Hg, respectively) compared with amlodipine 10 mg (15.6 mm Hg), valsartan 320 mg (13.3 mm Hg), and placebo (8.8 mm Hg). Diastolic BP response rates (<90 mm Hg or a ≥10‐mm Hg decrease from baseline) ranged from 88% to 91% with the higher doses of 5/320 mg, 10/160 mg, and 10/320 mg, compared with 41% to 49% with placebo. 17
Combination therapy is a necessary consideration in a large number of patients with hypertension; more than two thirds of patients with target organ involvement are not controlled by a single drug. 5 Major hypertension treatment guidelines recommend combination therapy as possible initial treatment for patients with severe elevations in BP and for those at greater risk of cardiovascular events, such as patients with multiple cardiovascular risk factors, obese patients, patients with diabetes mellitus or chronic renal disease, and the elderly. 5 , 12 , 18 , 19
The current subgroup analyses from 2 studies of combination therapy with amlodipine + valsartan demonstrate that the combination provides consistent efficacy and tolerability in patients regardless of hypertension stage, age, or race. Consistent with the results from the overall population, both systolic and diastolic BP were lowered to a greater extent with the majority of the doses of combination therapy compared with monotherapy in patients with stage 2 hypertension, in elderly patients (65 years or older), and in black patients. These findings are clinically significant because of the need to achieve BP goals in patients with more severe BP elevations (ie, stage 2 hypertension) and in elderly and black patients who may have other concomitant cardiovascular risk factors. These patient subgroups will typically require more than 1 antihypertensive agent to achieve BP control. 5
In addition to providing an improved efficacy profile compared with monotherapy, combination therapy with many different classes of antihypertensive agents—including diuretics +β‐blockers or angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers, and angiotensin‐converting enzyme inhibitors + calcium channel blockers—is generally well tolerated. In this study population, the combination of amlodipine + valsartan was well tolerated; the incidence of adverse events overall was 44.1% with combination therapy compared with 39.8% with valsartan monotherapy, 45.7% with amlodipine, and 38.3% with placebo. 17 Results of the current pooled analyses for adverse events in patient subgroups defined by hypertension stage, age, and race paralleled those for the population as a whole. Adverse events overall were reported at similar rates in patients with stage 1 and stage 2 hypertension. Incidence rates for adverse events were slightly greater in younger patients compared with older patients and in black patients compared with white patients. The finding of a lower incidence of adverse events in the elderly subgroup is similar to that observed in the Hypertension Detection and Follow‐Up Program, 20 where only 29.8% of patients 60 to 69 years of age experienced side effects compared with 34.1% to 38.0% of patients in younger subgroups. The overall incidence of adverse events with the calcium channel blocker/angiotensin II receptor blocker combination in each subgroup was generally comparable to monotherapy. The most common adverse events reported in each of the patient subgroups were similar, namely peripheral edema, headache, nasopharyngitis, and upper respiratory tract infection.
An analysis of data from some observational studies suggests that initial monotherapy with a renin‐angiotensin system blocker is associated with higher adherence rates than therapy with a diuretic or β‐blocker, 21 although outcome data with diuretic‐based therapy indicate a reduction in cardiovascular events similar to that achieved by other regimens. 22 Improving patient adherence is particularly important in difficult‐to‐control patients, since there is less than ideal overall adherence to antihypertensive therapy even in clinical trials. 16 , 21 , 23 Achieving target BP levels is important to prevent cardiovascular morbidity and mortality associated with hypertension, particularly in blacks and the elderly, who are at increased risk for the sequelae of hypertension. 10 , 16 Data from many trials with different medications indicate that achieved BP levels may be the major determinant of outcome.
LIMITATIONS OF THE STUDY
A limitation to the subgroup analyses reported herein is the small number of patients in some of the subgroups; for example, black and elderly patients. The resultant wider confidence intervals of the estimated mean BP reductions in these specific subgroups limits the ability to detect a clear dose‐response effect as observed in the overall population. Another potential limitation of these analyses is that patients were not stratified by subgroup at randomization. Since baseline characteristics were generally comparable among treatment groups within each subgroup, however, no significant impact on the interpretation of the data would be expected.
In conclusion, amlodipine + valsartan combination therapy was associated with greater BP‐lowering effects in patient subgroups, including those with stage 2 hypertension, the elderly, and blacks, compared with each respective monotherapy and placebo in 2 randomized, double‐blind, placebo‐controlled studies. These findings are consistent with the primary efficacy analysis results from the overall study populations. Combination regimens were generally well tolerated by all patient subgroups.
Acknowledgment and disclosures: The authors thank Landmark Programs, Inc, New York, NY, for providing editorial support. This study was supported by Novartis Pharmaceuticals Corporation, East Hanover, NJ. Robert Glazer, MD, Margaret Wernsing, BS, and Joseph Yen, PhD, are employees of Novartis Pharmaceuticals Corporation.
References
- 1. Fields LE, Burt VL, Cutler JA, et al. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398–404. [DOI] [PubMed] [Google Scholar]
- 2. Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. [DOI] [PubMed] [Google Scholar]
- 3. Ong KL, Cheung BM, Man YB, et al. Prevalence, awareness, treatment, and control of hypertension among United States adults, 1999–2004. Hypertension. 2007;49:69–75. [DOI] [PubMed] [Google Scholar]
- 4. American Heart Association . Heart Disease and Stroke Statistics—2006 Update. Dallas, TX: American Heart Association; 2006. [Google Scholar]
- 5. The Seventh Report of the Joint National Committee on Prevention . Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 6. Wolf‐Maier K, Cooper RS, Kramer H, et al. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension. 2004;43:10–17. [DOI] [PubMed] [Google Scholar]
- 7. Franklin SS. Hypertension in older people: part 1. J Clin Hypertens (Greenwich). 2006;8:444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferdinand KC. Managing cardiovascular risk in minority patients. J Natl Med Assoc. 2005;97:459–466. [PMC free article] [PubMed] [Google Scholar]
- 9. Hertz RP, Unger AN, Cornell JA, et al. Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med. 2005;165:2098–2104. [DOI] [PubMed] [Google Scholar]
- 10. Ferdinand KC, Saunders E. Hypertension‐related morbidity and mortality in African Americans: why we need to do better. J Clin Hypertens (Greenwich). 2006;8(suppl 1):21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levine RS, Foster JE, Fullilove RE, et al. Black‐white inequalities in mortality and life expectancy, 1933–1999: implications for Healthy People 2010. Public Health Rep. 2001;116:474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guidelines Committee . 2003 European Society of Hypertension—European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–1053. [DOI] [PubMed] [Google Scholar]
- 13. Cifkova R, Erdine S, Fagard R, et al. Practice guidelines for primary care physicians: 2003 ESH/ESC hypertension guidelines. J Hypertens. 2003;21:1779–1786. [DOI] [PubMed] [Google Scholar]
- 14. Weber MA. Creating a combination antihypertensive regimen: what does the research show? J Clin Hypertens (Greenwich). 2003;5(suppl 3):12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sica DA. Rationale for fixed‐dose combinations in the treatment of hypertension: the cycle repeats. Drugs. 2002;62:443–462. [DOI] [PubMed] [Google Scholar]
- 16. Elliott WJ. Optimizing medication adherence in older persons with hypertension. Int Urol Nephrol. 2003;35:557–562. [DOI] [PubMed] [Google Scholar]
- 17. Philipp T, Smith TR, Glazer R, et al. Two randomized, double blind, multicenter, multifactorial, placebo‐controlled, parallel‐group studies to evaluate the efficacy and safety of valsartan and amlodipine combined and alone in hypertensive patients [published online ahead of print April 2, 2007]. Clin Ther. doi:10.1016/j.clinthera.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 18. Douglas JG, Bakris GL, Epstein M, et al., for the Hypertension in African Americans Working Group . Management of high blood pressure in African Americans: consensus statement of the Hypertension in African Americans Working Group of the International Society on Hypertension in Blacks. Arch Intern Med. 2003;163:525–541. [DOI] [PubMed] [Google Scholar]
- 19. Arauz‐Pacheco C, Parrott MA, Raskin P, for the American Diabetes Association . Hypertension management in adults with diabetes. Diabetes Care. 2004;27(suppl 1):S65–S67. [DOI] [PubMed] [Google Scholar]
- 20. Curb JD, Borhani NO, Blaszkowski TP, et al. Long‐term surveillance for adverse effects of antihypertensive drugs. JAMA. 1985;253:3263–3268. [PubMed] [Google Scholar]
- 21. Payne KA, Esmonde‐White S. Observational studies of antihypertensive medication use and compliance: is drug choice a factor in treatment adherence? Curr Hypertens Rep. 2000;2:515–524. [DOI] [PubMed] [Google Scholar]
- 22. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 23. Osterberg L, Blaschke T. Drug therapy: adherence to medication. N Engl J Med. 2005;353:487–497. [DOI] [PubMed] [Google Scholar]


