Abstract
Atenolol and metoprolol succinate, dosed once daily, have different pharmacokinetic profiles. This study tests the hypothesis that differences that are especially noted in the early morning period, when cardiovascular risk is highest, in 24‐hour blood pressure (BP) control exist between these 2 β‐blockers. This was a small, randomized open‐label study with blinded end point evaluation in 36 hypertensive patients. All participants received hydrochlorothiazide 12.5 mg for 2 weeks before randomization to either 50 mg atenolol or metoprolol succinate given every morning; both treatments were force‐titrated to 100 mg/d at 4 weeks. The primary end point was the change in early morning ambulatory systolic BP. Early morning (12 am–6 am) systolic BP differences were 3±14 mm Hg with atenolol vs −7±8 mm Hg with metoprolol succinate (P=.03). The overall 24‐hour changes in systolic BP were 1±15 mm Hg with atenolol vs −9±11 mm Hg with metoprolol (P=.03). In conclusion, metoprolol succinate was more effective in sustaining 24‐hour and early morning BP reductions compared with atenolol in a small group of hypertensive patients also treated with once‐daily low‐dose hydrochlorothiazide. It is possible that differences in outcome between atenolol‐based and other therapies may be the result of inadequate dosing of atenolol, a medication that may not be effective for the entire 24‐hour period.
Hypertension is a risk factor for coronary artery disease, myocardial infarction, stroke, congestive heart failure, end‐stage renal disease, and peripheral vascular disease. 1 Several previous studies have reported that circadian variation of blood pressure (BP) is more pronounced in hypertensive than normotensive persons and that BP variability may contribute to target organ damage in hypertension. 2 , 3 While office BP readings have been used to assess risk in numerous longitudinal studies, several lines of evidence suggest that ambulatory BP monitoring (ABPM) is superior to office BP measurement in predicting cardiovascular events and mortality. 4 , 5
Pharmacologic treatment of hypertension is known to decrease the risk of cardiovascular complications. 1 Although casual BP assessment has been used to evaluate outcomes in most clinical trials, ABPM has been reported to be a better predictor of treatment‐related regression of target organ damage. 6 The results of ABPM provide reliable evaluations of BP over 24 hours and information on whether antihypertensive treatment attenuates BP fluctuations throughout daytime and nighttime. A natural extension of these data are that “total” 24‐hour BP control should be a treatment goal. 7 This is particularly important for early morning BP peaks; this is the period in which risk of cardiovascular events is highest. 8 It has been proposed that ABPM should be used routinely in the evaluation of antihypertensive compounds. 9
β‐BLOCKERS
β‐Blockers are one of the oldest antihypertensive classes, with more than 40 years of use in treatment of hypertension. 10 Among the most widely used agents within the class are the β1‐selective compounds atenolol and metoprolol. Metoprolol is available as metoprolol tartrate (immediate release) and metoprolol succinate (controlled, long‐acting release) formulations. It is well known that once‐daily compared with 2‐ or 3‐times‐daily medication administration may improve patient adherence and outcome. 11
At present, atenolol is the most commonly used β‐blocker. Because of differences in pharmacokinetic profile and a relatively shorter half‐life of atenolol compared with metoprolol succinate, 12 , 13 a difference in 24‐hour, and more specifically early morning, BP control might be expected if each of these medications is given once daily in the morning. Previous data support the notion that once‐daily atenolol does not provide adequate BP control during the nighttime and early morning periods. 14 , 15 Studies comparing once‐daily administration of atenolol and metoprolol succinate report either no difference in office BP values after the last dose 16 or lower office BP values with metoprolol, 17 but data from trials using ABPM have not been available. The aim of this study was to evaluate the effects of once‐daily atenolol and metoprolol succinate extended‐release (ER) dosed in the morning on 24‐hour BP control, focusing on nighttime and early morning BP control.
METHODS
Participants
The present single‐center study was an open‐label prospective randomized trial with blinded end point evaluation. The trial compared the use of once‐daily atenolol with metoprolol succinate ER in patients with hypertension who (1) were aged 20 to 75 years; (2) had stage 1 or 2 hypertension (treated with a maximum of 2 drugs) and a systolic BP (SBP) level ≥140 mm Hg at baseline; and (3) consented to participate in the study by signing an informed consent form. Exclusion criteria included (1) uncontrolled hypertension, defined as SBP >180 mm Hg and diastolic BP (DBP) >100 mm Hg; (2) requirement of ≥3 drugs to control BP; (3) recent (<12 months) stroke, myocardial infarction, or cardiovascular surgery; (4) second‐or third‐degree heart block, without a pacemaker; (5) concomitant refractory angina pectoris; (6) use of ophthalmic β‐blockers; (7) history of bronchial asthma; (8) presence of renal disease (serum creatinine level >1.4 mg/dL); (9) evidence of hepatic disease as determined by any one of the following: serum glutamic oxaloacetic transaminase or serum glutamic pyruvic transaminase levels at least twice as high as normal at visit 1, a history of hepatic encephalopathy, a history of esophageal varices, or a history of portocaval shunt; (10) body mass index (BMI) >39 kg/m2; (11) psychiatric illness; and (12) current substance (illegal drugs) abuse.
The study was conducted in accordance with Good Clinical Practices/International Conference on Harmonization guidelines and US 21 Code of Federal Regulations Part 50: Protection of Human Subjects and Part 56: Institutional Review Boards. All participants provided written informed consent approved by the Rush University Institutional Review Board before study initiation.
Study Protocol
Participants were initially evaluated at a screening visit (visit 1). Those who met all the inclusion and exclusion criteria had their antihypertensive medications stopped and were started on hydrochlorothiazide 12.5 mg once daily.
Two weeks after the screening visit (visit 2), participants were reevaluated; if their BP level was still ≥140/90 mm Hg, baseline 24‐hour ambulatory BP measurement was performed. Patients were then randomized to receive 50 mg of atenolol or metoprolol succinate ER once daily in addition to hydrochlorothiazide 12.5 mg every morning. Patients were instructed to take hydrochlorothiazide and the study drug at the same time every morning, within a window of 6 am to 10 am. Participants whose office BP level was <140/90 mm Hg at this visit were instructed to continue use of hydrochlorothiazide 12.5 mg/d and to return to the clinic in 2 weeks for the BP reevaluation.
After randomization, patients returned to the clinic for 3 more visits at 4‐week intervals. At each of these visits, patients were assessed for safety and efficacy variables. At visit 3 (4 weeks after randomization), if a participant's heart rate was >50 beats per minute (bpm), their regimen was force‐titrated to include metoprolol succinate ER 100 mg (once daily) or atenolol 100 mg (once daily). In addition, if the heart rate was <50 beats per minute and the patient was asymptomatic, treatment was also force‐titrated; however, if the patient was symptomatic, he or she was withdrawn from the study. Heart rate was evaluated as a safety and not an efficacy variable.
BP Measurement
Office Blood Pressure Measurement. At each visit, a total of 3 office BP measurements were taken with the auscultatory method according to the relevant recommendations. 1 Measurements were taken in a seated position using a standard mercury sphygmomanometer with an appropriate cuff size 24 hours after the last study medication was administered. Before the first measurement, participants remained at rest in the seated posture for at least 5 minutes, then 3 readings were taken in the non‐dominant arm at 2‐minute intervals. The average of the 3 BP measurements was used for treatment decisions. It should be noted that all office readings were performed at trough drug level (ie, just before the next dose of drug).
Ambulatory Blood Pressure Measurement. Ambulatory BP was monitored with Spacelabs 90217 devices (Spacelabs Corp, Redmond, WA) at visits 2, 4, and 5. The monitor recorded ambulatory BP 3 times an hour between 6 am and 10 pm and hourly between 10 pm and 6 am. Readings were used for the analysis only if ≥80% of measurements were valid, with no more than 2 invalid nonconsecutive readings during the daytime hours (6 am–10 pm) and no more than 1 invalid nighttime reading hour (10 pm–6 am). The final ABPM reading on each occasion occurred roughly 24 hours after the monitor was placed and morning study medications were administered.
Laboratory Analyses. Biochemical parameters measured during this trial included kidney function with a blood urea nitrogen and serum creatinine value, as well as electrolytes. In addition, baseline electrocardiography was performed and a complete blood cell count was done.
Statistical Analysis
The primary efficacy variable was the difference in nighttime SBP on ABPM values between groups at study end. The sample included a minimum of 50% African Americans. A sample size of 50 patients, 25 in each group, was calculated to provide 90% power to detect a 4‐mm Hg difference in nighttime SBP between groups. Because of logistic reasons, 36 patients were recruited but only 30 completed the ABPM recording for the final visit. While this reduces the power of the conclusions, there was still about an 80% power present to detect the aforementioned difference in early morning SBP.
Statistical analysis was performed using SAS/STAT software version 9.1 (SAS Institute, Inc, Cary, NC). Analysis was done by intention to treat. Baseline differences between the groups were tested using chi‐square tests for categoric measures, such as sex and race, and t tests for continuous measures, such as age and BMI. Paired t tests were conducted to test within‐drug differences in SBP, DBP, and heart rate from visit 2 to visit 5. Differences in SBP, DBP, and heart rate between the metoprolol and atenolol groups were tested at each visit for both ABPM and office readings using t tests. ABPM readings were averaged over the first 24 hours, 12 am to 6 am, 6 am to 9 am, and 6 am to 12 pm, when available. In addition, changes between baseline (visit 2) and the final visit (visit 5) were computed for both the ABPM and office BP measurement, and t tests were constructed to test whether there was a difference in changes from visit 2 to visit 5 between the 2 treatments. A P value <.05 (2‐tailed) was considered statistically significant. All data are expressed as mean ± SD).
RESULTS
In total, 146 patients were screened to find 36 evaluable patients. Baseline demographic data and office BP levels, stratified by treatment group, are presented in Table I. No significant difference between groups was present. Ambulatory BP and heart rate data at baseline are presented in Table II . No electrolyte abnormalities were noted, and all participants had left ventricular hypertrophy as assessed by electrocardiographic criteria. Two of 36 participants (5.5%) were lost to follow‐up, and 4 (11.1%) failed to complete ABPM at the final visit secondary to refusing to wear the monitor.
Table I.
Baseline Demographics and Office Blood Pressure Levels in the 2 Groups
| Atenolol | Metoprolol a | P Value | |
|---|---|---|---|
| No. | 18 | 18 | |
| Sex, women/total | 6/18 | 6/18 | 1.00 |
| Race, African American/total | 12/18 | 9/18 | .31 |
| Age, y | 57±7 | 58±9 | .62 |
| BMI, kg/m2 | 31±4 | 31±5 | .81 |
| SBP, mm Hg | 152±10 | 147±6 | .06 |
| DBP, mm Hg | 95±10 | 90±11 | .14 |
| Heart rate, bpm | 75±9 | 75±10 | 1.00 |
| aThe preparation of extended‐release metoprolol succinate was used. Values are mean ± SD unless otherwise indicated. Abbreviations: BMI, body mass index; bpm, beats per minute; DBP, diastolic blood pressure; SBP, systolic blood pressure. | |||
Table II.
Baseline Ambulatory Blood Pressure Levels and Heart Rates
| Atenolol (n=18) | Metoprolol a (n=18) | P Value | |
|---|---|---|---|
| SBP, mm Hg | |||
| 12 pm–6 pm | 147±9 | 138±13 | .03 |
| 6 pm–12 am | 146±6 | 135±16 | .01 |
| 12 am–6 am | 133±9 | 127±14 | .12 |
| 6 am–12 pm | 148±11 | 140±16 | .07 |
| 24 hours | 144±6 | 136±12 | .01 |
| DBP, mm Hg | |||
| 12 pm–6 pm | 91±9 | 85±11 | .10 |
| 6 pm–12 am | 87±11 | 81±11 | .09 |
| 12 am–6 am | 79±9 | 75±12 | .23 |
| 6 am–12 pm | 91±11 | 85±12 | .18 |
| 24 hours | 88±9 | 83±10 | .09 |
| Heart rate, bpm | |||
| 12 pm–6 pm | 79±12 | 71±9 | .02 |
| 6 pm–12 am | 77±14 | 70±10 | .08 |
| 12 am–6 am | 71±13 | 64±10 | .09 |
| 6 am–12 pm | 76±13 | 67±9 | .02 |
| 24 hours | 76±12 | 68±8 | .02 |
| aThe preparation of extended‐release metoprolol succinate was used. Values are mean ± SD. Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure. | |||
Office BP values decreased in both groups between baseline and 12 weeks (−11±21/−6±8 mm Hg in the atenolol group; P=.052 for SBP and P=.005 for DBP and −16±13/−8±8 mm Hg in the metoprolol group; P=.0002 for SBP and P=.0021 for DBP). There was no significant difference in office BP reduction between the atenolol and the metoprolol succinate ER groups (P=.41 for SBP and P=.58 for DBP, respectively) (Table III). Similarly, heart rate measured in the office decreased significantly between baseline and end of the study, without significant differences between the 2 groups (Table III).
Table III.
Differences in Office Blood Pressure Readings and Heart Rate Between Visit 2 and Visit 5
| Atenolol (n=17) | Metoprolol a (n=15) | P Value | |
|---|---|---|---|
| SBP, mm Hg | −11±21 | −16±13 | .41 |
| DBP, mm Hg | −6±8 | −8±8 | .58 |
| Heart rate, bpm | −11±12 | −9±16 | .68 |
| aThe preparation of extended‐release metoprolol succinate was used. Values are mean ± SD unless otherwise indicated. Abbreviations: bpm, beats per minute; DBP, diastolic blood pressure; SBP, systolic blood pressure. | |||
In contrast to office BP, ambulatory BP recordings exhibited a different pattern in the 2 groups (Table IV and Figure). Overall 24‐hour changes between baseline and 12 weeks in the atenolol and metoprolol succinate ER groups were 1±15 vs −9±11 mm Hg (P=.03) for SBP and −1±9 vs −6±6 mm Hg (P=.14) for DBP, respectively. These differences in 24‐hour SBP readings were the result of differences in nighttime and early morning (12 am–6 am) SBP values (3±14 mm Hg with atenolol vs −7±8 mm Hg with metoprolol; P=.03). Ambulatory DBP in the 2 groups followed a trend similar to that of SBP, but the DBP differences between groups were smaller and thus not significant. A similar pattern of changes in ambulatory SBP and DBP in the 2 groups was observed between baseline and the intermediate visit 4, at 8 weeks after randomization (data not shown). During the treatment period, 42 adverse events in the 2 groups were reported (Table V).
Table IV.
Differences in Ambulatory Blood Pressurea and Heart Rate Between Visit 2 and Visit 5
| Atenolol (n=16) | Metoprolol b (n=14) | P Value | |
|---|---|---|---|
| SBP, mm Hg | |||
| 12 pm–6 pm | −1±19 | −10±14 | .18 |
| 6 pm–12 am | 4±20 | −11±16 | .04 |
| 12 am–6 am | 3±14 | −7±8 | .03 |
| 6 am–12 pm | 6±19 | −4±14 | .15 |
| 24 hours | 1±15 | −9±11 | .03 |
| DBP, mm Hg | |||
| 12 pm–6 pm | −2±14 | −6±8 | .30 |
| 6 pm–12 am | 1±13 | −5±11 | .23 |
| 12 am–6 am | 0±10 | −5±7 | .21 |
| 6 am–12 pm | 1±13 | −1±11 | .57 |
| 24 hours | −1±9 | −6±6 | .14 |
| Heart rate, bpm | |||
| 12 pm–6 pm | −5±10 | −5±5 | .87 |
| 6 pm–12 am | −2±10 | −7±9 | .20 |
| 12 am–6 am | −1±10 | −4±6 | .32 |
| 6 am–12 pm | −1±10 | −2±6 | .79 |
| 24 hours | −3±8 | −6±4 | .21 |
| a≥80% of the readings were captured by ambulatory blood pressure monitoring. bThe preparation of extended‐release metoprolol succinate was used. Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure. | |||
Figure.
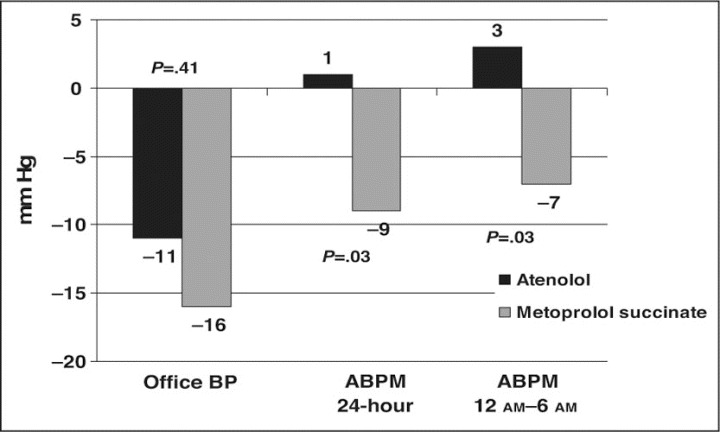
Mean changes in office and ambulatory systolic blood pressure (BP) between baseline (visit 2) and the end of the treatment period (visit 5). ABPM indicates ambulatory blood pressure monitoring.
Table V.
Frequency of Reported Adverse Events by Treatment (42 Events Reported)
| Adverse Event | Atenolol | Metoprolol a |
|---|---|---|
| Upper respiratory infection | 4 | 4 |
| Fatigue | 5 | 3 |
| Headaches | 2 | 1 |
| Dizziness | 2 | 2 |
| Rash | 2 | 0 |
| Perspiration | 0 | 2 |
| Dyspnea | 0 | 2 |
| Blurred vision | 2 | 0 |
| Urinary tract infection | 0 | 2 |
| Vomiting | 1 | 0 |
| Sexual dysfunction | 1 | 0 |
| Muscle cramps | 1 | 0 |
| Muscle aches | 0 | 1 |
| Minor chest pain | 0 | 1 |
| Diarrhea | 1 | 0 |
| Edema | 0 | 1 |
| Irregular heart rate | 1 | 0 |
| Gastrointestinal symptoms | 0 | 1 |
| aThe preparation of extended‐release metoprolol succinate was used. Note: One participant in the metoprolol group experienced a severe adverse event (hospitalized for shortness of breath). | ||
DISCUSSION
The present study was designed to compare the effects of once‐daily atenolol and metoprolol succinate ER on 24‐hour BP control in hypertensive patients. The results indicate differences in mean 24‐hour BP control between once‐daily atenolol and once‐daily metoprolol succinate ER, both dosed in the morning, when added to hydrochlorothiazide therapy. These differences were not detected by casual office BP measurement. These between‐group differences in 24‐hour ambulatory BP control were mainly observed by differences in nighttime (12 am–6 am) systolic BP. In the 2 groups, 24‐hour DBP exhibited a similar pattern, but to a lesser and nonsignificant degree.
The circadian variation of BP is more pronounced in hypertensive than normotensive persons; this BP variability contributes to target organ damage. 2 , 3 BP control over a 24‐hour period, and particularly during nighttime and early morning periods, should be the goal of antihypertensive treatment. 7 Office BP readings provide limited information about 24‐hour BP control, as they only capture BP levels from a small window of time. In contrast, ABPM gives reliable information on BP levels and the efficacy of antihypertensive treatment throughout the day and night. As previously noted, ABPM has been shown to be a better predictor of cardiovascular morbidity and mortality 4 , 5 as well as treatment‐related regression of target organ damage. 6
Previous data on the 24‐hour efficacy of once‐daily atenolol are conflicting. In one study, the use of atenolol (50–100 mg) and acebutolol (400–800 mg) given once daily at 9 am was compared. 14 The 2 drugs provided comparable average 24‐hour decreases, but during the final 6 hours of the dosing interval (3 am–9 am), acebutolol showed greater BP reductions than atenolol. This final 6‐hour effect of atenolol was less than that observed during the first 18 hours of the day. In a more recent study, atenolol 50 mg was compared with perindopril 8 mg, felodipine 10 mg, or hydrochlorothiazide 50 mg for 2 months. 15 All drugs significantly lowered 24‐hour SBP, but the decrease with atenolol was less than with the other drugs. In other studies, however, once‐daily atenolol in larger doses up to 100 mg has been shown to achieve greater decreases in DBP throughout the 24‐hour period than quinapril 20 mg/d 18 and during the daytime when compared with nebivolol in doses up to 5 mg. 19
Studies that have evaluated the pharmacokinetic and pharmacodynamic properties of atenolol and metoprolol succinate ER demonstrate that the plasma concentration‐time profiles were consistent with atenolol over the 24‐hour dose interval; a longer period during which plasma concentration exceeded 50% of the maximum concentration was noted with metoprolol succinate ER. 12 , 13 In addition, the longer‐acting metoprolol succinate ER achieves a lower BP over the 24‐hour period compared with shorter‐acting metoprolol tartrate (immediate‐release formulation). 20 In a previous study comparing once‐daily administration of 50 mg of atenolol and metoprolol succinate, the reductions of SBP and DBP at rest were significantly greater in the metoprolol group. 17 In a separate study, however, no differences were noted in office BP 24 hours after the last dose of 50 mg of atenolol or 100 mg of metoprolol succinate. 16 In the present study, we also noted no difference in office SBP and DBP between equivalent doses of atenolol and metoprolol given on background hydrochlorothiazide therapy. In this BP study, however, there were significant mean differences of 10 mm Hg in 24‐hour and nighttime SBP in favor of metoprolol succinate ER.
An important question raised by our findings is whether atenolol in dosages usually given, with its apparent lack of 24‐hour BP coverage, is an appropriate comparator in outcome trials. In past years, atenolol has been the most frequently used “active treatment” comparator in major cardiovascular outcome trials such as the Anglo‐Scandinavian Cardiac Outcomes Trial (ASCOT) 21 and the Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) study 22 ; it represents the most commonly prescribed once‐daily β‐blocker. Most trials, except for the International Verapamil SR and Trandolapril Study (INVEST), report poorer cardiovascular outcomes with atenolol‐based treatment. In INVEST, however, almost half of the participants received twice‐daily atenolol; the lack of 24‐hour effect would have been corrected by twice‐daily dosing. 23 It may, therefore, be the less effective BP‐lowering effects of once‐daily atenolol, rather than specific medication differences, that account for the cardiovascular outcome differences. Despite some data that suggest that the use of once‐daily atenolol 100 mg will result in nighttime BP lowering, this medication probably should be given as 50 mg bid.
We acknowledge that there are a number of limitations in our study findings. Although this study followed a randomized design and the analysis was carried out by intention to treat, it was not double‐blinded; however, it included blinded end point evaluations, as the investigators performing the office BP measurements and interpreting the ABPM results were unaware of the patient's treatment. As mentioned above, the final study population was estimated to have an 80% power to detect a 4‐mm Hg difference between groups in nighttime SBP , which was the primary outcome. The study was not powered at the same level to detect the respective changes in DBP , however. Last, there were differences in baseline office BP levels between groups during the afternoon and evening periods, favoring the chance of a greater reduction in the atenolol group. This suggests the possibility that this group is more difficult to treat; however, this was not our primary outcome. Pulse rate was reduced to a similar degree in both groups. Thus, we do not feel that this biases our results.
CONCLUSIONS
In the presence of background thiazide diuretic treatment, there are differences in 24‐hour BP control between once‐daily administration of atenolol and metoprolol succinate ER in hypertensive patients. These between‐group differences are mostly the result of disparate early morning BP readings; this is the time when the incidence of cardiovascular events is highest. 24 This inability of once‐daily atenolol to achieve sustained reductions in BP throughout daytime and nighttime could be responsible for the differences in observed benefits when this agent is compared with other therapies.
Disclosure:
Dr Bakris has received an investigator‐initiated grant from AstraZeneca.
References
- 1. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. [DOI] [PubMed] [Google Scholar]
- 2. Parati G, Pomidossi G, Albini F, et al. Relationship of 24‐hour blood pressure mean and variability to severity of target‐organ damage in hypertension. J Hypertens. 1987;5(1):93–98. [DOI] [PubMed] [Google Scholar]
- 3. Mancia G, Parati G, Hennig M, et al. Relation between blood pressure variability and carotid artery damage in hypertension: baseline data from the European Lacidipine Study on Atherosclerosis (ELSA). J Hypertens. 2001;19(11):1981–1989. [DOI] [PubMed] [Google Scholar]
- 4. Staessen JA, Thijs L, Fagard R, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators . JAMA. 1999;282(6):539–546. [DOI] [PubMed] [Google Scholar]
- 5. Ohkubo T, Hozawa A, Nagai K, et al. Prediction of stroke by ambulatory blood pressure monitoring versus screening blood pressure measurements in a general population: the Ohasama study. J Hypertens. 2000;18(7):847–854. [DOI] [PubMed] [Google Scholar]
- 6. Mancia G, Zanchetti A, Agabiti‐Rosei E, et al. Ambulatory blood pressure is superior to clinic blood pressure in predicting treatment‐induced regression of left ventricular hypertrophy. SAMPLE Study Group . Study on Ambulatory Monitoring of Blood Pressure and Lisinopril Evaluation. Circulation. 1997;95(6):1464–1470. [DOI] [PubMed] [Google Scholar]
- 7. Parati G, Ulian L, Santucciu C, et al. Blood pressure variability, cardiovascular risk and antihypertensive treatment. J Hypertens Suppl. 1995;13(4):S27–S34. [DOI] [PubMed] [Google Scholar]
- 8. Muller JE. Circadian variation in cardiovascular events. Am J Hypertens. 1999;12(2, pt 2):35S–42S. [DOI] [PubMed] [Google Scholar]
- 9. White WB. The evaluation of antihypertensive therapy using 24‐h ambulatory monitoring technology. Blood Press Monit. 2000;5(suppl 2):S13–S17. [DOI] [PubMed] [Google Scholar]
- 10. Sarafidis PA, Bakris GL. Are beta blockers passé for the treatment of hypertension? J Clin Hypertens (Greenwich). 2006;8(4):239–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Freis ED. Improving treatment effectiveness in hypertension. Arch Intern Med. 1999;159(21):2517–2521. [DOI] [PubMed] [Google Scholar]
- 12. Blomqvist I, Westergren G, Sandberg A, et al. Pharmacokinetics and pharmacodynamics of controlled‐release metoprolol: a comparison with atenolol. Eur J Clin Pharmacol. 1988;33(suppl):S19–S24. [DOI] [PubMed] [Google Scholar]
- 13. Darmansjah I, Wong E, Setiawati A, et al. Pharmacokinetic and pharmacodynamic properties of controlled release (CR/ZOK) metoprolol in healthy Oriental subjects: a comparison with conventional formulations of metoprolol and atenolol. J Clin Pharmacol. 1990;30(2 suppl):S39–S45. [DOI] [PubMed] [Google Scholar]
- 14. Neutel JM, Schnaper H, Cheung DG, et al. Antihypertensive effects of beta‐blockers administered once daily: 24‐hour measurements. Am Heart J. 1990;120(1):166–171. [DOI] [PubMed] [Google Scholar]
- 15. Morgan TO, Anderson A. Different drug classes have variable effects on blood pressure depending on the time of day. Am J Hypertens. 2003;16(1):46–50. [DOI] [PubMed] [Google Scholar]
- 16. Chen MF, Yang CY, Chen WJ, et al. A double‐blind comparison of once‐daily metoprolol controlled‐release and atenolol in the treatment of Chinese patients with mild to moderate hypertension. Cardiovasc Drugs Ther. 1995;9(3):401–406. [DOI] [PubMed] [Google Scholar]
- 17. Klein G, Berger J, Olsson G, et al. A double‐blind comparison of metoprolol CR/ZOK 50 mg and atenolol 50 mg once daily for uncomplicated hypertension. J Clin Pharmacol. 1990;30(2 suppl):S72–S77. [DOI] [PubMed] [Google Scholar]
- 18. Lacourcière Y, Lefebvre J, Provencher P, et al. Comparison of quinapril and atenolol as single drugs or in combination with hydrochlorothiazide in moderate to severe hypertensives, using automated ambulatory monitoring. Br J Clin Pharmacol. 1993;35(2):121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poirier L, Cleroux J, Nadeau A, et al. Effects of nebivolol and atenolol on insulin sensitivity and haemodynamics in hypertensive patients. J Hypertens. 2001;19(8):1429–1435. [DOI] [PubMed] [Google Scholar]
- 20. Aquilante CL, Terra SG, Schofield RS, et al. Sustained restoration of autonomic balance with long‐ but not short‐acting metoprolol in patients with heart failure. J Card Fail. 2006;12(3):171–176. [DOI] [PubMed] [Google Scholar]
- 21. Dahlöf B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the AngloScandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA): a multicentre randomised controlled trial. Lancet. 2005;366(9489):895–906. [DOI] [PubMed] [Google Scholar]
- 22. Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995–1003. [DOI] [PubMed] [Google Scholar]
- 23. Pepine CJ, Handberg EM, Cooper‐DeHoff RM, et al. A calcium antagonist vs a non‐calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil‐Trandolapril Study (INVEST): a randomized controlled trial. JAMA. 2003;290(21):2805–2816. [DOI] [PubMed] [Google Scholar]
- 24. Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370(9594):1219–1229. [DOI] [PubMed] [Google Scholar]


