Abstract
The secondary analysis of the Irbesartan/Hydrochlorothiazide Blood Pressure Reductions in Diverse Patient Populations (INCLUSIVE) clinical trial investigated whether baseline demographic and clinical variables are predictive of different degrees of blood pressure reduction following an angiotensin II receptor blocker/diuretic treatment regimen. Irbesartan/hydrochlorothiazide and other angiotensin receptor blocker combinations with a diuretic have been shown to be effective in reducing systolic blood pressure in a diverse patient population previously uncontrolled on monotherapy. Ordinary least squares regression analysis was performed on the intent‐to‐treat population of the INCLUSIVE study to identify variables predictive of variations in blood pressure changes in response to irbesartan/hydrochlorothiazide combination therapy. Higher baseline systolic blood pressure, female sex, type 2 diabetes, and statin therapy were found to be predictive of additional blood pressure lowering with this combination. The impact of higher baseline systolic blood pressure and diabetic state on changes in systolic blood pressure were diminished in female patients compared with male patients. In conclusion, a significant correlation may exist between certain clinical/demographic characteristics and the extent of the therapeutic response with irbesartan/hydrochlorothiazide treatment.
Cardiovascular disease (CVD), such as stroke, congestive heart failure, peripheral arterial disease, and coronary heart disease, presents a major health problem for developed countries. In the United States (US), 1 in 3 adults has some form of CVD. 1 CVD has been reported to be an underlying cause or contributory factor in 37.3% of all deaths in the US. 1 CVD also carries a substantial economic burden: the direct and indirect costs due to lost productivity from cardiovascular morbidity and mortality are predicted to reach $403.1 billion in 2006. 1
Hypertension is the single most important modifiable risk factor for CVD. 2 , 3 High blood pressure (BP) is present in about 70% of persons who have a first myocardial infarction or stroke or in whom congestive heart failure was diagnosed. 1 The relationship between diastolic BP (DBP), systolic BP (SBP), and CVD is continuous, and the findings of prospective observational studies suggest that for hypertensive or even normotensive individuals, a lower BP confers a lower risk of stroke and coronary heart disease. 4 No evidence has been found for a threshold of cardiovascular risk down to an SBP of 115 mm Hg. 5 The relationship between BP and cardiovascular events is independent of cholesterol levels and other cardiovascular risk factors.
Nonmodifiable risk factors for hypertension include age, sex, and ethnicity. There are age‐related pathophysiologic changes in the circulatory system, resulting in a linear increase in SBP. 2 , 6 The impact of age is such that an individual who is normotensive at age 55 has a 90% lifetime risk of developing hypertension. 7 There is a higher prevalence of hypertension and poorer health outcomes in women as they grow older compared with men. 8 , 9 Although, until the age of 45, men have a higher prevalence of hypertension than women of equivalent age, the incidence of hypertension increases rapidly in postmenopausal women. 10 Prevalence of hypertension can also vary in different racial groups. For example, it is high in African American patients 11 , 12 and the disease is likely to be more severe in this population than in Caucasians. By contrast, age‐adjusted prevalence of hypertension is lower among Hispanic patients than among black patients or non‐Hispanic white patients; the high incidence of hypertension‐associated mortality detected in this ethnic group may be attributed to poor awareness of hypertension, inadequate treatment, and/or poor control. 13
Other major modifiable risk factors for hypertension include obesity and type 2 diabetes. Obesity is now regarded as reaching epidemic proportions in the US, with >32.3% of the adult population being overweight or clinically obese. 14 Hypertension is twice as prevalent in diabetic than in nondiabetic individuals, being detected in 20% to 60% of patients with type 2 diabetes, 15 and serves as a multiplicative risk factor for CVD. In patients with type 2 diabetes, 86% of deaths are due to CVD. 16 , 17 Chronic renal disease is another frequent complication of type 2 diabetes, with diabetic nephropathy the leading cause of end‐stage renal disease in the US. 18
Control of BP is important, with any reduction associated with a decreased likelihood of CVD mortality and morbidity. 5 , 19 For example, a decrease in DBP of 10 mm Hg is associated with a 56% reduction in stroke and a 37% reduction in coronary heart disease, 4 but decreases in BP as small as 2 mm Hg have also been shown to reduce the risk of CVD. 20 The Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) 2 recommends a target SBP/DBP of <140/90 mm Hg in the absence of confounding risk factors. Additional risk factors require more rigorous control of BP. In the presence of type 2 diabetes, the SBP/DBP goal recommended by both JNC 7 and the American Diabetes Association is <130/80 mm Hg. 2 , 21 The JNC 7 guidelines recognize that many patients will require more than 1 class of antihypertensive agent when untreated SBP/DBP is >20/10 mm Hg of these target values, and these levels should be treated initially with 2 different classes of antihypertensives taken either as separate prescriptions or as a fixed‐dose combination. 2 The latter may have the advantage of enhancing patient adherence to treatment. These guidelines are especially relevant to other high‐risk patients and are consistent with those of the National Kidney Foundation and the International Society of Hypertension in Blacks. 22
Dual therapy with the angiotensin II receptor blocker (ARB) irbesartan and the thiazide diuretic hydrochlorothiazide (HCTZ) as well as combinations of other ARBs and diuretics, ACE inhibitors or β‐blockers and diuretics, and ACE inhibitors and calcium channel blockers have been shown to reduce BP in hypertensive patients. 23 , 24 , 25 , 26 Little is known, however, about whether the presence of specific factors influences the extent of the therapeutic response to some of these combination therapies.
The objective of this secondary analysis of the Irbesartan/Hydrochlorothiazide Blood Pressure Reductions in Diverse Patient Populations (INCLUSIVE) study data was to determine whether specific baseline demographic or clinical variables were predictors of SBP lowering in response to ARB/diuretic combination therapy.
MATERIALS AND METHODS
Full details of the study design and population have been previously published. 27 Brief details are provided below.
Study Population
Eligible patients included men and women aged 18 years and older, of self‐identified race/ethnic group, with uncontrolled SBP (140–159 mm Hg; 130–159 mm Hg for patients with type 2 diabetes) after ≥4 weeks of treatment with antihypertensive monotherapy. Excluded were patients with severe or secondary hypertension, significant concomitant disease, hypersensitivity to study medication, and patients receiving insulin. 27 At least 100 patients were recruited into each of the following subpopulations: elderly (65 years and older), African American, Hispanic/Latino, type 2 diabetes, and the metabolic syndrome.
Study Design
The INCLUSIVE trial was a multicenter, prospective, open‐label, single‐arm, 4‐phase study with titration to SBP. 27 The study was conducted at 119 sites in the US between July 2003 and August 2004. Previous antihypertensive monotherapy was discontinued and replaced by placebo for 4 to 5 weeks. Subsequently, forced titration was performed with the administration of HCTZ 12.5 mg for 2 weeks, followed by irbesartan/HCTZ 150/12.5 mg for 8 weeks, and then irbesartan/HCTZ 300/25 mg for 8 weeks. Mean seated trough SBP/DBP (3 readings at 2‐minute intervals; 8 am±2 hours) was determined using an automated, validated device (Omron HEM‐705CP; Omron Healthcare Inc, Deerfield, IL). 28 The qualifying SBP criterion for entry into the placebo, HCTZ 12.5‐mg, and irbesartan/HCTZ 150/12.5‐mg phases was 140 to 179 mm Hg (130–179 mm Hg for type 2 diabetic patients) and for the irbesartan/HCTZ 300/25‐mg phase the entry criterion was 120 to 179 mm Hg for all patients. The qualifying DBP criterion for all phases of the study was 70 to 109 mm Hg. Patients who did not meet these BP criteria at the start of each phase were withdrawn. Patients were withdrawn from the study if goal BP was achieved or because BP levels were outside the stated ranges.
The study protocol was approved by the institutional review board/ethics committee of each participating site. Informed consent was obtained from each patient.
Study Parameters
The primary efficacy end point of the study was the mean change in SBP from baseline (end of placebo phase) to week 18 (study end). In this secondary analysis, statistical models were constructed with available data from the intent‐to‐treat (ITT) population to identify the effect of baseline demographic or clinical variables on the changes in SBP from baseline to week 18.
Statistics
Mean, standard deviation, standard error, median, and minimum and maximum values were calculated for the continuous variables. Ordinary least squares regression was employed to identify factors that explain variations in SBP change from baseline to week 18 in the ITT population, which included patients with ≥1 valid SBP recordings after taking at least 1 dose of irbesartan/HCTZ 150/12.5 mg at week 10 of the study. The last observation carried forward was used to calculate the change from baseline in SBP in patients who were withdrawn from the study because of achievement of goal level before week 18. Model parameter estimates were tested at an α level of 0.05. Tolerance and variance inflation factors were employed to assess levels of collinearity and multicollinearity. DFBETAs were used to identify influential cases using Belsley, Kuh, and Welsch's criterion of DFBETA ≥2/n1/2 as the operational definition of a numerically influential case. 29
RESULTS
The proportion of patients with DBP <90 mm Hg and with DBP <90 mm Hg and SBP >140 mm Hg (>130 mm Hg for patients with diabetes) during the treatment regimen is given in Table I.
Table I.
Proportion of Patients With DBP <90 mm Hg and SBP >140 mm Hg, or >130 mm Hg for Patients With Diabetes, During the Treatment Regimen
| Patients With DBP <90 mm Hg | Patients With DBP <90 mm Hg and SBP >140 mm Hg (>130 mm Hg for Patients With Diabetes) | |
|---|---|---|
| Baseline | 40.08% (295 of 736) | 94.20% (278 of 295) |
| Week 2 | 47.15% (347 of 736) | 93.4% (324 of 347) |
| Week 10 | 72.33% (494 of 683) | 43.12% (213 of 494) |
| Week 18 | 81.39% (599 of 736) | 26.90% (161 of 599) |
| Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure. | ||
Significant changes in SBP from baseline to week 18 were observed for the ITT population (n=736) and across subgroups (Figure). In the overall ITT population, there was a decrease in SBP level from baseline to week 18 of 21.5 mm Hg. Significant reductions in SBP levels were observed across a diverse range of subgroups, with a slightly larger reduction observed in older patients (23.0 mm Hg), Hispanic/Latino patients (22.9 mm Hg), and women (22.9 mm Hg). SBP reductions were also observed at weeks 2 (2.9±10.5 mm Hg) and 10 (15.1±12.5 mm Hg).
Figure.
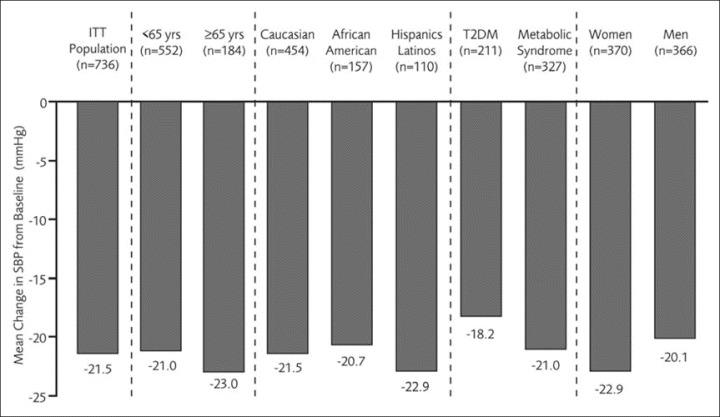
Systolic blood pressure (SBP) changes at week 18, by subgroup. ITT indicates intent‐to‐treat; T2DM, type 2 diabetes mellitus.
The adverse event profile of irbesartan/HCTZ in the safety population (n=1005) was good and similar in all patient subgroups. Overall, the incidence of adverse events was 55%: placebo (24%), HCTZ 12.5 mg (17%), irbesartan/HCTZ 150/12.5 mg (27%), and irbesartan/HCTZ 300/25 mg (26%). The most frequent adverse event was headache, which had a higher incidence with placebo (5%) compared with active treatment (1%–2%).
The greatest reductions in SBP from baseline to week 18 were observed in patients with a high baseline SBP level (Table II); each additional 1‐mm Hg elevation in baseline SBP was associated with a mean (± SE) greater reduction in SBP of 0.9±0.1 mm Hg (P<.0001). Diabetic patients had a mean greater reduction of 5.0±1.2 mm Hg compared with nondiabetic patients (P<.0001). Two significant interaction terms were identified: SBP and female sex, and diabetes and female sex. The impact of both of these parameters was diminished in women (Table II). The total female population, however, had on average a 3.0±0.9 mm Hg greater reduction than men (P=.002). Patients administered statins had a mean greater reduction of 2.1±1.0 mm Hg compared with patients not receiving statins. The duration of therapy also affected changes in SBP: each additional 10 days of treatment was associated with a mean reduction of 0.6±0.1 mm Hg.
Table II.
Predictors of Systolic Blood Pressure Response in the INCLUSIVE Study (n=728)
| Predictive Variables | P Value | Nonsignificant Variables |
|---|---|---|
| Higher systolic blood pressure baseline | .0001 | Ethnicity |
| Effect greater for men than women | .01 | Age (continuous variable) |
| Female sex | .002 | Elderly (65 y and older) |
| Type 2 diabetes mellitus | .0001 | History of coronary artery disease |
| Effect greater for diabetic men than diabetic women | .04 | History of cardiovascular disease |
| Statin use | .03 | Lipid levels |
| Duration of treatment | .0001 | Glucose levels |
| Metabolic syndrome | ||
| Antiplatelet therapy use | ||
| Waist circumference | ||
| Baseline body mass index | ||
| Abbreviation: INCLUSIVE, Irbesartan/Hydrochlorothiazide Blood Pressure Reductions in Diverse Patient Populations. | ||
Ethnicity, age (younger than 65 years vs 65 years and older), the presence of the metabolic syndrome, a history of coronary artery disease, a history of CVD, lipid and glucose levels, concurrent antiplatelet use, waist circumference, and baseline body mass index were identified as nonsignificant predictors of SBP control. Mean reductions from baseline were significantly similar (P<.001) in each of these subgroups throughout the entire 18‐week study.
DISCUSSION
The INCLUSIVE clinical trial demonstrated that irbesartan/HCTZ was effective and well tolerated in a range of patients previously uncontrolled on relatively short‐term monotherapy. Significant reductions in SBP from baseline were observed for the ITT population and in various subgroups (Figure). Irbesartan/HCTZ and other ARB/diuretic fixed combinations have previously been shown to have good safety profiles and to be well tolerated in a heterogeneous population of patients with hypertension. 24 , 25 , 26 , 27 This secondary analysis of the INCLUSIVE study employed statistical models to identify any demographic and clinical factors that may explain variations in SBP response with irbesartan/HCTZ.
The analysis found that higher baseline SBP is associated with a greater decrease in SBP at week 18. A previous open‐label, observational study in 31,793 hypertensive patients with type 2 diabetes demonstrated that switching from previous antihypertensive therapy to irbesartan (alone or in combination with HCTZ) produced a mean reduction in SBP of 22.5 mm Hg after 3 months of treatment and that the reductions in BP were greatest in patients who had a high baseline BP. 30 These findings are similar to those noted in other studies—the higher the initial BPs, the greater the degree of BP lowering.
Generally, female hypertensive patients displayed a greater decrease in SBP than male hypertensive patients but were less susceptible than men to the influence of baseline SBP on reductions in SBP at week 18. It has been proposed that efficacy of non‐pharmacologic interventions in lowering BP may vary between the sexes; women may find it more difficult to lose weight and possibly respond more favorably to dietary sodium reduction. 31 Whether women and men respond differently to antihypertensive therapy and require different antihypertensive treatments has been questioned. 8 Some previous studies have reported no sex difference in response to therapy, 32 whereas others have indicated differences in renin‐angiotensin system function 33 and greater sensitivity in women to renin‐angiotensin system blockade. 34 Synergism between ARBs and estrogen may underlie these effects. Postmenopausal women are at particular risk for hypertension and CVD morbidity and mortality compared with men of a comparable age 10 , 35 and are at greater risk for CVD mortality than their male counterparts if hypertension is uncontrolled. 9 Studies with irbesartan/HCTZ report that there were no sex‐related differences in pharmacokinetics observed in healthy adults or sex difference in half‐life or accumulation in hypertensive patients, although somewhat higher plasma concentrations of the ARB were observed in women than in men (11% to 44%). 36
This secondary analysis also demonstrated that patients with type 2 diabetes achieved significant SBP decreases from baseline at week 18. SBP lowering was more pronounced in men than in women. Management of BP in type 2 diabetic patients is important to prevent microvascular and macrovascular damage. 16 Reductions in BP and BP goal achievement, however, are often more difficult in patients with diabetes compared with patients without diabetes. 37 , 38 , 39 Combination therapy in type 2 diabetic patients has been advocated by both JNC 7 and the American Diabetes Association, 21 and expert consensus recommends that BP treatment include either an ARB or angiotensin‐converting enzyme (ACE) inhibitor with a thiazide diuretic. A β‐blocker/diuretic combination has also been proven to be effective in reducing cardiovascular complications in patients with type 2 diabetes if BP is lowered. 16 The use of several ARBs has been shown to provide effective BP control, and to slow down progression of renal disease in type 2 diabetic patients with incipient or overt nephropathy. 40 , 41 There is also evidence from outcome studies that the use of ARBs may result in fewer cases of new‐onset type 2 diabetes in nondiabetic hypertensive patients when compared with other medications such as β‐blockers or diuretics. 42
In this population, African American, Hispanic/Latino, or Caucasian ethnicity was not found to be predictive of a differential effect in SBP change. HCTZ has long been regarded as the cornerstone of antihypertensive treatment in African American patients. Data from this and other studies 12 indicate that this ethnic group also responds to a combination of an ARB or an ACE inhibitor with a diuretic.
Concurrent treatment of dyslipidemia with statins was shown to result in some additional lowering of SBP. Studies have suggested that statins may have BP‐lowering effects independent of their action on cholesterol levels, possibly acting to down‐regulate the angiotensin type 1 receptor expression; this may act synergistically with ARBs or ACE inhibitors. 43 , 44 , 45
A further finding of this analysis was that continued treatment with irbesartan/HCTZ was associated with additional reductions in SBP at week 18: each additional 10 days of treatment with irbesartan/HCTZ was associated with a mean reduction of 0.6±0.1 mm Hg. Given that dosage increases are temporal, we could not separate the effects of dosage increase and treatment duration on BP. The latter is, at least in part, a proxy for the former. Nonetheless, the model would be incorrectly specified if some variable capturing the effects of time on trial was not included. In addition, the effects of full dosing are likely to be underestimated since 20% (148 patients) of the ITT population did not complete the full 18 weeks of the study and, as such, did not receive the increased study dosage and/or the increased dosage for the full 8 weeks. The continued decrease in BP over time, however, suggests long‐term benefits of treatment with combination therapy not only in terms of BP control, but also in the possible prevention of target organ damage. Also of interest is the fact that diuretic use resulted in fewer adverse effects than placebo.
CONCLUSIONS
The INCLUSIVE study demonstrated that irbesartan/HCTZ was effective and well tolerated in a broad range of patients of differing ages, ethnicities, and concomitant disease. Higher baseline SBP, female sex, type 2 diabetes, and concurrent statin therapy were predictive of additional BP lowering with combination therapy. A causal relationship may exist between certain baseline demographic and clinical characteristics and the extent of therapeutic response to ARB/diuretic therapy.
Disclosure:
This clinical study was sponsored by the Bristol‐Myers Squibb Sanofi‐Synthelabo Partnership.
References
- 1. Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113(6):e85–e151. [DOI] [PubMed] [Google Scholar]
- 2. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. [DOI] [PubMed] [Google Scholar]
- 3. He J, Whelton PK. Elevated systolic blood pressure as a risk factor for cardiovascular and renal disease. J Hypertens Suppl. 1999;17(2):S7–S13. [PubMed] [Google Scholar]
- 4. MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335(8692):765–774. [DOI] [PubMed] [Google Scholar]
- 5. Lewington S, Clarke R, Qizilbash N, et al. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. [DOI] [PubMed] [Google Scholar]
- 6. Benetos A, Waeber B, Izzo J, et al. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. Am J Hypertens. 2002;15(12):1101–1108. [DOI] [PubMed] [Google Scholar]
- 7. Vasan RS, Larson MG, Leip EP, et al. Assessment of frequency of progression to hypertension in non‐hypertensive participants in the Framingham Heart Study: a cohort study. Lancet. 2001;358(9294):1682–1686. [DOI] [PubMed] [Google Scholar]
- 8. McBride SM, Flynn FW, Ren J. Cardiovascular alteration and treatment of hypertension: do men and women differ? Endocrine. 2005;28(2):199–207. [DOI] [PubMed] [Google Scholar]
- 9. Gudmundsson LS, Johannsson M, Thorgeirsson G, et al. Hypertension control as predictor of mortality in treated men and women, followed for up to 30 years. Cardiovasc Drugs Ther. 2005;19(3):227–235. [DOI] [PubMed] [Google Scholar]
- 10. Kearney PM, Whelton M, Reynolds K, et al. Worldwide prevalence of hypertension: a systematic review. J Hypertens. 2004;22(1):11–19. [DOI] [PubMed] [Google Scholar]
- 11. Ashaye MO, Giles WH. Hypertension in Blacks: a literature review. Ethn Dis. 2003;13(4):456–462. [PubMed] [Google Scholar]
- 12. Douglas JG, Bakris GL, Epstein M, et al. Management of high blood pressure in African Americans: consensus statement of the Hypertension in African Americans Working Group of the International Society on Hypertension in Blacks. Arch Intern Med. 2003;163(5):525–541. [DOI] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention . Hypertension‐related mortality among Hispanic subpopulations—United States, 1995–2002. Morb Mortal Wkly Rep. 2006;55(7):177–180. [PubMed] [Google Scholar]
- 14. Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–1555. [DOI] [PubMed] [Google Scholar]
- 15. Arauz‐Pacheco C, Parrott MA, Raskin P. The treatment of hypertension in adult patients with diabetes. Diabetes Care. 2002;25(1):134–147. [DOI] [PubMed] [Google Scholar]
- 16. Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321(7258):412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Howard BV, Best LG, Galloway JM, et al. Coronary heart disease risk equivalence in diabetes depends on concomitant risk factors. Diabetes Care. 2006;29(2):391–397. [DOI] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention . Incidence of end‐stage renal disease among persons with diabetes—United States 1990–2002. Morb Mortal Wkly Rep. 2005;54(43):1097–1100. [PubMed] [Google Scholar]
- 19. Whelton PK, He J, Appel LJ, et al. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288(15):1882–1888. [DOI] [PubMed] [Google Scholar]
- 20. Cook NR, Cohen J, Hebert PR, et al. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155(7):701–709. [PubMed] [Google Scholar]
- 21. American Diabetes Association . Standards of medical care in diabetes. Diabetes Care. 2005;28(suppl 1):S4–S36. [PubMed] [Google Scholar]
- 22. Douglas JG. Clinical guidelines for the treatment of hypertension in African Americans. Am J Cardiovasc Drugs. 2005;5(1):1–6. [DOI] [PubMed] [Google Scholar]
- 23. Raskin P, Guthrie R, Flack J, et al. The long‐term antihypertensive activity and tolerability of irbesartan with hydrochlorothiazide. J Hum Hypertens. 1999;13(10):683–687. [DOI] [PubMed] [Google Scholar]
- 24. Chrysant SG. Fixed combination therapy of hypertension: focus on valsartan/hydrochlorothiazide combination (Diovan/HCT). Expert Rev Cardiovasc Ther. 2003;1(3):335–343. [DOI] [PubMed] [Google Scholar]
- 25. Chrysant SG, Chrysant GS. Antihypertensive efficacy of olmesartan medoxomil alone and in combination with hydrochlorothiazide. Expert Opin Pharmacother. 2004;5(3):657–667. [DOI] [PubMed] [Google Scholar]
- 26. Bobrie G, Delonca J, Moulin C, et al. A home blood pressure monitoring study comparing the antihypertensive efficacy of two angiotensin II receptor antagonist fixed combinations. Am J Hypertens. 2005;18(11):1482–1488. [DOI] [PubMed] [Google Scholar]
- 27. Neutel JM, Saunders E, Bakris GL, et al. The efficacy and safety of low‐ and high‐dose fixed combinations of irbesartan/hydrochlorothiazide in patients with uncontrolled systolic blood pressure on monotherapy: the INCLUSIVE trial. J Clin Hypertens (Greenwich). 2005;7(10):578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O'Brien E, Mee F, Atkins N, et al. Evaluation of three devices for self‐measurement of blood pressure according to the revised British Hypertension Society Protocol: the Omron HEM‐705CP, Philips HP5332, and Nissei DS‐175. Blood Press Monit. 1996;1(1):55–61. [PubMed] [Google Scholar]
- 29. Belsley DA, Kuh E, Welsch D. Regression Diagnostics. New York, NY: Wiley; 1980. [Google Scholar]
- 30. Strutz F, Bramlage P, Paar WD. Effect of three months' treatment with irbesartan on blood and pulse pressure of hypertensive type 2 diabetic patients: open, observational study in 31,793 patients. Curr Med Res Opin. 2005;21(9):1433–1440. [DOI] [PubMed] [Google Scholar]
- 31. Lewis CE. Characteristics and treatment of hypertension in women: a review of the literature. Am J Med Sci. 1996;311(4):193–199. [DOI] [PubMed] [Google Scholar]
- 32. Blood pressure studies in 14 communities . A two‐stage screen for hypertension. JAMA. 1977;237(22):2385–2391. [DOI] [PubMed] [Google Scholar]
- 33. Miller JA, Anacta LA, Cattran DC. Impact of gender on the renal response to angiotensin II. Kidney Int. 1999;55(1):278–285. [DOI] [PubMed] [Google Scholar]
- 34. Miller JA, Cherney DZ, Duncan JA, et al. Gender differences in the renal response to renin‐angiotensin system blockade. J Am Soc Nephrol. 2006;17(9):2554–2560. [DOI] [PubMed] [Google Scholar]
- 35. Welty FK. Women and cardiovascular risk. Am J Cardiol. 2001;88(7B):48J–52J. [DOI] [PubMed] [Google Scholar]
- 36. Bristol‐Myers Squibb Sanofi‐Synthelabo Partnership. Avalide US prescribing information. http://www.avalide.com. Accessed August 1, 2006.
- 37. McLean DL, Simpson SH, McAlister FA, et al. Treatment and blood pressure control in 47,964 people with diabetes and hypertension: a systematic review of observational studies. Can J Cardiol. 2006;22(10):855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lindholm LH, Ibsen H, Dahlof B, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):1004–1010. [DOI] [PubMed] [Google Scholar]
- 39. Sowers JR, Neutel JM, Saunders E, et al. Antihypertensive efficacy of Irbesartan/HCTZ in men and women with the metabolic syndrome and type 2 diabetes. J Clin Hypertens (Greenwich). 2006;8(7):470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. [DOI] [PubMed] [Google Scholar]
- 41. Parving HH, Lehnert H, Brochner‐Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870–878. [DOI] [PubMed] [Google Scholar]
- 42. Aksnes TA, Reims HM, Kjeldsen SE, et al. Antihypertensive treatment and new‐onset diabetes mellitus. Curr Hypertens Rep. 2005;7(4):298–303. [DOI] [PubMed] [Google Scholar]
- 43. Milionis HJ, Liberopoulos EN, Achimastos A, et al. Statins: another class of antihypertensive agents? J Hum Hypertens. 2006;20(5):320–335. [DOI] [PubMed] [Google Scholar]
- 44. Nickenig G. Should angiotensin II receptor blockers and statins be combined? Circulation. 2004;110(8):1013–1020. [DOI] [PubMed] [Google Scholar]
- 45. Sarafidis PA, Kanaki AI, Lasaridis AN. Statins and blood pressure: is there an effect or not? J Clin Hypertens (Greenwich). 2007;9(6):460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]


