Abstract
Consumption of large quantities of liquorice can cause hypokalemia and hypertension. These effects are associated with increased cortisol‐mediated activation of renal mineralocorticoid receptors and hypoaldosteronism. The authors describe a patient with long‐standing hypokalemia and uncontrolled hypertension related to excessive ingestion of liquorice. The case highlights the importance of obtaining a detailed dietary history, especially considering the increasing use of liquorice‐containing foods, teas, and herbal products. The authors also discuss secondary causes of hypertension, focusing on pseudohyperaldosteronism.
Excessive dietary intake of liquorice can cause a syndrome mimicking hypermineralocorticoidism, characterized by hypertension, hypokalemia, alkalosis, low renin activity, and hypoaldosteronism. 1 , 2 , 3 , 4 The active ingredient in liquorice, glycyrrhizin (glycyrrhizic acid, glycyrrhizinate, glycyrrhetinic acid), induces pseudohyperaldosteronism by inhibiting the 11β‐hydroxysteroid dehydrogenase type 2 (11β‐HSD2), which converts active glucocorticoid cortisol to locally inactive cortisone. 5 This inhibition results in activation of renal mineralocorticoid receptors by cortisol. The net effect of renal mineralocorticoid receptor activation is Na+ reabsorption and K+ excretion with transient hypernatremia, persistent hypokalemia, and metabolic alkalosis, leading to a phenotype similar to that of the syndrome of apparent mineralocorticoid excess. 4 , 6 We report the case of a patient with resistant hypertension and hypokalemia who had been consuming excessive quantities of liquorice daily for 4 years following smoking cessation. The significance of this case relates to the fact that liquorice is increasingly being used in herbal medicines and teas as a laxative and as a flavoring agent in candies, chewing gums, breath fresheners, and food products. Considering the growing use of liquorice and the potential adverse clinical effects, physicians are encouraged to obtain detailed dietary and drug histories when patients present with hypokalemia and hypertension.
CASE REPORT
A 55‐year‐old woman was referred to the hypertension clinic by her family doctor for uncontrolled resistant hypertension. She had been diagnosed with hypertension 2 years previously. Despite multiple antihypertensive drugs, her blood pressure remained elevated (148–180/80–90 mm Hg). At the time of presentation to the clinic, she was taking the maximal recommended doses of an angiotensinconverting enzyme (ACE) inhibitor (enalapril) and a calcium channel blocker (amlodipine) and 12.5 mg of a thiazide diuretic. Risk factors for hypertension included a positive family history for cardiovascular disease and hypertension, postmenopause status, and ex‐smoker status. Other than the antihypertensive drugs, she was not taking any medication or herbal products. The patient exercised regularly, denied excessive alcohol intake, and consumed a “healthy” diet. On examination, casual sitting blood pressure was 151/85 mm Hg (by automatic blood pressure monitor) and 158/82 mm Hg (manual). Except for a left carotid bruit, the physical examination results were normal. The dosage of the thiazide diuretic was increased to 25 mg/d. One week later, routine laboratory investigation revealed hypokalemia (plasma K+, 2.4 mmol/L; normal, 3.3–5.1 mmol/L) and hypernatremia (plasma Na+, 148 mm Hg; normal, 135–145 mmol/L). Serum chloride was 99 mmol/L (normal, 101–111 mmol/L), carbon dioxide was 33 mmol/L (normal, 22–32 mmol/L), and anion gap was 14 mmol/L (normal, 5–12 mmol/L).
Because of the hypokalemia, the diuretic therapy was discontinued, K+ supplements were prescribed, and the patient was advised to consume a K+‐rich diet. Two weeks after stopping the diuretic, K+ was 2.7 mmol/L and Na+ was 145 mmol/L. Blood pressure remained uncontrolled (150/100 mm Hg) and the patient was examined for hyperaldosteronism. Plasma aldosterone (upright) was <31 pmol/L (normal, 111–860 pmol/L) and plasma renin (upright) was 4.2 ng/L (normal, 3–33 ng/L). In light of hypokalemia, hypernatremia, and reduced plasma aldosterone levels, a diagnosis of pseudohyperaldosteronism was made. A detailed dietary history revealed that since the patient stopped smoking 4 years ago, she started eating large quantities of liquorice daily. She always enjoyed eating liquorice and as a child frequently sucked the root of the liquorice plant (Glycyrrhiza glabra) and often consumed European/Dutch liquorice (which contains natural liquorice root extract). Since quitting smoking, she consumed 200 to 250 g of black liquorice daily, of various types, including liquorice ropes, liquorice candy pieces, and Dutch liquorice. Based on the manufacture's information, all of these products contained some natural liquorice root extract. The patient was advised to stop eating liquorice and to continue the K+‐rich diet and K+ supplementation. Three months later, K+ was 3.8 mmol/L, and K+ supplements were stopped. Eighteen months after presentation to the hypertension clinic and after stopping liquorice consumption, her blood biochemical profile was normal, blood pressure was controlled at 123/77 mm Hg (by automatic blood pressure monitor) and 128/75 mm Hg (manual) on low doses of perindopril and amlodipine, and she was clinically well.
DISCUSSION
Hypokalemia, hypernatremia, and hypertension are classically associated with mineralocorticoid excess, 1 , 2 , 3 , 4 , 5 , 6 and our initial diagnosis in the patient was hyperaldosteronism. Plasma aldosterone and renin levels were suppressed, however, and accordingly the possibility of primary aldosteronism was excluded and a diagnosis of pseudohyperaldosteronism was made. This was further considered in light of the chronic excessive consumption of liquorice, a well‐known cause of pseudohyperaldosteronism. Other causes of pseudohyperaldosteronism that were considered included endocrinopathies, such as Cushing's syndrome and ectopic corticotropin syndrome, 7 and genetic causes of which 3 monogenetic types of mineralocorticoid hypertension have been identified: Liddle's syndrome, glucocorticoid‐remediable hypertension, and apparent mineralocorticoid excess, an autosomal recessive disorder with mutations in the 11β‐HSD2 gene. 8 , 9 , 10 Clinical signs and the biochemical profile of the patient did not support any of these possibilities. Since hypokalemia improved and blood pressure was controlled following cessation of liquorice ingestion, our diagnosis of liquorice‐induced hypertension was confirmed.
Liquorice extracts and its principle component, glycyrrhizin, are extensively used in foods, tobacco, and both traditional and herbal medicine (Table). 11 The sweet taste of the root comes from glycyrrhizin, which is reputed to be 50 times sweeter than refined sugar. As a result, there is a high level of use of liquorice and glycyrrhizin in North America, with an estimated consumption of 0.027–3.600 mg glycyrrhizin/kg/d. 11 The World Health Organization suggested that consumption of 100 mg/d would be unlikely to cause adverse effects. 12 The Dutch Nutrition Information Bureau advised against a daily glycyrrhizin consumption in excess of 200 mg/d. 13 The glycyrrhizin content can vary by as much as 30‐fold from one confectionery product to another. 11 The original liquorice candy was made from dried root of the licorice plant; they looked like plain wooden sticks, were soft, and were popular with Dutch children. Today's popular liquorice candy is usually black and chewy and is packaged in coiled ropes, tubes, or as candy pieces. The black color is due to the use of carbon black as the food‐coloring agent. It is flavored with anise oil and the extract of the roots of the liquorice plant, as well as other artificial flavoring agents. Red liquorice resembles liquorice sticks but does not have the extract of the liquorice root and hence does not contain glycyrrhizin. Ingredients of commonly consumed liquorice candy are available at www.licorice.org.
Table.
US Food and Drug Administration Limitations for the Use of Liquorice and Its Derivatives in Foods
| Food Category | Maximum Allowable Levels in Foods | Functional Use |
|---|---|---|
| Baked goods | 0.05 | Flavor enhancer |
| Flavoring agent | ||
| Alcoholic beverages | 0.1 | Flavor enhancer |
| Flavoring agent | ||
| Surface active agent | ||
| Nonalcoholic beverages | 0.15 | Flavor enhancer |
| Flavoring agent | ||
| Surface active agent | ||
| Chewing gum | 1.1 | Flavor enhancer |
| Flavoring agent | ||
| Hard candy | 16.0 | Flavor enhancer |
| Flavoring agent | ||
| Soft candy | 3.1 | Flavor enhancer |
| Flavoring agent | ||
| Herbs and seasonings | 0.15 | Flavor enhancer |
| Flavoring agent | ||
| Plant protein products | 0.15 | Flavor enhancer |
| Flavoring agent | ||
| Vitamin or mineral dietary supplements | 0.5 | Flavor enhancer |
| Flavoring agent | ||
| Values are expressed as the percentage of glycyrrhizin content. Data from World Health Organization. 12 | ||
The active molecule of liquorice that influences mineralocorticoid metabolism is glycyrrhizin. Hence, artificial liquorice flavoring agents not containing glycyrrhizin would not influence mineralocorticoid metabolism. Glycyrrhizin constitutes 10% to 25% of licorice root extract and is considered the primary active ingredient. Glycyrrhizin is a saponin compound comprising a triterpenoid aglycone, glycyrrhetic acid (glycyrrhetinic acid, enoxolone), conjugated to a disaccharide of glucuronic acid. Both glycyrrhizin and glycyrrhetic acid can exist in the 18α ‐ and 18β‐stereoisomers. 14 , 15 As a tribasic acid, glycyrrhizin can form a variety of salts and occurs naturally in licorice root as the calcium and potassium salts.
Initially, it was thought that the mineralocorticoid actions of liquorice were mediated through competitive binding of glycyrrhizin binding to the mineralocorticoid receptor, because glycyrrhetic acid has a structural resemblance to aldosterone. 16 , 17 It has now been confirmed, however, that glycyrrhetic acid does not have a direct effect on renal function but that it influences glucorticoid metabolism by inhibiting 11β‐HSD2, which prevents conversion of active cortisol to inactive cortisone (Figure). 5 , 16 , 17 , 18 , 19 This results in an unrestricted activation of mineralocorticoid receptors by cortisol, the concentration of which is 100 to 1000 times higher than that of aldosterone, leading to increased sodium retention, exaggerated renal potassium loss, low renin and aldosterone levels, and hypertension. The clinical profile of liquorice‐induced pseudohyperaldosteronism is similar to the syndrome of apparent mineralocorticoid excess.
Figure.
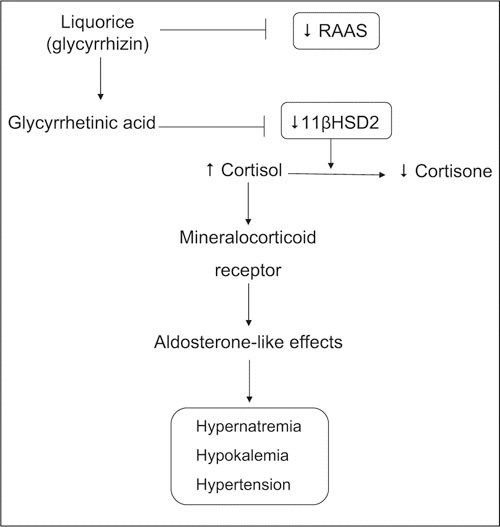
Schematic demonstrating mechanisms whereby liquorice induces hypertension. Liquorice contains glycyrrhizin, which is converted to glycyrrhetinic acid in the bowel. Glycyrrhetinic acid inhibits activation of 11β‐hydroxysteroid dehydrogenase 2 (11β‐HSD2), an enzyme that converts active cortisol to inactive cortisone. 11β‐HSD2 is expressed in the renal cortex and the salivary glands. Decreased activation of 11β‐HSD2 results in elevated cortisol levels and increased binding to renal mineralocorticoid receptors. Mineralocorticoid receptor activation leads to Na+ reabsorption and renal K+ loss and consequent hypernatremia and hypokalemia. This is associated with elevated blood pressure. Liquorice (glycyrrhizin) may also inhibit the renin‐angiotensin‐aldosterone system (RAAS) by reducing renin secretion. ↑ indicates increase effect; ↓, decrease effect.
In addition to hypokalemia and hypertension, excessive liquorice intake has been associated with rhabdomyolysis, muscle paralysis, respiratory impairment, hypertensive emergencies, hyperparathyroidism, encephalopathy, and acute renal failure. 20 , 21 , 22 , 23 , 24 , 25 Susceptibility to adverse actions of glycyrrhetinic acid is variable. Usually, clinically relevant effects become evident when glycyrrhetinic acid ingestion exceeds 400 mg/d. Essential hypertension, use of diuretics, and salt sensitivity amplify mineralocorticoid actions of liquorice. 26 , 27 , 28 , 29 Recent data indicate that the renin‐angiotensin‐aldosterone system is more responsive to liquorice in men than in women, suggesting a sex effect, possibly related to actions on estrogens and testosterone, as previously reported. 27 , 30 , 31 In an acute study, effects of daily confectionary licorice consumption on electrolyte status and the renin‐angiotensin‐aldoster‐one axis were evaluated in healthy participants. 32 Volunteers ate 100 to 200 g of licorice‐containing candy daily for 1 to 4 weeks and were monitored for blood pressure, plasma aldosterone, plasma renin activity, plasma angiotensin II, plasma electrolytes, and urinary electrolyte and aldosterone concentrations. 32 Licorice was prematurely withdrawn in 50% of the 14 participants because of hypokalemia or edema. Electrolyte imbalance was demonstrated in most participants, and there was a significant reduction in the levels of plasma renin activity, angiotensin II, and plasma and urinary aldosterone concentrations. These values returned to control levels within 1 to 2 weeks after licorice withdrawal. Participants with the most prolonged renin‐angiotensin‐aldosterone axis suppression had the most severely affected hypokalemia and edema that necessitated their early withdrawal from the study. 32 It has also been shown that excessive liquorice consumption increases plasma concentrations of atrial natriuretic peptide, possibly as a reflection of sodium and water retention. 33 Other risk factors for developing liquorice‐induced pseudohyperaldosteronism include old age and chronic inflammatory conditions. 21 , 22
Liquorice raises blood pressure with a linear dose‐response relationship. Even doses as low as 50 g of liquorice (75 mg of glycyrrhetinic acid) consumed daily for 2 weeks can cause a significant rise in blood pressure. 31 This knowledge, together with the fact that there is increasing use of liquorice in herbal products, teas, breath fresheners, chewing gums, and food products, 34 , 35 emphasizes the importance of obtaining a detailed dietary and drug history from patients. Hypokalemia, hypertension, and hypoaldosteronism should alert the physician of liquorice‐induced pseudoaldosteronism, a condition that could result in medical emergencies. 21
References
- 1. Farese RV Jr, Biglieri EG, Shackleton CH, et al. Licorice‐induced hypermineralocorticoidism. N Engl J Med. 1991;325(17):1223–1227. [DOI] [PubMed] [Google Scholar]
- 2. Mumoli N, Cei M. Licorice‐induced hypokalemia. Int J Cardiol. 2007 Feb 21; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 3. Van Uum SH. Liquorice and hypertension. Neth J Med. 2005;63(4):119–120. [PubMed] [Google Scholar]
- 4. Palermo M, Quinkler M, Stewart PM. Apparent mineralocorticoid excess syndrome: an overview. Arq Bras Endocrinol Metabol. 2004;48(5):687–696. [DOI] [PubMed] [Google Scholar]
- 5. Van Uum SH, Lenders JW, Hermus AR. Cortisol, 11beta‐hydroxysteroid dehydrogenases, and hypertension. Semin Vasc Med. 2004;4(2):121–128. [DOI] [PubMed] [Google Scholar]
- 6. Walker BR, Edwards CR. Licorice‐induced hypertension and syndromes of apparent mineralocorticoid excess. Endocrinol Metab Clin North Am. 1994;23:359–377. [PubMed] [Google Scholar]
- 7. Gomez‐Sanchez CE, Gomez‐Sanchez EP, Yamakita N. Endocrine causes of hypertension. Semin Nephrol. 1995;15(2):106–115. [PubMed] [Google Scholar]
- 8. Dluhy RG, Anderson B, Harlin B, et al. Glucocorticoid‐remediable aldosteronism is associated with severe hypertension in early childhood. J Pediatr. 2001;138(5):715–720. [DOI] [PubMed] [Google Scholar]
- 9. Lifton RP. Genetic dissection of human blood pressure variation: common pathways from rare phenotypes. Harvey Lect. 20042005;100:71–101. [PubMed] [Google Scholar]
- 10. Armanini D, Calo L, Semplicini A. Pseudohyper‐aldosteronism: pathogenetic mechanisms. Crit Rev Clin Lab Sci. 2003;40(3):295–335. [DOI] [PubMed] [Google Scholar]
- 11. Isbrucker RA, Burdock GA. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul Toxicol Pharmacol. 2006;46(3):167–192. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization . Evaluation of certain food additives. World Health Organ Tech Rep Ser. 2005;928:1–156. [PubMed] [Google Scholar]
- 13. Fenwick GR, Lutomski J, Nieman C. Liquorice, Glycyrrhiza glabra L.—Composition, uses and analysis. Food Chem. 1990;38(2):119–143. [Google Scholar]
- 14. Wang ZY, Athar M, Bickers DR. Licorice in foods and herbal drugs: chemistry, pharmacology, toxicology, and uses. In: Mazza G, Oomah BD, eds. Herbs, Botanicals and Teas. Lancaster, PA: Technomic Publishing Co. Inc; 2000:321–353. [Google Scholar]
- 15. Eisenbrand G. Glycyrrhizin. Mol Nutr Food Res. 2006;50(11):1087–1088. [DOI] [PubMed] [Google Scholar]
- 16. Card WI, Mitchell W, Strong JA, et al. Effects of liquorice and its derivatives on salt and water metabolism. Lancet. 1953;1(14):663–668. [DOI] [PubMed] [Google Scholar]
- 17. Quinkler M, Stewart PM. Hypertension and the cortisolcortisone shuttle. J Clin Endocrinol Metab. 2003;88(6):2384–2392. [DOI] [PubMed] [Google Scholar]
- 18. Draper N, Stewart PM. 11beta‐hydroxysteroid dehydrogenase and the pre‐receptor regulation of corticosteroid hormone action. J Endocrinol. 2005;186(2):251–271. [DOI] [PubMed] [Google Scholar]
- 19. Stewart PM, Wallace AM, Valentino R, et al. Mineralocorticoid activity of liquorice: 11‐beta‐hydroxysteroid dehydrogenase deficiency comes of age. Lancet. 1987;2(8563):821–824. [DOI] [PubMed] [Google Scholar]
- 20. Yasue H, Itoh T, Mizuno Y, et al. Severe hypokalemia, rhabdomyolysis, muscle paralysis, and respiratory impairment in a hypertensive patient taking herbal medicines containing licorice. Intern Med. 2007;46(9):575–578. [DOI] [PubMed] [Google Scholar]
- 21. Breidthardt T, Namdar M, Hess B. A hypertensive urgency induced by the continuous intake of a herbal remedy containing liquorice. J Hum Hypertens. 2006;20(6):465–466. [DOI] [PubMed] [Google Scholar]
- 22. Janse A, Van Iersel M, Hoefnagels WH, et al. The old lady who liked liquorice: hypertension due to chronic intoxication in a memory‐impaired patient. Neth J Med. 2005;63(4):149–150. [PubMed] [Google Scholar]
- 23. Russo S, Mastropasqua M, Mosetti MA, et al. Low doses of liquorice can induce hypertension encephalopathy. Am J Nephrol. 2000;20:145–148. [DOI] [PubMed] [Google Scholar]
- 24. Hussain RM. The sweet cake that reaches parts other cakes can't Postgrad Med J. 2003;79(928):115–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saito T, Tsuboi Y, Fujisawa G, et al. An autopsy case of licorice‐induced hypokalemic rhabdomyolysis associated with acute renal failure: special reference to profound calcium deposition in skeletal and cardiac muscle. Nippon Jinzo Gakkai Shi. 1994;36(11):1308–1314. [PubMed] [Google Scholar]
- 26. Sigurjonsdottir HA, Manhem K, Axelson M, et al. Subjects with essential hypertension are more sensitive to the inhibition of 11 beta‐HSD by liquorice. J Hum Hypertens. 2003;17(2):125–131. [DOI] [PubMed] [Google Scholar]
- 27. Megia A, Herranz L, Martin‐Almendra MA, et al. Angiotensin I‐converting enzyme levels and renin‐aldoster‐one axis recovery after cessation of chronic licorice ingestion. Nephron. 1993;65(2):329–330. [DOI] [PubMed] [Google Scholar]
- 28. Ferrari P, Sansonnens A, Dick B, et al. In vivo 11beta‐HSD‐2 activity: variability, salt‐sensitivity, and effect of licorice. Hypertension. 2001;38(6):1330–1336. [DOI] [PubMed] [Google Scholar]
- 29. Sigurjonsdottir HA, Axelson M, Johannsson G, et al. The liquorice effect on the RAS differs between the genders. Blood Press. 2006;15(3):169–172. [DOI] [PubMed] [Google Scholar]
- 30. Armanini D, Mattarello MJ, Fiore C, et al. Licorice reduces serum testosterone in healthy women. Steroids. 2004;69(11–12):763–766. [DOI] [PubMed] [Google Scholar]
- 31. Sigurjónsdóttir HA, Franzson L, Manhem K, et al. Liquorice‐induced rise in blood pressure: a linear dose‐response relationship. J Hum Hypertens. 2001;15(8):549–552. [DOI] [PubMed] [Google Scholar]
- 32. Epstein MT, Espiner EA, Donald RA, et al. Effect of eating liquorice on the renin‐angiotensin aldosterone axis in normal subjects. BMJ. 1977;1:488–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Forslund T, Fyhrquist F, Froseth B, et al. Effects of licorice on plasma atrial natriuretic peptide in healthy volunteers. J Intern Med. 1989;225:95–99. [DOI] [PubMed] [Google Scholar]
- 34. De Klerk GJ, Nieuwenhuis MG, Beutler JJ. Hypokalaemia and hypertension associated with use of liquorice flavoured chewing gum. BMJ. 1997;314(7082):731–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olukoga A, Donaldson D. Liquorice and its health implications. J R Soc Health. 2000;120(2):83–89. [DOI] [PubMed] [Google Scholar]


