Abstract
This double‐blind, multicenter, randomized placebo‐controlled study evaluated the antihypertensive efficacy and safety of nebivolol, a selective β1‐adrenoreceptor blocker with vasodilating effects, in patients with mild to moderate hypertension (sitting diastolic blood pressure [SiDBP] ≥95 mm Hg and ≤109 mm Hg). A total of 909 patients were randomized to receive placebo or nebivolol 1.25, 2.5, 5, 10, 20, or 40 mg once daily for up to 84 days. The primary end point was the change in trough SiDBP from baseline to study end. Nebivolol significantly reduced trough SiDBP (8.0–11.2 mm Hg compared with 2.9 mm Hg with placebo; P<.001) and trough sitting systolic blood pressure (a 4.4–9.5‐mm Hg decrease compared with a 2.2‐mm Hg decrease with placebo; P≤.002). The overall adverse event experience was similar in the nebivolol (46.1%) and placebo (40.7%) groups (P=.273). Once‐daily nebivolol is an effective antihypertensive in mild to moderate hypertensive patients.
The clinical benefits of β‐blockers are well established in the treatment of hypertension and cardiovascular (CV) disease. Adverse events (AEs) and adverse metabolic effects associated with the use of traditional β‐blockers may be a concern, however. 1 , 2 β‐blocker use may result in an undesirable increase in peripheral vascular resistance in hypertensive patients. 3 Some patients may therefore not be able to fully realize the clinical benefits of β‐blockers due to the limitations of some of the currently available agents.
Several studies have documented that β‐blockers, as a class, are a heterogeneous group with differences in pharmacodynamic and pharmacokinetic effects of potentially significant clinical relevance. 4 Since hypertension generally requires lifelong therapy, antihypertensive treatment strategies with β‐blockers should provide clinical benefits and be effective and well tolerated to ensure long‐term patient compliance.
Nebivolol is a new β‐adrenergic‐blocking drug that combines a high degree of β1 selectivity with endothelium‐dependent vasodilating effects without sympathomimetic activity. 5 , 6 , 7 In randomized clinical trials, nebivolol has been shown to lower blood pressure (BP) in a dose‐dependent manner in patients with hypertension. 8 , 9 Most of the clinical experience with nebivolol has been in patients outside of the United States, however. Therefore, the objective of this study was to evaluate the efficacy and safety of monotherapy with nebivolol in a US population with mild to moderate hypertension.
METHODS
Study Population
Male and female patients aged 18 years or older with mild to moderate hypertension, defined as mean sitting diastolic BP (SiDBP) ≥95 mm Hg and ≤109 mm Hg, were eligible to participate in the study. Patients were not included if they had secondary or malignant hypertension; body mass index (BMI) ≥35 kg/m2; bronchospasm, bradycardia, or any other known contraindication to β‐blocker therapy; uncontrolled diabetes mellitus (hemoglobin A1c≥10%); recent (within 6 months) myocardial infarction or stroke; heart failure; hemodynamically significant valvular heart disease; clinically significant thyroid, renal, or hepatic dysfunction; peripheral vascular disease; positive pregnancy test result; or previous exposure to nebivolol.
Patients were stratified across all treatment arms by nebivolol metabolism based on oxidative genotype (poor vs extensive metabolizers), history of diabetes mellitus, self‐reported race (black vs nonblack), age (younger than 65 years vs 65 years or older), and sex.
Study Design
This double‐blind, randomized, placebo‐controlled parallel‐group study was conducted at 70 sites in the United States. A central institutional review board approved the protocol, and all patients provided written informed consent before undergoing any study‐related procedure. The study was conducted in accordance with the general principles of the Declaration of Helsinki and was in compliance with the regulations set forth in the US Code of Federal Regulations and the Good Clinical Practice Guidelines.
At the screening visit, patients were examined and a medical history was obtained to determine patients' eligibility for enrollment in the study. Following screening, all patients entered a 4‐week, single‐blind placebo run‐in/washout phase. Patients previously on antihypertensive medication were allowed an additional 2‐week single‐blind placebo run‐in/washout. At the end of the placebo run‐in/washout period (day 0), baseline and demographic characteristics were recorded and eligible patients were randomized to receive placebo or once‐daily nebivolol 1.25, 2.5, 5, 10, 20, or 40 mg in a double‐blind manner for 84 days. Patients in the 40‐mg nebivolol group were initiated at 30 mg once daily; the dosage was increased to 40 mg once daily after 2 weeks, only if their sitting heart rate (HR) at trough was >55 beats per minute (bpm). Patients returned to the study unit for assessments on days 14, 28, 56, and 84 of the double‐blind treatment period, at which time BP and HR were measured, compliance with study medication was monitored, and use of concomitant medications was recorded. Clinical laboratory parameters were measured at screening, at randomization, and at day 84.
Concomitant therapy with oral and ophthalmic β‐blockers, angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, diuretics, and α1‐receptor blockers was prohibited during the single‐blind run‐in phase and during the double‐blind treatment period.
Primary and Secondary End Points
The primary efficacy end point was change from baseline to day 84 in mean SiDBP at trough (24±2 hours post‐previous morning's dose). Secondary efficacy end points included changes from baseline to day 84 in mean sitting systolic BP (SiSBP) at trough, mean SiDBP and SiSBP at peak (2–3 hours postdose), and mean supine and standing diastolic BP (DBP) and systolic BP (SBP) at trough and peak. Another efficacy variable was the responder rates of treatment groups, defined as the proportion of patients with an SiDBP <90 mm Hg at the end of the study or an absolute reduction of ≥10 mm Hg in SiDBP from baseline.
Efficacy and Safety Assessments
BP was measured at trough and peak using an automatic sphygmomanometer and appropriately sized cuff in the supine, sitting, and standing positions. Three separate measurements were taken 2 minutes apart in the same arm: first after the patient had been at rest in the supine position for at least 5 minutes, then after sitting for 1 minute, and finally after standing for 1 minute. The mean of 3 readings in each position was calculated and recorded. Trough BP and HR measurements were taken at screening and randomization and on days 14, 28, 56, and 84 of the treatment period; peak BP and HR measurements were taken at randomization and on days 28 and 84 of the treatment period.
Safety was assessed by clinical review, vital signs (including HR), 12‐lead electrocardiograms (ECGs), and clinical laboratory evaluations including chemistry panel, hematologic profile, and urinalysis. All AEs occurring during the study were documented as to type, onset, duration, intensity, and relation to study drug.
Sample Size and Statistical Methods
Based on a standard deviation of 7.2 mm Hg for DBP reduction, it was estimated that a sample size of 59 patients per treatment arm would have 90% power to detect a 4.4‐mm Hg difference in DBP between any of the nebivolol dose groups and placebo; estimates indicated that 122 patients would be needed to detect with 90% power a 3‐mm Hg difference in DBP between the nebivolol doses of 5, 10, 20, and 40 mg. To account for a 20% dropout rate, 75 patients each were randomized in the placebo and nebivolol 1.25‐ and 2.5‐mg groups and 150 patients each in the nebivolol 5‐, 10‐, 20‐, and 40‐mg groups. The study was analyzed using the intent‐to‐treat (ITT) approach and included all randomized patients who took at least 1 dose of study medication. The last observation carried forward method was used in the case of missing data. Two‐sided statistical tests were performed, with a significance level of .05.
The patients' demographic characteristics and vital signs at baseline were summarized and compared between the treatment groups. Data for continuous variables were compared using analysis of variance overall F test. For categoric variables, the observed frequencies were compared using a chi‐square test. Changes in BP from baseline to the last visit (day 84) were compared between treatment groups using an analysis of covariance (ANCOVA) model with treatment as a main effect and baseline BP and dichotomous variables as covariates. The primary statistical method was a step‐down dose‐response trend test using a linear contrast in the ANCOVA. The 40‐mg dose was studied for safety purposes and therefore was not included in the step‐down trend test for efficacy. Response rates were analyzed using a logistic regression model with responder as the response variable and baseline DBP and dichotomous variables as covariates; the frequencies were compared using Wald chi‐square test.
For continuous safety variables, the change from baseline was tested with a step‐down method similar to that used for the efficacy variables, using an ANCOVA model with treatment and baseline covariates, except that linear contrasts comprised all treatment groups, including the 40‐mg group. For categoric safety variables, the P value was based on the Cochran‐Mantel‐Haenszel test adjusted for dichotomous baseline covariates. The overall AE incidences were compared for each individual nebivolol dose group and for all nebivolol groups combined vs placebo.
RESULTS
Disposition of Patients and Baseline Characteristics
At the end of the single‐blind placebo run‐in phase, 913 patients were randomized to double‐blind treatment. Four randomized patients failed to take any study medication; the IT population comprised 909 patients who were distributed as follows: placebo, n=81; nebivolol 1.25 mg, n=83; nebivolol 2.5 mg, n=82; nebivolol 5 mg, n=165; nebivolol 10 mg, n=166; nebivolol 20 mg, n=166; and nebivolol 40 mg, n=166 (all doses once daily). Of the 166 patients randomized to nebivolol 40 mg, 147 patients (88.6%) had their dosage increased from the initial 30‐mg once‐daily to a 40‐mg once daily dose, and 19 patients remained at a 30‐mg dose. Compliance, determined by the amount of patients' returned medication by study personnel at each clinic visit, ranged from 95.5% to 98.7% in the IT population overall.
A total of 777 patients (85.5%) completed the study: 82.7% in the placebo group and 85.7% in the nebivolol groups combined. The discontinuation rates ranged from 10.2% to 19.9% across the nebivolol dose groups, compared with 17.3% in the placebo group. The most common reasons for not completing the study were withdrawn consent (5.3%), AEs (2.5%), and loss to follow‐up (2.5%).
Treatment groups were comparable with respect to demographics and baseline characteristics (Table I). Overall, the majority of patients were men (57.0%), nonblack (85.5%), and younger than 65 years (78.8%). It is noteworthy that 9.7% of the patients were diabetic and 43.9% of all patients were obese (BMI ≥30 kg/m2).
Table I.
Baseline Patient Characteristics by Treatment
| Parameter | Nebivolol Dose | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n=81) | 1.25 mg (n=83) | 2.5 mg (n=82) | 5 mg (n=166) | 10 mg (n=166) | 20 mg (n=166) | 40 mg (n=166) | Total (N=909) | |
| Age, y, mean (SD) | 56.0 (11.6) | 55.5 (11.5) | 53.4 (12.3) | 54.9 (11.8) | 55.2 (12.5) | 54.1 (11.6) | 54.3 (11.6) | 54.7 (11.8) |
| Younger than 65 y, No. (%) | 64 (79.0) | 65 (78.3) | 68 (82.9) | 132 (80.0) | 125 (75.3) | 134 (80.7) | 128 (77.1) | 716 (78.8) |
| Aged 65 y or older, No. (%) | 17 (21.0) | 18 (21.7) | 14 (17.1) | 33 (20.0) | 41 (24.7) | 32 (19.3) | 38 (22.9) | 193 (21.2) |
| Sex, No. (%) | ||||||||
| Male | 46 (56.8) | 46 (55.4) | 53 (64.6) | 96 (58.2) | 93 (56.0) | 92 (55.4) | 92 (55.4) | 518 (57.0) |
| Female | 35 (43.2) | 37 (44.6) | 29 (35.4) | 69 (41.8) | 73 (44.0) | 74 (44.6) | 74 (44.6) | 391 (43.0) |
| Race, No. (%) | ||||||||
| Black | 11 (13.6) | 12 (14.5) | 13 (15.9) | 23 (13.9) | 23 (13.9) | 25 (15.1) | 25 (15.1) | 132 (14.5) |
| Nonblack | 70 (86.4) | 71 (85.5) | 69 (84.1) | 142 (86.1) | 143 (86.1) | 141 (84.9) | 141 (84.9) | 777 (85.5) |
| Diabetes mellitus, No. (%) | 7 (8.6) | 9 (10.8) | 10 (12.2) | 11 (6.7) | 17 (10.2) | 14 (8.4) | 20 (12.0) | 88 (9.7) |
| Body mass index, kg/m2, mean (SD) | 29.1 (4.2) | 29.3 (4.2) | 29.4 (3.9) | 29.2 (3.7) | 29.0 (4.1) | 28.7 (4.1) | 29.4 (3.9) | 29.1 (4.0) |
| <30, No. (%) | 44 (54.3) | 43 (51.8) | 45 (54.9) | 91 (55.2) | 102 (61.4) | 101 (60.8) | 84 (50.6) | 501 (56.1) |
| ≥30, No. (%) | 37 (45.7) | 40 (48.2) | 37 (45.1) | 74 (44.8) | 64 (38.6) | 65 (39.2) | 82 (49.4) | 399 (43.9) |
Efficacy
All nebivolol treatments effectively lowered BP. The least‐squares (LS) mean reductions in trough SiDBP and SiSBP from baseline to final visit (day 84) were significantly greater with nebivolol dosages ranging from 1.25 to 20 mg once daily than with placebo (SiDBP, P<.001; SiSBP, P=.002 for nebivolol 1.25 mg; P<.001 for all other doses) (Table II). Placebo‐subtracted LS mean reductions in trough SiDBP and SiSBP ranged from 8.3 mm Hg and 11.7 mm Hg, respectively, at maximum doses, with little difference, however, in SBP effect from 2.5 to 20 mg (Figure 1).
Table II.
Effect of Nebivolol on Blood Pressure and Heart Rate in Patients With Mild to Moderate Hypertension
| Variable | Nebivolol Dose | ||||||
|---|---|---|---|---|---|---|---|
| Placebo | 1.25 mg | 2.5 mg | 5 mg | 10 mg | 20 mg | 40 mg | |
| Trough SiDBP, mm Hg | |||||||
| No. | 81 | 83 | 82 | 165 | 166 | 166 | 166 |
| Baseline mean | 100.3 | 98.9 | 99.8 | 99.6 | 99.5 | 99.4 | 99.3 |
| Mean change (SD) | −3.2 (7.7) | −8.0 (7.7) | −8.7 (7.7) | −8.6 (8.0) | −9.4 (8.1) | −9.9 (8.7) | −11.3 (8.3) |
| LS mean change from baseline (SE)a | −2.9 (1.1) | −8.0 (1.1) | −8.5 (1.1) | −8.4 (1.0) | −9.2 (0.9) | −9.8 (0.9) | −11.2 (0.9) |
| Step‐down trend test P value | <.001 | <.001 | <.001 | <.001 | <.001 | ||
| Trough SiSBP, mm Hg | |||||||
| No. | 81 | 83 | 82 | 165 | 166 | 166 | 166 |
| Baseline mean | 154.9 | 152.2 | 150.1 | 152.6 | 155.8 | 151.9 | 153.1 |
| Mean change (SD) | −4.7 (12.2) | −7.8 (12.0) | −9.0 (14.7) | −9.9 (11.6) | −10.7 (13.2) | −11.5 (14.8) | −13.6 (13.0) |
| LS mean change from baseline (SE)a | +2.2 (1.9) | −4.4 (1.9) | −6.3 (1.9) | −5.9 (1.6) | −7.0 (1.6) | −6.5 (1.6) | −9.5 (1.5) |
| Step‐down trend test P value | .002 | <.001 | <.001 | <.001 | <.001 | ||
| Trough sitting heart rate, bpm | |||||||
| No. | 67 | 69 | 68 | 148 | 136 | 145 | 149 |
| Baseline mean | 73.9 | 72.3 | 73.5 | 72.7 | 71.5 | 73.3 | 71.7 |
| Mean change (SD) | 0.2 (8.7) | −2.7 (8.9) | −4.3 (8.1) | −6.5 (9.0) | −6.5 (8.0) | −9.7 (8.5) | −9.8 (7.8) |
| LS mean change from baseline (SE)a | +2.4 (1.1) | −1.4 (1.1) | −2.4 (1.1) | −4.9 (0.9) | −5.5 (0.9) | −7.9 (0.9) | −8.9 (0.9) |
| Step‐down trend test P value | .002 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| Abbreviations: bpm, beats per minute; LS, least‐squares; SiDBP, sitting diastolic blood pressure; SiSBP, sitting systolic blood pressure. aFrom an analysis of covariance with factor, treatment, and covariates baseline blood pressure, nebivolol metabolism rate, diabetes status, sex, race, and age group. | |||||||
Figure 1.
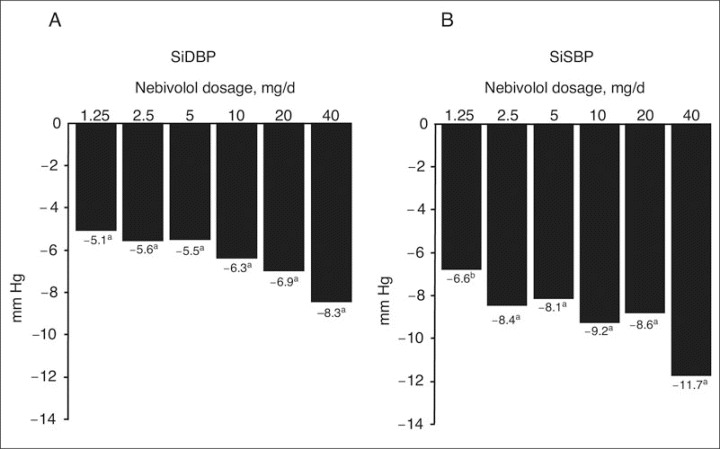
Placebo‐subtracted least‐squares mean reductions in trough sitting diastolic blood pressure (SiDBP) (A) and trough sitting systolic blood pressure (SiSBP) (B) from baseline to study end. aP<.001 vs placebo. bP=.002 vs placebo.
Nebivolol also significantly decreased peak SiDBP (LS mean reductions from baseline of 9.1 to 13.9 mm Hg vs 5.4 mm Hg with placebo; P=.005 for nebivolol 1.25 mg; P<.001 for all other doses) and peak SiSBP (LS mean reductions from baseline of 7.6–14.0 mm Hg vs 3.1 mm Hg with placebo; P=.029 for nebivolol 1.25 mg; P=.015 for nebivolol 2.5 mg; P<.001 for all other doses) in a dose‐dependent manner. The reductions in SBP and DBP at trough and peak in both the supine and standing positions for all doses of nebivolol were also significantly better than with placebo except for standing and supine peak SBP with the 1.25‐mg dose (data not shown).
To demonstrate the duration of action of nebivolol, trough‐to‐peak ratios were calculated. All doses of nebivolol induced a sustained effect throughout the 24‐hour interval. The trough‐to‐peak ratios derived from LS mean reductions in SiDBP from baseline to study end were 0.87, 0.84, 0.78, 0.79, 0.74, and 0.80 for nebivolol 1.25, 2.5, 5, 10, 20, and 40 mg, respectively. The placebo‐subtracted trough‐to‐peak ratios derived from mean reductions in SiDBP from baseline to study end were ≥0.9 for all doses of nebivolol, confirming that once‐daily dosing was effective.
After 84 days of treatment, significantly higher percentages of patients were responders (ie, patients with SiDBP ≤90 mm Hg or a reduction of ≥10 mm Hg from baseline) in all nebivolol treatment groups compared with placebo (P=.008 for nebivolol 1.25 mg; P=.001 for nebivolol 2.5 mg; P<.001 for all other doses) (Figure 2).
Figure 2.
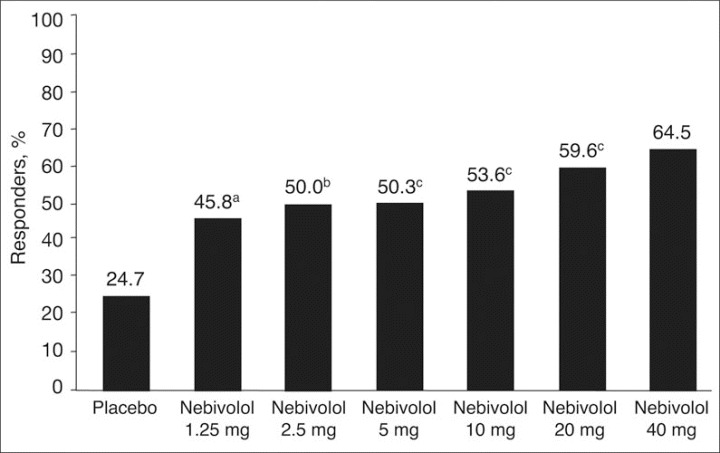
Responder rates by treatment, defined as average trough sitting diastolic blood pressure ≤90 mm Hg at study end or a decrease of ≥10 mm Hg. aP=.008 vs placebo. bP=.001 vs placebo. cP<.001 vs placebo.
Mean HR (measured at trough in the sitting position) decreased from the relevant baseline during treatment with nebivolol in a dose‐related manner, and this reduction was significantly different from placebo (P=.002 for nebivolol 1.25 mg; P<.001 for all other doses) (Table II). Reductions in standing and supine HR were comparable, although HR reductions in the standing position were generally greater, ranging from −4.6 bpm for nebivolol 1.25 mg to −12.0 bpm for nebivolol 40 mg. The corresponding sitting and supine HR reductions at peak were similar to trough values and likewise significant. At peak, standing HR reductions were comparable to sitting and supine reductions (data not shown).
Safety
During the double‐blind treatment period, treatment emergent AEs were reported in 382 (46.1%) of the 828 patients on nebivolol and 33 (40.7%) of 81 patients on placebo and were mainly mild to moderate in intensity. There was no significant difference between the placebo‐ and nebivolol‐treated groups in the incidence of AEs (P=.273). However, AEs tended to increase slightly by dose, ranging from 34.9% in the 1.25‐mg treatment group to 50.6% in the 40‐mg group. When each nebivolol group was evaluated separately, only patients treated with nebivolol 20 or 40 mg had a significantly higher incidence of AEs (P<.044 and P<.009, respectively) than patients in the placebo group. The most frequently reported treatment‐emergent AEs are summarized in Table III. Headache was the most common treatment‐related AE experienced by 6% to 9% of nebivolol‐treated patients and 7.4% of the placebo‐treated patients. Of note, the incidence of AEs commonly associated with β‐blocker use was low in the nebivolol groups combined, including fatigue (3.6% vs 2.5% with placebo), erectile dysfunction (0.2%), decreased libido (0.1%), bradycardia (0.7%), and depression (0.2%). No dose‐dependent trends were observed for any type of AE.
Table III.
Summary of Most Frequently Reported Treatment‐Emergent AEs
| AE | Nebivolol Dose | Total (Nebivolol (n=828) | ||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n=81) | 1.25 mg (n=83) | 2.5 mg (n=82) | 5 mg (n=165) | 10 mg (n=166) | 20 mg (n=166) | 40 mg (n=166) | ||
| Any event | 33 (40.7) | 29 (34.9) | 36 (43.9) | 73 (44.2) | 76 (45.8) | 84 (50.6) | 84 (50.6) | 382 (46.1) |
| Headache | 6 (7.4) | 6 (7.2) | 5 (6.1) | 12 (7.3) | 10 (6.0) | 11 (6.6) | 15 (9.0) | 59 (7.1) |
| Fatigue | 2 (2.5) | 1 (1.2) | 5 (6.1) | 4 (2.4) | 3 (1.8) | 9 (5.4) | 8 (4.8) | 30 (3.6) |
| Nasopharyngitis | 6 (7.4) | 2 (2.4) | 4 (4.9) | 5 (3.0) | 3 (1.8) | 5 (3.0) | 5 (3.0) | 24 (2.9) |
| Diarrhea NOS | 2 (2.5) | 1 (1.2) | 2 (2.4) | 4 (2.4) | 5 (3.0) | 6 (3.6) | 5 (3.0) | 23 (2.8) |
| Dizziness | 3 (3.7) | 1 (1.2) | 4 (4.9) | 2 (1.2) | 2 (1.2) | 5 (3.0) | 9 (5.4) | 23 (2.8) |
| Increased CRP level | 1 (1.2) | 1 (1.2) | 5 (6.1) | 3 (1.8) | 4 (2.4) | 5 (3.0) | 4 (2.4) | 22 (2.7) |
| Nausea | 1 (1.2) | 0 | 2 (2.4) | 2 (1.2) | 5 (3.0) | 4 (2.4) | 2 (1.2) | 15 (1.8) |
| Sinusitis NOS | 0 | 1 (1.2) | 0 | 2 (1.2) | 3 (1.8) | 4 (2.4) | 3 (1.8) | 13 (1.6) |
| URT infection NOS | 2 (2.5) | 0 | 1 (1.2) | 2 (1.2) | 1 (0.6) | 4 (2.4) | 5 (3.0) | 13 (1.6) |
| Peripheral edema | 0 | 2 (2.4) | 1 (1.2) | 2 (1.2) | 3 (1.8) | 1 (0.6) | 1 (0.6) | 10 (1.2) |
| Dyspepsia | 2 (2.5) | 0 | 1 (1.2) | 2 (1.2) | 1 (0.6) | 3 (1.8) | 1 (0.6) | 8 (1.0) |
| Bronchitis NOS | 0 | 2 (2.4) | 0 | 1 (0.6) | 1 (0.6) | 2 (1.2) | 1 (0.6) | 7 (0.8) |
| Cough | 1 (1.2) | 3 (3.6) | 2 (2.4) | 1 (0.6) | 1 (0.6) | 0 | 0 | 7 (0.8) |
| Abbreviations: AE, adverse event; CRP, C‐reactive protein; NOS, not otherwise specified; URT, upper respiratory tract. | ||||||||
No deaths occurred during the course of the study. There were 12 patients who had a serious AE (SAE) during the double‐blind treatment period: 11 in the nebivolol group and 1 in the placebo group. Of the 11 SAEs in the nebivolol group, 2 were considered possibly drug‐related: 1 patient on nebivolol 20 mg had an abnormal ECG with inferior T wave changes, and another patient on nebivolol 40 mg had an abnormal ECG with ST changes. Both resolved spontaneously without discontinuation of study drug treatment. There were no increases in orthostatic hypotension with nebivolol compared with placebo.
There were few laboratory parameters with any significant changes during the study. There were no significant changes in any of the laboratory parameters associated with CV risk (total cholesterol, low‐density lipoprotein cholesterol, triglycerides, or glucose; data not shown), except for high‐density lipoprotein cholesterol (HDL‐C), which showed statistically significant decreases from baseline to study end for nebivolol (LS mean changes from baseline ranging from 0.383–1.887 mg/dL across the nebivolol dose groups). Increases in serum uric acid and phosphorous were statistically significant for nebivolol doses ≥5 mg (placebo‐subtracted changes ranged from 0.24–0.31 mg/dL) and ≥10 mg (placebo‐subtracted increases ranged from 0.07–0.17 mg/dL), respectively. The number of patients with values shifting from normal at baseline to outside the normal range at study end was not statistically significant for these parameters, however. There were also statistically significant changes from baseline to study end in hematologic laboratory values at nebivolol doses of 5 to 40 mg. Specifically, the mean placebo‐subtracted changes at these doses ranged from −0.11 to −0.15 g/dL for hemoglobin and from −0.41% to −0.70% for hematocrit. The magnitude of these reductions as well as the changes in HDL‐C may not be clinically relevant. Among the 10 patients treated with nebivolol who had clinically significant low hematocrit and/or hemoglobin levels at the end of the study, all had demonstrated below‐normal values at baseline; there was no evidence of a dose‐related effect on these parameters.
DISCUSSION
The results of the present dose‐ranging study indicate that once‐daily nebivolol is an effective antihypertensive agent in patients with mild to moderate hypertension. The decrease in SBP and DBP observed with all doses of nebivolol was significantly greater than that observed with placebo. High response rates were achieved in patients treated with nebivolol, ranging from approximately 46% to 65%. Nebivolol treatment also showed safety and tolerability comparable to placebo overall, except at high doses, with incidences of AEs and withdrawal rates comparable to those of placebo over the duration of this trial. The trough‐to‐peak ratios indicate that BP control was sustained for the full 24‐hour period and was devoid of wide trough‐to‐peak variation, an important safety aspect for a once‐daily antihypertensive drug.
The strengths of the current study include the randomized controlled trial design and the composition of the study population: 43.0% were women, 14.5% were black, 21.2% were aged 65 years or older, 9.7% were diabetic, and 43.9% were obese. Thus, the patients studied can be regarded as representative of the general US hypertensive population. 10 , 11 , 12 , 13
The findings of this study are of further interest because of nebivolol's pharmacologic profile, which involves both highly selective blockade of the β1‐adrenergic receptor and endothelium‐dependent vasodilating action. 14 , 15 The selectivity of nebivolol at clinically relevant doses for the β1‐adrenoceptor was shown to be 321‐fold higher in human myocardial tissue than for the β2‐adrenoceptor. This is higher than other β‐blockers currently available. Metoprolol, for example, demonstrated only a 74‐fold higher β1‐ vs β2‐adrenoceptor selectivity ratio, 14 and atenolol was shown to have 2 to 4 times less β1‐ vs β‐adrenoceptor selectivity than nebivolol. 16 Studies in normotensive and hypertensive persons have noted that nebivolol modulates nitric oxide release and its bioavailability within the vascular bed, thereby promoting vasodilation and reducing peripheral vascular resistance. 6 , 15 In addition, a number of studies have consistently shown that nebivolol preserves left ventricular function, whereby it increases stroke volume and cardiac output and maintains cardiac chronotropism during exertion. 15 , 17 As a result, nebivolol possesses a beneficial hemodynamic profile that distinguishes it from many cardioselective β‐blockers.
The magnitude of the effects of nebivolol on BP observed in this study were comparable with those observed in an earlier nebivolol dose‐ranging study. 8 In a 4‐week, randomized, double‐blind placebo‐controlled study conducted in 509 patients with essential hypertension (DBP ≥95 mm Hg) in Europe and the United States, nebivolol at dosages of 1, 2.5, 5, and 10 mg once daily produced baseline and placebo‐corrected reductions at trough in mean supine SBP/DBP of −3.7/−2.7, −5.5/−3.8, −6.1/−5.9, and −5.1/−6.9 mm Hg, respectively. Response rates, defined as an achieved supine DBP at trough of ≤90 mm Hg and/or a reduction of ≥10 mm Hg, ranged from 38% to 57% with the above dosages. The population of this study was also heterogeneous and comparable to that of the present study where nebivolol at 1.25 to 10 mg once daily produced baseline and placebo‐corrected reductions in trough SiSBP/SiDBP ranging from −6.6/−5.1 mm Hg to −9.2/−6.3 mm Hg, respectively. In other European trials, nebivolol has consistently demonstrated antihypertensive efficacy similar to that of other β1‐adrenergic receptor‐selective β‐blockers 18 , 19 , 20 and of other major classes of antihypertensive agents, including angiotensin‐converting enzyme inhibitors, 21 , 22 calcium channel blockers, 23 , 24 and angiotensin receptor blockers. 25
Interestingly, the reported frequencies of sexual dysfunction and central nervous system AEs, such as fatigue and depression, were not statistically different from those found with placebo. 26 The low incidence of adverse effects with nebivolol may be attributable to its β1‐selectivity and its nitric oxide‐mediated vasodilatory effects. 26 , 27 , 28 All doses of nebivolol significantly decreased HR in a dose‐dependent manner compared with placebo. Nebivolol was not associated with adverse metabolic effects or clinically significant adverse changes in laboratory parameters associated with CV risk, although the small decrease in HDL‐C may warrant further investigation.
The good tolerability with nebivolol found in the present study is also consistent with previous studies showing a low AE rate of only 0.5% in 5740 patients with mild hypertension 29 and a lack of significant adverse metabolic effects with nebivolol for serum lipids, carbohydrate metabolism, and insulin resistance. 30 , 31 , 32 A meta‐analysis was conducted of 10 published European studies that compared the efficacy, safety, and tolerability of nebivolol with that of other β1‐adrenergic receptor‐selective β‐blockers, including atenolol, metoprolol, and bisoprolol, in a total of 1122 patients. 26 The meta‐analysis found that 5.7% fewer patients experienced AEs with nebivolol than with these other agents. These authors pooled 3 studies and demonstrated that total AEs were 30% lower and drug‐related AEs were 62% lower with nebivolol compared with the other β‐blockers. 26 Taken together, the results suggest that nebivolol may have a favorable tolerability profile compared with that of other cardioselective β‐blockers such as atenolol, metoprolol, and bisoprolol.
Overall, the efficacy and safety results of monotherapy with nebivolol in this trial, when combined with findings of previous studies, suggest that nebivolol is as effective as other widely used antihypertensive therapies in lowering BP with a satisfactory AE profile.
CONCLUSIONS
Treatment of hypertension should not only aim at reducing BP but also at preventing or delaying hypertension‐related CV disease. Actions other than efficacy that may affect long‐term outcomes should be considered when choosing an antihypertensive agent.
Nebivolol, a novel, long‐acting selective β1‐adrenoreceptor blocker with a unique hemodynamic profile, is an effective antihypertensive agent in patients with mild to moderate hypertension. It is also well tolerated, with a low incidence of AEs such as fatigue, depression, and erectile dysfunction that are associated with many β‐blockers.
Acknowledgment and disclosures:
Editorial assistance was provided by Advantage Communications, New York, NY. This study was supported by Bertek Pharmaceuticals Inc (now part of Mylan Laboratories Inc). The authors disclose the following affiliations: Dr Weiss, Speakers' Bureau, AstraZeneca, Kos, Novartis, and Pfizer; Dr Weber, Consultant/Speakers' Bureau/Advisory Boards, Bristol‐Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Forest Laboratories, Merck, Novartis, Pfizer, and Sanofi‐Aventis; Dr Carr, Speakers' Bureau, AstraZeneca and Merck. Dr Carr also discloses that Southern Clinical Research and Management, Inc, of which he is director, has received funding from Abbott, Amgen, Encysive, Gilead Sciences, Merck, Novartis, Pfizer, Roche, Sanofi‐Aventis, and Takeda for research he conducted.
References
- 1. Gress TW , Nieto FJ , Shahar E , et al , for the Atherosclerosis in Communities Study . Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus . N Engl J Med . 2000. ; 342 : 905 – 912 . [DOI] [PubMed] [Google Scholar]
- 2. Ko DT , Hebert PR , Coffey CS , et al. β‐Blocker therapy and symptoms of depression, fatigue, and sexual dysfunction . JAMA . 2002. ; 288 : 351 – 357 . [DOI] [PubMed] [Google Scholar]
- 3. Man in't Veld AJ , Van den Meiracker AH , Schalekamp MA . Do beta‐blockers really increase peripheral vascular resistance? Review of the literature and new observations under basal conditions . Am J Hypertens . 1988. ; 1 : 91 – 96 . [DOI] [PubMed] [Google Scholar]
- 4. Weber MA . The role of the new beta‐blockers in treating cardiovascular disease . Am J Hypertens . 2005. ; 18 ( 12 , pt 2 ): 169S – 176S . [DOI] [PubMed] [Google Scholar]
- 5. Van de Water A , Janssens W , Van Neuten J , et al. Pharmacological and hemodynamic profile of nebivolol, a chemically novel, potent, and selective β1‐adrenergic antagonist . J Cardiovasc Pharmacol . 1988. ; 11 : 552 – 563 . [DOI] [PubMed] [Google Scholar]
- 6. Tzemos N , Lim PO , MacDonald TM . Nebivolol reverses endothelial dysfunction in essential hypertension: a randomized, double‐blind, crossover study . Circulation . 2001. ; 104 : 511 – 514 . [DOI] [PubMed] [Google Scholar]
- 7. Ritter JM . Nebivolol: endothelium‐mediated vasodilating effect . J Cardiovasc Pharmacol . 2001. ; 38 ( suppl 3 ): S13 – S16 . [DOI] [PubMed] [Google Scholar]
- 8. Van Nueten L , Dupont AG , Vertommen C , et al. A dose‐response trial of nebivolol in essential hypertension . J Hum Hypertens . 1997. ; 11 : 139 – 144 . [DOI] [PubMed] [Google Scholar]
- 9. McNeely W , Goa KL . Nebivolol in the management of essential hypertension: a review . Drugs . 1999. ; 57 : 633 – 651 . [DOI] [PubMed] [Google Scholar]
- 10. Hajjar I , Kotchen TA . Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000 . JAMA . 2003. ; 290 : 199 – 206 . [DOI] [PubMed] [Google Scholar]
- 11. Fields LE , Burt VL , Cutler JA , et al. The burden of adult hypertension in the United States 1999 to 2000: a rising tide . Hypertension . 2004. ; 44 : 398 – 404 . [DOI] [PubMed] [Google Scholar]
- 12. Must A , Spadano J , Coakley EH , et al. The disease burden associated with overweight and obesity . JAMA . 1999. ; 282 : 1523 – 1529 . [DOI] [PubMed] [Google Scholar]
- 13. Sowers JR , Epstein M , Frohlich ED . Diabetes, hypertension, and cardiovascular disease: an update . Hypertension . 2001. ; 37 : 1053 – 1059 . [DOI] [PubMed] [Google Scholar]
- 14. Bristow MR , Nelson P , Minobe W , et al. Characterization of β1‐adrenergic receptor selectivity of nebivolol and various other beta‐blockers in human myocardium . Am J Hypertens . 2005. ; 18 ( 5 ): A51 – A52 . [Google Scholar]
- 15. Zanchetti A . Clinical pharmacodynamics of nebivolol: new evidence of nitric oxide‐mediated vasodilating activity and peculiar haemodynamic properties in hypertensive patients . Blood Press Suppl . 2004. ; 1 : 17 – 32 . [DOI] [PubMed] [Google Scholar]
- 16. Pauwels PJ , Gommeren W , Van Lommen G , et al. The receptor binding profile of the new antihypertensive agent nebivolol and its stereoisomers compared with various β‐adrenergic blockers . Mol Pharmacol . 1988. ; 34 : 843 – 851 . [PubMed] [Google Scholar]
- 17. Kamp O , Sieswarda GT , Visser CA . Comparison of effects on systolic and diastolic left ventricular function of nebivolol versus atenolol in patients with uncomplicated essential hypertension . Am J Cardiol . 2003. ; 92 : 344 – 348 . [DOI] [PubMed] [Google Scholar]
- 18. Van Nueten L , Taylor FR , Robertson JI . Nebivolol vs atenolol and placebo in essential hypertension: a double‐blind randomised trial . J Hum Hypertens . 1998. ; 12 : 135 – 140 . [DOI] [PubMed] [Google Scholar]
- 19. Uhlíš O , Fejfuša M , Havránek K , et al. Nebivolol versus metoprolol in the treatment of hypertension . Drug Invest . 1991. ; 3 ( suppl 1 ): 107 – 110 . [Google Scholar]
- 20. Czuriga I , Riecansky I , Bodnar J , et al , for the NEBIS Investigators Group . Comparison of the new cardioselective beta‐blocker nebivolol with bisoprolol in hypertension: the Nebivolol, Bisoprolol Multicenter Study (NEBIS) . Cardiovasc Drugs Ther . 2003. ; 17 : 257 – 263 . [DOI] [PubMed] [Google Scholar]
- 21. Lacourcière Y , Lefebvre J , Poirier L , et al. A double‐blind crossover comparison of nebivolol and lisinopril in the treatment of ambulatory hypertension . Am J Ther . 1994. ; 1 : 74 – 80 . [DOI] [PubMed] [Google Scholar]
- 22. Van Nueten L , Rishøj Nielsen M , Vertommen C , et al. Nebivolol versus enalapril in essential hypertension: a long‐term double‐blind comparative trial . Acta Clin Belg . 1999. ; 54 : 19 – 25 . [DOI] [PubMed] [Google Scholar]
- 23. Van Nueten L , Lacourcière Y , Vyssoulis G , et al. Nebivolol versus nifedipine in the treatment of essential hypertension: a double‐blind, randomized, comparative trial . Am J Ther . 1998. ; 5 : 237 – 243 . [DOI] [PubMed] [Google Scholar]
- 24. Mazza A , Gil‐Extremera B , Maldonato A , et al. Nebivolol vs amlodipine as first‐line treatment of essential arterial hypertension in the elderly . Blood Press . 2002. ; 11 : 182 – 188 . [DOI] [PubMed] [Google Scholar]
- 25. Van Bortel LM , Bulpitt CJ , Fici F . Quality of life and antihypertensive effect with nebivolol and losartan . Am J Hypertens . 2005. ; 18 : 1060 – 1066 . [DOI] [PubMed] [Google Scholar]
- 26. Ambrosioni E , Borghi C . Tolerability of nebivolol in head‐to‐head clinical trials versus other cardioselective β‐blockers in the treatment of hypertension: a meta‐analysis . High Blood Press Cardiovasc Prev . 2005. ; 12 : 27 – 35 . [Google Scholar]
- 27. Prichard BN , Cruickshank JM , Graham BR . Beta‐adrenergic blocking drugs in the treatment of hypertension . Blood Press . 2001. ; 10 : 366 – 386 . [DOI] [PubMed] [Google Scholar]
- 28. López‐Sendón J , Swedberg K , McMurray J , et al , and the Task Force on Beta‐Blockers of the European Society of Cardiology. Expert consensus document on β‐adrenergic receptor blockers . Eur Heart J . 2004. ; 25 : 1341 – 1362 . [DOI] [PubMed] [Google Scholar]
- 29. Cleophas TJ , Agrawal R , Lichtenthal A , et al. Nationwide efficacy‐safety study of nebivolol in mildly hypertensive patients . Am J Ther . 2006. ; 13 : 192 – 197 . [DOI] [PubMed] [Google Scholar]
- 30. Fogari R , Zoppi A , Lazzari P , et al. Comparative effects of nebivolol and atenolol on blood pressure and insulin sensitivity in hypertensive subjects with type II diabetes . J Hum Hypertens . 1997. ; 11 : 753 – 757 . [DOI] [PubMed] [Google Scholar]
- 31. Pesant Y , Marc‐Aurèle J , Bielmann P , et al. Metabolic and antihypertensive effects of nebivolol and atenolol in normometabolic patients with mild‐to‐moderate hypertension . Am J Ther . 1999. ; 6 : 137 – 147 . [DOI] [PubMed] [Google Scholar]
- 32. Poirier L , Cléroux J , Nadeau A , et al. Effects of nebivolol and atenolol on insulin sensitivity and haemodynamics in hypertensive patients . J Hypertens . 2001. ; 19 : 1429 – 1435 . [DOI] [PubMed] [Google Scholar]


