Abstract
Blood pressure (BP) control at recently established goals of <130/80 mm Hg is often difficult to achieve in diabetic patients. This work examines the effect of pioglitazone on 24‐hour ambulatory BP monitoring in patients with type 2 diabetes and difficult‐to‐control hypertension. Twenty‐seven participants with difficult‐to‐control hypertension (defined as ambulatory BP monitoring ≥125/75 mm Hg) taking antihypertensive medications (mean, 4.1±0.8 drugs) were enrolled in an open, prospective, blinded end point study of add‐on therapy with pioglitazone 30 to 45 mg for 20 weeks. After 20 weeks of treatment, 24‐hour ambulatory BP monitoring showed significant reductions (from 144±13 to 136±16 mm Hg systolic BP and from 79±9 to 76±10 mm Hg diastolic BP [P=.001]). Treatment was also associated with improvements in insulin sensitivity and glycemic and lipid profile. These findings suggest that pioglitazone could be a therapeutic option in diabetics who still have elevated BP values in spite of receiving treatment with at least 3 antihypertensive drugs.
Type 2 diabetes mellitus (DM) and hypertension (HT) are clinical diagnoses that are frequently associated and that significantly increase cardiovascular risk. 1 , 2 , 3 , 4 In recent years, evidence has indicated that when DM is present, blood pressure (BP) control goals for treatment should be different from those in nondiabetic patients. It has also been demonstrated that better control of BP is more effective in reducing cardiovascular events in the diabetic hypertensive patient than in nondiabetics. 5 , 6 , 7 Thus, several scientific societies (the American Diabetes Association [ADA]; the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [JNC 7]; the European Society of Hypertension/European Society of Cardiology [ESH/ESC]) recommend decreasing BP to <130/80 mm Hg in diabetic patients. 8 , 9 , 10
It is especially difficult to achieve these BP control values in clinical practice. The frequency with which so‐called difficult‐to‐control HT (BP >130 and/or 80 mm Hg with ≥3 antihypertensive drugs, one of which is a diuretic) 11 occurs in hypertensive diabetic patients is high.
The frequent coexistence of HT and DM suggests the need to search for a common etiopathogenic mechanism. Presently, it is considered that insulin resistance (IR) is the common feature of many metabolic and nonmetabolic diseases, such as type 2 DM, abdominal obesity, HT, and dyslipidemia, which also tend to be grouped into the so‐called metabolic syndrome. 12 Several studies have found an association between treatment‐resistant HT in these patients and IR. 13 , 14 , 15
Thiazolidinediones or glitazones are a type of antidiabetic agent that decreases IR in the muscle, liver, and adipose tissue. By reducing IR and decreasing insulin requirements, these drugs improve glycemic control 16 , 17 , 18 , 19 and probably preserve β‐cell function. 16 , 20 , 21 Thus, glitazones are currently used as antidiabetic drugs. Increasingly, studies seem to demonstrate that glitazones may have other beneficial effects on the cardiovascular system in addition to glycemic control, such as reduction of triglycerides (TGs), increase in high‐density lipoprotein (HDL) cholesterol, and decrease of C‐reactive protein (CRP) levels in plasma. 17 , 18 , 19 , 22 , 23 , 24
A recent meta‐analysis of the effect of thiazolidinediones on BP has reported that these drugs produce small decreases in systolic and diastolic BP in patients with or without HT and with or without DM. 25 No study, however, has assessed the effect of these drugs in diabetic patients with difficult to‐treat HT. Thus, the primary purpose of this study was to use ambulatory monitoring to assess the effect on BP of the addition of pioglitazone to the antihypertensive treatment of patients with poorly controlled BP and type 2 DM. In addition, this study aimed to assess the effect of pioglitazone on other cardiovascular risk factors, including lipid and glycemic parameters.
PATIENTS AND METHODS
Patients
Thirty type 2 diabetic patients (20 men and 10 women) with poorly controlled HT diagnosed on 24‐hour ambulatory BP monitoring (ABPM) were selected. All the patients were receiving treatment with at least 3 antihypertensive drugs used in adequate doses, one of which was a diuretic. All were diagnosed as having type 2 DM according to ADA 2004 criteria. The study protocol was reviewed and approved by the ethics committee of the Hospital Clínico San Carlos, Madrid. All patients gave their informed consent before participating in the study. One of the patients withdrew his consent during follow‐up. Another was withdrawn from the study because of treatment noncompliance and another because of acute symptoms of heart failure that disappeared when the drug was withdrawn. Thus, the final study group included 27 patients with poorly controlled HT and type 2 DM (19 men and 8 women).
Study Protocol
All the patients who came to the HT unit of the Hospital Clínico San Carlos between January 2 and December 31, 2004, with the diagnosis of type 2 DM and who had poorly controlled HT with values >130/80 mm Hg at several clinic visits in spite of receiving treatment with at least 3 antihypertensive drugs in adequate doses underwent ABPM to confirm the diagnosis of poorly controlled HT.
A physical examination and blood analysis were conducted in patients in whom this diagnosis was confirmed; they were excluded from the study if they had any of the following: type 1 diabetes; known or suspected causes of secondary HT; heart failure (New York Heart Association class I to IV); unstable chest angina; serum creatinine level ≥2 mg/dL; renal transplant or current use of dialysis; alanine aminotransferase (ALT) or aspartate aminotransferase in serum >1.5 times the upper limit of normal; clinical signs or symptoms of liver disease; hemoglobin or hematocrit levels below the lower limit of normal; signs or symptoms of substance abuse; or serious end‐stage disease.
Patients who fulfilled all the inclusion criteria and none of the exclusion criteria as defined in the protocol were included in an open, prospective, blinded end point study initiating treatment with pioglitazone 30 mg once a day. Hypoglycemic treatment was modified according to previous treatment and glycated hemoglobin A1c (HbA1c) levels. Antihypertensive treatment and lipid‐lowering medication (if any) were maintained constant during the study. Furthermore, the patients were advised to maintain their physical activity and dietary habits during the study.
The patients came to a 12‐week assessment visit in which the appearance of adverse reactions to treatment was evaluated. The pioglitazone tablets were counted to verify therapeutic compliance. BP was measured and HbA1c was also measured to adjust treatment with pioglitazone, increasing the dose to 45 mg in patients with HbA1c level >6.5%.
After 20 weeks of treatment, the patients came to the clinic once again to undergo all the tests that were conducted on the initial visit, including a new 24‐hour ABPM.
Assessments
Ambulatory Monitoring of BP. Spacelab 90202 ABPM (Redmond, WA) was used. The mean BP obtained during ABPM was the principal criterion of the study to assess the effect of pioglitazone on the BP. The ambulatory pressure monitors were programmed beforehand to record BP every 15 minutes during the day (7 am to 11 pm) and every 20 minutes at night (11 pm to 7 am). The readings were used for the analysis only if ≥70% of the measurements were valid. BP threshold used to diagnose a diabetic patient as having difficult‐to‐treat HT was ≥125/75 mm Hg in the 24‐hour average ABPM. Mean systolic and diastolic BP were calculated over the entire 24‐hour daytime and nighttime period. The patients carried out their normal daily activities and took all medication in the usual way while the ABPM was being performed.
Measurement of IR. IR was measured with the homeostasis model assessment (HOMA). The HOMA‐IR index was calculated using the following formula: baseline insulin (µU/mL) × baseline glucose (mmol/L) / 22.5. 26 Its value was calculated at the onset of the study before initiation of treatment with pioglitazone and at the end of follow‐up. The HOMA‐IR index has been shown to have a strong inverse correlation with insulin sensitivity measured with the hyperinsulinemic‐euglycemic clamp (gold standard) and is considered a reliable index of IR in clinical studies. 26 , 27 , 28 It was considered that a patient had IR when the HOMA index values were ≥3.8. 29
Biochemical Analyses. All laboratory analyses were performed by automatic procedures in the central laboratories of the Hospital Clínico San Carlos of Madrid. Blood samples were obtained from an antecubital vein without compression in all patients after 12 hours of fasting to measure levels of hemoglobin, hematocrit, HbA1c, apolipoprotein B, sodium and potassium; hepatic and renal function; and other routine laboratory parameters. Plasma glucose level was determined in duplicate by a glucose‐oxidase method adapted to Autoanalyzer (Hitachi 704, Boehringer Mannheim, Germany). Total serum cholesterol, TG, and HDL cholesterol were determined by enzymatic methods using commercial kits (Boehringer Mannheim, Germany). Low‐density lipoprotein (LDL) cholesterol was calculated by the Friedewald formula. Serum insulin concentrations were determined by radioimmunoassay (Linco Research Inc, St Louis, MO). Serum CRP levels were measured by high‐sensitivity assay (Beckman Coulter). Urinary albumin excretion was assessed with nephelometry (Beckman Array System).
Statistical Analyses. The statistical analysis was conducted with SPSS version 12.0 (SPSS Inc, Chicago, IL). Data are presented as mean ± SD. To compare baseline values with those at the end of observation, the Student t test for paired data or Wilcoxon signed ranges test was used when necessary, depending on the normality or not of the distribution. Correlation coefficients were calculated using Spearman ordinal correlation coefficient. Null hypothesis was rejected in those tests with an α error <0.05.
RESULTS
Table I shows the baseline demographic data of the participants. The mean age of the patients was 65.7±8.38 years, and 66.7% were men. At baseline, 15 patients (55.6%) had IR defined by HOMA ≥3.8. There was a statistically significant relationship between the HOMA values and presence of abdominal obesity (P=.03).
Table I.
Characteristics of the Study Participants at Baseline
| All Participants | |
|---|---|
| No. | 27 |
| Age, y | 65.7±8.38 |
| Duration of hypertension, y | 13±10 |
| Duration diabetes mellitus, y | 4±5 |
| Height, m | 160.8±8.3 |
| Weight, kg | 81.5 ±12.7 |
| Body mass index, kg/m2 | 31.5±4.4 |
| Obesity (body mass index >30 kg/m2), % | 66.7 |
| Abdominal circumference, cm | 105.6±10.4 |
| Abdominal obesity (men >102 cm; women >88 cm), % | 74.1 |
| Patients treated with ADO at baseline, No. | 17 |
| Insulin resistance (homeostasis model assessment ≥3.8), % (No.) | 55.6 (15) |
| Patients treated ADO at baseline, No. | 11 |
| Patients treated with non‐ADO at baseline, No. | 4 |
| Glycosylated hemoglobin | 6.56±0.89 |
| Patients treated with ADO at baseline, No. (range) | 6.8 (5–8.3) |
| Patients treated with non‐ADO at baseline, No. (range) | 6.15 (4.9–7) |
| Values are mean ± SD unless otherwise indicated. Abbreviation: ADO, antidiabetic oral drug. | |
All patients were receiving treatment with antihypertensive medication and had been treated with ≥3 antihypertensive drugs for at least 4 weeks before being included in the study. The mean number of drugs used per patient was 4.14±0.86 (range, 3–6). The percentages of patients treated with each type of antihypertensive drug were as follows: diuretics, 100%; angiotensin receptor blockers, 88.9%;angiotensin‐converting enzyme inhibitors, 63%; calcium antagonists, 70.4%; β‐blockers, 29.6%; α‐blockers, 51.9%; spironolactone, 7.4%; and monoxidin, 7.4%. A total of 70.4% patients were on treatment with statins, 22.2% with antiplatelet agents, and 11.1% with anticoagulants.
At baseline, 63% of patients were receiving treatment with oral antidiabetics (none were treated with insulin). Patients without antidiabetic drug treatment had an HbA1c level <7%; 6 had been recently diagnosed. All patients initiated treatment with pioglitazone 30 mg, and the dose was increased to 45 mg in 40.7% of patients at week 12.
Adding pioglitazone to the antihypertensive treatment for 20 weeks induced a significant decrease in systolic and diastolic BP in the 24‐hour ABPM period (Table II; Figure 1). In addition, a significant mean decrease of 15±18/5±9 mm Hg was observed in BP taken in the clinic (Figure 1). A clinically significant increase in patients who reached BP control values (18.5%) (<130/80 mm Hg in clinic BP and <125/75 mm Hg in 24‐hour ABPM) was noted.
Table II.
Clinic and Ambulatory BP Before and After Treatment With Pioglitazone
| N=27 | Onset | Week 12 | Week 20 | P Value (Week 20 vs Onset) |
|---|---|---|---|---|
| Clinic SBP, mm Hg | 160±18 | 151±20a | 144±16 | <001 |
| Clinic DBP, mm Hg | 82±10 | 79±14b | 77±9 | .01 |
| ABPM, 24‐h | ||||
| SBP, mm Hg | 144±13 | 136±16 | .001 | |
| DBP, mm Hg | 79±9 | 76±10 | .001 | |
| ABPM, daytime | ||||
| SBP, mm Hg | 147±13 | 138±16 | .001 | |
| DBP, mm Hg | 82±8 | 78±10 | .000 | |
| ABPM, nighttime | ||||
| SBP, mm Hg | 139±16 | 132±18 | .01 | |
| DBP, mm Hg | 74±11 | 71±12 | .03 | |
| Values are expressed as mean ± SD. a P=.01 vs onset. b P=.156 vs onset. Abbreviations: ABPM, ambulatory BP monitoring; BP, blood pressure; DBP, diastolic BP; SBP, systolic BP. | ||||
Figure 1.
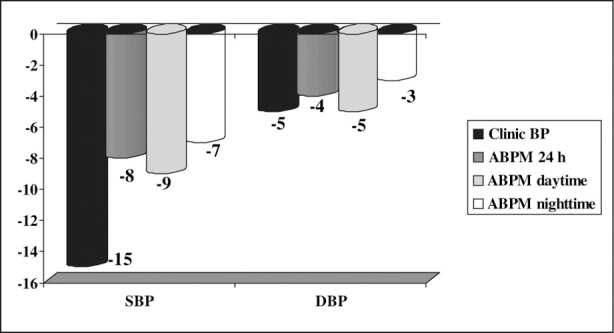
Blood pressure (BP) mean change (mm Hg) before and after treatment with pioglitazone. SBP indicates systolic BP; DBP, diastolic BP; ABPM, ambulatory BP monitoring.
While the use of pioglitazone resulted in a significant reduction in nighttime BP, the percentage of patients with a nondipper BP pattern (<10% decline in nocturnal mean relative to the diurnal mean BP) was not changed at the end of the study (16 patients at baseline vs 17 at the end of the study; P=1).
Mean BP decreases on 24‐hour ABPM were greater in the group of patients with IR at the onset of the study than in the patients without IR at baseline (Figure 2).
Figure 2.
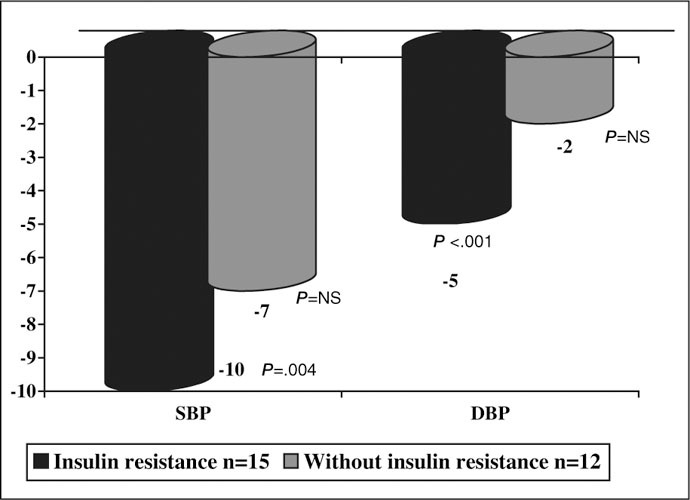
Blood pressure (BP) mean change (mm Hg) in ambulatory BP monitoring before and after treatment with pioglitazone for subgroups. SBP indicates systolic BP; DBP, diastolic BP; NS, not significant.
Treatment with pioglitazone led to a significant increase in insulin sensitivity in the entire study group and a decrease in fasting insulin levels, HOMA index, and percentage of patients with IR between onset and the study end (from 55.6% to 24%; P=.012). This was accompanied by improvement in the patients' glycemic profile. A decrease in HbA1c values (from 6.56±0.89% to 6.29±0.77%; P=.057) was achieved with treatment; although these changes did not reach statistical significance, 17.1% more patients on therapy reached DM control HbA1c values <6.5% compared with baseline (from 44.6% to 61.5%) (Table III).
Table III.
Glucose, Insulin, HbA1c, and HOMA Before and After Treatment With Pioglitazone
| N=27 | Onset | Week 12 | Week 20 | P Value (Week 20 vs Onset) |
|---|---|---|---|---|
| HOMA | 4.47±2.78 | 2.85±1.37 | .001 | |
| HOMA ≥3.8, % | 55.6 | 24 | .012 | |
| Insulin, µU/mL | 11.1±5.54 | 8±3.06 | .001 | |
| Glucose, mg/dL | 151.5±38.85 | 142.37±35.74a | 139.48±30.84 | .042 |
| HbA1c, % | 6.56±0.89 | 6.50±0.97b | 6.29±0.77 | .057 |
| Values are expressed as mean ± SD unless otherwise indicated. Abbreviations: HbA1c, glycosylated hemoglobin; HOMA, homeostasis model assessment. a P=.153 vs onset. b P=.679 vs onset. | ||||
Treatment with pioglitazone caused a significant decrease in TG values and a significant increase in HDL cholesterol values, and a greater percentage of patients reached the target HDL cholesterol value (>45 mg/dL). Pioglitazone had a neutral effect on total cholesterol, LDL cholesterol, and apolipoprotein B (Table IV).
Table IV.
Lipid Levels Before and After Treatment With Pioglitazone
| N=27 | Onset | Week 20 | P Value |
|---|---|---|---|
| Total cholesterol, mg/dL | 184.89±41.52 | 187.15±31.62 | NS |
| LDL cholesterol, mg/dL | 100.48±24.68 | 103.43±26.69 | NS |
| HDL cholesterol, mg/dL | 47.7±10.41 | 56±10.57 | <.001 |
| HDL cholesterol >45 mg/dL, % | 53.8 | 92.3 | .002 |
| Triglycerides, mg/dL | 208.3±304.9 | 135.12±66.67 | .016 |
| Triglycerides <150 mg/dL, % | 65.4 | 73.1 | NS |
| Apolipoprotein B, mg/dL | 100.26±19.22 | 91.81±19.86 | NS |
| Values are expressed as mean ± SD unless otherwise indicated. Abbreviations: HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; NS, not significant. | |||
A nonsignificant decrease in the albumin‐creatinine ratio was observed (from 247.27±652.42 to 165.97±414.60 mg/g; P=.078). A nonsignificant decrease was also observed in the high‐sensitivity CRP values (from 0.36±0.3 to 0.26±0.21 mg/dL; P=.153).
There were no hypoglycemic episodes during the study. Two participants reported symptoms suggestive of heart failure at the end of follow‐up and were brought to the emergency department. Neither required hospitalization or needed to discontinue treatment with pioglitazone. Clinical symptoms resolved in 1 of the participants when the angiotensin receptor blocker prescribed was changed to candesartan. In the other patient, the acute administration of intravenous diuretics was required without subsequent modifications in home treatment. The echocardiogram obtained in the emergency department showed preserved ejection fraction.
None of the participants had significant elevations in hepatic parameters (defined as increases of 1.5 in the baseline level). There were significant decreases from baseline levels to end values of ALT (from 26.44±15.63 to 21.93±9.99 U/L; P=.018) and γ‐glutamyl transferase (from 32.61±13.73 to 26.22±9.64 U/L; P=.044).
There were no significant changes in weight or body mass index after 20 weeks of treatment. There was a reduction, however, in the patients' abdominal circumference at the end of follow‐up (from 105.65±10.44 to 103.95±10.24 cm; P=.008).
A small but significant decrease was observed in hemoglobin levels (from 14.21±1.06 to 13.85±1.15 g/dL; P=.013) and in hematocrit levels (from 42±3.06% to 41.2±3.63%; P=.049).
DISCUSSION
The main result of the study is that adding pioglitazone to antihypertensive treatment of patients with difficult‐to‐control HT and type 2 DM produces a significant reduction in BP as determined by ABPM during the 24‐hour period. A significant improvement in insulin sensitivity and lipid and glycemic profiles was also noted.
Recently, several studies with troglitazone (before it was withdrawn from the market), rosiglitazone, and pioglitazone have reported decreases in BP in patients with or without diabetes and with or without HT at baseline (Table V). The results of the present study, the first conducted in patients with difficult‐to‐control HT as well as the first using ABPM to assess the effect of glitazones on BP, show similar decreases in diastolic BP and greater changes in systolic BP, measured both in the clinic and by ABPM, compared with those found in most of the previous studies. The exception was found in the study by Li and colleagues, 30 in which a decrease of 17 mm Hg in systolic pressure measured in the clinic in hypertensive, overweight, or obese patients was observed. When the results of this study are interpreted, however, it must be taken into account that antihypertensive treatment was discontinued 2 weeks before treatment with rosiglitazone was initiated. In the present study, not only was treatment not discontinued but the significant reductions in BP values were obtained in a group of patients taking multiple medications (mean of 4.14±0.86 antihypertensive drugs).
Table V.
Effect of Glitazones on BP in Several Studies in Patients With or Without Diabetes or Hypertension
| DM Without Hypertension | Hypertension Without DM | DM Plus Hyptertension | ||
|---|---|---|---|---|
| Clinic BP | Pioglitazone | −9.4/4.4 mm Hg 19 | −7.9/8 mm Hg 38 | −10/8 mm Hg (HT subgroup) 18 |
| Rosiglitazone | −12/6 mm Hg 32 | −17/11 mm Hg 30 | −6.1/4.2 mm Hg 40 | |
| ABPM, 24‐h | Pioglitazone | |||
| Rosiglitazone | −3.5/2.7 mm Hg 32 | −4/5 mm Hg 39 | −5.4/4.1 mm Hg 35 | |
| Abbreviations: ABPM, ambulatory blood pressure monitoring; BP, blood pressure; DM, diabetes mellitus. | ||||
In spite of the recognized importance of controlling BP in diabetic patients, generally fewer than 20% of patients achieve BP <130/80 mm Hg. 31 In this study, when pioglitazone was added to the antihypertensive treatment, significant decreases in BP were achieved. Five patients (18.5%) achieved BP control as presently defined. These results have relevance if we consider that they have been obtained in patients who are not only diabetic but whose BP could not be controlled with at least 3 antihypertensive drugs. The decrease in BP was greater among patients who had initial IR (more than half of the sample). This suggests that IR may play a role in poor response to antihypertensive treatment.
Glitazones, whether as monotherapy or in combination with other oral antidiabetics, have been shown to improve the glycemic profile of patients with type 2 diabetes. In our study, pioglitazone decreased fasting plasma glucose levels by about 11.6 mg/dL and HbA1c levels by 0.26%. Although the decreases in glycosylated hemoglobin levels were slightly less than those observed in previous studies with pioglitazone, in which baseline levels of HbA1c were ≥7%, 16 , 17 , 18 , 19 our results agree with the results from the only study 17 that examined the effect of pioglitazone in patients who had lower baseline HbA1c values (between ≥6.5% and <7%).
The results of our study agree with the beneficial effect of glitazones (especially pioglitazone) on the lipid profile of diabetic patients described previously in the literature. 22 , 23 A significant mean decrease in triglyceride values of approximately 68.11 mg/dL was observed with a significant increase of the HDL cholesterol values of 7.65 mg/dL, without significant modifications of the total cholesterol and LDL cholesterol levels.
It has been described that glitazones have antiinflammatory effects; their capacity to decrease CRP levels has been demonstrated in several studies. In the present study, treatment with pioglitazone was associated with a nonsignificant decrease in high‐sensitivity CRP values. These results may be due to the initial lower values observed (0.36±0.3 mg/dL) compared with those of other studies 23 , 24 and with the fact that a high percentage of patients (70.4%) were receiving treatment with statins, drugs that have also been demonstrated to decrease CRP‐2 values. 19
Treatment with pioglitazone was associated with a nonsignificant decrease in the albumin‐creatinine ratio. All patients had the renin‐angiotensin system blocked; this could have decisively influenced this result and clearly differentiates our study from other previous trials in which glitazones have been reported to decrease urinary albumin excretion in diabetic patients. In these studies, patients receiving angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, β‐blockers, or calcium antagonists were excluded. 32
Pioglitazone was generally well tolerated. There were no cases of hypoglycemia. Of the 27 patients who completed the study protocol, 2 patients had symptoms suggestive of heart failure. Neither, however, required hospitalization or changes in their usual medication, including pioglitazone, to resolve their symptoms. In the remaining patients, no peripheral edema related to treatment was observed. Although it is recommended to closely monitor transaminases in treatment with glitazones, not only were there no elevations of the hepatic transaminases in the current study but there was also a significant decrease in the ALT and γ‐glutamyl transferase values. These decreases, described in previous studies, 17 , 18 , 33 , 34 may be due to a decrease in fat and IR at the hepatic level.
Piogliotazone has also been associated with weight gain of between 0.5 and 3 kg, which is not associated with an increase in abdominal circumference or with decreased efficacy of the drug to decrease glucose values. Thus, weight gain may not be associated with an increase in cardiovascular risk. 18 , 35 In this study, treatment with pioglitazone was not associated with significant weight gain; rather, a decrease in abdominal circumference was observed. Other studies have reported that improvement of insulin sensitivity is related to a modification of body fat distribution (ie, reducing intrahepatic and visceral fat and increasing peripheral subcutaneous fat). 36 , 37
In the present study, a small but significant decrease in hemoglobin and hematocrit values was observed. These decreases, described previously in other studies, may be related to increased plasma volume and have rarely been associated with any significant hematologic clinical effects. 16 , 35
This pilot study has several limitations that must be acknowledged. First, it was a 5‐month nonrandomized and observational intervention study with a small number of patients (27 in the final sample). The HOMA index was used to assess IR. Although this index is not a gold standard to measure IR, it is a simple method that has been shown to correlate well with euglycemic clamp results. Finally, the study population is made up of patients with difficult‐to‐control HT who were also diabetic. We chose the design of this study with this in mind because we aimed to focus on the effects of pioglitazone in patients in whom the drug is used as therapy in usual clinical practice. Therefore, we are not aware of whether a similar group of nondiabetic patients would respond with a similar reduction in BP values.
CONCLUSIONS
Pioglitazone added to antihypertensive treatment of patients with difficult‐to‐control HT and type 2 DM has been shown to achieve a significant decrease in ambulatory BP values. An increased number of patients achieved target BP values. Treatment with pioglitazone was also associated with improvements in glucose and lipid profiles. Until randomized studies are performed in patients with difficult‐to‐treat HT without DM, it seems that pioglitazone may be a therapeutic option in diabetic patients who still have elevated BP values in spite of receiving treatment with at least 3 antihypertensive drugs.
References
- 1. Simonson DC. Etiology and prevalence of hypertension in diabetic patients. Diabetes Care. 1988;11(10):821–827. [DOI] [PubMed] [Google Scholar]
- 2. Hypertension and Diabetic Study (HDS) . Prevalence of hypertension in newly presenting type 2 patients and the association with risk factors for cardiovascular and diabetic complications. J Hypertens. 1993;11:309–317. [DOI] [PubMed] [Google Scholar]
- 3. Tarnow L, Rossing P, Gall MA, et al. Prevalence of arterial hypertension in diabetic patients before and after the JNC V. Diabetes Care. 1994;17:1247–1251. [DOI] [PubMed] [Google Scholar]
- 4. Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. UK Prospective Diabetes Study (UKPDS) Group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 6. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351:1755–1762. [DOI] [PubMed] [Google Scholar]
- 7. The Heart Outcomes Prevention Evaluation Study Investigators . Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes: results of the HOPE study and micro‐HOPE substudy. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- 8. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27:S5–S10. [DOI] [PubMed] [Google Scholar]
- 9. The Seventh Report of the Joint National Committee on Prevention . Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2571. [DOI] [PubMed] [Google Scholar]
- 10. 2003 European Society of Hypertension‐European Society of Cardiology guidelines for management of arterial hypertension. J Hypertens. 2003;21:1011–1053. [DOI] [PubMed] [Google Scholar]
- 11. Moser M, Setaro JF. Clinical practice. Resistant or difficult‐to‐control hypertension. N Engl J Med. 2006;355:385–392. [DOI] [PubMed] [Google Scholar]
- 12. Grundy SM, Brewer B, Cleeman JI, et al. American Heart Association . National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. [DOI] [PubMed] [Google Scholar]
- 13. Martell N, Rodriguez‐Cerrillo M, Grobbee DE, et al. High prevalence of secondary hypertension and insulin resistance in patients with refractory hypertension. Blood Press. 2003;12:149–154. [DOI] [PubMed] [Google Scholar]
- 14. Modan M, Halkin H, Almog S, et al. Hyperinsulinemia. A link between hypertension obesity and glucose tolerance. J Clin Invest. 1985;75:809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Isaksson H, Cederholm T, Jansson E, et al. Therapy resistant hypertension associated with central obesity, insulin resistance and large muscle fibre area. Blood Press. 1993;2:46–52. [DOI] [PubMed] [Google Scholar]
- 16. Aronoff S, Rosenblatt S, Braithwaite S, et al. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6‐month randomized placebo‐controlled dose‐response study. The Pioglitazone 001 study group . Diabetes Care. 2000;23:1605–1611. [DOI] [PubMed] [Google Scholar]
- 17. Herz M, Johns D, Reviriego J, et al. A randomised, double‐blind, placebo‐controlled, clinical trial of the effects of pioglitazone on glycemic control and dyslipidemia in oral antihyperglycemic medication‐naïve patients with type 2 diabetes mellitus. Clin Ther. 2003;25:1074–1095. [DOI] [PubMed] [Google Scholar]
- 18. Gerber P, Lubben G, Heusler S, et al. Effects of pioglitazone on metabolic control and blood pressure: a randomised study in patients with type 2 diabetes mellitus. Curr Med Res Opin. 2003;19:532–539. [DOI] [PubMed] [Google Scholar]
- 19. Langenfeld MR, Forst T, Hohberg C, et al. Pioglitazone decreases carotid intima‐media thickness independently of glycemic control in patients with type 2 diabetes mellitus. Results from a controlled randomized study. Circulation. 2005;111:2525–2531. [DOI] [PubMed] [Google Scholar]
- 20. Lebovitz HE, Dole JF, Patwardhan R, et al; Rosiglitazone Clinical Trials Study Group . Rosiglitazone monotherapy is effective in patients with type 2 diabetes. J Clin Endocrinol Metab. 2001;86(1):280–288. [DOI] [PubMed] [Google Scholar]
- 21. Ovalle F, Bell DSH. Thiazolidinedione induced recovery of pancreatic cell function. Diabetes. 2000;49(suppl 1):A120. [Google Scholar]
- 22. Chiquette E, Ramirez G, Defronzo R. A meta‐analysis comparing the effect of thiazolidinediones on cardiovascular risk factors. Arch Intern Med. 2004;164(19):2097–2104. [DOI] [PubMed] [Google Scholar]
- 23. Goldberg RB, Kendall DM, Deeg MA, et al, for the GLAI Study Investigators . A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005;28:1547–1554. [DOI] [PubMed] [Google Scholar]
- 24. Haffner SM, Greenberg AS, Weston WM, et al. Effects of rosiglitazone treatment on non‐traditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation. 2002;106(6):679–684. [DOI] [PubMed] [Google Scholar]
- 25. Qayyum R, Adomaityte JA. Meta‐analysis of the effect of thiazolidinediones on blood pressure. J Clin Hypertens. 2006;8:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity. Diabetes Care. 2000;23(1):57–63. [DOI] [PubMed] [Google Scholar]
- 27. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 28. Kang ES, Yun YS, Park SW, et al. Limitation of the validity of the homeostasis model assessment as an index of insulin resistance in Korea. Metabolism. 2005;54(2):206–211. [DOI] [PubMed] [Google Scholar]
- 29. Ascaso JF, Romero P, Real JT, et al. Insulin resistance quantification by fasting insulin plasma values and HOMA index in a non‐diabetic population. Med Clin (Barc). 2001;117(14):530–533. [DOI] [PubMed] [Google Scholar]
- 30. Li GW, Wang JP, Li CM, et al. Antihypertensive effect of rosiglitazone in non‐diabetic essential hypertension. Zhonghua Nei Ke Za Zhi. 2004;43(12):907–910. [PubMed] [Google Scholar]
- 31. Bruno G, Merletti F, Bargero G, et al. Changes over time in the prevalence and quality of care of type 2 diabetes in Italy: the Casale Monteferrato Surveys, 1988 and 2000. Nutr Metab Cardiovasc Dis. 2007 Feb 21; Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32. Bakris G, Viberti G, Weston WM, et al. Rosiglitazone reduces urinary albumin excretion in type II diabetes. J Hum Hypertens. 2003;17(1):7–12. [DOI] [PubMed] [Google Scholar]
- 33. Tan MH, Baksi A, Krahulec B, et al. Comparison of pioglitazone and glicazide in sustaining glycemic control over 2 years in patients with type 2 diabetes. Diabetes Care. 2005;28(3):544–550. [DOI] [PubMed] [Google Scholar]
- 34. Ono M, Ikegami H, Fujisawa T, et al. Improvement of liver function parameters in patients with type 2 diabetes treated with thiazolinediones. Metabolism. 2005;54(4):529–532. [DOI] [PubMed] [Google Scholar]
- 35. Sarafidis PA, Lasaridis AN, Nilsson PM, et al. Ambulatory blood pressure reduction after rosiglitazone treatment in patients with type 2 diabetes and hypertension correlates with insulin sensitivity increase. J Hypertens. 2004;22(9):1769–1777. [DOI] [PubMed] [Google Scholar]
- 36. Miyazaki Y, Mahankali A, Matsuda M, et al. Effects of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2002;87(6):2784–2791. [DOI] [PubMed] [Google Scholar]
- 37. Virtanen KA, Hallsten K, Parkkola R, et al. Different effects of rosiglitazone and metformin on adipose tissue glucose uptake in type 2 diabetes. Diabetes. 2003;52(2):283–290. [DOI] [PubMed] [Google Scholar]
- 38. Fullert S, Schneider F, Haak E, et al. Effects of pioglitazone in nondiabetic patients with arterial hypertension: a double‐blind, placebo‐controlled study. J Clin Endocrinol Metab. 2002;87(12):5503–5506. [DOI] [PubMed] [Google Scholar]
- 39. Raji A, Seely E, Bekins SA, et al. Rosiglitazone improves insulin sensitivity and lowers blood pressure in hypertensive patients. Diabetes Care. 2003;26(1):172–178. [DOI] [PubMed] [Google Scholar]
- 40. Yosefy C, Magen E, Kiselevich A, et al. Rosiglitazone improves, while glibenclamide worsens blood pressure control in treated hypertensive diabetic and dyslipidemic subjects via modulation of insulin resistance and sympathetic activity. J Cardiovasc Pharmacol. 2004;44(2):215–222. [DOI] [PubMed] [Google Scholar]


