Abstract
Background:
In a survey of students at our institution, suturing was the most desired workshop for simulation; however, cost, quality, and availability of skin pads is often prohibitive for suturing workshops. In-hospital fabrication may be utilized to manufacture non-commercial, high-fidelity, and low-cost simulation models. We describe the production, value, and face validation of our simulated skin model.
Materials and Methods:
Using an in-hospital fabrication laboratory, we have developed a model for skin and subcutaneous tissue. Our model uses a variety of commercially available materials to simulate the epidermis, dermis, subcutaneous fat, fascia, and muscle. A cost analysis was performed by comparing it to other commonly used commercial skin models. Expert surgeons assessed the material characteristics, durability, and overall quality of our model in comparison to other commercial models.
Results:
The materials cost of our novel skin pad model was 30.9% of the mean cost of five different commonly used foam and silicone based commercial skin models. This low-cost model is more durable than the commercial models, does not require skin pad holders, and is of higher fidelity than the commercial products. In addition to skin closure, our model may be used to simulate fascial closure or fasciotomy.
Conclusion:
Model creation using in-hospital workspaces is an effective strategy to decrease cost while improving quality of surgical simulation. Our methods for creation of an inexpensive and high-fidelity skin pad may be purposed for several soft tissue models.
Keywords: Simulation, Suturing, Skin Model, Cost, Fidelity, Surgical Training
INTRODUCTION
There is a great deal of variability in basic surgical skills amongst interns entering surgical residency due to a range of exposures during medical school1,2. In a survey of students at our institution, suturing was the most desired workshop for simulation; however, accommodating on-demand skills training for large classes of medical students is challenging and dependent on several local factors. Common barriers to the implementation of simulation training programs are cost and limited access to training facilities3. In-hospital model fabrication may help reduce simulation costs by utilizing wholesale-priced component materials for model making. It also provides model makers the benefit of collaboration with educators to customize models for specific learning needs and maintain quality of the models.
Traditional models to simulate skin, such as cadaver skin, animal skin, and fruit peels have been considered to have high fidelity; however, these biologic models can be costly, non-durable, require specialized storage, or be subject to cumbersome institutional protocols4. In addition to durability, biosimilar silicone is a great alternative to biologic material due to silicone’s characteristic mechanical shear modulus, friction, and deformability, similar to that of human soft tissue5. Simulation models made from foam and silicone are available commercially, but the literature describing non-commercial, low-cost, and high-fidelity simulation skin models remains scarce6.
Fidelity of simulation may be difficult to define for partial task trainers and is not always correlated with more efficacious learning or higher skill performance7-9. Models with high physical resemblance may curb learner disbelief and increase learner engagement because of the anatomic accuracy and mechanical properties afforded by biosimilar silicone models. The purpose of this study is to describe how to fabricate our silicone-based skin model, perform a value analysis of the model, and conduct an expert face validation of the model’s fidelity.
MATERIALS AND METHODS
Simulation Model Creation
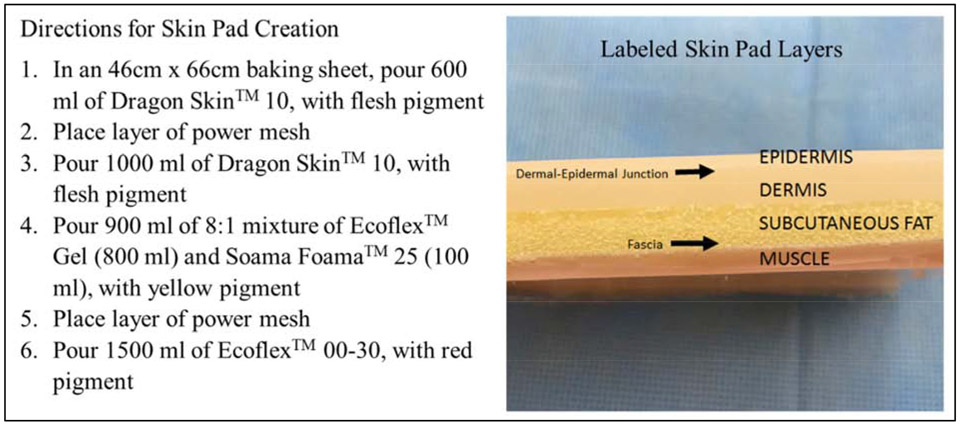
This study was approved by the University of Texas Medical Branch Institutional Review Board and a waiver of signed informed consent was obtained. Prototype development occurred in a dedicated in-hospital fabrication laboratory, the Maker Health Space, with the intent of utilizing the model in our surgical simulation training laboratory. Our layered model simulates the epidermis, dermis, dermal-epidermal junction, subcutaneous fat, fascia, and muscle using Smooth-On® platinum-based silicone materials including Dragon Skin™, Eco-Flex™, Soama Foama™, Silc Pig™ (Smooth-On, Easton, PA), and power mesh, a meshed fabric of 90% nylon and 10% spandex (Figure 1). Silicone layers are allowed to cure at room temperature for twenty minutes in between pours. These products are mixed on a baking sheet as described to create one large batch of skin pads that are then cut into 30 smaller pads. Each batch of 30 pads requires 20 minutes of intermittent labor from one individual, not including several silicone curing steps that require additional time.
Figure 1.
Photograph of Skin Pad Layers and Directions for Model Creation
Cost Analysis
The cost of five commercially available skin pads (models A, B, C, D, and E), all previously used in our simulation training laboratory, were analyzed based on manufacturer pricing. This cost analysis does not include the additional cost of necessary skin pad holders which are required to mount and hold models A, B, C, and D. The cost analysis of our novel skin model does not include mixing containers, molds, labor costs, or costs incurred during the prototype development phase. Materials costs for fabrication was based on publicly available manufacturer pricing that may differ from what an institution would pay.
Expert Assessment of Fidelity
Only experts, defined as resident or attending surgeons in either general or plastic surgery, participated in assessing the fidelity of the novel skin model. Experts were blinded to which skin pad they were assessing and performed two separate tasks followed by an anonymous survey that was filled out after each task. The first task was to perform two simple interrupted stitches and a running subcuticular stitch on an unused 4 cm incision made on the fabricated skin model and commercial models A, B, and C. This survey asked the expert to rank the quality of the models based on the characteristics of density, elasticity/stretch, surface texture, and anatomic accuracy from 1(best and most-like human skin) to 4(worst). The second task was to perform a running subcuticular stitch on each model using a separate 4 cm incision that had been previously closed with 20 running subcuticular stitches to simulate previous use of the model. Experts ranked the durability and overall quality of the models from 1(best and most-like human skin) to 4(worst).
Statistical Analysis
Expert survey rankings are displayed as median scores and descriptive statistics were performed using the Whitney-Mann U test. Significance level was set with an alpha value of 0.05. Statistical analysis was performed using Excel 2016 (Microsoft Corporation, Redmond, WA).
RESULTS
Simulation Model Creation
We modified several iterations of the skin model based of feedback received from experts, none of which participated in the face validation of the model, before testing the one presented here. The 100cm2 skin pad is relative to the size of commercially available skin models and does not require a skin pad holder. Each model was produced in a total of 95 minutes.
Cost Analysis
The cost of production for our fabricated model is $8.80 per 100 cm2 pad. Mean cost of the fabricated model is 30.9% of the mean cost of all commercial models, and Table 1 shows the comparison of relative cost, per same unit size, by each commercial model.
Table 1.
Cost Analysis of Commercial Models
| Commercial Model |
Model Surface Area (cm2) | Absolute Cost | Relative Percentage Cost |
|---|---|---|---|
| A | 89 | $17.50 | 225% |
| B | 100.8 | $19.50 | 222% |
| C | 87.7 | $30.00 | 391% |
| D | 161.3 | $17.00 | 120% |
| E | 108.4 | $63.00 | 662% |
Comparison of relative cost of fabricated model to five commercial models. Absolute cost and surface area in square centimeters of each model are included. The fabricated model had an absolute cost of $8.80 and a model surface area of 100 cm2.
Validation of Model
A total of fifteen experts completed our survey. Five experts were faculty surgeons and ten experts were surgical residents in post graduate year ranging from 1 to 5. The results of the survey are presented in Table 2. The fabricated model showed only a significant difference in the durability characteristic compared to commercial model A. Our fabricated model had a significantly higher ranking in all characteristics and overall quality when compared to commercial model B. Compared to commercial model C, our model had significantly higher rankings for durability, anatomic accuracy, and overall quality.
Table 2.
Results of Expert Assessment of Skin Models
| Fabricated Model |
Commercial Models |
P- values | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | ||
| Density | 2 | 3 | 4 | 2 | 0.59 | <0.05 | 1 |
| Elasticity/Stretch | 2 | 2 | 4 | 2 | 0.51 | <0.05 | 0.35 |
| Surface Texture | 2 | 2 | 4 | 2 | 0.68 | <0.05 | 0.55 |
| Anatomic Accuracy | 1 | 2 | 4 | 3 | 0.14 | <0.05 | <0.05 |
| Durability | 1 | 2 | 4 | 3 | <0.05 | <0.05 | <0.05 |
| Overall Quality | 2 | 3 | 4 | 2 | 0.25 | <0.05 | <0.05 |
Comparison of fabricated model to three commercial models based on ranking of material characteristics and overall quality from expert survey (n=15). Median values expressed with lowest rank corresponding to highest quality in the designated characteristic.
p values obtained when comparing fabricated model to commercial model A
p values obtained when comparing fabricated model to commercial model B
p values obtained when comparing fabricated model to commercial model C
DISCUSSION
Our in-hospital fabricated skin model is a fraction of the cost of commercially available skin pads and thought to be more durable than all concomitantly tested commercial skin pads. This suggests that the value of the fabricated skin pad exceeds the value reflected in the cost analysis data, as our models do not need to be replaced, due to wear, as often as commercial pads. Additionally, our fabricated skin model was rated by expert surgeons to simulate the characteristics of density, elasticity/stretch, surface texture, and anatomic accuracy better than commercial model B that we currently use in our surgical simulation center.
The existence of a Maker Health Space at our institution allowed us to use knowledge from expert model makers and some materials were available for use in prototype models. This knowledge included understanding the tensile strength, Shore hardness, shear and elastic modulus of the various silicone rubbers available. This was advantageous during the prototyping phase of the model to hasten both the design and production process, lowering the cost of prototyping. The use of power mesh allows for increased anatomic accuracy by demarcating the border of the dermis and epidermis. This aids in teaching the functional task of placing sutures at the dermal-epidermal junction, as would be important in a subcutaneous stitch that is commonly performed in surgery. The subcutaneous fat layer contains foam with low tensile strength that does not hold suture well, while the power mesh augments the dermal layer’s ability to hold suture. This exemplifies the utilization of material qualities for simulating the characteristics of real tissue.
Our fabricated skin pad may be used for procedural training in fasciotomy and fascial closure. This is a unique feature of our model and a functional advantage over commercial skin pads which only simulate epidermis, dermis, and subcutaneous fat. Additionally, future projects could use a combination of the materials used here for other soft tissue simulation models, such as those involving more detailed muscle layers or skin covering. Our skin pad may also be modified to exclude the layers for subcutaneous fat, fascia, and muscle, to only include dermal and epidermal layers, which would further decrease the material cost.
Because our fabricated skin pad is cost effective, students and residents may be provided with a fabricated skin pad for their personal use. We believe this will increase independent practice secondary to increased accessibility. This allows for earlier exposure to suture skills training during medical school, which has been shown to translate into earlier technical proficiency during residency training10. Moreover, due to the fidelity and increased durability of our fabricated skin pads, the quality of scheduled suturing training sessions has improved because students are no longer using worn-out commercial models. And so, in addition to being a cheaper alternative to purchasing commercial products, in-hospital fabrication of simulation models allows quality to remain the same or better than commercial products. With improved quality and lower cost, our model provides better overall value than commercial alternatives.
We acknowledge several limitations to our study including the small number of expert participants. Due to difficulty with objective measurement of model durability, our assessment of durability is subjective but descriptive of this characteristic. Also, the cost to prototype our model was not included in comparing costs to commercially available skin pads. These costs are hard to account for because initial prototypes utilized variable quantities of materials owned by the Maker Health Space. Labor costs vary widely among centers and were not included in overall model cost; however, labor times were short and thus labor costs remain low with production.
While there are other commercial models than the ones we evaluated, we chose to assess models that have been previously used in our simulation lab. The commercial models chosen are widely available; however, it is difficult to acquire objective data as to their popularity and utilization by others.
CONCLUSIONS
Model creation using in-hospital workspaces is an effective strategy to decrease cost while improving quality of surgical simulation. Our methods for creation of an inexpensive and high-fidelity skin pad may be easily purposed for use in several soft tissue models.
HIGHLIGHTS.
Self-fabrication of simulation models is an effective strategy to decrease cost of surgical simulation while improving quality
Our inexpensive and high-fidelity skin model may be purposed for myriad soft tissue models
Acknowledgements
We would like to acknowledge Kristen Kahrig for her assistance with model fabrication and acquisition of model pricing data.
Funding:
KJH is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number T32DK007639. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Sachdeva A, et al. “Variability in the Clinical Skills of Residents Entering Training Programs in Surgery.” Surgery, vol. 118, no. 2, 1995, pp. 300–309. [DOI] [PubMed] [Google Scholar]
- 2.Davis R, et al. “Surgical and Procedural Skills Training at Medical School – a National Review.” International Journal of Surgery, vol. 12, no. 8, 2014, pp. 877–882. [DOI] [PubMed] [Google Scholar]
- 3.Hosny SG, et al. “Barriers to the Implementation and Uptake of Simulation-Based Training Programs in General Surgery: a Multinational Qualitative Study.” Journal of Surgical Research, vol. 220, 2017, pp. 419–426. [DOI] [PubMed] [Google Scholar]
- 4.Wong K, et al. “Banana Fruit: An ‘Appealing’ Alternative for Practicing Suture Techniques in Resource-Limited Settings.” American Journal of Otolaryngology, vol. 39, no. 5, 2018, pp. 582–584. [DOI] [PubMed] [Google Scholar]
- 5.Sparks JL, et al. “Use of Silicone Materials to Simulate Tissue Biomechanics as Related to Deep Tissue Injury.” Advances in Skin & Wound Care, vol. 28, no. 2, February. 2015, pp. 59–68. [DOI] [PubMed] [Google Scholar]
- 6.Zendejas B, et al. “Cost: The missing outcome in simulation-based medical education research: A systematic review.” Surgery, vol. 153, no.2, 2013, pp. 160–176. [DOI] [PubMed] [Google Scholar]
- 7.Shen Z, et al. “A Novel Clinical-Simulated Suture Education for Basic Surgical Skill: Suture on the Biological Tissue Fixed on Standardized Patient Evaluated with Objective Structured Assessment of Technical Skill (OSATS) Tools.” Journal of Investigative Surgery, vol. 31, no. 4, 21 June 2017, pp. 333–339. [DOI] [PubMed] [Google Scholar]
- 8.Massoth C, et al. “High-Fidelity Is Not Superior to Low-Fidelity Simulation but Leads to Overconfidence in Medical Students.” BMC Medical Education, vol. 19, no. 1, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamstra SJ, et al. “Reconsidering Fidelity in Simulation-Based Training.” Academic Medicine, vol. 89, no. 3, 2014, pp. 387–392. [DOI] [PubMed] [Google Scholar]
- 10.Gershuni V, et al. “Retention of Suturing and Knot-Tying Skills in Senior Medical Students after Proficiency-Based Training: Results of a Prospective, Randomized Trial.” Surgery, vol. 154, no. 4, 2013, pp. 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]