Abstract
The blood pressure (BP) goals set by hypertension management guidelines (<140/90 mm Hg in uncomplicated hypertension; <130/80 mm Hg in type 2 diabetes or kidney disease) are not being achieved in a high proportion of patients, partly because monotherapy is insufficient in many patients. In particular, patients with uncontrolled moderate or severe hypertension and/or associated cardiovascular risk factors remain at high risk for cardiovascular events and hypertensive emergency. In recognition of the urgency of treating moderate and severe hypertension, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) advocates the initial use of 2‐drug therapies in patients with systolic BP levels >20 mm Hg above goal or diastolic BP level >10 mm Hg above goal. Regimens should usually include a thiazide diuretic and, for patients with diabetes or kidney disease, an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker. Recently, clinical trial data have shown that first‐step antihypertensive treatment of moderate and severe hypertension with carefully chosen fixed‐dose combinations provides a high rate of BP goal achievement, a simplified dosing regimen, and superior tolerability compared with monotherapy.
Hypertension is a highly prevalent disease, affecting more than 65 million adult Americans, or one‐third of the adult population. 1 , 2 As one of the most important modifiable risk factors for cardiovascular disease, a steep, continuous, consistent, and independent positive relationship exists between blood pressure (BP) and both cardiovascular morbidity and mortality. 3 , 4 , 5 , 6 , 7 Across the BP range from 115/75 to 185/115 mm Hg, the risk of death from ischemic heart disease and stroke increases linearly for individuals in all age groups between 40 and 89 years. 3 For those aged between 40 and 69 years, mortality from ischemic heart disease and stroke doubles for every 20‐mm Hg increase in systolic BP (SBP) or 10‐mm Hg increase in diastolic BP (DBP). 3 Thus, patients with uncontrolled moderate or severe hypertension (SBP >160 mm Hg or DBP >100 mm Hg) are likely to experience poor cardiovascular outcomes. In particular, exposure to severely elevated BP levels is associated with considerable immediate risk of hypertensive urgency (ie, DBP ≥120 mm Hg) or hypertensive emergency, which also includes rapidly evolving end‐organ damage. The latter often results in hospitalization for events such as congestive heart failure, intracranial hemorrhage, rupture of aneurysms, and progression of hypertensive retinopathy and nephropathy. 8 , 9 , 10
In practice, the BP goals set by hypertension management guidelines (<140/90 mm Hg in patients with uncomplicated hypertension and <130/80 mm Hg in patients with concomitant type 2 diabetes or kidney disease) are not being achieved in approximately one‐half of treated patients, and greater efforts are needed to improve BP control and decrease cardiovascular risk on a nationwide scale. One important explanation of the poor hypertension control rates is that monotherapy is insufficient to achieve BP goals in many patients. This was reinforced by recent findings from the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), 11 which found that diuretic, calcium channel blocker, and angiotensin‐converting enzyme (ACE) inhibitor treatment arms were similar in reducing coronary heart disease outcomes in more than 33,000 study participants with stage 1 or 2 hypertension and at least 1 additional risk factor for coronary heart disease. At the end of 5 years of therapy, however, BP levels in 34% of all study participants were not at the goal of <140/90 mm Hg despite >45% of study participants receiving step 2, step 3, and/or other antihypertensive therapy. This review examines the rationale for greater use of antihypertensive fixed‐dose combination therapy for the first‐step treatment of patients with moderate and severe hypertension, a practice increasingly adopted by hypertension specialists. In addition, we review available safety and efficacy data from clinical trials of potential combination therapies in patients with moderate and severe hypertension.
CARDIOVASCULAR RISK IN PATIENTS WITH MODERATE AND SEVERE HYPERTENSION
Continual exposure to moderately or severely elevated BP levels is associated with a substantial increase in the risk of cardiovascular events. 3 , 4 , 5 , 6 , 7 Severe hypertension can even lead to hypertensive crises. 9 , 12 , 13 , 14 Two early studies, the Veterans Administration Cooperative (VA Coop) trial 12 , 13 and one by the National Heart, Lung, and Blood Institute (NHLBI), 14 demonstrated a relatively high incidence of hypertensive emergencies among patients presenting with severe hypertension. Of importance, the 2 studies also showed that reduction of BP with first‐step 2‐ or 3‐drug therapy provided rapid and nearly complete prevention of such emergencies. In the landmark VA Coop study, 143 patients had baseline DBP levels of 115 to 129 mm Hg (mean, 121 mm Hg). 12 , 13 A total of 27 patients in the placebo group experienced severe hypertensive complications over 1.3 years, or 1 crisis per 4 patient‐years. In contrast, none of the patients taking multiple‐drug antihypertensive treatment developed these complications; thus, the trial was terminated. Furthermore, the between‐group difference was observed early in the trial; the numbers of complications in the placebo compared with active treatment groups were 5 vs 0 at 2 months and 9 vs 0 at 6 months.
The NHLBI study enrolled 87 patients with a mean DBP level of 109.5 mm Hg. 14 The excess risk of morbid events in 42 placebo‐treated patients was 13 cases over 2 years, which translated to 1 event per 7 patient‐years. In the placebo group, there were 5 cases of retinopathy (grade 3 or worse), 8 patients who developed congestive heart failure, and 4 incidences of severe headache. In contrast, none of the patients taking combination antihypertensive therapy experienced these adverse events. Again, the benefit of multiple‐drug treatment over placebo was observed early in the trial.
Data from recent large clinical trials support the hypothesis that the time it takes to reach target BP levels influences cardiovascular outcomes in high‐risk patients. 15 , 16 , 17 The Valsartan Antihypertensive Long‐Term Use Evaluation (VALUE) study 15 compared BP‐lowering and clinical event rates between patients treated with the angiotensin receptor blocker (ARB) valsartan and the calcium channel blocker amlodipine as well as between those in whom immediate or delayed BP control was achieved. About 15,000 patients with a mean BP level of 155/88 mm Hg were followed up for approximately 4.2 years. Early BP control, independent of the drug type used, was associated with significantly lower rates of combined cardiac events, stroke, myocardial infarction, and mortality. The authors of the VALUE study suggested that BP goals need to be reached within a relatively short time (weeks rather than months), at least in patients who are at high cardiovascular risk. Additional data from the Study on Cognition and Prognosis in the Elderly (SCOPE) 16 and the Systolic Hypertension in Europe (Syst‐Eur) 17 trial suggest that delays of 3 months to 2 years in starting antihypertensive therapy can increase the risk of certain cardiovascular end points, especially stroke.
It is becoming apparent from large‐scale clinical trials that a 3‐ or 4‐mm Hg difference in BP often results in a disproportionately large reduction in clinical event rates. 6 , 11 , 15 , 18 , 19 , 20 In a meta‐analysis of 9 major prospective observational studies, prolonged reductions in DBP of 5, 7.5, and 10 mm Hg were associated with 34%, 46%, and 56% fewer strokes and 21%, 29%, and 37% lower incidences of coronary heart disease, respectively. 4 Likewise, in 14 prospective observational studies, a 5‐ to 6‐mm Hg reduction in DBP was associated with 35% to 40% fewer strokes and a 20% to 25% lower rate of coronary heart disease. 5
RATIONALE FOR FIRST‐STEP ANTIHYPERTENSIVE COMBINATION THERAPY
For maximal cardiovascular protection, hypertension management guidelines recommend that BP is controlled to a level of <140/90 mm Hg in patients with uncomplicated hypertension and <130/80 mm Hg in patients with concomitant type 2 diabetes or kidney disease. 21 , 22 , 23 , 24 , 25 , 26 However, between 2003 and 2004, only 37% of adult Americans with hypertension had BP controlled to <140/90 mm Hg, and the target of <130/80 mm Hg was achieved in 37% of those with type 2 diabetes. 2 Similar low rates of hypertension control have been reported worldwide. 27 Furthermore, although data are limited, it is likely that the percentage of patients with severe hypertension in whom goal BP is achieved is even lower.
There are several possible reasons for the current undermanagement of hypertension. These include poor patient compliance with medication, resulting mainly from adverse effects and lack of convenient dosing 28 ; reluctance of physicians to more rapidly modify or titrate initially chosen therapy to obtain BP control 29 , 30 ; and suboptimal attention to many of the sociologic factors that impact BP control. 31 Thus, tolerability and a convenient dosing/titration drug regimen are clearly important factors when choosing antihypertensive therapy. Another important explanation of the current low rates of hypertension control is that monotherapy fails to achieve BP goals in many patients. Hypertension is a multifactorial disease, and BP target will be reached in only 40% to 50% of patients taking any single antihypertensive agent targeting one mechanism. 32 , 33 Evidence of physician inertia was displayed in a recent survey that showed that >30% of patients were continued on the same antihypertensive therapy despite elevated BP. 34
Compared with the use of 2 separate drugs, fixed‐dose combinations offer simpler dosing regimens that favor treatment compliance and persistence. The first‐step use of fixed‐dose antihypertensive combination therapy has several benefits compared with monotherapy. Fixed‐dose combinations produce additive or synergistic BP‐lowering efficacy, providing the potential for greater BP response rates. 35 , 36 , 37 , 38 , 39 Of importance, early normalization of BP may greatly reinforce the motivation of patients to adhere to lifelong treatment. 28 , 40 Fixed‐dose combinations also provide a convenient dosing regimen, with fewer pills and a simplified titration process, thereby further increasing the potential for patient compliance and BP goal achievement. In addition, careful selection of antihypertensive drug combinations can attenuate adverse events. For example, diuretic‐induced potassium depletion can be offset by the concomitant administration of an ACE inhibitor or ARB. 41 , 42
GUIDELINES FOR THE TREATMENT OF MODERATE AND SEVERE HYPERTENSION
Hypertension management guidelines have developed classification systems that assign different thresholds of BP to arbitrary levels of hypertension severity. 22 , 23 , 25 For example, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) 22 classified BP thresholds as normal, prehypertension, stage 1 hypertension, and stage 2 hypertension (Table I). For stage 1 hypertension (SBP, 140–159 mm Hg or DBP, 90–99 mm Hg), JNC 7 recommends both lifestyle modification and antihypertensive monotherapy. In recognition of the urgency of treating moderate and severe hypertension, the guidelines advocate the initial use of 2 antihypertensive drugs in patients with an SBP level ≥160 mm Hg (>20 mm Hg above goal) or a DBP level ≥100 mm Hg (>10 mm Hg above goal).
Table I.
Classification of BP According to JNC 7 22
| BP Classification | SBP, mm Hg | DBP, mm Hg | JNC 7 Treatment Recommendation |
|---|---|---|---|
| Normal | <120 | and <80 | — |
| Prehypertension | 120–139 | or 80–89 | Lifestyle modification |
| Stage 1 hypertension | 140–159 | or 90–99 | Lifestyle modification and possible medication monotherapy (thiazide, ARB, ACE inhibitor, β‐blocker, calcium channel blocker) |
| Stage 2 hypertension | ≥160 | or ≥100 | Lifestyle modification and 2‐drug therapy (thiazide plus ARB, ACE inhibitor, β‐blocker, calcium channel blocker) |
| Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BP, blood pressure; DBP, diastolic BP; JNC 7, Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; SBP, systolic BP. | |||
Other hypertension management guidelines also recommend treatment with 2 or more antihypertensive agents in patients whose BP is above goal by a certain amount (Table II). 21 , 22 , 23 , 24 , 26 The National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines advocate combination therapy in patients with SBP levels >20 mm Hg over goal, according to the stage of chronic kidney disease and cardiovascular disease. 21 The consensus statement of the Hypertension in African Americans Working Group recommends antihypertensive combinations in patients with SBP levels ≥15 mm Hg above goal or DBP levels ≥10 mm Hg above goal; these lower thresholds reflect the somewhat higher cardiovascular risk observed in blacks than in patients of other races. 24 Guidelines issued by the European Society of Hypertension/European Society of Cardiology and the American Diabetes Association also endorse multiple drug therapy in high‐risk patients, according to BP and cardiovascular complications. 23 , 26
Table II.
Recommendations for Initial Antihypertensive Therapy
| Hypertension Management Guidelines | BP Levels Requiring First‐Step Combination Antihypertensive Treatment |
|---|---|
| JNC 7 22 | SBP >20 mm Hg or DBP >10 mm Hg above goal |
| National Kidney Foundation K/DOQI 21 | SBP >20 mm Hg above goal, according to the stage of chronic kidney disease and cardiovascular disease risk |
| Consensus statement of the Hypertension in African Americans Working Group of the ISHIB 24 | African Americans with SBP ≥15 mm Hg or DBP ≥10 mm Hg above goal |
| ESH/ESC 23 | High‐risk patients according to BP and cardiovascular disease complications |
| ADA 26 | Patients with type 2 diabetes and BP >130/80 mm Hg |
| Abbreviations: ADA, American Diabetes Association; BP, blood pressure; DBP, diastolic BP; ESH/ESC, European Society of Hypertension/European Society of Cardiology; ISHIB, International Society on Hypertension in Blacks; JNC 7, Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; K/DOQI, Kidney Disease Outcomes Quality Initiative; SBP, systolic BP. | |
The choice of initial combination therapy can significantly influence BP‐lowering efficacy, goal attainment, tolerability, and long‐term patient compliance. JNC 7 recommends that one of the drugs in the combination should usually be a thiazide diuretic, such as hydrochlorothiazide (HCTZ), and the other should be an ACE inhibitor, ARB, β‐blocker, or calcium channel blocker. 22 Appropriate choice of antihypertensive drug combinations can have potential benefits that may be independent of BP lowering. Angiotensin II is the primary agent of the renin‐angiotensin‐aldosterone system (RAAS), and in addition to having an effect on BP, it plays a central role in the pathophysiology of cardiovascular disease and target organ damage. The RAAS‐blocking agents, ACE inhibitors, and ARBs inhibit the harmful effects of angiotensin II. Thus, these classes of antihypertensive agents may provide cardiovascular protection in addition to their BP‐lowering effects, 43 , 44 , 45 , 46 , 47 and they are recommended by hypertension management guidelines for treatment of patients with type 2 diabetes or kidney disease. 21 , 22 , 24 , 48
CLINICAL EXPERIENCE WITH FIRST‐STEP ANTIHYPERTENSIVE COMBINATION THERAPY IN MODERATE AND SEVERE HYPERTENSION
The BP‐lowering efficacy and safety of first‐step JNC 7‐recommended antihypertensive medications in patients with moderate or severe hypertension have been examined in several clinical trials (Table III). 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 Overall, data support the recommendations for wider use of this approach in these patients. A more detailed summary of studies in patients with moderate and severe hypertension follows.
Table III.
BP‐Lowering Efficacy and Percentage of Patients in Whom BP Goals Were Achieved With First‐Step Antihypertensive Combination Treatment With ARBs and ACE Inhibitors in Some Clinical Trials of Patients With Moderate or Severe Hypertension
| Trial | Design | Patients | Baseline BP, mm Hg | Drugs/Doses | BP Reductions From Baseline, mm Hg | Patients in Whom BP Goal Was Achieved | |
|---|---|---|---|---|---|---|---|
| SBP | DBP | ||||||
| Severe Hypertension at Baseline (SBP ≥180 mm Hg or DBP ≥110 mm Hg) | |||||||
| Neutel et al 49 | Multicenter, randomized (2:1), double‐blind, forced‐titration, 7‐week | Untreated (DBP ≥110 mm Hg) or uncontrolled (DBP ≥100 mm Hg) severe hypertension (N=697) | 172/113 | Irbesartan/HCTZ 150/12.5 mg FDC force‐titrated to 300/25 mg FDC after week 1 | −30.8 | −24.0 | DBP <90 mm Hg (week 5) 47.2%a |
| Irbesartan 150 mg force‐titrated to 300 mg after week 1 | −21.1 | −19.3 | 33.2% | ||||
| Salerno et al 50 | Multicenter, randomized, double‐blind, titration, 6‐week | Uncontrolled severe hypertension (≥110 and ≤220 mm Hg) (N=585) | 171/113 | Losartan/HCTZ 50/12.5 mg FDC (to 100/25 mg at week 4 if necessary) | −25.1a | −17.8a | DBP <90 mm Hg 31%a |
| Losartan 50 mg (to 100–150 mg at week 2–4 if necessary) | −14.1 | −11.9 | 12.6% | ||||
| Moderate Hypertension at Baseline (SBP 160–179 or DBP 100–109 mm Hg) | |||||||
| Rump et al 51 | Multicenter, randomized, double‐blind, 12‐week | Untreated (≥160/≥110 to ≤120 mm Hg) or uncontrolled (DBP 90–110 mm Hg) moderate to severe hypertension (N=766) | 170/104 | Olmesartan/HCTZ 20/12.5 mg | −29.3 | −17.6 | <l40/90 mm Hg 43.2% |
| Losartan/HCTZ 50/12.5 mg | −24.9 | −16.5 | 32.1% | ||||
| Lacourcière et al 52 | Randomized, double‐blind, 8‐week | Stage 2 or 3 systolic hypertension (SBP ≥160 and ≤200 mm Hg) (N=774) | 168/93 | Valsartan/HCTZ 160/0 mg force‐titrated to 160 mg/25 mg after week 4 | −28.3 | −10.1 | SBP <140 mm Hg or ≥20 mm Hg decrease 75.0%b |
| Valsartan/HCTZ 160/0 mg force‐titrated to 160 mg/12.5 mg after week 4 | −27.9 | −10.2 | 74.4%b | ||||
| Valsartan 80 mg force‐titrated to 160 mg after week 4 | −20.7 | −6.6 | 56.9% | ||||
| Severe Hypertension at Baseline (SBP ≥180 mm Hg or DBP ≥110 mm Hg) | |||||||
| Lapuerta et al 53 | Multicenter, randomized (3:1:1), double‐blind, forced‐titration, 12‐week | Untreated (SBP 160–180 mm Hg and DBP <110 mm Hg, or DBP 100–110 mm Hg and SBP 130–180 mm Hg) or uncontrolled (SBP 150–180 mmHg and DBP <110 mm Hg, or DBP 95–110 mm Hg and SBP 130–180 mm Hg) moderate hypertension (N=538) | 162/98 | Irbesartan/HCTZ 150/12.5 mg FDC force‐titrated to 300/25 mg FDC after week 2 | (week 8) −27.1 | (week 8) −14.6 | BP <l40/90 mm Hg (week 8) 53.4%c |
| Irbesartan 150 mg force‐titrated to 300 mg after week 2 | −22.0 | −11.6 | 40.6% | ||||
| HCTZ 12.5 mg force‐titrated to 25 mg after week 2 | −15.7 | −7.3 | 20.2% | ||||
| Gradman et al 54 | Multicenter, randomized (2:2:1), double‐blind, placebo‐controlled, 8‐week | Moderate to severe hypertension (DBP 105–115 mm Hg) (N=446) | Losartan/HCTZ 100/25 mg, 160/108 | Losartan/HCTZ 100/25 mg | −21.8 | −17.5a | DBP <90 mm Hg or ≥10 mm Hg decrease 86.7% |
| Losartan/HCTZ 50/12.5 mg, 160/108 | Losartan/HCTZ 50/12.5 mg | −18.3 | −15.2a | 78.9% | |||
| Placebo, 161/108 | Placebo | −4.7 | −8.5 | 50% | |||
| Lenz et al 55 | Multicenter, double‐blind, 8‐week | Moderate to severe hypertension (DBP ≥105 and ≤120 mm Hg) (N=368) | 171/110 | Quinapril/HCTZ 10/12.5 mg titrated to 20/25 mg after week 4 | −27.1 | −19.5 | 72% |
| Quinapril 10 mg titrated to 20 mg after week 4 | −19.7 | −17.0 | 66% | ||||
| HCTZ 12.5 mg titrated to 25 mg after week 4 | −20.4 | −17.2 | 70% | ||||
| Cushman et al 56 | Multicenter, randomized, double‐blind, placebo‐controlled, 12‐week | Stage 1–3 hypertension (DBP 95–115 mm Hg) (N=891) | DBP, 102 | Enalapril/diltiazem 5/180 mg, titrated as needed | −9.0b | −8.3b | DBP <90 mm Hg or ≥10 mm Hg decrease 77% |
| Enalapril/diltiazem 5/120 mg, titrated as needed | −7.9b | −7.6b | 77% | ||||
| Enalapril 5 mg | −5.7 | −5.7 | |||||
| Diltiazem 180 mg | −3.2 | −6.0 | |||||
| Diltiazem 120 mg | −2.1 | −5.1 | |||||
| Placebo | 1.0 | −3.3 | |||||
| a P<.001; P<.05; c P<.0001. Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BP, blood pressure; DBP, diastolic BP; FDC, fixed‐dose combination; HCTZ, hydrochlorothiazide; SBP, systolic BP. | |||||||
Once‐daily irbesartan combined with HCTZ as well as other ARB/thiazide and ACE inhibitor/thiazide combinations have been reported to have BP‐lowering efficacy and safety in patients with mild to moderate hypertension. 41 , 57 , 58 , 59 , 60 In a large trial of patients with severe hypertension, rapid BP control that occurs over weeks was demonstrated with irbesartan/thiazide fixed‐dose combination therapy compared with irbesartan monotherapy (Table III). 49 Untreated patients with DBP levels ≥110 mm Hg or patients with DBP levels ≥100 mm Hg after at least 4 weeks of antihypertensive monotherapy were enrolled in this 7‐week, multicenter, randomized, double‐blind parallel‐group study. Randomization was 2:1 to initial irbesartan/HCTZ 150/12.5 mg fixed‐dose combination forced‐titrated to 300/25 mg after week 1 (n=468), or irbesartan 150 mg forced‐titrated to 300 mg after week 1 (n=229). At week 5, irbesartan/HCTZ provided greater BP reductions from baseline (31/24 vs 21/19 mm Hg), and a trough DBP <90 mm Hg was achieved in a higher rate of patients than with irbesartan monotherapy (primary end point, 47% vs 33%; P=.0005). The BP goal of <140/90 mm Hg was achieved in 35% of patients taking combination therapy and 19% of those taking monotherapy at week 5. The greater reductions from baseline in both DBP and SBP with irbesartan/HCTZ over irbesartan monotherapy were observed at all time points examined (Figure). As a result, patients treated with the combination experienced significantly less exposure to severe levels of DBP; the difference was 26 patient‐weeks for every 100 patients treated (P=.004). 61 Furthermore, patients treated with irbesartan/HCTZ were significantly less likely than those taking irbesartan to have severe hypertension (SBP ≥180 mm Hg or DBP ≥110 mm Hg) after 5 weeks of treatment (5.4% vs 13.8%; P=.0003).
Figure.
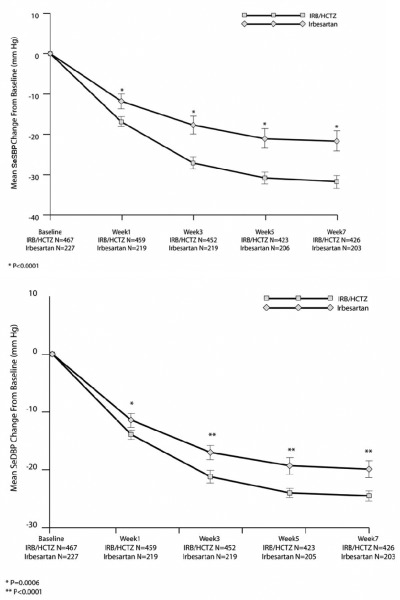
Mean change from baseline in seated systolic and diastolic blood pressure (SeSBP/SeDBP) during 7 weeks of double‐blind treatment with initial fixed‐dose irbesartan (IRB)/hydrochlorothiazide (HCTZ) vs irbesartan monotherapy in patients with severe hypertension. Data from Neutel et al. 49
The forced‐titration design of this study addressed the primary safety concern of hypertension management (ie, that lowering BP too quickly may lead to hypotension, dizziness, and syncope). Indeed, despite the more rapid and aggressive BP reductions in patients taking irbesartan/HCTZ combination treatment, patients had a superior adverse event profile than did those taking irbesartan monotherapy. 49 The combined incidence of prespecified adverse events (hypotension, dizziness, syncope, headache, hypokalemia/hyperkalemia) was lower with irbesartan/HCTZ than with irbesartan (8.8% vs 11.5%); only headache (4.3% vs 6.6%) and dizziness (3.6% vs 4.0%) occurred with an incidence of >1%. There was no syncope during the study, and hypotension was rare in the combination therapy and monotherapy groups (0.6% vs 0%). There were no treatment‐related serious adverse events and no deaths. Furthermore, the discontinuation rate for adverse events was low (1.9% and 2.2% for the combination therapy and monotherapy groups, respectively).
A trial of initial fixed‐dose irbesartan/HCTZ was recently completed in patients with moderate hypertension, in which the combination was significantly more effective than monotherapy with either component (Table III). 53 Eligible patients had either untreated hypertension (BP 160–180/<110 mm Hg or 130–180/100–110 mm Hg) or uncontrolled hypertension on at least 4 weeks of antihypertensive monotherapy (BP 150–180/<110 mm Hg or 130–180/95–110 mm Hg). Patients were randomized 3:1:1 to irbesartan/HCTZ 150/12.5 mg fixed‐dose combination force‐titrated to 300/25 mg after week 2 (n=328), irbesartan 150 mg force‐titrated to 300 mg after week 2 (n=106), or HCTZ 12.5 mg force‐titrated to 25 mg after week 2 (n=104). The fixed‐dose combination produced significantly greater reductions in SBP at week 8 (primary end point) compared with either monotherapy regimen, with a mean difference of 5.0 and 11.3 mm Hg compared with irbesartan and HCTZ, respectively (P=.0016 and P<.0001). Goal was achieved in significantly more patients treated with irbesartan/HCTZ (53.4%) than in irbesartan (40.6%; P=.025) or HCTZ (20.2%; P<.0001) recipients. Irbesartan/HCTZ showed comparable safety to either irbesartan alone or HCTZ alone, with total and prespecified adverse events occurring in 47% and 11%, 45% and 7%, and 39% and 7% of patients, respectively.
Fixed‐dose losartan/HCTZ was more effective than monotherapy in 585 patients with severe hypertension (DBP ≥110 mm Hg and SBP ≤220 mm Hg and taking at least 3 antihypertensive medications at screening) (Table III). 50 In the 6‐week, double‐blind, randomized multicenter study, patients received either losartan/HCTZ 50/12.5 mg (titrated to 100/25 mg at week 4, as necessary) or losartan 50 mg (titrated to 100 mg at week 2 and to 150 mg at week 4, as necessary). In more patients on combination treatment, a target DBP level of <90 mm Hg was reached, compared with those on monotherapy at 4 weeks (primary end point, 19.6% vs 9.9%; P=.002) and at 6 weeks (31% vs 12.6%; P<.001). The overall adverse event rate was lower with the ARB‐based combination than with monotherapy (43% vs 53%). Incidences of first‐dose adverse experiences and adverse events of special interest (hypotension, syncope, dizziness, increased serum creatinine level) were low and did not differ between the 2 treatment groups. There was also a decreased incidence of renal adverse events (increased serum creatinine level) in the combination group compared with the monotherapy group (0.5% vs 1.1%). No drug‐related adverse events or deaths occurred during the study.
A 12‐week, multicenter, randomized double‐blind trial demonstrated effective BP lowering and goal achievement with both losartan/HCTZ and olmesartan/HCTZ in 613 patients with moderate to severe hypertension that was either newly diagnosed (mean DBP ≥110 to ≤120 mm Hg and mean SBP ≥160 mm Hg) or inadequately controlled (DBP 90–110 mm Hg), despite using at least 1 antihypertensive agent (Table III). 51 Both ARB‐based combinations were well tolerated. There were no treatment‐related serious adverse events or deaths. The most frequent adverse events in the olmesartan/HCTZ and losartan/HCTZ groups, respectively, were dizziness (5.4% and 3.5%) and headache (2.9% and 3.8%).
Valsartan 160 mg force‐titrated after 4 weeks to either valsartan/HCTZ 160/25 mg or 160/12.5 mg provided improved BP lowering and response rates than valsartan monotherapy in patients with stage 2 or 3 systolic hypertension in an 8‐week, randomized, double‐blind parallel‐group trial (Table III). 52 Again, the significantly greater antihypertensive efficacy with combination therapies did not adversely affect tolerability; adverse event rates were similar with valsartan (28%), valsartan/HCTZ 160/12.5 mg (29%), and valsartan/HCTZ 160/25 mg (34%).
CONCLUSIONS
Hypertension is not being controlled in approximately one‐half of treated patients, and this relates to multiple factors, including insufficient BP‐lowering efficacy of monotherapy in many patients. Clinical experience affords sufficient reason not to delay aggressive treatment in patients with moderate or severe hypertension who are at high risk for cardiovascular events and hypertensive emergency. Combination therapy decreases BP more effectively and more rapidly than single‐agent therapy, leading to fewer changes in medications and visits to the health care provider. It also minimizes the risk of adverse events through the use of lower doses of the constituent agents than might be needed as monotherapy to achieve the same reduction in BP. Simplicity, tolerability, convenience, and cost‐effectiveness should encourage patient compliance with the treatment regimen. These benefits of fixed‐dose combinations point to an important role as first‐step therapy, not only for lowering BP but also for reducing the incidence of cardiovascular‐associated morbidity and mortality.
Fixed‐dose combinations appropriate for the initiation of therapy should usually include a thiazide diuretic plus an ARB or an ACE inhibitor. For patients with hypertension and type 2 diabetes or kidney disease, one of the agents should be an ACE inhibitor or an ARB. First‐step use of fixed‐dose antihypertensive combinations might help to decrease cardiovascular disease and target organ damage on a population‐wide scale. Although there are patients whose high BP can be controlled on monotherapy, there is currently no means of identifying these individuals from the total population of hypertensives. The use of low‐dose combination therapy would not harm such patients; in fact, the risk‐benefit ratio would be expected to shift in favor of the combination approach by providing even better BP lowering with fewer adverse effects. The benefits of prescribing fixed‐dose combinations as initial therapy are already well recognized by hypertension experts; thus, primary care physicians may wish to consider this more effective approach for hypertension management in the future.
Acknowledgement and disclosure:
This publication was supported by Bristol‐Myers Squibb/Sanofi Pharmaceutical Partnership. The authors would like to acknowledge the editorial and data collection support of Sharon Rayner, Envision Pharma, Horsham, UK. Dr Norris is supported in part by National Institutes of Health grants MD00182, RR11145, and RR019234. Keith Norris, MD, has no conflicts of interest. Joel M. Neutel, MD, is a member of the Speakers' Bureaus for Novartis, Bristol‐Myers Squibb/Sanofi Pharmaceutical Partnership, Boehringer Ingelheim, Pfizer, Forest, Biovail, and Sankyo.
References
- 1. Fields LE, Burt VL, Cutler JA, et al. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398–404. [DOI] [PubMed] [Google Scholar]
- 2. Ong KL, Cheung BMY, Man YB, et al. Prevalence, awareness, treatment and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. [DOI] [PubMed] [Google Scholar]
- 3. Lewington S, Clarke R, Qizilbash N, et al, for the Prospective Studies Collaboration . Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 4. MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. [DOI] [PubMed] [Google Scholar]
- 5. Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short‐term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335:827–838. [DOI] [PubMed] [Google Scholar]
- 6. Vasan RS, Larson MG, Leip EP, et al. Impact of high‐normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. [DOI] [PubMed] [Google Scholar]
- 7. Grimm RH Jr, Cohen JD, Smith WM, et al. Hypertension management in the Multiple Risk Factor Intervention Trial (MRFIT). Six‐year intervention results for men in special intervention and usual care groups . Arch Intern Med. 1985;145:1191–1199. [PubMed] [Google Scholar]
- 8. Zampaglione B, Pascale C, Marchisio M, et al. Hypertensive urgencies and emergencies. Prevalence and clinical presentation. Hypertension. 1996;27:144–147. [DOI] [PubMed] [Google Scholar]
- 9. Preston RA, Baltodano NM, Cienki J, et al. Clinical presentation and management of patients with uncontrolled, severe hypertension: results from a public teaching hospital. J Hum Hypertens. 1999;13:249–255. [DOI] [PubMed] [Google Scholar]
- 10. Flanigan JS, Vitberg D. Hypertensive emergency and severe hypertension: what to treat, who to treat, and how to treat. Med Clin North Am. 2006;90:439–451. [DOI] [PubMed] [Google Scholar]
- 11. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic. The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 12. Effects of treatment on morbidity in hypertension . Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA. 1967;202:1028–1034. [PubMed] [Google Scholar]
- 13. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213:1143–1152. [PubMed] [Google Scholar]
- 14. Wolff FW, Lindeman RD. Effects of treatment in hypertension. Results of a controlled study. J Chronic Dis. 1966;19:227–240. [DOI] [PubMed] [Google Scholar]
- 15. Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. [DOI] [PubMed] [Google Scholar]
- 16. Lithell H, Hansson L, Skoog I, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double‐blind intervention trial. J Hypertens. 2003;21:875–886. [DOI] [PubMed] [Google Scholar]
- 17. Staessen JA, Thijisq L, Fagard R, et al. Effects of immediate versus delayed antihypertensive therapy on outcome in the Systolic Hypertension in Europe Trial. J Hypertens. 2004;22:847–857. [DOI] [PubMed] [Google Scholar]
- 18. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351:1755–1762. [DOI] [PubMed] [Google Scholar]
- 19. Heart Outcomes Prevention Evaluation Study Investigators . Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO‐HOPE substudy. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- 20. The Heart Outcomes Prevention Evaluation Study Investigators . Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. N Engl J Med. 2000;342:145–153. [DOI] [PubMed] [Google Scholar]
- 21. National Kidney Foundation . K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Kidney Disease Outcomes Quality Initiative (K/DOQI). Am J Kidney Dis. 2004;43(5 suppl 1):S1–S290. [PubMed] [Google Scholar]
- 22. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 23. Guidelines Committee . 2003 European Society of Hypertension‐European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–1053. [DOI] [PubMed] [Google Scholar]
- 24. Douglas JG, Bakris GL, Epstein M, et al. Management of high blood pressure in African Americans: consensus statement of the Hypertension in African Americans Working Group of the International Society on Hypertension in Blacks. Arch Intern Med. 2003;163:525–541. [DOI] [PubMed] [Google Scholar]
- 25. World Health Organization, International Society of Hypertension Writing Group . 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–1992. [DOI] [PubMed] [Google Scholar]
- 26. Standards of medical care in diabetes—2007. Diabetes Care. 2007;30(suppl 1):S4–S41. [DOI] [PubMed] [Google Scholar]
- 27. Wolf‐Maier K, Cooper RS, Kramer H, et al. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension. 2004;43:10–17. [DOI] [PubMed] [Google Scholar]
- 28. Neutel JM, Smith DH. Improving patient compliance: a major goal in the management of hypertension. J Clin Hypertens (Greenwich). 2003;5:127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huse DM, Roht LH, Alpert JS, et al. Physicians' knowledge, attitudes, and practice of pharmacologic treatment of hypertension. Ann Pharmacother. 2001;35:1173–1179. [DOI] [PubMed] [Google Scholar]
- 30. Oliveria SA, Lapuerta P, McCarthy BD, et al. Physician‐related barriers to the effective management of uncontrolled hypertension. Arch Intern Med. 2002;162:413–420. [DOI] [PubMed] [Google Scholar]
- 31. Martins D, Norris K. Hypertension treatment in African Americans: physiology is less important than sociology. Cleve Clin J Med. 2004;71:735–743. [DOI] [PubMed] [Google Scholar]
- 32. Materson BJ, Reda DJ, Cushman WC, et al. Single‐drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. N Engl J Med. 1993;328:914–921. [DOI] [PubMed] [Google Scholar]
- 33. Materson BJ, Reda DJ, Cushman WC, for the Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents . Department of Veterans Affairs single‐drug therapy of hypertension study. Revised figures and new data. Am J Hypertens. 1995;8:189–192. [DOI] [PubMed] [Google Scholar]
- 34. Moser M, Franklin SS. Hypertension management: results of a new national survey for the Hypertension Education Foundation: Harris Interactive. J Clin Hypertens (Greenwich). 2007;9:316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sica DA. Rationale for fixed‐dose combinations in the treatment of hypertension: the cycle repeats. Drugs. 2002;62:443–462. [DOI] [PubMed] [Google Scholar]
- 36. Gavras I, Rosenthal T. Combination therapy as first‐line treatment for hypertension. Curr Hypertens Rep. 2004;6:267–272. [DOI] [PubMed] [Google Scholar]
- 37. Neutel JM, Smith DH, Weber MA. Low‐dose combination therapy: an important first‐line treatment in the management of hypertension. Am J Hypertens. 2001;14:286–292. [DOI] [PubMed] [Google Scholar]
- 38. Prisant LM. Fixed low‐dose combination in first‐line treatment of hypertension. J Hypertens Suppl. 2002;20(suppl 1):S11–S19. [PubMed] [Google Scholar]
- 39. Skolnik NS, Beck JD, Clark M. Combination antihypertensive drugs: recommendations for use. Am Fam Physician. 2000;61:3049–3056. [PubMed] [Google Scholar]
- 40. Waeber B. Combination therapy with ACE inhibitors/angiotensin II receptor antagonists and diuretics in hypertension. Expert Rev Cardiovasc Ther. 2003;1:43–50. [DOI] [PubMed] [Google Scholar]
- 41. Kochar M, Guthrie R, Triscari J, et al. Matrix study of irbesartan with hydrochlorothiazide in mild‐to‐moderate hypertension. Am J Hypertens. 1999;12:797–805. [DOI] [PubMed] [Google Scholar]
- 42. McGill JB, Reilly PA. Telmisartan plus hydrochlorothiazide versus telmisartan or hydrochlorothiazide monotherapy in patients with mild to moderate hypertension: a multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group trial. Clin Ther. 2001;23:833–850. [DOI] [PubMed] [Google Scholar]
- 43. Karalliedde J, Viberti G. Evidence for renoprotection by blockade of the renin‐angiotensin‐aldosterone system in hypertension and diabetes. J Hum Hypertens. 2006;20:239–253. [DOI] [PubMed] [Google Scholar]
- 44. Izuhara Y, Nangaku M, Inagi R, et al. Renoprotective properties of angiotensin receptor blockers beyond blood pressure lowering. J Am Soc Nephrol. 2005;16:3631–3641. [DOI] [PubMed] [Google Scholar]
- 45. Ruggenenti P, Perna A, Remuzzi G. ACE inhibitors to prevent end‐stage renal disease: when to start and why possibly never to stop: a post hoc analysis of the REIN trial results. Ramipril Efficacy in Nephropathy. J Am Soc Nephrol. 2001;12:2832–2837. [DOI] [PubMed] [Google Scholar]
- 46. Tylicki L, Larczynski W, Rutkowski B. Renal protective effects of the renin‐angiotensin‐aldosterone system blockade: from evidence‐based approach to perspectives. Kidney Blood Press Res. 2005;28:230–242. [DOI] [PubMed] [Google Scholar]
- 47. Giunti S, Cooper M. Management strategies for patients with hypertension and diabetes: why combination therapy is critical. J Clin Hypertens (Greenwich). 2006;8:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005;28(suppl 1):S4–S36. [PubMed] [Google Scholar]
- 49. Neutel JM, Franklin SS, Oparil S, et al. Efficacy and safety of irbesartan/HCTZ combination therapy as initial treatment for rapid control of severe hypertension. J Clin Hypertens (Greenwich). 2006;8:850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Salerno CM, Demopoulos L, Mukherjee R, et al. Combination angiotensin receptor blocker/hydrochlorothiazide as initial therapy in the treatment of patients with severe hypertension. J Clin Hypertens (Greenwich). 2004;6:614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rump LC, Ambrosioni E, Burnier M, et al. Initial combination therapy with olmesartan/hydrochlorothiazide in moderate‐to‐severe hypertension. J Hum Hypertens. 2006;20:299–301. [DOI] [PubMed] [Google Scholar]
- 52. Lacourcière Y, Poirier L, Hebert D, et al. Antihypertensive efficacy and tolerability of two fixed‐dose combinations of valsartan and hydrochlorothiazide compared with valsartan monotherapy in patients with stage 2 or 3 systolic hypertension: an 8‐week, randomized, double‐blind, parallel‐group trial. Clin Ther. 2005;27:1013–1021. [DOI] [PubMed] [Google Scholar]
- 53. Lapuerta P, Ptaszynska A. Irbesartan/HCTZ as initial treatment in patients with moderate hypertension. Presented at: European Society of Hypertension/European Society of Cardiology; June 1216, 2006; Madrid, Spain. Poster 493. [Google Scholar]
- 54. Gradman AH, Brady WE, Gazdick LP, et al. A multicenter, randomized, double‐blind, placebo‐controlled, 8‐week trial of the efficacy and tolerability of once‐daily losartan 100 mg/hydrochlorothiazide 25 mg and losartan 50 mg/hydrochlorothiazide 12.5 mg in the treatment of moderate‐to‐severe essential hypertension. Clin Ther. 2002;24:1049–1061. [DOI] [PubMed] [Google Scholar]
- 55. Lenz T, Schulte KL, Wagner B, et al. Quinapril, hydrochlorothiazide, and combination in patients with moderate to severe hypertension. Eur Heart J. 1994;15:940–946. [DOI] [PubMed] [Google Scholar]
- 56. Cushman WC, Cohen JD, Jones RP, et al. Comparison of the fixed combination of enalapril/diltiazem ER and their monotherapies in stage 1 to 3 essential hypertension. Am J Hypertens. 1998;11:23–30. [DOI] [PubMed] [Google Scholar]
- 57. Bristol‐Myers Squibb Sanofi‐Synthelabo Partnership Avalide Web site. http://www.avalide.com. Accessed September 22, 2006.
- 58. Rosenstock J, Rossi L, Lin CS, et al. The effects of irbesartan added to hydrochlorothiazide for the treatment of hypertension in patients non‐responsive to hydrochlorothiazide alone. J Clin Pharm Ther. 1998;23:433–440. [DOI] [PubMed] [Google Scholar]
- 59. Howe P, Phillips P, Saini R, et al. The antihypertensive efficacy of the combination of irbesartan and hydrochlorothiazide assessed by 24‐hour ambulatory blood pressure monitoring. Irbesartan Multicenter Study Group . Clin Exp Hypertens. 1999;21:1373–1396. [DOI] [PubMed] [Google Scholar]
- 60. Raskin P, Guthrie R, Flack J, et al. The long‐term antihypertensive activity and tolerability of irbesartan with hydrochlorothiazide. J Hum Hypertens. 1999;13:683–687. [DOI] [PubMed] [Google Scholar]
- 61. Franklin SS, Bhaumik A, Ptaszynska A. Early reduction of exposure to severe blood pressure levels with irbesartan/HCTZ as a first‐line treatment of severe hypertension. Presented at: European Society of Hypertension/European Society of Cardiology; June 1216, 2006; Madrid, Spain. Poster 492. [Google Scholar]


