Abstract
This post‐hoc analysis of the Treating to New Targets (TNT) study evaluated the joint effects of managing low‐density lipoprotein cholesterol (LDL‐C) and systolic blood pressure (SBP) on cardiovascular outcomes. Patients (N=9739) with clinically evident, stable coronary heart disease (CHD) were randomized to atorvastatin 10 or 80 mg/d. The primary end point was occurrence of a first major cardiovascular event. At 3 months' follow‐up, patients were stratified according to SBP (<140 mm Hg vs ≥140 mm Hg) and tertiles of LDL‐C. At 4.9 years' median follow‐up, the rate of major cardiovascular events was reduced most in patients with lower LDL‐C (P<.001) and in patients with SBP <140 mm Hg (P=.014). A 42% relative risk reduction was observed for patients in the lowest LDL‐C tertile with an SBP <140 mm Hg, compared with patients in the highest LDL‐C tertile with an SBP ≥140 mm Hg. The effect of lower SBP on stroke was most pronounced in the lowest LDL‐C tertile.
The association between serum cholesterol and blood pressure (BP) with cardiovascular disease (CVD) is widely acknowledged, 1 , 2 and effective treatment of elevated low‐density lipoprotein cholesterol (LDL‐C) and BP levels reduces the morbidity and mortality associated with CVD. 3 , 4 , 5 In addition, the positive correlation between serum cholesterol and BP with CVD is noted over a wide range of values 2 ; even modest reductions in LDL‐C and/or BP can bring about significant reductions in CVD outcomes. 6
Controlled trials have demonstrated the benefits of statin use in lowering LDL‐C and preventing CVD events. 3 , 7 , 8 , 9 Recent studies have shown that more intensive cholesterol lowering results in better angiographic and clinical outcomes in high‐risk patients. 10 , 11 , 12 , 13 , 14 , 15 , 16 Consequently, US 17 and European 18 treatment guidelines now recommend an LDL‐C treatment goal of <100 mg/dL (2.6 mmol/L) for patients at high risk for coronary heart disease (CHD) (as well as for those with CHD). It has been suggested that in patients at very high risk for CHD, an even more aggressive target of <70 mg/dL (1.8 mmol/L) should be adopted. 19
However, even intensive LDL‐C lowering does not eliminate cardiovascular events; epidemiologic studies have demonstrated that outcomes are determined by individual cardiovascular risk factors and the interactions between them. 2 , 20 Hypertension frequently coexists with dyslipidemia, 21 , 22 and patients with both conditions are at greater risk for cardiovascular events than those with either condition alone. 2 , 23
Clinical trials have demonstrated the potential benefits of simultaneously controlling multiple cardiovascular risk factors. 24 , 25 In one study, which followed 686 middle‐aged men with treated hypertension for up to 12 years, a combined reduction in BP and cholesterol resulted in a marked decrease in CVD or CHD in comparison with lowering either systolic blood pressure (SBP) or cholesterol alone. 24 cases in which cholesterol was not reduced, the benefits of reduction, as assessed by comparing the CHD and CVD events between quartiles of in‐study SBP, were modest. 24 Indeed, treatment guidelines now recommend an integrated approach to reducing overall cardiovascular risk by managing both elevated BP and dyslipidemia, as well as other risk factors, using a combination of therapeutic lifestyle changes and medications. 17 , 18
The Treating to New Targets (TNT) study evaluated whether intensive statin treatment improves cardiovascular outcomes in comparison with moderate statin therapy in patients at high risk for CVD and/or with stable CHD. In the intensive treatment arm, LDL‐C lowering to an average of 77 mg/dL (2.0 mmol/L) was associated with a 22% relative reduction and an absolute reduction of 2.2% in risk of major cardiovascular events compared with the less intensive treatment, which achieved an average LDL‐C of 101 mg/dL (2.6 mmol/L). 11
The purpose of this post‐hoc analysis of the TNT study was to assess whether cardiovascular outcomes varied across differing LDL‐C levels and between normal or high SBP during follow‐up, as well as whether the best outcomes occurred among patients with lower SBP and LDL‐C.
METHODS
The design, protocol, end points, and results of the TNT study have been published previously. 11 , 26 In brief, men and women aged 35 to 75 years with stable CHD (ie, persons at high risk) received open‐label atorvastatin 10 mg/d for 8 weeks. After this run‐in period, 10,001 patients in whom an LDL‐C <130 mg/dL (3.4 mmol/L) was reached with atorvastatin 10 mg/d were randomized to double‐blind therapy with atorvastatin, 10 or 80 mg/d. Patients were followed for a median of 4.9 years. The primary end point was time to the first occurrence of a major cardiovascular event, defined as CHD death, nonfatal non‐procedure‐related myocardial infarction (MI), resuscitated cardiac arrest, and fatal or nonfatal stroke. 11
For this analysis, we stratified the total patient cohort into tertiles based on on‐treatment LDL‐C levels (≤73 mg/dL, 74–94 mg/dL, ≥95 mg/dL) and into 2 strata according to SBP (<140 mm Hg vs ≥140 mm Hg), both measured after 3 months of the double‐blind period (Figure 1). Clinical outcomes and safety data were compared between the 2 SBP strata and across tertiles of LDL‐C at 3 months. Two components of the primary end point were analyzed separately as secondary end points: CHD death and nonfatal non‐procedure‐related MI, and fatal or nonfatal stroke. Mean SBP levels were relatively constant throughout the follow‐up period. Patients continued with their antihypertensive treatments (including β‐blockers, angiotensin‐converting enzyme inhibitors, antiplatelet agents, angiotensin receptor blockers, aspirin, calcium channel blockers, and diuretics) throughout the treatment period.
Figure 1.

Study design. aOnly patients who had both an LDL‐C and SBP measurement at 3 months were included in this post‐hoc study. CHD indicates coronary heart disease; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; CV, cardiovascular; MI, myocardial infarction.
Statistical Analyses
Statistical analyses were performed with patients who received at least 1 dose of the study drug and for whom SBP and LDL‐C measurements were available at 3 months of follow‐up (n=9739). The impact of reducing LDL‐C and/or SBP on the occurrence of end points were assessed by univariate, bivariate, and multivariate analyses using Cox proportional hazard (PH) models. Based on the model, the estimated hazard ratios and the corresponding 95% confidence intervals were reported.
The unadjusted and adjusted effects of LDL‐C and/or SBP at 3 months were evaluated by univariate and bivariate Cox PH models, respectively. For the univariate analysis, either the LDL‐C level alone or SBP the value alone was included as the only term in the model. The adjusted effects of LDL‐C and of SBP, controlling for one or the other, were assessed by a bivariate Cox PH analysis including both terms in the model. In both the univariate and the bivariate analyses, LDL‐C was included in the model either as a continuous variable (ie, a 1‐mg/dL increase) or a categorical variable (tertile 1, ≤73 mg/dL; tertile 2, 74–94 mg/dL; tertile 3, ≥95 mg/dL); SBP was included as either a continuous variable (per 1‐mm Hg increase) or a categorical variable (<140 or ≥140 mm Hg).
For each end point studied, the adjusted effects of LDL‐C and SBP at 3 months of double‐blind treatment were assessed by multivariate analysis, controlling for those covariates found to be significant predictors in the Cox PH model. Both LDL‐C and SBP were included in the model as continuous or categorical variables. The following 3 categories of covariates were assessed:
-
1
Baseline LDL‐C and SBP (both on continuous scales).
-
2
Demographics: age, sex, race, past smoking status, weight, and body mass index (BMI).
-
3
Comorbidities: MI, coronary artery bypass graft, coronary angioplasty, cerebrovascular accident, angina, peripheral vascular disease, hypertension, arrhythmia, congestive heart failure, and diabetes.
Only those covariates found to be significant in both univariate and multivariate analyses were included in the final Cox PH model. The interactions between LDL‐C and SBP at 3 months of double‐blind treatment were assessed in both bivariate and multivariate analyses.
For all analyses, 2‐sided P values of <.05 were regarded as significant. All analyses were performed using SAS statistical software (version 8.12, SAS Institute Inc, Cary, NC).
RESULTS
Patient Population
Of the 10,001 patients randomized, 9739 patients (97.4%) had both LDL‐C and SBP measured at 3 months and were eligible for inclusion in this post‐hoc analysis (Table I).
Table I.
Baseline Characteristics of Randomized Patients by LDL‐C/SBP Subsets
| Baseline characteristic | LDL‐C Tertile 1 (≤73 mg/dL) (n=3321) (2854/467)a | LDL‐C Tertile 2 (74–94 mg/dL) (n=3142) (1461/1681)a | LDL‐C Tertile 3 (≥95 mg/dL) (n=3276) (546/2730)a | |||
|---|---|---|---|---|---|---|
| SBP <140 mm Hg (n=2325) | SBP ≥140 mm Hg (n=996) | SBP <140 mm Hg (n=2153) | SBP ≥140 mm Hg (n=989) | SBP <140 mm Hg (n=2134) | SBP ≥140 mm Hg (n=1142) | |
| Age, y | 60.6±8.8 | 64.0±7.9 | 59.9±8.9 | 63.9±8.1 | 58.9±9.0 | 62.6±8.1 |
| Men | 1922 (82.7) | 795 (79.8) | 1773 (82.3) | 752 (76.0) | 1745 (81.8) | 900 (78.8) |
| White | 2211 (95.1) | 927 (93.1) | 2032 (94.4) | 931 (94.1) | 1994 (93.4) | 1070 (93.7) |
| SBP, mm Hg | 125.3±14.6 | 142.1±16.4 | 125.2±13.9 | 142.3±15.5 | 125.3±14.0 | 142.0±16.3 |
| DBP, mm Hg | 75.8±8.8 | 81.4±9.2 | 76.3±8.9 | 81.3±9.5 | 76.6±9.1 | 81.9±9.9 |
| Body mass index, kg/m2 | 28.0±4.2 | 28.7±4.4 | 28.4±4.5 | 29.0±4.5 | 28.6±4.6 | 29.1±5.2 |
| Taking antihypertensive medications | 2229 (95.9) | 957 (96.1) | 2041 (94.8) | 943 (95.3) | 2041 (95.6) | 1095 (95.9) |
| Current smoker | 318 (13.7) | 98 (9.8) | 273 (12.7) | 95 (9.6) | 360 (16.9) | 156 (13.7) |
| Ex‐smoker | 1439 (61.9) | 659 (66.2) | 1405 (65.3) | 630 (63.7) | 1315 (61.6) | 709 (62.1) |
| Systemic hypertension | 1084 (46.6) | 697 (70.0) | 980 (45.5) | 695 (70.3) | 1018 (47.7) | 803 (70.3) |
| History of diabetes mellitus | 321 (13.8) | 219 (22.0) | 277 (12.9) | 175 (17.7) | 267 (12.5) | 203 (17.8) |
| Metabolic syndrome | 1200 (51.6) | 644 (64.7) | 1111 (51.6) | 626 (63.3) | 1146 (53.7) | 717 (62.8) |
| Myocardial infarction | 1428 (61.4) | 523 (52.5) | 1306 (60.7) | 526 (53.2) | 1278 (59.9) | 623 (54.5) |
| Cardiovascular history | ||||||
| Angina | 1886 (81.1) | 839 (84.2) | 1747 (81.1) | 814 (82.3) | 1735 (81.3) | 915 (80.1) |
| Cerebrovascular accident | 98 (4.2) | 51 (5.1) | 105 (4.9) | 63 (6.4) | 102 (4.8) | 84 (7.4) |
| Peripheral vascular disease | 223 (9.6) | 136 (13.6) | 242 (11.2) | 138 (13.9) | 218 (10.2) | 181 (15.8) |
| Congestive heart failure | 158 (6.8) | 54 (5.4) | 182 (8.4) | 88 (8.9) | 162 (7.6) | 105 (9.2) |
| Arrhythmia | 431 (18.5) | 188 (18.9) | 396 (18.4) | 190 (19.2) | 365 (17.1) | 213 (18.6) |
| Coronary revascularization | 1992 (85.7) | 855 (85.8) | 1874 (87.0) | 869 (87.9) | 1814 (85.0) | 986 (86.3) |
| Coronary angioplasty | 1299 (55.9) | 524 (52.6) | 1200 (55.7) | 532 (53.8) | 1178 (55.2) | 540 (47.3) |
| Coronary bypass | 1010 (43.4) | 474 (47.6) | 975 (45.3) | 503 (50.9) | 965 (45.2) | 608 (53.2) |
| Mean lipid levels, mg/dL | ||||||
| LDL‐C | 88.0±15.0 | 87.3±15.0 | 96.7±15.9 | 96.9±15.3 | 108.0±15.8 | 107.7±15.5 |
| Total cholesterol | 164.0±21.6 | 165.7±22.2 | 173.2±22.1 | 174.5±22.5 | 185.7±22.3 | 186.5±22.3 |
| Triglycerides | 146.8±69.7 | 151.8±73.4 | 149.3±69.4 | 145.3±63.2 | 154.9±70.6 | 154.9±77.8 |
| HDL‐C | 46.8±10.7 | 48.2±11.6 | 46.8±10.6 | 48.6±11.4 | 46.9±10.6 | 48.2±11.5 |
| Abbreviations: DBP, diastolic blood pressure; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure. Values are mean ± SD or No. (%). aNumber of patients: atorvastatin 80 mg/atorvastatin 10 mg. | ||||||
Overall, the mean age of the study population was 61.0 years, the mean SBP/diastolic BP at baseline was 130.7/78.0 mm Hg, and the mean LDL‐C at baseline was 97.5 mg/dL (2.5 mmol/L). At month 3, mean SBP was 121.6 mm Hg in the <140 mm Hg group and 149.7 mm Hg in the ≥140 mm Hg group. There was no difference in the proportion of patients receiving antihypertensive medication between the 2 SBP groups. Overall, >95% of patients were receiving antihypertensive drug therapy (Table I). Mean LDL‐C values for each tertile were as follows: 60.5 mg/dL (1.6 mmol/L) for tertile 1, 83.9 mg/dL (2.2 mmol/L) for tertile 2, and 113.2 mg/dL (2.9 mmol/L) for tertile 3. Patients in LDL‐C tertile 1 with an SBP <140 mm Hg had the lowest BMI, and that tertile had the lowest proportion of patients with peripheral vascular disease (Table I). Across all LDL‐C tertiles, as expected, patients whose SBP was ≥140 mm Hg were more likely to have a history of hypertension, diabetes, or peripheral vascular disease and to have had a cerebrovascular accident than those with SBP <140 mm Hg. For all LDL‐C tertiles, patients with SBP ≥140 mm Hg were older than those with SBP <140 mm Hg. Mean lipid levels within each LDL‐C tertile were similar in patients with SBP <140 mm Hg in comparison with those with SBP ≥140 mm Hg.
Efficacy Outcomes Primary Efficacy Assessment: Major Cardiovascular Events
After a median 4.9 years of follow‐up, the benefits of LDL‐C lowering on the primary outcome of major cardiovascular events were evident in patients whose SBP was above or below 140 mm Hg. The effect was most pronounced in patients in the lowest LDL‐C tertile (tertile 1), with 7.8% of patients experiencing a cardiovascular event compared with 9.6% and 11.6% of patients in tertiles 2 and 3, respectively (P<.001; absolute difference of 1.8% and 3.8%, respectively). The rate of cardiovascular events was lower in each LDL‐C tertile for patients with SBP <140 mm Hg compared with ≥140 mm Hg at month 3 (Figure 2). SBP <140 mm Hg at month 3 was predictive of additional reduction in cardiovascular event rates (both <140 mm Hg vs ≥140 mm Hg; P=.014, and per 1‐mm Hg increase, P=.007), independent of the effects of lower on‐treatment LDL‐C. Among patients who had the most optimally controlled LDL‐C and SBP (LDL‐C tertile 1 and SBP <140 mm Hg), 7.1% experienced a major cardiovascular event compared with 12.3% of patients who had the least controlled LDL‐C and SBP (LDL‐C tertile 3 and SBP ≥140 mm Hg; an absolute difference of 5.2%). Hence, compared with patients in the highest LDL‐C tertile with an SBP ≥140 mm Hg, 42% of major cardiovascular events were prevented with optimal control of both SBP and LDL‐C. Bivariate analysis showed there was no evidence of significant interaction (P=.46) between LDL‐C and SBP at 3 months in their association with major cardiovascular events. Lower SBP levels (Figure 2) accounted for decreases of 2.2%, 1.1%, and 1.2% in LDL‐C tertiles 1, 2, and 3, respectively.
Figure 2.
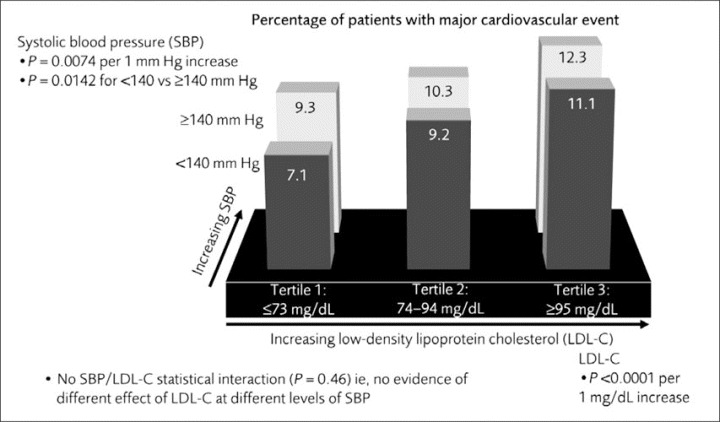
Rate of major cardiovascular events (primary end point) by low‐density lipoprotein cholesterol (LDL‐C) tertiles and systolic blood pressure (SBP) <140 mm Hg or ≥140 mm Hg at month 3.
Secondary Efficacy Assessments: Components of the Primary End Point
Analysis of individual components of the primary end point demonstrated similar results. Overall across both SBP categories, 5.6% of patients in LDL‐C tertile 1 experienced CHD death or nonfatal, non‐procedure‐related MI compared with 6.9% and 9.0% of patients whose LDL‐C levels fell with‐in LDL‐C tertiles 2 and 3, respectively. There was a significant relationship between LDL‐C (both as a continuous or categorical variable) and CHD death and nonfatal, non‐procedure‐related MI (P<.001). In all 3 LDL‐C tertiles, the event rate was lower in patients with <140 mm Hg compared with ≥140 mm Hg (Figure 3). However, SBP was not predictive of additional reduction of this end point (P=.195) above the effect of lower LDL‐C.
Figure 3.
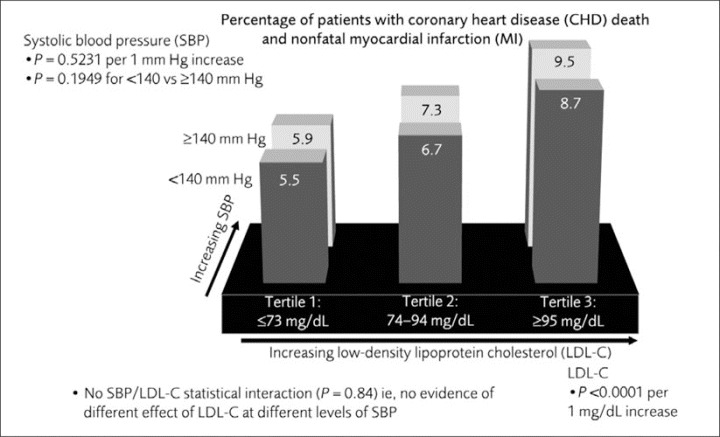
Rate of coronary heart disease death and nonfatal, non‐procedure‐related myocardial infarction (MI) by low‐density lipoprotein cholesterol (LDL‐C) tertiles and systolic blood pressure (SBP) <140 mm Hg or ≥140 mm Hg at month 3.
The pattern of fatal or nonfatal stroke was similar. LDL‐C lowering was associated with a continuous and significant reduction (P=.040), although the effect did not reach statistical significance when LDL‐C was assessed as tertiles (P=.073). The correlation between SBP <140 mm Hg and reduced rate of stroke was most pronounced in the lowest LDL‐C tertile (Figure 4). Of importance, SBP (both <140 mm Hg vs ≥140 mm Hg and per 1 mm Hg) was predictive of additional significant reduction in stroke (P=.001 and P<.001, respectively) independent of the effect of lower on‐treatment LDL‐C. Although there was no statistically significant interaction, the effect of lower LDL‐C was pronounced among patients with SBP <140 mm Hg but not evident among those with SBP ≥140 mm Hg.
Figure 4.
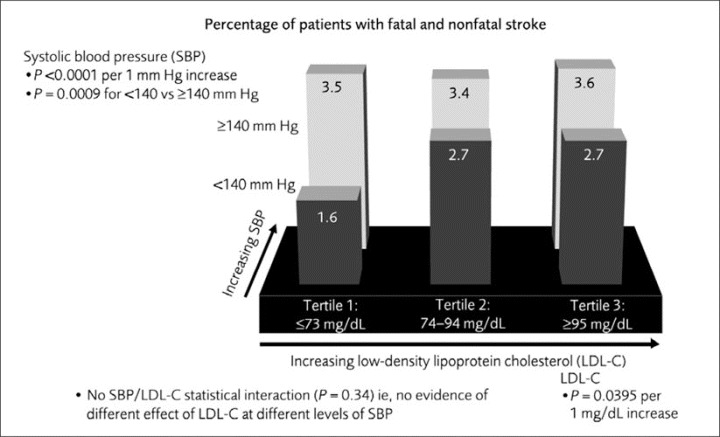
Rate of fatal and nonfatal stroke by low‐density lipoprotein cholesterol (LDL‐C) tertiles and systolic blood pressure (SBP) <140 mm Hg or ≥140 mm Hg at month 3.
While there was no statistically significant trend for mortality across subsets, mortality due to any cause and due to noncardiovascular causes was, as expected, numerically lowest among patients with the lowest on‐treatment SBP and LDL‐C levels. All‐cause mortality rates for LDL‐C tertiles 1, 2, and 3 were 4.5%, 5.2%, and 5.6%, respectively, compared with 6.2%, 6.5%, and 5.9% for patients with SBP <140 mm Hg and ≥140 mm Hg, respectively. Noncardiovascular mortality rates by LDL‐C tertiles 1, 2, and 3 were 2.3%, 2.6%, and 2.9%, respectively, compared with 3.4%, 3.3%,and 2.9% for patients with SBP <140 mm Hg and ≥140 mm Hg, respectively. Cardiovascular death rates were also lower with lower levels of on‐treatment BP and LDL‐C. Cardiovascular mortality rates by LDL‐C tertiles 1, 2, and 3 were 2.1%,2.5%, and 2.7%, respectively, vs 2.8%, 3.1%, and 3.0% for patients with SBP <140 mm Hg and ≥140 mm Hg, respectively. There were no significant differences among the subsets for all‐cause mortality, noncardiovascular mortality, or cardiovascular deaths. Analysis of suicide, cancer deaths, and hemorrhagic stroke demonstrated no significant trend across LDL‐C tertiles or SBP cohorts.
Multivariate Analysis
Multivariate analysis adjusting for significant demographic variables and comorbidities identified low LDL‐C and low SBP as independent predictors of improved cardiovascular outcomes. LDL‐C per 1‐mg/dL increase was a strong predictor of worse outcomes for the occurrence of major cardiovascular events (P<.001) and for the tested components of this end point (P<.001 for CHD death or nonfatal, non‐procedure‐related MI and P=.052 for fatal or nonfatal stroke). Elevated SBP (per 1 mm Hg increase) was predictive of a worse outcome for the occurrence of fatal and nonfatal stroke (P=.015).
When baseline LDL‐C and SBP were included as covariates in the categorical analyses, LDL‐C tertile had a statistically significant impact on major cardiovascular events (P<.001 for overall tertile effect). When individual components of the end point were assessed, LDL‐C tertile was found to be a significant predictor of CHD death and nonfatal, non‐procedure‐related MI (P<.001 for overall tertile effect) but did not have a significant impact on the occurrence of fatal and nonfatal stroke (P=.120). After adjusting for baseline LDL‐C and SBP, SBP category (<140 mm Hg vs ≥140 mm Hg) did not significantly affect the primary end point or any of the tested components of the end point.
Safety and Tolerability
Overall, 95.7% of patients reported an adverse event (AE); treatment‐associated AEs were present in 31.1% of patients. There were no patterns or consistent differences in treatment‐associated AE rates among the 6 LDL‐C/SBP subsets (Table II). The rate of withdrawals due to AEs associated with treatment was also similar across these subsets. There was no apparent association between the incidence of muscle‐related adverse effects (eg, myalgia) and LDL‐C concentration (by tertile) or SBP category. Only 1 persistent elevation in creatine kinase (defined as 2 consecutive measurements obtained 4 to 10 days apart that were ≥10 times the upper limit of the normal range) was reported. None of the 5 cases of rhabdomyolysis were attributed by the investigators to the study drug (other clinical information about these cases has been reported previously 11 ). Persistent elevations of liver enzymes were infrequent but slightly more prevalent at the lowest LDL‐C levels. For example, persistent increases in alanine aminotransferase and aspartate aminotransferase were reported in 0.9% of patients in LDL‐C tertile 1, 0.86% in tertile 2, and 0.46% in tertile 3.
Table II.
Patients With Adverse Events in Each of the 6 LDL‐C/SBP Subsets
| LDL‐C Tertile 1 (≤73 mg/dL) (n=3321) (2854/467)a | LDL‐C Tertile 2 (74–94 mg/dL) (n=3142) (1461/1681)a | LDL‐C Tertile 3 (≥95 mg/dL) (n=3276) (546/2730)a | ||||
|---|---|---|---|---|---|---|
| SBP <140 mm Hg (n=2325) | SBP ≥140 mm Hg (n=996) | SBP <140 mm Hg (n=2153) | SBP ≥140 mm Hg (n=989) | SBP <140 mm Hg (n=2134) | SBP ≥140 mm Hg (n=1142) | |
| Patients with adverse events, % | ||||||
| All‐cause | 95.7 | 97.1 | 94.7 | 97.2 | 95.0 | 96.4 |
| Treatment‐associated | 30.9 | 31.8 | 31.0 | 29.9 | 31.2 | 32.3 |
| Withdrawals due to adverse events, % | ||||||
| All | 8.4 | 9.7 | 7.4 | 7.8 | 9.5 | 9.9 |
| Treatment‐associated | 6.2 | 6.6 | 5.1 | 4.2 | 6.5 | 6.7 |
| Treatment‐associated myalgia | 4.5 | 5.0 | 4.7 | 4.1 | 5.1 | 4.9 |
| Persistentb CK >10× ULN | 0.0 | 0.0 | 0.0 | 0.0 | 0.05 | 0.0 |
| Persistentb ALT/AST >3× ULN | 1.2 | 0.20 | 1.0 | 0.61 | 0.47 | 0.44 |
| Abbreviations: ALT, alanine transaminase; AST, aspartate aminotransferase; CK, creatine kinase; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; ULN, upper limit of normal. aNumber of patients: atorvastatin 80 mg/atorvastatin 10 mg. bOccurring twice with 4–10 days. | ||||||
DISCUSSION
This post‐hoc analysis of the TNT study highlights that the best cardiovascular outcomes occur in high‐risk patients with the lowest LDL‐C and SBP. In this study, atorvastatin was well tolerated across all 3 LDL‐C tertiles and both SBP categories. An SBP <140 mm Hg at month 3 was predictive of additional significant reduction in the occurrence of major cardiovascular events, especially fatal and nonfatal stroke, independent of the effect of lower on‐treatment LDL‐C. Compared with patients in the highest LDL‐C tertile with SBP ≥140 mm Hg, 42% of major cardiovascular events were prevented in patients with the most optimally controlled LDL‐C and SBP levels.
This reflects an absolute benefit of 5.2% in the study's primary end point between patients with LDL‐C ≤73 mg/dL and SBP <140 mm Hg in comparison with those with LDL‐C ≥95 mg/dL and SBP ≥140 mm Hg. This is interesting considering that SBP was reasonably well controlled overall among this post‐hoc study population (baseline mean BP, 130.7/78.0 mm Hg).
The 2% decrease in the rate of major cardiovascular events between LDL‐C tertiles 3 (LDL‐C ≥95 mg/dL) and 2 (LDL‐C 74–94 mg/dL) and the 1.8% decrease between LDL‐C tertiles 2 (LDL‐C 74–94 mg/dL) and 1 (LDL‐C ≤73 mg/dL) demonstrates that over the range of LDL‐C values studied here there is no lower limit for the benefit of LDL‐C reduction on major cardiovascular events. This is broadly consistent with a recent meta‐analysis of statin trials that showed that for every 39‐mg/dL (1‐mmol/L) reduction in LDL‐C, there was a 1.2% absolute reduction in all‐cause mortality, a 1.0% absolute reduction in CHD mortality, a 2.4% absolute reduction in any major coronary event, and a 0.7% absolute reduction in fatal or nonfatal stroke, irrespective of the levels of LDL‐C at baseline. 3 Hence, even small reductions in LDL‐C may be of significant benefit regardless of the initial LDL‐C concentration in high‐risk patients. In the TNT study, a stepwise lowering of the rate of major cardiovascular events, accompanied by good tolerability, was observed across LDL‐C quintiles at 3 months of therapy. 14 This is consistent with observational studies that demonstrate a continuous graded relationship between serum cholesterol and the long‐term risk of CHD. 2 , 23 , 27
Atorvastatin 10 mg/d has been shown to decrease regional arterial stiffness in patients with diabetes. 28 Atorvastatin 80 mg/d has also been reported to improve systemic arterial compliance in persons with isolated systolic hypertension. 29 A beneficial effect of high‐dose atorvastatin on reducing arterial stiffness could potentially have contributed, in the present study, to the reduction in cardiovascular outcomes, particularly in patients with high SBP (≥140 mm Hg) at baseline.
The decrease in the rate of major cardiovascular events across all LDL‐C tertiles combined from 10.7%, with SBP ≥140 mm Hg to 9.1%, with SBP <140 mm Hg, at 3 months is consistent with recent trials and meta‐analyses, 5 , 30 which collectively demonstrate that relatively small differences in BP can lead to significant differences in the occurrence of cardiovascular events over a relatively short time period. 5 , 30 In the Valsartan Antihypertensive Long‐Term Use Evaluation (VALUE) trial, 30 amlodipine‐based treatment reduced mean SBP by 3.8 mm Hg more than valsartan‐based treatment over the first 3 months of the study; this may have accounted for a reduction of 2.4 strokes and 3.5 deaths per 1000 patients.
The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC) states that the risk of CVD begins at 115/75 mm Hg and doubles with each increment of 20/10 mm Hg. 4 Thus, at 135/85 mm Hg, a level now termed prehypertension, the risk is higher than at 115/75 mm Hg. Although a BP of 140/90 mm Hg is often used as the cutoff between normal and elevated BP, it is important to recognize that the risk of CVD associated with BP, like LDL‐C levels, is a continuum.
The impact of a 1‐mg/dL increase in LDL‐C on fatal or nonfatal stroke, although significant (P=.040; Figure 4), was not as strong as the effect of LDL‐C per 1 mg/dL on major cardiovascular events (Figure 2). Similarly, the impact of such LDL‐C increases on stroke was not as strong as that of a 1‐mm Hg increase in SBP (Figure 4). In fact, when BP levels were >140 mm Hg, there was no difference in event rates for stroke between LDL‐C tertiles (Figure 4), emphasizing the profound importance of reducing BP levels for stroke prevention. These observations were confirmed by the adjusted multivariate analysis.
The findings of this post‐hoc analysis indicate that combined, intensive treatment of BP and LDL‐C is important for optimizing the management of patients with stable CHD. The concomitant use of antidiabetic and antihypertensive medications did not influence the reduction in cardiovascular events found by optimizing SBP and LDL‐C. These observations are also likely to be applicable to patients with all forms of CVD. This message is echoed in a study by Wong and colleagues 31 that estimated the potential effect of optimizing lipids (LDL‐C to <100 mg/dL and high‐density lipoprotein cholesterol to ≥60 mg/dL) and BP (to <120/80 mm Hg) on the prevention of CHD events in patients with the metabolic syndrome. The analysis calculated that if LDL‐C was controlled to within recommended levels, only a modest decrease in the incidence of CHD events would be observed. However, with optimal control of lipids and BP, >80% of CHD events might theoretically be prevented. This is important considering that a recent observational study demonstrated that <10% of patients with hypertension and dyslipidemia are at the currently recommended therapeutic targets for BP and LDL‐C. 22 Recent evidence suggests that a therapy targeting elevated LDL‐C and BP simultaneously may be an effective means of addressing the cotreatment of these 2 cardiovascular risk factors. 32
CONCLUSIONS
On‐treatment LDL‐C and SBP have a significant predictive relationship with the risk of major cardiovascular events in patients with CHD, with the lowest incidence of events being seen among patients with the lowest LDL‐C (≤73 mg/dL) and controlled SBP (<140 mm Hg). Physicians should be aware that intensive management of both LDL‐C and BP is important in patients with stable CHD to reduce their overall risk of developing future major cardiovascular events.
Acknowledgments and disclosures:
This post‐hoc analysis was proposed by the authors of the paper. This study was funded by Pfizer Inc. Pfizer Inc. was involved in the design and conduct of the study; the collection, analysis, and interpretation of the data; and the preparation of the manuscript. The authors would like to acknowledge the assistance of Lisa Tarasenko and JoAnn B. Trainer (employees of Pfizer Inc), and Jon Edwards of Envision Pharma (a medical writer funded by Pfizer Inc), for their contributions to the development of this paper. Dr Kostis has received grant support from the National Institutes of Health, Pfizer Inc, Boehringer Ingelheim, and Kos Pharmaceuticals; he has served as consultant to Pfizer Inc, Daiichi‐Sankyo, Schering‐Plough, Merck, and Taisho and is on the Speakers' Bureau of Pfizer Inc, Sanofi‐Aventis, Bristol‐Myers Squibb, Merck, AstraZeneca, and Daiichi‐Sankyo. Dr Breazna is an employee of Pfizer Inc, and holds stock options in Pfizer Inc. Dr Deedwania has received honoraria for speaking engagements and consulting fees from Pfizer Inc, and AstraZeneca. Dr LaRosa has received honoraria for speaking engagements and consulting fees from Pfizer Inc, and Bayer.
References
- 1. The Pooling Project Research Group . Relationship of blood pressure, serum cholesterol, smoking habit, relative weight and ECG abnormalities to incidence of major coronary events: final report of the pooling project. J Chronic Dis. 1978;31:201–306. [DOI] [PubMed] [Google Scholar]
- 2. Neaton JD, Wentworth D. Serum cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease. Overall findings and differences by age for 316,099 white men. Multiple risk factor intervention trial research Group . Arch Intern Med. 1992;152:56–64. [PubMed] [Google Scholar]
- 3. Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 4. The seventh report of the Joint National Committee on prevention, Detection, evaluation, and treatment of high Blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 5. Turnbull F. Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: results of pro‐spectively‐designed overviews of randomised trials. Lancet. 2003;362:1527–1535. [DOI] [PubMed] [Google Scholar]
- 6. Emberson J, Whincup P, Morris R, et al. Evaluating the impact of population and high‐risk strategies for the primary prevention of cardiovascular disease. Eur Heart J. 2004;25:484–491. [DOI] [PubMed] [Google Scholar]
- 7. The Scandinavian Simvastatin Survival Study Group . Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 8. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and recurrent events trial investigators . N Engl J Med. 1996;335:1001–1009. [DOI] [PubMed] [Google Scholar]
- 9. The Long‐term intervention with pravastatin in ischaemic Disease (LIPID) Study Group . Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. [DOI] [PubMed] [Google Scholar]
- 10. De Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316. [DOI] [PubMed] [Google Scholar]
- 11. Larosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. [DOI] [PubMed] [Google Scholar]
- 12. Pedersen TR, Faergeman O, Kastelein JJ, et al. High‐dose atorvastatin vs usual‐dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–2445. [DOI] [PubMed] [Google Scholar]
- 13. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. [DOI] [PubMed] [Google Scholar]
- 14. Larosa JC, Grundy SM, Kastelein JJ, et al; TNT steering Committee and investigators . Safety and efficacy of atorvastatin‐induced very low‐density lipoprotein cholesterol levels in patients with coronary heart disease (a post hoc analysis of the treating to New targets [TNT] study). Am J Cardiol. 2007;100:747–752. [DOI] [PubMed] [Google Scholar]
- 15. Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid‐lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–1080. [DOI] [PubMed] [Google Scholar]
- 16. Wiviott SD, Cannon CP, Morrow DA, et al. Can low‐density lipoprotein be too low? The safety and efficacy of achieving very low low‐density lipoprotein with intensive statin therapy: a PROVE IT‐TIMI 22 substudy. J Am Coll Cardiol. 2005;46:1411–1416. [DOI] [PubMed] [Google Scholar]
- 17. Executive summary of the third report of the National Cholesterol education program (NCEP) expert panel on Detection, evaluation, and treatment of high Blood Cholesterol in Adults (Adult treatment panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 18. De Backer G, Ambrosioni E, Borch‐Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint task force of european and Other societies on Cardiovascular Disease prevention in Clinical practice . Eur Heart J. 2003;24:1601–1610. [DOI] [PubMed] [Google Scholar]
- 19. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol education program Adult treatment panel iii guidelines. Circulation. 2004;110:227–239. [DOI] [PubMed] [Google Scholar]
- 20. Jackson R, Lawes CM, Bennett DA, et al. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual's absolute cardiovascular risk. Lancet. 2005;365:434–441. [DOI] [PubMed] [Google Scholar]
- 21. O'Meara JG, Kardia SL, Armon JJ, et al. Ethnic and sex differences in the prevalence, treatment, and control of dyslipidemia among hypertensive adults in the GENOA study. Arch Intern Med. 2004;164:1313–1318. [DOI] [PubMed] [Google Scholar]
- 22. Wong ND, Lopez V, Tang SSK, et al. Prevalence, treatment, and control of combined hypertension and hypercholesterolemia in the united states. Am J Cardiol. 2006;98:204–208. [DOI] [PubMed] [Google Scholar]
- 23. Thomas F, Bean K, Guize L, et al. Combined effects of systolic blood pressure and serum cholesterol on cardiovascular mortality in young (<55 years) men and women. Eur Heart J. 2002;23:528–535. [DOI] [PubMed] [Google Scholar]
- 24. Samuelsson O, Wilhelmsen L, Andersson OK, et al. Cardiovascular morbidity in relation to change in blood pressure and serum cholesterol levels in treated hypertension. results from the primary prevention trial in Goteborg, sweden. JAMA. 1987;258:1768–1776. [PubMed] [Google Scholar]
- 25. Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. [DOI] [PubMed] [Google Scholar]
- 26. Waters DD, Guyton JR, Herrington DM, et al. Treating to New targets (TNT) study: does lowering low‐density lipoprotein cholesterol levels below currently recommended guidelines yield incremental clinical benefit. Am J Cardiol. 20049;3:154–158. [DOI] [PubMed] [Google Scholar]
- 27. Stamler J, Daviglus ML, Garside DB, et al. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long‐term coronary, cardiovascular, and all‐cause mortality and to longevity. JAMA. 2000;284:311–318. [DOI] [PubMed] [Google Scholar]
- 28. Shinohara K, Shoii T, Kimoto E, et al. Effect of atorvastatin on regional arterial stiffness in patients with type 2 diabetes mellitus. J Atheroscler Thromb. 2005;12:205–210. [DOI] [PubMed] [Google Scholar]
- 29. Ferrier KE, Muhlmann MH, Baquet JP, et al. Intensive cholesterol reduction lowers blood pressure and large artery stiffness in isolated systolic hypertension. J Am Coll Cardiol. 2002;39:1020–1025. [DOI] [PubMed] [Google Scholar]
- 30. Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. [DOI] [PubMed] [Google Scholar]
- 31. Wong ND, Pio JR, Franklin SS, et al. Preventing coronary events by optimal control of blood pressure and lipids in patients with the metabolic syndrome. Am J Cardiol. 2003;91:1421–1426. [DOI] [PubMed] [Google Scholar]
- 32. Blank R, Lasalle J, Reeves R, et al. Single‐pill therapy in the treatment of concomitant hypertension and dyslipidemia (the amlodipine/atorvastatin GEMINI study). J Clin Hypertens (Greenwich). 2005;7:264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]


