Abstract
To assess the strategy of increasing the dose of a diuretic compared with using an angiotensin receptor blocker in combination with a diuretic, the authors performed a multicenter, randomized, parallel group trial in hypertensive patients (baseline blood pressure [BP], 153/97 mm Hg) whose BP remained uncontrolled on initial low‐dose diuretic monotherapy (hydrochlorothiazide [HCTZ] 12.5 mg Hg). Patients with stage 1 and 2 hypertension were randomized to treatment with valsartan/HCTZ (160/12.5 mg) or to doubling of the HCTZ dose (25 mg). The primary end point was the percentage of patients whose clinic BP values were <140/90 mm Hg following 4 weeks of double‐blind therapy. A significantly higher proportion (P<.001) of hypertensive patients met BP control levels in the valsartan/HCTZ (160/12.5 mg) group compared with the HCTZ 25 mg group (37% vs 16%). Changes from baseline in BP were significantly greater (P<.001) for both systolic BP and diastolic BP in the combination therapy arm compared with the diuretic monotherapy arm (−12. 4/−7.5 mm Hg in valsartan/HCTZ 160/12.5 mg group vs −5.6/−2.1 mm Hg in HCTZ 25 mg group). Tolerability and adverse events were similar in the 2 treatment groups. This study suggests that in the management of hypertension, utilizing an angiotensin receptor blocker/diuretic combination was more effective in lowering BP and achieving BP goals when compared with increasing the dose of the diuretic.
There is substantial evidence that intensive control of blood pressure (BP) in patients with hypertension is required to produce the maximum reduction in clinical cardiovascular end points 1 , 2 ; published hypertension guidelines now advocate a target BP <140/90 mm Hg in patients with uncomplicated hypertension and <130/80 mm Hg in patients with any form of cardiovascular or kidney diseases. 3 , 4 Angiotensin receptor blockers (ARBs) alone or in fixed combinations with low‐dose (12.5 mg) hydrochlorothiazide (HCTZ) have been shown to be effective and well tolerated in clinical trials. 5 , 6 Clinical outcome studies have demonstrated that ARBs as well as angiotensin‐converting enzyme inhibitors (ACEIs) (usually given with a diuretic) reduce cardiovascular and cerebrovascular events and prolong survival in high‐risk hypertension, 7 , 8 heart failure, 9 and diabetic nephropathy. 10 , 11 Fewer patients develop new‐onset diabetes with a regimen that includes an ACEI or an ARB when compared with other agents.
Since the results of the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) and the publication of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, Treatment and of High Blood Pressure (JNC 7), trends in hypertension management have been to titrate doses of thiazide diuretics to improve BP control. 12 However, it is not clear how effective this strategy is compared with using 2 drugs either separately or in combination when the low‐dose diuretic has failed to achieve goal BP. A study comparing a fixed‐dose combination of an ARB with 12.5 mg of HCTZ to an upward titration of the thiazide diuretic was undertaken to determine the benefits and adverse effects of this strategy. Valsartan 160 mg plus HCTZ 12.5 mg and HCTZ 25 mg in patients with stage 1 or 2 hypertension were compared in a trial entitled Valsartan Hydrochlorothiazide Diuretic for Initial Control and Titration to Achieve Optimal Therapeutic Effect (Val‐DICTATE). A 16‐week follow‐up was performed with open‐label use of the combination therapy to further evaluate the impact of dose titration and usefulness of the ARB/diuretic strategy with a dihydropyridine calcium antagonist (amlodipine) as a third agent in the regimen.
METHODS
Study Design
This trial was a multicenter, randomized, active‐controlled, parallel group study to evaluate valsartan/HCTZ 160/12.5 mg in comparison with HCTZ 25 mg in the treatment of patients with hypertension uncontrolled by HCTZ 12.5 mg monotherapy. The study design is shown in Figure 1. The study was composed of 4 periods: (1) the screening period, (2) the baseline period to assess the antihypertensive effects of HCTZ at 12.5 mg/d (single‐blind), (3) the double‐blind treatment period, and (4) an open‐label treatment period. Following a 2‐ to 4‐week washout period for patients who were currently receiving antihypertensive therapy to establish baseline BP values, eligible patients were assigned to single‐blind treatment with 12.5 mg of HCTZ for 4 weeks. After 4 weeks of the HCTZ 12.5 mg, patients with uncontrolled hypertension (>140 and/or >90 mm Hg and <180/110 mm Hg) were randomized to double‐blind, double‐dummy treatment with either valsartan/HCTZ 160/12.5 mg/d or HCTZ 25 mg/d 4 weeks. If the seated clinic BP was <140/90 mm Hg at this time, the patient was withdrawn from the trial. Patients in whom a goal BP of <140/90 mm Hg, was not reached at the end of the third phase double‐blind treatment period entered an open‐label treatment phase with valsartan/HCTZ 160/25 mg for 4 weeks as shown in Figure 1. After 4 weeks at this dosage, if the BP was <140/90 mm Hg, the patient completed the study; if the BP was >140/90 mm Hg, the study drug was increased to valsartan/HCTZ 320/25 mg for 4 weeks (Figure 1, week 12). For patients whose BP was still >140/90 mm Hg, amlodipine 5 mg was added to the valsartan/HCTZ 320/25 mg for 4 weeks. If the BP was <140/90 mm Hg, the patient completed the study. Finally, at week 16, if the BP was <140/90 mm Hg, the patient completed the study, but if the BP was >140/90 mm Hg, the dose of amlodipine was increased to 10 mg and valsartan/HCTZ 320/25 mg was maintained. At week 20, treatment in all patients remaining in the study was discontinued and participants began to receive appropriate conventional treatment with scheduled follow‐up.
Figure 1.
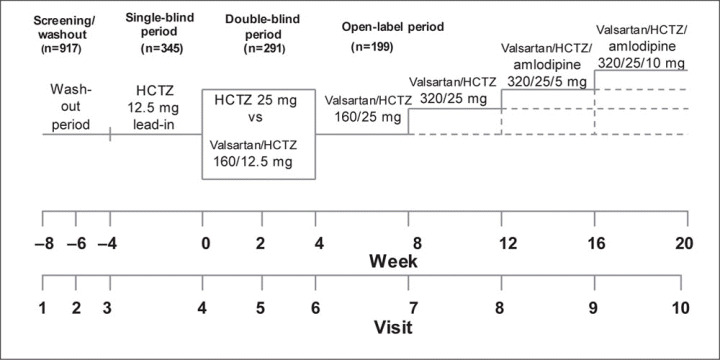
Study design of the Valsartan Hydrochlorothiazide Diuretic for Initial Control and Titration to Achieve Optimal Therapeutic Effect (Val‐DICTATE) trial. There were 4 treatment periods: screening/placebo washout (if receiving prior antihypertensive therapy); 4‐week, single‐blind treatment period with hydrochlorothiazide (HCTZ), 12.5 mg/d; 4‐week, double‐blind treatment period with valsartan/HCTZ 160/12.5 mg/d vs HCTZ 25 mg/d; and a 16‐week open‐label treatment period with increasing doses of valsartan/HCTZ with amlodipine depending on blood pressure responses.
Patient Population
Men and women with systemic hypertension aged 18 years or older were included if their average seated systolic BP was >150 mm Hg but <180 mm Hg and average seated diastolic BP was >95 mm Hg but <110 mm Hg following 4 weeks of single‐blind placebo treatment. Female patients who were postmenopausal or surgically sterile for 1 year or longer and women using effective contraceptive methods were eligible for study participation. Patients with history of stroke at any time; history of significant cardiac disease within the past 6 months, including unstable angina pectoris, arrhythmias, congestive heart failure, and myocardial infarction; known or suspected secondary hypertension; diabetes mellitus; and chronic kidney disease (serum creatinine >2.0 mg/dL, known renal artery stenosis) were excluded from the study.
Measurements of Efficacy and Safety Parameters
Office BP was measured in triplicate by a semiautomatic and independently validated device (Omron Healthcare, Vernon Hills, IL) in the seated position at all visits. Care was made to use the correct cuff/bladder size according to guidelines of the American Heart Association guidelines. 13 The pulse rate was measured in conjunction with the BP measurements at each visit. Study coordinators recorded times of medication dosing and BP measurements on the case report forms. Safety was assessed by the evaluation of adverse events and vital signs at each visit, and changes from baseline to the end of the study in laboratory parameters. All reported adverse events were categorized by body system and preferred term using the Medical Dictionary for Regulatory Activities. 14 The incidence of treatment‐emergent adverse effects in each treatment group was tabulated by severity and by relationship to study drug (as ascertained by the site study personnel). Treatment compliance was assessed by a physical count of returned study medications. Randomization codes and data about all study drugs dispensed to patients and all dosage changes were tracked using an interactive voice recorder system.
Statistical Analyses
The primary end points for assessing efficacy were the percentages of patients in whom goal BP was attained (<140/90 mm Hg) after 4 weeks of double‐blind treatment with valsartan/HCTZ compared with HCTZ. Treatment comparisons were made using the Cochran‐Mantel‐Haenszel chi‐squared test, adjusting for center effects. Similar tests were performed after 2 weeks of double‐blind therapy. In addition, changes from baseline in clinic diastolic BP and systolic BP measured 23 to 26 hours after dosing of study medication were assessed at week 4 using an analysis of covariance model with treatment and center as factors and baseline values as covariates to analyze and estimate treatment effects. In the case of patients withdrawing from the study before the completion of the 4‐week double‐blind treatment period, last‐observation‐carried‐forward principles were utilized.
The primary objective of the study was to demonstrate the differences between valsartan/HCTZ and HCTZ alone following 4 weeks of double‐blind therapy. With the expectation that the percentage of patients in whom BP control would be attained in the valsartan/HCTZ group would be 40% and the percentage of patients in whom BP control would be attained in the HCTZ group would be 20%, a sample size of 120 completed patients per treatment group would have 90% power to demonstrate at the 5% (2‐sided) level of significance that valsartan/HCTZ is superior to HCTZ alone. Assuming a 20% rate of premature discontinuation from the study, approximately 300 patients were needed to perform the study. The study was also required to enroll 370 patients in the single‐arm lead‐in phase to ensure that at least 300 patients would be eligible for randomization with a 90% probability. This number was based on the assumption that BP control would not be attained with HCTZ at a dosage of 12.5 mg/d in 85% of the patients enrolled at the lead‐in phase.
The comparability of patients in the 2 treatment groups was determined from the demographic data and baseline BP values. The primary end points as well as all secondary continuous variables were analyzed using an analysis of covariance model involving treatment group with baseline value as a covariate. Further adjustments were made for age, sex, and race for comparative effects of the 2 treatments. Treatment group comparisons were based on the least‐squares means obtained via the SAS general linear model procedure (SAS version 8.2 VMS operating system, Cary, NC). In addition, effects of age, sex, body mass index (BMI), and race on changes in BP were evaluated in subgroup analyses.
RESULTS
Baseline Characteristics of the Study Population
The baseline characteristics of all randomized patients in the 2 treatment arms are shown in Table I. For the entire patient population, the mean age was 53 years, with a greater percentage of males (62%), and the population was predominantly nonblack (78%) with baseline BP values of 153/97 mm Hg. There was a slightly lower proportion of females in the valsartan/HCTZ arm compared with the HCTZ monotherapy arm. In addition, there were significantly more black patients randomized to the valsartan/HCTZ arm than to the HCTZ monotherapy arm (26% vs 19%; P=.01). No other differences in baseline characteristics between the treatment arms were noted.
Table I.
Baseline Characteristics of the Patient Population
| Parameter | Valsartan/HCTZ 160/12.5 mg (n=146) | HCTZ 25 mg (n=145) | P Value |
|---|---|---|---|
| Age, y | 52.9±10.6 | 52.6±10.6 | .797 |
| Age groups | |||
| <65 y | 125 (85.6) | 128 (88.3) | .501 |
| >65 y | 21 (14.4) | 17 (11.7) | |
| Sex | |||
| Male | 94 (64.4) | 86 (59.3) | .372 |
| Female | 52 (35.6) | 59 (40.7) | |
| Race | |||
| Nonblack | 108 (74) | 118 (81) | .014 |
| Black | 38 (26) | 27 (19) | |
| Body mass index, kg/m2 | 31.9±5.7 | 33.0±7.2 | .151 |
| Seated systolic BP, mm Hg | 153.0±10.6 | 153.2±10.7 | .853 |
| Seated diastolic BP, mm Hg | 97.3±6.3 | 97.0±6.3 | .747 |
| Seated heart rate, beats/min | 75.1±9.9 | 76.2±10.4 | .350 |
| Abbreviations: BP, blood pressure; HCTZ, hydrochlorothiazide. Data are No. (%) or mean ± SD. | |||
Proportion of Patients in Whom BP Control Was Achieved
The effects of the 2 treatments on BP control are shown in Table II. As noted, goal BP was achieved in significantly more patients randomized to valsartan/HCTZ 160/12.5 mg at both weeks 2 and 4 compared with alone. The primary end point (week 4) showed a 20.7% greater rate of BP control with valsartan/HCTZ (P<.0001) compared with HCTZ alone. In addition, in patients treated with valsartan/HCTZ 160/12.5 mg, the reductions in trough clinic BP values (−12.4/−7.5 mm Hg) were significantly greater (P<.0001 for both systolic and diastolic BP) than those for patients treated with HCTZ 25 mg (−5.6/−2.1 mm Hg) (Figure 2). Similar findings occurred after only 2 weeks of double‐blind therapy (Table II, Figure 2).
Table II.
Proportion of Patients in Whom Trough BP <140/90 mm Hg Was Achieved
| Valsartan/HCTZ 160/12.5 mg | HCTZ 25 mg | ||||
|---|---|---|---|---|---|
| BP control criteria met | BP control criteria met | ||||
| Visit | Yes | No | Yes | No | |
| No. (%) | No. (%) | No. (%) | No. (%) | ||
| Week 2 | 35 (25) | 105 (75) | 20 (14) | 123 (86) | |
| Difference between groups | 9.0% | ||||
| 95% CI | 1.15%–20.9% | ||||
| P value | .019 | ||||
| Week 4 | 53 (36.6) | 92 (63.4) | 23 (15.9) | 122 (84.1) | |
| Difference between groups | 20.1% | ||||
| 95% CI | 10.2%–31.2% | ||||
| P value | <.0001 | ||||
| Open‐label extension therapy rates,a visit and therapy | |||||
| Week 8, valsartan/HCTZ 70 (36) | 160/25 mg | ||||
| Week 12, valsartan/HCTZ 320/25 mg | 43 (36) | ||||
| Week 16, valsartan/HCTZ 320/25 mg and amlodipine 5 mg | 32 (43) | ||||
| Week 20, valsartan/HCTZ 320/25 mg and amlodipine 10 mg | 22 (58) | ||||
| Abbreviations: BP, blood pressure; CI, confidence interval; HCTZ, hydrochlorothiazide. aControl rates were calculated based on the number of patients left at the visit; if BP control was achieved, patients were discontinued at the visit. Week 8, n=195; week 12, n=118; week 16, n=74; week 20, n=38. | |||||
Figure 2.
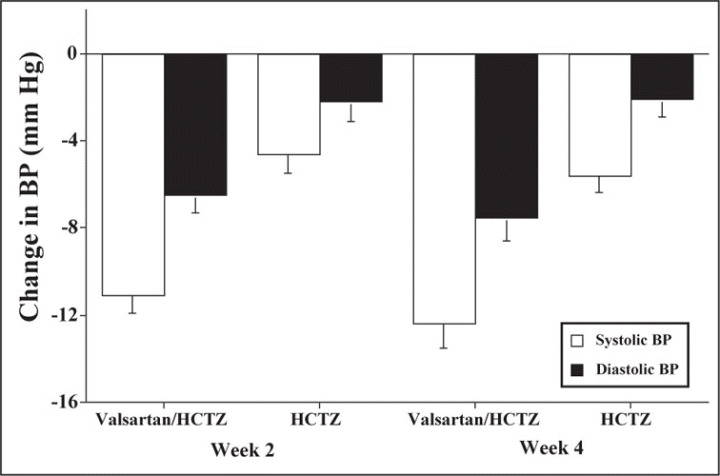
Changes from hydrochlorothiazide (HCTZ) 12.5 mg baseline period in seated, clinic systolic and diastolic blood pressure (BP) following 4 weeks of valsartan/HCTZ 160/12.5 mg vs HCTZ 25 mg/d. All changes were significantly larger (P<.001) with valsartan/HCTZ 160/12.5 mg.
The Impact of Age, Sex, Body Mass Index, and Race on BP and BP Control
Age. The impact of age (<65 or ≥65 years and <55 or ≥55 years) on reductions in BP for the 2 treatment groups is shown in Table III. In patients younger than 65 years and younger than 55 years, greater BP control rates occurred with valsartan/HCTZ compared with HCTZ alone. There were also larger reductions in BP with valsartan/HCTZ 160/12.5 mg vs HCTZ 25 mg in the younger patient group. The number of patients aged 65 years or older was quite small, and there were no differences in the proportion of patients whose BP was controlled with valsartan/HCTZ 160/12.5 mg compared with 25 mg HCTZ or in the changes from baseline in clinic BP. The patients older than 55 years had significantly larger reductions in BP with valsartan/HCTZ 160/12.5 compared with HCTZ 25 mg; BP control rates were not significantly different between treatment groups in those older than 55 years (P=.08).
Table III.
Effects of Age, Sex, BMI, and Ethnicity on Changes in Blood Pressure at Week 4
| Subgroup | Valsartan/HCTZ 160/12.5 mg | HCTZ 25 mg | P Value |
|---|---|---|---|
| Age <65 y, No. | 124 | 128 | |
| No. (%) in whom BP was controlled | 49 (39.5) | 20 (15.6) | <.0001 |
| Change in BP, mm Hg | –13/–8±12/9 | –6/–2±11/7 | <.0001 for both |
| Age ≥65 y, No. | 21 | 17 | |
| No. (%) in whom BP was controlled | 4 (19) | 3 (17.6) | .8672 |
| Change in BP, mm Hg | –6/–3±11/6 | –5/–1±13/8 | <.0001/.3083 |
| Age <55 y, No. | 85 | 83 | |
| No. (%) in whom BP was controlled | 35 (41.0) | 13 (16.0) | <.0002 |
| Change in BP, mm Hg | –13/–9±13/9 | –5/–2±10/8 | <.0001 for both |
| Age ≥ 55 y, No. | 59 | 58 | |
| No. (%) in whom BP was controlled | 18 (30.0) | 10 (16.4) | .080 |
| Change in BP, mm Hg | –11/–6±12/8 | –6/–3±13/6 | <.022/.009 |
| Male, No. | 93 | 86 | |
| No. (%) in whom BP was controlled | 29 (31.2) | 15 (17.4) | .035 |
| Change in BP, mm Hg | –12/–7±11/9 | –5/–1±11/7 | <.0001 for both |
| Female, No. | 52 | 59 | |
| No. (%) in whom BP was controlled | 24 (46.2) | 8 (13.6) | <.0001 |
| Change in BP, mm Hg | –13/–9±13/9 | –6/–3±11/7 | <.0001 for both |
| BMI <30 kg/m2, No. | 53 | 57 | |
| No. (%) in whom BP was controlled | 21 (39.6) | 11 (19.3) | <.0001 |
| Change in BP, mm Hg | –13/–8±13/8 | –6/–4±12/7 | .009/<.0001 |
| BMI >30 kg/m2, No. | 82 | 80 | |
| No. (%) in whom BP was controlled | 28 (34.1) | 12 (15) | .005 |
| Change in BP, mm Hg | –12/–7±13/9 | –5/–1±11/7 | <.0001 for both |
| Black, No. | 38 | 27 | |
| No. (%) in whom BP was controlled | 12 (13.6) | 5 (18.5) | .279 |
| Change in BP, mm Hg | –13/–19±13/9 | –6/–2±9/8 | <.0001 for both |
| White, No. | 87 | 111 | |
| No. (%) in whom BP was controlled | 35 (40.3) | 18 (16.2) | .0002 |
| Change in BP, mm Hg | –13/–8±12/9 | –5/–2±11/8 | <.0001 for both |
| Abbreviations: BMI, body mass index; BP, blood pressure. | |||
Sex. The impact of sex on reductions in BP in the 2 treatment groups is shown in Table III. No significant treatment‐by‐sex interaction was found for BP control rates or for changes in BP. The proportion of patients in whom BP control was achieved at 4 weeks was greater with valsartan/HCTZ 160/12.5 mg than with HCTZ 25 mg in both sexes. The adjusted mean changes from baseline for women (−13/−9 mm Hg) with valsartan/HCTZ were similar to the changes observed in men (−12/−7 mm Hg). The proportion of women in whom BP control was reached with valsartan/HCTZ also tended to be larger (46%) than the proportion of men (31%), and for both sexes the control rates were higher with valsartan/HCTZ 160/12.5 mg than with HCTZ 25 mg.
Body Mass Index. A high proportion of the study population in the Val‐DICTATE trial were overweight or obese (56%). There was no impact of BMI on the efficacy of valsartan/HCTZ 160/12.5 mg compared with HCTZ 25 mg (Table III). Similar reductions in BP and percentages of BP control occurred in the treatment groups both in patients with a BMI <30 km/m2 and those with a BMI >30 km/m2.
Race. The impact of race group (nonblack and black) on reductions in BP for the 2 treatment groups is shown in Table III. No significant treatment‐by‐race group interaction was found for changes in BP or in the percentage of patients in whom BP control was attained at 4 weeks. In addition, there were no significant overall differences found between the adjusted mean changes in BP for nonblack and black patients receiving valsartan/HCTZ 160/12.5 mg (−13/−8 vs −12/−7 mm Hg, respectively) or receiving HCTZ 25 mg (−5/−2 mm Hg vs −6/−2 mm Hg, respectively).
Open‐Label Period
A total of 199 patients entered the open‐label extension period of 16 weeks (Figure 1). The proportion of patients in whom a BP value <140/90 mm Hg was reached is shown in Table II and increased with each visit to a cumulative level of 91.4% by week 20. Changes from week 4 BP values are shown in Figure 3. All changes were significantly greater at each of the time intervals compared with at the end of the double‐blind period. For example, at week 16, the changes in BP were −15.5/−9.0 mm Hg with valsartan/HCTZ 320/25 mg with amlodipine 5 mg compared with the values obtained at week 4 while receiving valsartan/HCTZ 160/12.5 mg or HCTZ 25 mg.
Figure 3.
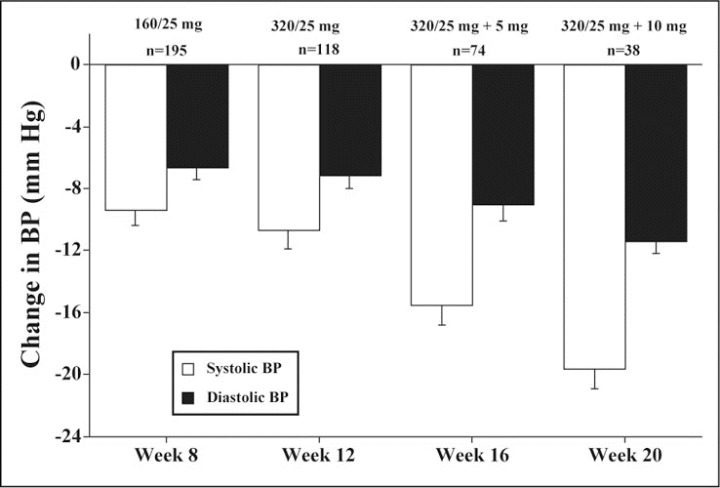
Reductions from week 4 during the open‐label extension period. All changes are significantly greater than week 4 values. Note that if blood pressure (BP) control (<140/90 mm Hg) was reached, patients were discontinued from the trial at that point. 160/25, valsartan‐HCTZ; 320/25, valsartan‐HCTZ; 320/25 + 5 mg, valsartan‐HCTZ + amlodipine; 320/25 + 10 mg, valsartan‐HCTZ + amlodipine
Adverse Effects
Of the 291 patients who were randomized to the study, 98 (34%) had at least one adverse event with treatment during the trial; 48 of 146 (33%) in the valsartan/HCTZ arm and 50 of 145 (34%) in the HCTZ arm (Table IV).
Table IV.
Adverse Events According to Type and Treatment Group (Including Double‐Blind Period and Extension)
| Valsartan/HCTZ 160/12.5 mg | HCTZ 25 mg | |
|---|---|---|
| No. studied | 146 | 145 |
| No. (%) with adverse event | 48 (32.9) | 50 (34.5) |
| Deaths | 0 (0) | 0 (0) |
| Serious adverse events | ||
| Coronary artery disease | 0 (0) | 1 (0.7) |
| Abdominal pain | 1 (0.5)a | |
| Tendon rupture | 1 (0.5)a | |
| Alcoholism | 1 (0.5)a | |
| Adverse event leading to drug discontinuation | ||
| Headache and fatigue | 1 (0.7)a | 0 (0) |
| Fatigue | 1 (0.7)a | 0 (0) |
| Cough | 1 (0.7)a | 0 (0) |
| Dizziness | 1 (0.7)a | 0 (0) |
| Alcohol abuse | 1 (0.7)a | 0 (0) |
| Rectal bleeding | 0 (0) | 1 (0.7) |
| Coronary artery disease | 0 (0) | 1 (0.7) |
| Chest pain | 0 (0) | 1 (0.7) |
| Nonserious adverse event typesb | ||
| Gastrointestinal disorders | 8 (5.5) | 5 (3.4) |
| Infections | 9 (6.2) | 14 (9.7) |
| Musculoskeletal disorders | 8 (5.5) | 3 (2.1) |
| Nervous system disorders | 9 (6.2) | 15 (10.3) |
| Psychiatric disorders | 5 (3.4) | 3 (2.1) |
| Renal/urinary disorders | 7 (4.8) | 1 (0.7) |
| Skin disorders | 3 (2.1) | 4 (2.8) |
| aOccurred during the open‐label extension period; b>2% incidence. | ||
There were no deaths reported during the study. Five patients experienced a serious adverse event: 1 during the HCTZ 12.5 mg run‐in (atrial fibrillation), 1 during the double‐blind treatment period, and 3 during the 16‐week extension period (Table IV). Overall, only 14 patients (4.8%) discontinued the study early: 6 patients in the valsartan/HCTZ 160/12.5 group and 8 patients in the HCTZ 25 mg group. Adverse events accounted for only 4 of these discontinuations, while 3 were due to lack of efficacy. The remainder were due to patient compliance issues and withdrawal of consent by study patients.
There were few laboratory parameters with any significant changes during the study. During the single‐blind lead‐in period (HCTZ, 12.5 mg/d), a reduction outside of the normal values in potassium was noted in 13 (4.5%) patients; increases in total cholesterol in 39 patients (13.4%), low‐density lipoprotein cholesterol in 31 patients (11.3%), triglycerides in 31 patients (10.7%), and uric acid in 24 patients (8.3%) were observed. During the double‐blind period, the proportion of patients with reductions in serum potassium was less with valsartan/HCTZ 160/12.5 mg (1.4%) compared with those receiving HCTZ 25 mg (6.4%), and the proportion of patients with an elevation in serum uric acid was lower with valsartan/HCTZ 160/12.5 mg (5.0%) compared with HCTZ 25 mg (8.6%).
DISCUSSION
Principal Findings
This clinical trial was designed to evaluate the efficacy of an ARB administered in combination with a low‐dose thiazide diuretic compared with the efficacy of a higher‐dose thiazide diuretic. The primary findings demonstrated that valsartan/HCTZ 160/12.5 mg lowered both the systolic and diastolic BP to a greater extent than did HCTZ 25 mg and that normal clinic BP values were attained in a higher proportion of patients (Table II and Figure 2). Following the initial 4 weeks of therapy with HCTZ at 12.5 mg/d, only 15% of patients had normalization of BP (<140/90 mm Hg). Normalization of BP was achieved in 37% of patients switched to valsartan/HCTZ 160/12.5 mg compared with 16% whose diuretic dosage was doubled to HCTZ 25 mg/d. The findings between these 2 treatment groups were predictable, in part, considering the known pharmacologic benefits of adding an ARB to thiazide diuretic therapy. 15 , 16 , 17 , 18 The present study adds information involving a strategy of switching to combination therapy in contrast to doubling the dose of diuretic monotherapy.
ARBs in Combination With HCTZ. Several fixed‐dose combination therapies of ARBs as well as ACEIs and diuretics have been used for the treatment of hypertension and have demonstrated a greater degree of BP lowering than either medication alone. 15 , 18 , 19 , 20 , 21 For example, in a large study by Benz and associates 15 involving 871 patients, reductions in BP with use of valsartan and HCTZ at doses of 160 and 12.5 mg, respectively, lowered BP 16/9 mm Hg more than placebo, compared with 10/5 mm Hg with valsartan 160 mg alone and 5/3 mm Hg with HCTZ alone. Because these changes were both clinically and statistically greater than the monotherapy components and occurred without excessive adverse events, it led to the approval of the fixed‐dose combination valsartan/HCTZ used in the present study. In another study, by Mallion and coworkers, 19 in 2002 patients whose BP was inadequately controlled with valsartan 160 mg, valsartan/HCTZ 160/12.5 mg induced a 4/2‐mm Hg greater reduction in BP than monotherapy, with an absolute rate of response of 65% compared with 50% with valsartan monotherapy.
In a trial similar in concept to that of Mallion and associates, 19 Rosenstock and colleagues 20 evaluated the antihypertensive efficacy and safety of adding the ARB irbesartan to HCTZ in patients whose BP was not adequately controlled by HCTZ alone. In this study, however, the background drug was HCTZ 25 mg/d rather than the ARB. After demonstrating a lack of BP control with the thiazide monotherapy, patients were randomized in a double‐blind fashion to the same dosage of diuretic or irbesartan 75 mg/d was added. As expected, by week 12 of treatment, BP decreased by 14.5/12.1 mm Hg with the combination treatment in contrast to 5.0/3.4 mm Hg in patients who remained on HCTZ treatment.
Higher Doses of Thiazide Diuretics With ARBs. One of the objectives of our study was to evaluate the clinical effects of doubling a thiazide diuretic dose compared with switching to an ARB and maintaining the dose of the thiazide diuretic. While several studies have shown improved efficacy of titrating the dosage of HCTZ to 25 mg/d, this comes at a possible metabolic cost of increases in low‐density lipoprotein cholesterol, glucose, and uric acid and reductions in potassium, all phenomena observed in the present study but of questionable clinical significance. However, incremental BP‐lowering effects have been observed with higher doses of HCTZ (ie, 25 mg) in combination with ARBs, 16 , 19 which has led to the development of combination formulations used in the extension portion of this trial. These combination formulations have fewer metabolic consequences than does HCTZ alone at 25 mg/d. For example, in the factorial design study by Benz and associates, 15 valsartan/HCTZ at a dose of 160/25 mg lowered BP by 22/15 mm Hg compared with 18/14 mm Hg with valsartan/HCTZ at a dose of 160/12.5 mg. In a study by McGill and Reilly, 6 the greatest effects from a large factorial design study with varying doses of telmisartan and HCTZ was seen with telmisartan/HCTZ at 160/25 mg (a dose not clinically available), which decreased BP by 25/18 mm Hg. In both of these earlier studies, 6 , 15 as the dose of the ARBs increased when added to HCTZ 25 mg/d, reductions in serum potassium induced by HCTZ were attenuated or negated. These findings support the use of combination agents with the higher dosages of HCTZ (ie, 25 mg/d) in patients with stage 2 hypertension.
The Importance of Achieving BP Control
Studies such as this clinical trial are important in establishing differences in the antihypertensive effects of drugs when patients are nonresponsive to treatment with low‐dose thiazide diuretics. As shown in Table III and Figure 2, regardless of race or sex, the use of valsartan/HCTZ 160/12.5 mg resulted in significantly greater reductions in BP compared with HCTZ 25 mg, by about 7/5 mm Hg at the end of 4 weeks. This difference may have important clinical implications. In a meta‐analysis involving 1 million adults in 60 prospective studies, the relationship between the reduction in BP and cardiovascular morbidity and mortality events was shown to be approximately log‐linear, and systolic BP differences of 20 mm Hg and diastolic BP differences of 10 mm Hg directly correlated to a 50% reduction in stroke mortality and in death rates for ischemic heart disease and other vascular deaths. 1 Certainly, as has been shown in ALLHAT 23 and VALUE 23 , greater reductions in BP induced by one pharmacologic regimen compared with another may have important clinical implications related to reductions in cardiovascular and cerebrovascular morbidity, even during a period of <6 months. Thus, the clinical practice strategy of changing to multiple‐drug or fixed‐dose combination therapy when the response to therapy with a low‐dose diuretic therapy is inadequate may be more appropriate than doubling the dose of the diuretic; the results of this study with an ARB/diuretic appear to confirm this concept.
Acknowledgments and disclosures:
This study was supported by a grant from Novartis Pharmaceuticals. The authors express their appreciation to Amie Grosso and Edite O'Hern for the expert assistance in project management. (ClinicalTrials.gov identifier: NCT00277472.) Research funding was provided to the University of Connecticut Hypertension Division by the National Institutes of Health and Novartis Pharmaceuticals.
References
- 1. Lewington S, Clarke R, Qizilbash N, et al; Prospective Studies Collaboration. Age‐specific relevance of usual BP to vascular mortality: a meta‐analysis of individual data for one million adults in 60 prospective studies. Lancet. 2002;14:1903–1913. [DOI] [PubMed] [Google Scholar]
- 2. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomized trial. Lancet. 1998;351:1755–1762. [DOI] [PubMed] [Google Scholar]
- 3. The Seventh Report of the Joint National Committee on prevention, detection, evaluation and treatment of high BP. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 4. 2003 European Society of Hypertension‐European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–1054. [DOI] [PubMed] [Google Scholar]
- 5. Ruddy MC, Kostis JB. Angiotensin receptor antagonists. In Oparil S, Weber MA, eds. Hypertension. Philadelphia, PA: WB Saunders Co; 2000:621–637. [Google Scholar]
- 6. McGill JB, Reilly PA. Telmisartan plus hydrochlorothiazide versus telmisartan or hydrochlorothiazide monotherapy in patients with mild to moderate hypertension: A multicenter, randomized, double‐blind, placebo‐controlled parallel‐group trial. Clin Ther. 2001;23:833–850. [DOI] [PubMed] [Google Scholar]
- 7. Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): a randomized trial against atenelol. Lancet. 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- 8. Weber MA, Julius S, Kjeldsen SE, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE trial. Lancet. 2004;363(9426):2049–2051. [DOI] [PubMed] [Google Scholar]
- 9. Cohn JN, Tognoni G; Valsartan Heart Failure Trial Investigators . A randomized trial of the angiotensin‐receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. [DOI] [PubMed] [Google Scholar]
- 10. Brenner BM, Cooper ME, deZeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. [DOI] [PubMed] [Google Scholar]
- 11. Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group . Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:857–860. [DOI] [PubMed] [Google Scholar]
- 12. Moser M. Update on the management of hypertension: recent clinical trials and the JNC 7. J Clin Hypertens (Greenwich). 2004;6(10 suppl 2):4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the subcommittee of professional and public education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. [DOI] [PubMed] [Google Scholar]
- 14. Medical Dictionary for Regulatory Activities Terminology (MedDRA). Version 3.3. Reston, VA: MedDRA MSSO, December 2000. [Google Scholar]
- 15. Benz JR, Black HR, Graff A, et al. Valsartan and hydrochlorothiazide in patients with essential hypertension. A multiple dose, double‐blind, placebo controlled trial comparing combination therapy with monotherapy. J Hum Hypertens. 1998;12:861–866. [DOI] [PubMed] [Google Scholar]
- 16. Kjeldsen SE, Os I, Hoieggen A, et al. Fixed‐dose combinations in the management of hypertension: defining the place of angiotensin receptor antagonists and hydrochlorothiazide. Am J Cardiovasc Drugs. 2005;5:17–22. [DOI] [PubMed] [Google Scholar]
- 17. Wagstaff AJ. Valsartan/hydrochlorothiazide: a review of its use in the management of hypertension. Drugs. 2006;66:1881–1901. [DOI] [PubMed] [Google Scholar]
- 18. Schmidt A, Adam SA, Kolloch R, et al. Antihypertensive effects of valsartan/hydrochlorothiazide combination in essential hypertension. Blood Press. 2001;10:230–237. [DOI] [PubMed] [Google Scholar]
- 19. Mallion JM, Carretta R, Trenkwalder P, et al. Valsartan/hydrochlorothiazide is effective in hypertensive patients inadequately controlled by valsartan monotherapy. Blood Press Suppl. 2003;1:36–43. [DOI] [PubMed] [Google Scholar]
- 20. Rosenstock J, Rossi L, Lin CS, et al. The effects of irbesartan added to hydrochlorothiazide for the treatment of hypertension in patients non‐responsive to hydrochlorothiazide alone. J Clin Pharm Ther. 1998;23:433–440. [DOI] [PubMed] [Google Scholar]
- 21. Lacourciere Y, Poirer L. Antihypertensive effects of two fixed‐dose combinations of losartan and hydrochlorothiazide versus hydrochlorothiazide monotherapy in subjects with ambulatory systolic hypertension. Am J Hypertens. 2003;16:1036–1042. [DOI] [PubMed] [Google Scholar]
- 22. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 23. Julius S, Kjeldsen SE, Weber M, et al; VALUE trial group . Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. [DOI] [PubMed] [Google Scholar]


