Abstract
Brachial systolic and pulse blood pressures (BPs) are better predictors of adverse cardiovascular (CV) events than diastolic BP in individuals older than 50 years. The principal cause of increased systolic and pulse BP is increased stiffness of the elastic arteries as a result of degeneration and hyperplasia of the arterial wall. Recent studies have shown that central BP, the pressure exerted on the heart, brain, and kidneys, is a better predictor of CV risk than brachial BP. As stiffness increases, reflected wave amplitude increases and augments pressure in late systole, producing an increase in left ventricular afterload and myocardial oxygen demand. Vasoactive drugs have little direct effect on large human elastic arteries but can markedly modify wave reflection by altering stiffness of the muscular arteries and changing pulse wave velocity of the reflected wave from the periphery to the heart. Vasodilators decrease the amplitude and increase the travel time (or delay) of the reflected wave, causing a generalized decrease in systolic BP. The decrease in systolic BP brought about by this mechanism is grossly underestimated when systolic BP is measured in the brachial artery.
The association between blood pressure (BP) components and major cardiovascular (CV) events is well documented in both normotensive and hypertensive individuals. 1 Numerous observational epidemiology studies and clinical trials have shown a strong positive, continuous, and graded association between brachial artery systolic and/or pulse BP and CV risk and adverse events in individuals older than 50 years. 2 Arterial diastolic BP has been found to be inversely (or negatively) related to coronary artery disease (CAD) and CV mortality. 2 In some large studies, the association of diastolic BP with all‐cause and CV mortality is reported as J‐ or U‐shaped in elderly participants. 3 CV disease and outcome are also related to invasively measured central aortic pulse BP, the pressure exerted on the heart, brain, and kidneys. 4 Since central systolic and pulse BP are related to changes in arterial effects 5 and can be measured easily, accurately, and noninvasively using a validated generalized transfer function and pulse wave analysis, 6 attention has been directed toward arterial stiffness, pulse wave velocity (PWV), and wave reflections as independent CV risk factors that predict adverse events and relate to outcome. 4 , 7 Independent studies have shown that central elastic arterial stiffness and systolic wave reflection amplitude are increased in older individuals 8 and in patients with hypertension, 9 CAD, 10 myocardial infarction, 11 heart failure, 12 end‐stage renal disease, 13 diabetes mellitus (types 1 and 2), 14 , 15 hypercholesterolemia, 16 hyperhomocysteinemia, 17 aldosteronism, 18 inflammation (and increased C‐reactive protein), 19 , 20 obstructive sleep apnea, 21 and macular degeneration. 22
Both systolic and pulse BPs markedly increase while diastolic and mean BPs slightly decrease as the pressure wave travels from the heart toward the periphery. 5 This difference between peripheral and central systolic BPs is greater in young patients with compliant elastic arteries and less in older patients with stiff elastic arteries. The “amplification” of the pressure pulse is due to greater stiffness of peripheral arteries and enhanced wave reflection amplitude, which depends on the difference between elastic moduli of the respective arteries and distance to major reflecting sites. 5 As a consequence, these 2 pulsatile BP components are greater in arteries of the extremities than in the central aorta. Amplification of the pressure wave depends on a number of variables, including age, PWV, exercise, vasoactive drugs, and heart rate and may explain why diastolic BP is a reliable predictor of CV risk in patients younger than 50 years. 23 This difference is also important because the major organs (brain, heart, and kidney) “see” central arterial BP and not brachial BP. Therefore, brachial systolic and pulse BPs measured with a sphygmomanometer in the arm are not always reliable measures of central aortic systolic and pulse BPs.
As might be expected, recent studies have shown that compared with brachial BP, central aortic BP is a better predictor of carotid intima‐media thickness, 24 restenosis after coronary angioplasty, 25 CAD severity, 26 and left ventricular (LV) mass 27 —an independent predictor of heart failure and CV mortality. 28 In patients with end‐stage renal disease 29 and hypertension 30 and in Native American patients, 7 central pulse BP is a better predictor of CV outcome than brachial pulse BP. It has been demonstrated that reduction of central aortic systolic and pulse BPs with vasodilator drugs is underestimated by measurements of brachial artery BP. 30 This disparity is due to the amplitude and timing of reflected pressure waves from the periphery to the heart, which depend on stiffness of the entire arterial tree and major reflection site distance. 5 Studies have also noted that β‐blockers have less effect on central BP than other pressure‐lowering agents. 31 Such studies have suggested that logical treatment for lowering systolic and pulse BPs should not only focus on arteriolar caliber and peripheral resistance, but also on arterial stiffness and wave reflections.
PWV IN ELASTIC AND MUSCULAR ARTERIES
The systemic arterial system serves as a conduit that delivers blood at high pressure to peripheral vascular beds to meet their metabolic requirements. This arterial system can be separated into 3 anatomical regions and, since the LV is a pulsatile pump, each region has a distinct and separate function: (1) the large elastic arteries (eg, aorta, carotid, iliac), which have a limited number of smooth muscle cells (SMCs), serve as a buffering reservoir that passively expands, stores the ejected blood during systole, and expels it into the peripheral circulation during diastole so that the capillaries receive a continuous flow of blood during the entire cardiac cycle; (2) the muscular arteries, especially those in the lower body (eg, femoral, popliteal, posterior tibial), by altering SMC tone, modify the speed of travel of pressure and flow waves along their length and determine when, during the cardiac cycle, the reflected waves arrive back at the heart; and (3) the arterioles, by changing their caliber, alter peripheral resistance and, therefore, aid in the maintenance of mean arterial BP. Permanent (or chronic) changes (eg, increased stiffness) in the central elastic arteries occur over an extended period and acute alterations in wall effects are passive, while changes in the muscular arteries and arterioles most often occur acutely; alterations in wall effects are active. 5
Active changes in arterial wall effects occur acutely in response to an intervention but will usually last only until the intervention is terminated. An increase in stiffness and PWV with age is virtually confined to the aorta and central, predominantly elastic, arteries (PWVe); there is little or no consistent change in stiffness and PWVm of the muscular arteries with age (Figure 1). 32 PWVe or central elastic artery stiffness increases exponentially and exceeds PWVm at about 60 years of age. This difference in PWV (or stiffness) between elastic and muscular arteries with advancing age has been referred to as a reversal of the arterial stiffness gradient. 33 Findings of increased arterial stiffness and elevated collagen content with age in the central but not the peripheral arteries of humans are consistent with the previously proposed theory that fracture of elastic load‐bearing elements of the arterial wall causes the wall to stretch so that stress is transferred to the stiff collagen elements in the wall, just as when an artery is acutely distended by a greater BP within. 5
Figure 1.
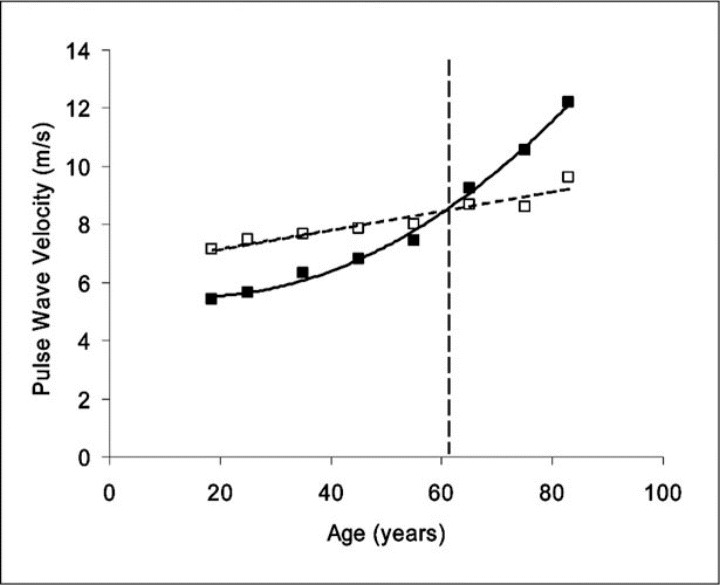
Age‐related changes in aortic (PWVe) and brachial artery (PWVm) transmission velocity in healthy human patients. Data are from the Anglo‐Cardiff Collaborative Trial (ACCT) study population. 8 Note the relatively linear relationship for PWVm (dotted line and open squares), compared with the curved relationship for PWVe (solid line and closed squares), which changes much more, 32 especially in patients 50 years and older. The broken vertical line represents the age at which the arterial stiffness gradient reverses.
PULSE WAVE REFLECTIONS AND AUGMENTATION INDEX
Early measurements of aortic impedance laid the groundwork for studies that separated measured pressure and flow waves into forward and backward (or reflected) waves, 34 making it possible to study wave reflection characteristics and their effects on pressure and flow wave contour. Roundtrip travel time of the pressure and flow wave from the heart to the periphery and back is directly related to major reflecting site distance and inversely related to PWV. 5 , 35 Increased arterial stiffness causes an increase in transmission velocity of both forward and reflected waves, which, in turn, causes the reflected wave to arrive earlier in the central aorta with greater amplitude and duration. These changes in wave reflection characteristics augment pressure and decrease flow in late systole. When reflection site distance from the heart is short, travel time of the reflected wave is decreased and, as a consequence, wave reflection amplitude is high and its systolic duration is long. These modifications in arterial effects and reflected wave characteristics cause an increase in systolic (and pulse) BP and LV force generation, which cause a reduction in stroke output. 5 Similar changes occur in the shape of pressure and flow waves when the elastic arteries stiffen. The reflected wave arrives at the heart early during systole because of increased PWV and decreased travel time of the reflected wave from the periphery.
Changes in arterial stiffness can alter systolic and pulse BP in the central arteries by 2 mechanisms, depending on whether the change occurs in the central elastic arteries or the peripheral muscular arteries (or both). In large elastic arteries, the wall becomes stiffer and dilated with age and hypertension and PWVe increases, while in peripheral muscular arteries, stiffness remains relatively constant and PWVm changes very little. 32 The early part of the central arterial pressure wave (the unaugmented pressure) with amplitude (Pi ‐ Pd) (Figure 2) is generated by the LV ejection wave. This forward‐traveling pressure wave is dependent on ascending aortic stiffness and is not influenced by wave reflections. Also, an increase in local arterial stiffness causes a decrease in diastolic pressure (Pd) that is not influenced by wave reflections. 5 Although these mechanisms increase systolic and pulse BP, the increase is minor compared with the increase caused by wave reflection. The later part of the measured pressure wave (the augmented pressure) with amplitude (Ps ‐ Pi) is generated by the reflected wave from the lower body arriving during systole and adding to the forward‐traveling pressure wave. 34 This augmentation of the measured pressure wave can be estimated as an augmentation index (AIa) and is dependent on the elastic effects of the entire arterial tree, the velocity of the forward and reflected waves, and distance to the major reflecting site. 5
Figure 2.

High‐fidelity recordings of ascending aortic pressure and flow waves in 2 middle‐aged men of similar height using a multisensor catheter; 1 patient (56 years of age) was normotensive (left) and the other (61 years of age) had isolated systolic hypertension (ISH)(right). Ascending aortic PWVe was 466 cm/s in the normotensive patient and 583 cm/s in the ISH patient. A distinct inflection point (Pi) can be identified in the normotensive patient but, in the ISH patient, the reflected pressure wave arrives early and merges with the incident wave so that Pi cannot be identified. Therefore, Pi is measured at the point on the pressure wave where peak flow occurs. These morphologic changes in pressure and flow waves are associated with a decrease in travel time (Tr) of the waves to the periphery and back to the heart and an increase in AIa from 32% to 40%. The different components of the ascending aortic pressure wave are shown at the left. Ps indicates peak systolic blood pressure; Pd, minimum diastolic blood pressure; Tr, the round‐trip travel time of the forward wave from the ascending aorta to the major reflecting site and back; ED, ejection duration (ED ‐ Tr is reflected wave systolic duration); Ps ‐ Pi, reflected wave amplitude; Pi ‐ Pd, forward wave amplitude; Ps ‐ Pd, pulse pressure; augmentation index, (Ps ‐ Pi)/(Ps ‐ Pd).
These mechanisms are the major cause of increasing systolic and pulse BP with advancing age and in patients with hypertension and other diseases that increase arterial stiffness. 8 , 32 The augmentation in systolic BP increases LV mass, force generation, tension‐time index, and myocardial oxygen demand, while the decrease in diastolic BP decreases diastolic pressure‐time index and coronary artery perfusion, causing a mismatch in ventricular/vascular coupling and an imbalance in the myocardial oxygen supply/demand ratio. 5
MORPHOLOGIC CHANGES IN AORTIC PRESSURE AND FLOW WAVES WITH AGE AND HYPERTENSION
Age and arterial disease including hypertension are associated with an increase in elastic artery stiffness, which causes profound changes in ascending aortic pressure and flow wave contours (Figure 2). Such changes are associated with increased LV afterload and hypertrophy 27 , 36 and are primarily attributed to changes in PWVe and amplitude and timing of pulse wave reflections from the lower body, resulting from asymptomatic elastic artery degeneration, dilation, and hyperplasia. 5 The major cause of death in individuals with elevated after‐load is from LV hypertrophy and damage to blood vessels in vital organs, causing myocardial infarction, stroke, kidney disease, and heart failure. 11 , 12 In young human patients, a definite diastolic wave occurs in the pressure wave following the incisura. This is explained on the basis of wave reflection from the lower body returning in diastole after aortic valve closure. By middle age (older than 50 years), the elastic arteries become stiffer and both forward and reflected waves travel faster so that the reflected wave arrives during LV ejection and markedly augments pressure in late systole. 35 In older patients (usually those with isolated systolic hypertension) with very stiff elastic arteries and increased PWVe, the reflected wave arrives shortly after aortic valve opening and merges with the forward wave so that no inflection point is seen and reflected wave amplitude and duration are greater 5 ; Pd decreases as stiffness increases. This is the type of pressure wave usually seen when PWVe exceeds PWVm and there is a reversal of the so‐called arterial stiffness gradient. In this type of waveform, the inflection point occurs at peak blood flow velocity and denotes the initial upstroke of the reflected pressure wave. 5 If pressure and flow velocity are simultaneously measured, Pi can be determined and AIa calculated (Figure 2).
The detrimental changes in arterial pressure contour and wave reflection characteristics caused by increased arterial stiffness place an extra workload on the left ventricle, causing it to generate more force to produce the stroke output. Sustained elevation in late systolic BP augmentation and the resulting LV hypertrophy are associated with progressive degenerative changes in the myocytes such that these weaken, developing less force with each contraction. The weakened hypertrophied fibers lengthen and the ventricle dilates, with augmented pressure and stroke output initially being maintained at greater muscle length and ventricular volume through the Frank‐Starling mechanism. The ejection fraction in these patients is usually >40%. Ultimately, compensation is lost and the left ventricle cannot generate the force necessary to overcome the late systolic augmented pressure. AIa and systolic (and pulse) pressure are therefore reduced and associated with a decrease in ejection duration (Figure 3) and stroke volume. The ejection fraction in these patients is usually ≤40%. 12 , 37
Figure 3.

Measured radial artery (left) and synthesized aortic pressure (right) waves recorded in a heart failure patient (53 years of age) before (top) and after heart transplant (bottom). The failing left ventricle could not generate enough force to overcome the late systolic pressure boost usually seen at this age. This reduced contractile force resulted in an abbreviated ejection duration (ED) (240 ms) and a low augmentation index (AIa). After heart transplant, the left ventricle could again generate the necessary force to overcome the late systolic pressure boost, which caused an increase in ED (309 ms) and AIa. Heart rate was similar before and after transplant (75 b/m). Sp, indicates systolic pressure; Dp, diastolic pressure; MP, mean pressure; PP, pulse pressure; HR, heart rate.
Although AIa is associated with arterial wall effects, it is not always a reliable surrogate measure of arterial stiffness in populations because it is influenced by other variables such as height, heart rate, and ejection duration. 5 , 8 AIa is, however, an accurate measure of wave reflection intensity, which is a very important component of LV afterload.
EFFECTS OF INTERVENTION ON ARTERIAL STIFFNESS, PWV, AND WAVE REFLECTIONS
When investigating changes (especially acute changes) in arterial wall effects and wave reflection characteristics that occur in response to a certain intervention, baseline and response measurements should be made at the same time of day because of diurnal variation. AIa is maximum in early morning, gradually diminishes until mid‐afternoon, and increases again somewhat thereafter. 38 Both long‐term cigarette smoking and coffee consumption increase PWVe and exert a detrimental effect on the amplitude and timing of wave reflection. 39 , 40 High‐dose combinations of vitamins C and E 41 and of ezetimibe and simvastatin 19 have beneficial effects on endothelial function and PWVe but do not change AIa in patients with essential hypertension or rheumatoid arthritis.
Studies using antihypertensive drugs to lower BP have drawn attention to their separate effects on arteries and arterioles. Arteriolar vasodilators, such as nitropriusside and α‐blockers, primarily increase arteriolar caliber (decrease tone) and thereby decrease peripheral resistance and mean arterial BP, while arterial vasodilators, such as glyceryl trinitrate, primarily relax SMC in muscular arteries and thereby decrease PWVm and stiffness. 42 Most currently available pressure‐lowering drugs, even vasodilators, have little direct action on the large elastic arteries and PWVe. Some recent reports suggest, however, that long‐term use of angiotensin‐converting enzyme (ACE) inhibitors that inhibit tissue ACE may have a direct beneficial affect on elastic artery stiffness in diabetic hypertensive patients. 43 In general, elastic artery stiffness passively decreases in response to a decrease in mean arterial BP caused by pressure‐lowering drugs or decreased cardiac output. Thus, vasodilator‐type antihypertensive drugs reduce central systolic and pulse BP by a dual mechanism: passive reduction in central elastic artery stiffness and active reduction in peripheral muscular artery stiffness. Although ACE inhibitors and β‐blockers have the same effect on PWVe, ACE inhibitors decrease AIa because they primarily decrease PWVm, while β‐blockers do not. Consequently, β‐blockers are less effective in lowering central BP. 31 , 44
As stated above, increased AIa is primarily caused by the changes in amplitude and timing of wave reflection as a result of increased PWVe with little change in PWVm. Acute modification of AIa is accomplished by the active effect of drugs on muscular arteries and the passive (pressure‐lowering) effect on elastic arteries. Vasodilator drugs such as ACE inhibitors, angiotensin receptors blockers, calcium channel blockers, nitrates, sildenafil, and aldosterone antagonists actively decrease arterial stiffness and PWVm and alter reflected wave characteristics through their action on SMC in the arterial wall, while diuretics passively decrease elastic artery stiffness and PWVe and alter wave reflection characteristics and BP by decreasing intravascular volume. 18 , 45 It has been suggested that long‐term use of diuretics may decrease peripheral resistance. 46 Although β‐blockers decrease PWVe and BP, they do not decrease wave reflection intensity. A hierarchy of wave reflection and central BP responses to such therapy has been reported: calcium channel blockers produced the most significant antihypertensive effect, followed by diuretics, ACE inhibitors, and β‐blockers. 45
The reduction in PWV (PWVe and/or PWVm) delays return of the reflected wave from the periphery to the heart and decreases its amplitude and systolic duration. These changes are associated with a decrease in the low‐frequency aortic input impedance moduli and a shift of the impedance moduli minimum to the left, indicating increased reflection site distance from the heart. 5 , 35 These modifications of the reflected wave characteristics decrease systolic and pulse BP, AIa, and LV force generation (Figure 4), which leads to regression of LV hypertrophy. 47 Furthermore, drug‐induced regression in LV mass may be achieved without any reduction in peripheral BP. 48
Figure 4.
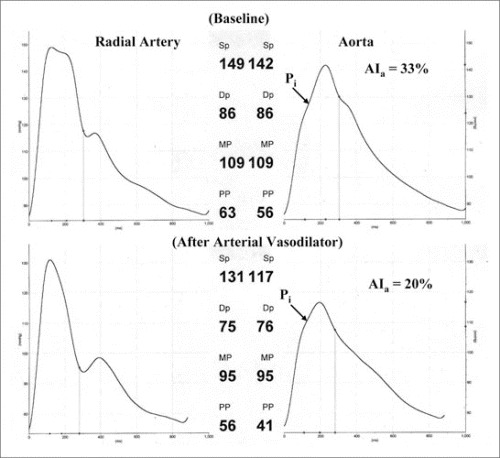
Measured radial artery (left) and synthesized aortic pressure (right) waves recorded in a hypertensive patient at baseline (top) and after treatment with the angiotensin‐converting enzyme inhibitor lisinopril (bottom). The vasodilator caused a delay in transmission velocity of the reflected wave from the periphery to the heart that resulted in a decline of augmented pressure (from 18 mm Hg to 8.0 mm Hg), augmentation index (AIa) (from 33% to 20%), and AIa@75 (from 25% to 16%). Reflected wave systolic duration decreased from 158 ms to 139 ms. Aortic systolic blood pressure decreased 25 mm Hg, while brachial systolic blood pressure was less sensitive, decreasing 18 mm Hg.
These changes suggest that the beneficial effects of these drugs are grossly underestimated by measurements of brachial artery cuff BP. Therefore, clinical trials such as the Heart Outcomes Prevention Evaluation (HOPE) 49 and the Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) 48 studies cannot conclude, with certainty, that the beneficial effects, including regression of LV hypertrophy, of the ACE inhibitor ramipril or the angiotensin receptors blocker losartan are independent of changes in arterial BP. This contention is supported by results from the Conduit Artery Function Evaluation (CAFE) 31 and the Preterax in Regression of Arterial Stiffness in a Controlled Double‐Blind Study (REASON) trials. 44 , 47 Newer agents capable of modifying the effects of the large elastic arteries may yet enter clinical practice. Alagebrium (or ALT‐711), a thiazolium‐based compound, is able to break advanced glycation end product‐related collagen cross‐links. This agent has been associated with reduced aortic stiffness and a decrease in central pulse BP in both animals and humans. 50 Likewise, the use of agents to retard or reverse the deposition of calcium in the large arteries, such as bisphosphonates, 51 may prove therapeutically useful. Agents such as these have the potential to improve elastic artery stiffness, which will decrease systolic BP by reducing wave reflection amplitude and increase diastolic BP by increasing elastic artery distensibility. These modifications in arterial effects and wave reflection characteristics are the ideal mode of action for antihypertensive treatment, especially in older patients with isolated systolic hypertension and in patients with CAD.
CONCLUSIONS
Compared with diastolic BP, brachial systolic and pulse BP are stronger predictors of CAD, myocardial infarction, congestive heart failure, stroke, end‐stage renal disease, and overall CV mortality in patients 50 years and older. The principal cause of increased systolic and pulse BP in the aged and those with hypertension and other vascular diseases is an increase in stiffness of the elastic arteries as a result of degeneration and hyperplasia of the arterial wall. An increase in stiffness also causes a decrease in diastolic BP, which inversely correlates with CAD and CV mortality in this age group. Furthermore, recent studies have shown that central BP, the pressure exerted on major internal organs, is a better predictor of CV risk than brachial BP. The central aortic pressure wave is composed of a forward‐traveling and a backward‐traveling reflected wave. It has been suggested that waveform dispersion, not reflection, may be the major determinant of aortic pressure waveform morphology 52 ; however, the main emphasis of this review is directed toward arterial stiffness and wave reflection. As stiffness increases, forward wave amplitude increases only a small amount, but reflected wave amplitude markedly increases and significantly augments LV and aortic pressure in late systole. These changes in arterial wall effects and wave reflection characteristics cause an increase in LV afterload and myocardial oxygen demand and a decrease in myocardial perfusion pressure, which induces an imbalance in the myocardial supply/demand ratio, especially in patients who have hypertrophied hearts with CAD.
An increase in systolic BP increases arterial wall circumferential stress, which promotes fatigue and dilation and the development of endothelial dysfunction and atherosclerosis. Sustained elevation in systolic BP and persistent LV hypertrophy are associated with progressive degenerative changes in LV contractile force. Ultimately, the ventricle cannot generate the force necessary to overcome the late systolic augmented pressure. When this occurs, all components of BP are reduced, augmented pressure and AIa are markedly decreased, and ejection duration is abbreviated.
Vasoactive drugs have little direct effect on large human elastic arteries but can markedly modify wave reflection amplitude and AIa by altering stiffness of the muscular arteries and changing PWV of the reflected wave from the periphery to the heart. Vasodilators decrease the amplitude and increase the travel time (or delay) of the reflected wave, causing a generalized decrease in systolic BP and arterial wall stress and an increase in ascending aortic blood flow during late systole. The decrease in central systolic BP brought about by this mechanism is grossly underestimated when BP is measured in the brachial artery.
References
- 1. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. [DOI] [PubMed] [Google Scholar]
- 2. Haider AW, Larson MG, Franklin SS, et al. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003;138(1):10–16. [DOI] [PubMed] [Google Scholar]
- 3. Lewington S, Clarke R, Qizilbash N, et al. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. [DOI] [PubMed] [Google Scholar]
- 4. Chirinos JA, Zambrano JP, Chakko S, et al. Relation between ascending aortic pressures and outcomes in patients with angiographically demonstrated coronary artery disease. Am J Cardiol. 2005;96(5):645–648. [DOI] [PubMed] [Google Scholar]
- 5. Nichols WW, O'Rourke MF. McDonald's Blood Flow in Arteries: Theoretic, Experimental and Clinical Principles. 5th ed. London, England: Edward Arnold; 2005. [Google Scholar]
- 6. Pauca AL, O'Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38(4):932–937. [DOI] [PubMed] [Google Scholar]
- 7. Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure. The Strong Heart Study. Hypertension. 2007;50(1):197–203. [DOI] [PubMed] [Google Scholar]
- 8. McEniery CM, Yasmin, Hall IR, et al. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo‐Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol. 2005;46(9):1753–1760. [DOI] [PubMed] [Google Scholar]
- 9. Wallace SM, Yasmin, McEniery CM, et al. Isolated systolic hypertension is characterized by increased aortic stiffness and endothelial dysfunction. Hypertension. 2007;50(1):228–233. [DOI] [PubMed] [Google Scholar]
- 10. Weber T, Auer J, O'Rourke MF, et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109(2):184–189. [DOI] [PubMed] [Google Scholar]
- 11. Hirai T, Sasayama S, Kawasaki T, et al. Stiffness of systemic arteries in patients with myocardial infarction. A noninvasive method to predict severity of coronary atherosclerosis. Circulation. 1989;80(1):78–86. [DOI] [PubMed] [Google Scholar]
- 12. Weber T, Auer J, Lamm G, et al. Arterial stiffness, central blood pressures, and wave reflections in cardiomyopathy‐implications for risk stratification. J Card Fail. 2007;13(5):353–359. [DOI] [PubMed] [Google Scholar]
- 13. London GM, Marchais SJ, Guerin AP. Arterial stiffness and function in end‐stage renal disease. Adv Chronic Kidney Dis. 2004;11(2):202–209. [DOI] [PubMed] [Google Scholar]
- 14. Wilkinson IB, MacCallum H, Rooijmans DF, et al. Increased augmentation index and systolic stress in type 1 diabetes mellitus. QJM. 2000;93(7):441–448. [DOI] [PubMed] [Google Scholar]
- 15. Brooks BA, Molyneaux LM, Yue DK. Augmentation of central arterial pressure in Type 2 diabetes. Diabet Med. 2001;18(5):374–380. [DOI] [PubMed] [Google Scholar]
- 16. Wilkinson IB, Prasad K, Hall IR, et al. Increased central pulse pressure and augmentation index in subjects with hypercholesterolemia. J Am Coll Cardiol. 2002;39(6):1005–1011. [DOI] [PubMed] [Google Scholar]
- 17. Tayama J, Munakata M, Yoshinaga K, et al. Higher plasma homocysteine concentration is associated with more advanced systemic arterial stiffness and greater blood pressure response to stress in hypertensive patients. Hypertens Res. 2006;29(6):403–409. [DOI] [PubMed] [Google Scholar]
- 18. Mahmud A, Feely J. Aldosterone‐to‐renin ratio, arterial stiffness, and the response to aldosterone antagonism in essential hypertension. Am J Hypertens. 2005;18(1):50–55. [DOI] [PubMed] [Google Scholar]
- 19. Maki‐Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol. 2007;50(9):852–858. [DOI] [PubMed] [Google Scholar]
- 20. Kullo IJ, Seward JB, Bailey KR, et al. C‐reactive protein is related to arterial wave reflection and stiffness in asymptomatic subjects from the community. Am J Hypertens. 2005;18(8):1123–1129. [DOI] [PubMed] [Google Scholar]
- 21. Phillips C, Hedner J, Berend N, et al. Diurnal and obstructive sleep apnea influences on arterial stiffness and central blood pressure in men. Sleep. 2005;28(5):604–609. [DOI] [PubMed] [Google Scholar]
- 22. Sato E, Feke GT, Appelbaum EY, et al. Association between systemic arterial stiffness and age‐related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2006;244(8):963–971. [DOI] [PubMed] [Google Scholar]
- 23. Wilkinson IB, Franklin SS, Hall IR, et al. Pressure amplification explains why pulse pressure is unrelated to risk in young subjects. Hypertension. 2001;38(6):1461–1466. [DOI] [PubMed] [Google Scholar]
- 24. Boutouyrie P, Bussy C, Lacolley P, et al. Association between local pulse pressure, mean blood pressure, and large‐artery remodeling. Circulation. 1999;100(13):1387–1393. [DOI] [PubMed] [Google Scholar]
- 25. Nakayama Y, Tsumura K, Yamashita N, et al. Pulsatility of ascending aortic pressure waveform is a powerful predictor of restenosis after percutaneous transluminal coronary angioplasty. Circulation. 2000;101(5):470–472. [DOI] [PubMed] [Google Scholar]
- 26. Jankowski P, Kawecka‐Jaszcz K, Czarnecka D, et al. Ascending aortic, but not brachial blood pressure‐derived indices are related to coronary atherosclerosis. Atherosclerosis. 2004;176(1):151–155. [DOI] [PubMed] [Google Scholar]
- 27. Hashimoto J, Imai Y, O'Rourke MF. Indices of pulse wave analysis are better predictors of left ventricular mass reduction than cuff pressure. Am J Hypertens. 2007;20(4):378–384. [DOI] [PubMed] [Google Scholar]
- 28. Brown DW, Giles WH, Croft JB. Left ventricular hypertrophy as a predictor of coronary heart disease mortality and the effect of hypertension. Am Heart J. 2000;140(6):848–856. [DOI] [PubMed] [Google Scholar]
- 29. Safar ME, Blacher J, Pannier B, et al. Central pulse pressure and mortality in end‐stage renal disease. Hypertension. 2002;39(3):735–738. [DOI] [PubMed] [Google Scholar]
- 30. O'Rourke MF, Kelly RP, Avolio AP, et al. Effects of arterial dilator agents on central aortic systolic pressure and on left ventricular hydraulic load. Am J Cardiol. 1989;63(19):38I–44I. [DOI] [PubMed] [Google Scholar]
- 31. Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure‐lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113(9):1213–1225. [DOI] [PubMed] [Google Scholar]
- 32. McEniery CM, Wilkinson IB, Avolio AP. Age, hypertension and arterial function. Clin Exp Pharmacol Physiol. 2007;34(7):665–671. [DOI] [PubMed] [Google Scholar]
- 33. Mitchell GF, Parise H, Benjamin EJ, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43(6):1239–1245. [DOI] [PubMed] [Google Scholar]
- 34. Westerhof N, Sipkema P, Van Den Bos GC, et al. Forward and backward waves in the arterial system. Cardiovasc Res. 1972;6(6):648–656. [DOI] [PubMed] [Google Scholar]
- 35. Murgo JP, Westerhof N, Giolma JP, et al. Aortic input impedance in normal man: relationship to pressure wave forms. Circulation. 1980;62(1):105–116. [DOI] [PubMed] [Google Scholar]
- 36. Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322(22):1561–1566. [DOI] [PubMed] [Google Scholar]
- 37. Westerhof N, O'Rourke MF. Haemodynamic basis for the development of left ventricular failure in systolic hypertension and for its logical therapy. J Hypertens. 1995;13(9):943–952. [DOI] [PubMed] [Google Scholar]
- 38. Papaioannou TG, Karatzis EN, Papamichael CM, et al. Circadian variation of arterial pressure wave reflections. Am J Hypertens. 2006;19(3):259–263. [DOI] [PubMed] [Google Scholar]
- 39. Jatoi NA, Jerrard‐Dunne P, Feely J, et al. Impact of smoking and smoking cessation on arterial stiffness and aortic wave reflection in hypertension. Hypertension. 2007;49(5):981–985. [DOI] [PubMed] [Google Scholar]
- 40. Vlachopoulos C, Panagiotakos D, Ioakeimidis N, et al. Chronic coffee consumption has a detrimental effect on aortic stiffness and wave reflections. Am J Clin Nutr. 2005;81(6):1307–1312. [DOI] [PubMed] [Google Scholar]
- 41. Plantinga Y, Ghiadoni L, Magagna A, et al. Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am J Hypertens. 2007;20(4):392–397. [DOI] [PubMed] [Google Scholar]
- 42. Stokes GS, Barin ES, Gilfillan KL. Effects of isosorbide mononitrate and AI inhibition on pulse wave reflection in hypertension. Hypertension. 2003;41(2):297–301. [DOI] [PubMed] [Google Scholar]
- 43. Tropeano AI, Boutouyrie P, Pannier B, et al. Brachial pressure‐independent reduction in carotid stiffness after long‐term angiotensin‐converting enzyme inhibition in diabetic hypertensives. Hypertension. 2006;48(1):80–86. [DOI] [PubMed] [Google Scholar]
- 44. Asmar RG, London GM, O'Rourke ME, et al. Improvement in blood pressure, arterial stiffness and wave reflections with a very‐low‐dose perindopril/indapamide combination in hypertensive patient: a comparison with atenolol. Hypertension. 2001;38(4):922–926. [DOI] [PubMed] [Google Scholar]
- 45. Morgan T, Lauri J, Bertram D, et al. Effect of different antihypertensive drug classes on central aortic pressure. Am J Hypertens. 2004;17(2):118–123. [DOI] [PubMed] [Google Scholar]
- 46. Levenson J, Gariepy J, Megnien JL, et al. Diuretics and arteriolar resistance and arterial compliance in human hypertension. Eur Heart J. 1992;13 suppl G:48–52. [DOI] [PubMed] [Google Scholar]
- 47. De Luca N, Asmar RG, London GM, et al; REASON Project Investigators . Selective reduction of cardiac mass and central blood pressure on low‐dose combination perindopril/indapamide in hypertensive subjects. J Hypertens. 2004;22(8):1623–1630. [DOI] [PubMed] [Google Scholar]
- 48. Devereux RB, Dahlof B, Gerdts E, et al. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation. 2004;110(11):1456–1462. Epub 2004 Aug 23. [DOI] [PubMed] [Google Scholar]
- 49. Mathew J, Sleight P, Lonn E, et al. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin‐converting enzyme inhibitor ramipril. Circulation. 2001;104(14):1615–1621. [DOI] [PubMed] [Google Scholar]
- 50. Kass DA, Shapiro EP, Kawaguchi M, et al. Lakatta EG. Improved arterial compliance by a novel advanced glycation end‐product crosslink breaker. Circulation. 2001;104(13):1464–1470. [DOI] [PubMed] [Google Scholar]
- 51. Ariyoshi T, Eishi K, Sakamoto I, et al. Effect of etidronic acid on arterial calcification in dialysis patients. Clin Drug Investig. 2006;26(4):215–222. [DOI] [PubMed] [Google Scholar]
- 52. Hope SA, Tay DB, Meredith IT, et al. Waveform dispersion, not reflection, may be the major determinant of aortic pressure Instant CME Credit www.ash‐us.org/highlights wave morphology. Am J Physiol Heart Circ Physiol. 2005;289(6):H2497–H2502. [DOI] [PubMed] [Google Scholar]


