Abstract
A post hoc pooled analysis of 2 multicenter, randomized, double‐blind, active‐controlled force‐titration studies assessed the antihypertensive efficacy and tolerability of 7 to 8 weeks' once‐daily fixed‐dose irbesartan/hydrochlorothiazide (HCTZ) 300/25 mg in 796 stage 1 or 2 hypertensive patients according to age (65 years or older or younger than 65) (n=121 or 675) and presence or absence of obesity (n=378 or 414), type 2 diabetes (n=99 or 697), and high World Health Organization‐defined cardiovascular risk (n=593 or 202). Systolic/diastolic blood pressure reductions (27–31/16–22 mm Hg) were similar regardless of age, obesity, and type 2 diabetes status and were greater in high‐ vs low‐risk patients. Dizziness (2.0%–3.7%), hypotension (0%–0.7%), and syncope (0%) were rare and not centered in any subgroup. There was no hypotension in the elderly or in type 2 diabetics. Irbesartan/HCTZ provided consistent blood pressure lowering and tolerability regardless of age, obesity, and type 2 diabetes and greater efficacy in patients with high cardiovascular risk.
Elevated blood pressure (BP) frequently coexists with other cardiovascular (CV) risk factors, such as advanced age, obesity, and type 2 diabetes, all of which compound the CV risk of hypertension. 1 , 2 , 3 The result is a diverse population of high‐risk patients who require antihypertensive treatment to reduce their chance of experiencing a CV event, stroke, or target‐organ damage. The presence of CV risk factors, however, may influence the response of individual patients to antihypertensive treatment regimens. Therefore, it is important to assess whether the BP‐lowering efficacy and tolerability of recommended agents is consistent in subgroups of high‐risk patients.
Inadequate control of BP is still leaving patients at risk of CV disease, particularly those patients with multiple CV risk factors. In the United States, the BP goals set by hypertension management guidelines (<140/90 mm Hg; <130/80 mm Hg in patients with type 2 diabetes or renal dysfunction) 1 , 2 , 3 are currently being achieved in only one‐half of treated patients and only one‐third of the population. 4 , 5 Similar low rates of hypertension control have been reported worldwide. 6 Furthermore, although data are limited, the percentage of patients with stage 2 hypertension achieving goal BP may be even lower. Thus, in most patients, treatment guidelines advocate combination therapy to achieve goal BP with any one of several medications (ie, an angiotensin receptor blocker [ARB], angiotensin‐converting enzyme [ACE] inhibitor, or β‐blocker combined with a thiazide diuretic, hydrochlorothiazide [HCTZ], or a calcium channel blocker with an ACE inhbitor). 1 , 2 , 3 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 An ARB‐based combination, irbesartan/HCTZ, is one such treatment that has demonstrated antihypertensive efficacy and tolerability in the management of hypertension. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 Although previous clinical trials of this medication have included a broad range of patients with hypertension, the consistency of BP‐lowering efficacy and tolerability has not yet been examined in double‐blind controlled studies involving large subgroups of high‐risk patients.
A post hoc pooled analysis was performed on 2 similar, randomized, double‐blind active‐controlled trials involving 796 patients with stage 1 or 2 hypertension treated for up to 12 weeks with first‐step fixed‐dose irbesartan/HCTZ 150/12.5 mg, force‐titrated to 300/25 mg. 24 , 25 Pooling these 2 trials produced sufficiently large patient subgroups to assess BP‐lowering efficacy and tolerability according to age (65 years or older or younger than 65) and the presence or absence of obesity, type 2 diabetes, and high CV risk according to World Health Organization (WHO) criteria. 2 The latter includes the additional risk factors of smoking, elevated serum cholesterol level, target‐organ damage, renal disease, and family history of CV disease. Thus, the patients included in the present analysis represent the more difficult‐to‐treat patients with hypertension seen in clinical practice. Importantly, the 2 trials had a similar design, including major inclusion and exclusion criteria (except BP levels), irbesartan/HCTZ dosing, and efficacy evaluation at weeks 7 and 8.
METHODS
Study Design and Patients
This was a post hoc analysis of data pooled from 2 first‐step, multicenter, randomized, double‐blind, active‐controlled force‐titration studies of patients aged 18 years and older with hypertension. The methods of these trials have been published previously. 24 , 25 Briefly:
-
•
Study 1 was a 7‐week trial in patients with seated diastolic BP (SeDBP) ≥110 mm Hg. Following a 7‐day single‐blind placebo lead‐in period, randomization was 2:1 to irbesartan/HCTZ 150/12.5‐mg fixed‐dose combination force‐titrated to 300/25 mg after week 1 (n=468), or irbesartan 150 mg force‐titrated to 300 mg after week 1 (n=229).
-
•
Study 2 was a 12‐week trial in patients with a seated systolic BP (SeSBP) level 160 to 180 mm Hg or SeDBP level 100 to 110 mm Hg. Following a 21‐day single‐blind placebo wash‐out period, patients were randomized 3:1:1 to irbesartan/HCTZ 150/12.5‐mg fixed‐dose combination force‐titrated to 300/25 mg after week 2 (n=328), irbesartan 150 mg force‐titrated to 300 mg after week 2 (n=106), or HCTZ 12.5 mg force‐titrated to 25 mg after week 2 (n=104).
Patients were excluded if their BP level was above certain limits (study 1, SeSBP ≥220 mm Hg or SeDBP ≥130 mm Hg; study 2, SeSBP ≥180 mm Hg or SeDBP ≥110 mm Hg) or if they had known or suspected secondary hypertension or any condition that required more immediate BP lowering. All study medication was taken once daily between 6 am and 11 am, except on the morning of a study visit so that BP could be measured at trough (24±3 hours following the last dose of study medication).
A total of 185 study centers in the United States, Canada, Germany, France, the Netherlands, Belgium, Russia, and Israel randomized patients in study 1. In study 2, 135 study centers randomized patients in the United States, Canada, Germany, and France. All centers received prior ethics committee and/or institutional review board approval, and the trials were conducted in accordance with the ethical principles of the current Declaration of Helsinki. All patients gave written informed consent before study enrollment.
This pooled analysis focused only on patients who had available efficacy data at week 7 (study 1) or week 8 (study 2) and who received fixed‐dose irbesartan/HCTZ. Those patients who received monotherapy in the 2 studies were not included, since the goal was to assess whether the BP‐lowering efficacy and tolerability of this medication was consistent in subgroups of high‐risk patients. These high‐risk patients were pooled and analyzed overall and divided into the following subgroups: (1) those aged 65 years or older or those younger than 65 years; (2) those with and without obesity (body mass index [BMI] ≥30 kg/m2); (3) those with and without type 2 diabetes; and (4) those with and without high CV risk defined as patients meeting WHO‐defined criteria for either “high” or “very high” CV risk. 2 Patients could belong to more than one of these subgroups.
Efficacy and Safety End Points Following Fixed‐Dose Irbesartan/HCTZ 300/25 mg
Efficacy evaluations were conducted on all randomized patients. End points included the mean change from baseline to week 7 (study 1) or week 8 (study 2) in SeSBP and SeDBP in the overall pooled hypertensive population and in the pooled hypertension subgroups above. In addition, the proportion of patients achieving SeDBP control (<90 mm Hg) and dual SeSBP/SeDBP control (<140/90 mm Hg; <130/80 mm Hg in the subgroups with and without type 2 diabetes only) were assessed.
All randomized patients who took at least 1 dose of double‐blind study medication were included in the safety analysis. The incidence and severity of all adverse events occurring during double‐blind treatment were reported for the pooled patients. These included adverse events that were considered to be treatment‐related, serious, and the cause of discontinuation from either study. The incidence of prespecified adverse events was reported, including dizziness, hypotension, syncope, headache, hyperkalemia, hypokalemia, and serum potassium level >6.0 mmol/L.
Statistical Analysis
Baseline characteristics were descriptively summarized. Mean changes from baseline in BP were determined, and 95% confidence intervals were calculated for between‐subgroup differences using a t test. Rates for BP control were expressed as frequency counts and percentages with 95% confidence intervals for within subgroups.
Mean systolic BP (SBP) reductions from baseline were adjusted using an analysis of covariance model for baseline age, BMI, type 2 diabetes, sex, race, cholesterol, target organ damage, and acute coronary syndrome.
RESULTS
Patient Characteristics
The 2 trials included a combined total of 796 patients who received irbesartan/HCTZ 300/25‐mg fixed‐dose combination therapy. The numbers of patients in the pooled hypertension subgroups were as follows: (1) aged 65 years or older (n=121) and younger than 65 years (n=675); (2) with (n=378) and without (n=414) obesity; (3) with (n=99) and without (n=697) type 2 diabetes; and (4) with (n=593) and without (n=202) high CV risk.
Baseline patient demographics and characteristics of the pooled hypertensive population are shown in Table I. Mean ± SD SBP was 167.5±15.5 mm Hg and mean diastolic BP (DBP) was 106.8±9.9 mm Hg. Pooled patients had a mean age of 53.4±10.9 years, and 121 (15%) patients were aged 65 years or older. Most patients were white (84%), and 58% were male.
Table I.
Baseline Demographics and Characteristics of Patients Pooled From 2 First‐Step, Multicenter, Randomized, Double‐Blind, Active‐Controlled Studies of Stage 1 or 2 Hypertension
| Parameter | Pooled (N=796) |
|---|---|
| Male | 457 (58) |
| Race/ethnic groupa | |
| Caucasian | 666 (84) |
| African American | 117 (15) |
| Asian | 9 (1) |
| Other | 4 (1) |
| Age | |
| Mean (SD), y | 53.4 (10.9) |
| Proportion aged 65 y or older | 121 (15) |
| Duration of hypertension, y | |
| Mean (SD) | 7.0 (7.7) |
| Range | 0–48.9 |
| SBP, mm Hg | |
| Mean (SD) | 167.5 (15.5) |
| Range | 127.7–221.7 |
| DBP, mm Hg | |
| Mean (SD) | 106.8 (9.9) |
| Range | 66.0–131.6 |
| Other cardiovascular risk factors | |
| Body weight, kg | |
| Mean (SD) | 88.8 (20.0) |
| Range | 46.0–164.3 |
| BMI, mean (SD), kg/m2 | 30.6 (5.9) |
| BMI ≥30 kg/m2 | 378 (48) |
| Type 2 diabetes | 99 (13) |
| High WHO‐defined cardiovascular risk | 593 (75) |
| Values are expressed as No. (%). aPatients may have been categorized into more than 1 group. Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; WHO, World Health Organization. | |
Baseline BP levels were not affected by the presence or absence of obesity (165.4/107.1 mm Hg vs 169.1/106.5 mm Hg) or of type 2 diabetes (168.6/104.2 mm Hg vs 167.2/107.1 mm Hg). In contrast, patients aged 65 years or older had somewhat higher SBP and lower DBP than those younger than 65 years (173.5/101.0 vs 166.2/107.8 mm Hg). In addition, patients at high CV risk had more severe hypertension at baseline than did patients at low CV risk (169.5/109.8 mm Hg vs 161.0/98.2 mm Hg). In all patient subgroups, baseline BP was above the recommended goal of 140/90 mm Hg, by 21 to 34 mm Hg for SBP and by 8 to 20 mm Hg for DBP, with the greatest differences between baseline and goal observed in patients with high CV risk (SBP and DBP) and the elderly (SBP only).
Mean Changes in SeSBP and SeDBP From Baseline
In the overall pooled population, mean ± SD reductions from baseline to week 7/8 were 29.9±15.9 for SBP and 20.4±10.2 for DBP. Fixed‐dose irbesartan/HCTZ 300/25‐mg treatment provided similar SBP and DBP mean reductions from baseline in the patient subgroups divided by age (65 years or older or younger than 65 years), and the presence or absence of obesity and type 2 diabetes (Figure). Reductions from baseline for these subgroups ranged from 26.7 to 30.6 mm Hg for SBP and from 17.0 to 20.9 mm Hg for DBP. In patients with high CV risk, irbesartan/HCTZ 300/25 mg produced greater BP reductions from baseline than in patients with low CV risk (30.4/22.1 mm Hg vs 28.4/15.6 mm Hg). When adjusted for baseline age, BMI, type 2 diabetes, sex, race, cholesterol, target organ damage, and acute coronary syndrome, mean reductions in SBP were significantly higher in patients aged 65 years or older than in those younger than 65 years (−24.6 vs −21.0 mm Hg; difference −3.6 mm Hg; P=.015) (Table II). Obesity and type 2 diabetes status had no effect on adjusted mean reductions in SBP.
Figure.
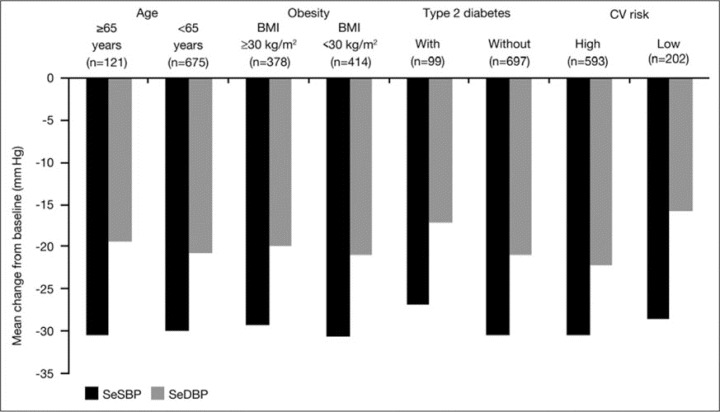
Mean reductions from baseline in seated systolic blood pressure (SeSBP) and seated diastolic blood pressure (SeDBP) following fixed‐dose irbesartan/hydrochlorothiazide 300/25‐mg treatment in pooled hypertensive patients according to age group (65 years or older and younger than 65 years) and obesity, type 2 diabetes, and high cardiovascular (CV) risk status. BMI indicates body mass index.
Table II.
Effect of Age, Obesity, and Type 2 Diabetes Status on Mean SeSBP Following 7 to 8 Weeks' Fixed‐Dose Irbesartan/Hydrochlorothiazide 300/25 mg in Pooled Hypertensive Patientsa
| Patient subgroup | Baseline, No. | SeSBP Adjusted Mean Change From Baseline | |||
|---|---|---|---|---|---|
| Mean (SD) | Difference | P Value | 95% CI | ||
| Age | |||||
| 65 y or older | 121 | −24.6 (1.5) | −3.6 | .015 | −6.4 to –0.7 |
| Younger than 65 y | 675 | −21.0 (1.8) | |||
| Obesity | |||||
| BMI ≥30 kg/m2 | 378 | −22.9 (1.6) | |||
| BMI <30 kg/m2 | 414 | −22.7 (1.5) | −0.2 | .826 | −2.3 to 1.8 |
| Type 2 diabetes | |||||
| With type 2 diabetes | 99 | –21.4 (1.9) | |||
| Without type 2 diabetes | 697 | −24.1 (1.5) | 2.7 | .082 | −0.3 to 5.8 |
| aData were adjusted using an analysis of covariance model, for baseline age, body mass index (BMI), type 2 diabetes, sex, race, cholesterol, target organ damage, and acute coronary syndrome. Abbreviations: CI, confidence interval; SeSBP, seated systolic blood pressure. | |||||
BP Control Rates
The proportions of patients in whom BP control was achieved with irbesartan/HCTZ 150/12.5 mg force‐titrated to 300/25 mg in the pooled hypertensive patient subgroups are shown in Table III. DBP control rates ranged from 51.5% to 72.7%, and rates of dual SBP/DBP control ranged from 35.4% to 56.9%. The BP control rates were similar in patients with and without obesity (DBP control 57.1% vs 62.3%; SBP/DBP control 46.8% vs 42.0%). For the other patient subgroups studied, however, some differences were observed in the rates of patients achieving BP control. Patients aged 65 years or older had higher rates of DBP control (72.7% vs 57.5%) and lower rates of dual SBP/DBP control (37.2% vs 45.5%) than did those younger than 65 years. In patients at low CV risk, higher rates of both DBP control (72.3% vs 55.6%) and SBP/DBP control (56.9% vs 40.0%) were achieved than in patients at high CV risk. The rate of achieving SBP/DBP <140/90 mm Hg was lower in patients with type 2 diabetes than without (35.4% vs 45.5%). Rates of control to the lower target of <130/80 mm Hg were poor, both in patients with and without type 2 diabetes (12.1% and 13.2%, respectively).
Table III.
Proportion of Patients With Blood Pressure Control Following Fixed‐Dose Irbesartan/Hydrochlorothiazide 300/25 mg in Pooled Hypertensive Patients
| Patient Subgroup | Baseline, No. | Controlled, No. (%) | 95% CI | Controlled, No. (%) | 95% CI |
|---|---|---|---|---|---|
| SeDBP <90 mm Hg | SeSBP <140 mm Hg and SeDBP <90 mm Hg | ||||
| Age | |||||
| 65 y or older | 121 | 88 (72.7) | 64.8–80.7 | 45 (37.2) | 28.6–45.8 |
| Younger than 65 y | 675 | 388 (57.5) | 53.8–61.2 | 307 (45.5) | 41.7–49.2 |
| Obesity | |||||
| BMI ≥30 kg/m2 | 378 | 216 (57.1) | 52.2–62.1 | 177 (46.8) | 41.8–51.9 |
| BMI <30 kg/m2 | 414 | 258 (62.3) | 57.7–67.0 | 174 (42.0) | 37.3–46.8 |
| Type 2 diabetes | |||||
| With type 2 diabetes | 99 | 51 (51.5) | 41.7–61.4 | 35 (35.4) | 25.9–44.8 |
| Without type 2 diabetes | 697 | 425 (61.0) | 57.4–64.6 | 317 (45.5) | 41.8–49.2 |
| Cardiovascular risk | |||||
| High | 593 | 330 (55.6) | 51.7–59.6 | 237 (40.0) | 36.0–43.9 |
| Low | 202 | 146 (72.3) | 66.1–78.5 | 115 (56.9) | 50.1–63.8 |
| SeDBP <80 mm Hg | SeSBP <130 mm Hg and SeDBP <80 mm Hg | ||||
| Type 2 diabetes | |||||
| With type 2 diabetes | 99 | 21 (21.2) | 13.2–29.3 | 12 (12.1) | 5.7–18.6 |
| Without type 2 diabetes | 697 | 156 (22.4) | 19.3–25.5 | 92 (13.2) | 10.7–15.7 |
| Abbreviations: BMI, body mass index; CI, confidence interval; SeDBP, seated diastolic blood pressure; SeSBP, seated systolic blood pressure. | |||||
Safety and Tolerability
Adverse event rates were similar regardless of age and the presence or absence of obesity, type 2 diabetes, and high CV risk (35%–43%) (Table IV). Adverse events that were reported at an incidence of ≥3% in any patient subgroup were headache (2.9%–5.6%), dizziness (2.0%–3.7%), upper respiratory tract infection (0.8%–4.0%), nasopharyngitis (1.0%–3.0%), and back pain (0%–3.5%). In addition to dizziness and headache, other prespecified adverse events were hypotension (0%–0.8%) and syncope (no occurrences). There were no incidences of hypotension in patients aged 65 years or older, in patients with a BMI ≥30 kg/m2, or in patients with type 2 diabetes.
Table IV.
Adverse Event Profile by Cardiovascular Risk, Age, Obesity, and Type 2 Diabetes Status (All Treated Patients)
| Adverse Event | Overall (n=796) | Age | Obesity | Type 2 Diabetes | Cardiovascular Risk | ||||
|---|---|---|---|---|---|---|---|---|---|
| 65 y or Older (n=121) | Younger than 65 y (n=675) | BMI <30 kg/m 2 (n=414) | BMI ≥30 kg/m 2 (n=378) | With (n=99) | Without (n=697) | High (n=593) | Low (n=202) | ||
| All | 294 (36.9) | 46 (38.0) | 248 (36.7) | 144 (34.8) | 148 (39.2) | 37 (37.4) | 257 (36.9) | 208 (35.1) | 86 (42.6) |
| Treatment‐related | 100 (12.6) | 16 (13.2) | 84 (12.4) | 51 (12.3) | 48 (12.7) | 14 (14.1) | 86 (12.3) | 73 (12.3) | 27 (13.4) |
| ≥3% Incidence in any hypertension subgroup | |||||||||
| Headache | 33 (4.1) | 4 (3.3) | 29 (4.3) | 12 (2.9) | 21 (5.6) | 4 (4.0) | 29 (4.2) | 26 (4.4) | 7 (3.5) |
| Dizziness | 26 (3.3) | 3 (2.5) | 23 (3.4) | 12 (2.9) | 14 (3.7) | 2 (2.0) | 24 (3.4) | 20 (3.4) | 6 (3.0) |
| URT infection | 19 (2.4) | 1 (0.8) | 18 (2.7) | 5 (1.2) | 14 (3.7) | 2 (2.0) | 17 (2.4) | 11 (1.9) | 8 (4.0) |
| Nasopharyngitis | 17 (2.1) | 3 (2.5) | 14 (2.1) | 9 (2.2) | 8 (2.1) | 1 (1.0) | 16 (2.3) | 11 (1.9) | 6 (3.0) |
| Back pain | 11 (1.4) | 2 (1.7) | 9 (1.3) | 6 (1.4) | 5 (1.3) | 0 | 11 (1.6) | 4 (0.7) | 7 (3.5) |
| Prespecified | |||||||||
| Headache | 33 (4.1) | 4 (3.3) | 29 (4.3) | 12 (2.9) | 21 (5.6) | 4 (4.0) | 29 (4.2) | 27 (4.6) | 11 (5.4) |
| Dizziness | 26 (3.3) | 3 (2.5) | 23 (3.4) | 12 (2.9) | 14 (3.7) | 2 (2.0) | 24 (3.4) | 21 (3.5) | 6 (3.0) |
| Serum potassium >6.0 mmol/L | 7 (0.9) | 1 (0.8) | 6 (0.9) | 5 (1.2) | 2 (0.5) | 0 | 7 (1.0) | 5 (0.8) | 2 (1.0) |
| Hyperkalemia/increased serum potassium | 3 (0.4) | 0 | 3 (0.4) | 1 (0.3) | 2 (0.5) | 0 | 3 (0.4) | 3 (0.5) | 2 (1.0) |
| Hypokalemia/decreased serum potassium | 3 (0.4) | 0 | 3 (0.4) | 1 (0.3) | 2 (0.5) | 1 (1.0) | 2 (0.3) | 4 (0.7) | 2 (1.0) |
| Hypotension | 3 (0.4) | 0 | 3 (0.4) | 3 (0.7) | 0 | 0 | 3 (0.4) | 5 (0.8) | 1 (0.5) |
| Syncope | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Values are expressed as No. (%). Abbreviations: BMI, body mass index; URT, upper respiratory tract. | |||||||||
Treatment‐related adverse events were reported with comparable incidences across all subgroups of patients divided by age (65 years or older or younger than 65 years) and the presence or absence of obesity, type 2 diabetes, and high CV risk (12.3%–14.1%) (Table IV). The incidence of serious adverse events was 1.5% (n=3) in the low CV risk group and 0.7% (n=4) in the high CV risk group. Only one of these events was considered probably related to treatment (hypokalemia in a patient in the low‐risk group whose potassium level was 3.2 mEq/L).
DISCUSSION
Patients with hypertension require rapid BP lowering to recommended goals to avoid the increased risk of CV events that is associated with delayed or failed BP goal achievement. 26 In recognition of the need for ≥2 antihypertensive agents to reach BP goals, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) 1 and European Society of Hypertension/European Society of Cardiology (ESH/ESC) 7 guidelines recommend the initial use of 2‐drug therapy in patients with stage 2 hypertension.
In this pooled analysis of 2 randomized, double‐blind, active‐controlled trials, the antihypertensive efficacy and tolerability of fixed‐dose irbesartan/HCTZ 300/25‐mg treatment for 7 to 8 weeks was consistent in patients with stage 1 or 2 hypertension divided according to age (65 years or older or younger than 65 years) and the presence or absence of obesity and type 2 diabetes. Patients with WHO‐defined high CV risk had higher baseline levels of BP and experienced greater BP reductions with treatment than low‐risk patients. At baseline, mean SBP in the patient subgroups was 21 to 34 mm Hg above the recommended goal of 140 mm Hg, and mean DBP was 8 to 20 mm Hg above the goal of 90 mm Hg. Thus, the BP reductions produced by irbesartan/HCTZ in this analysis (27–31 mm Hg for SBP and 16–22 mm Hg for DBP) were clinically relevant in all patient subgroups.
Individuals who are elderly or obese or have type 2 diabetes or high CV risk due to multiple coexisting risk factors each represent substantial proportions of patients with hypertension seen in clinical practice. 1 , 2 , 3 Previous studies have suggested that ARB/HCTZ may represent an effective treatment option for reducing SBP and DBP in a broad range of patients with hypertension. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 In a previous multicenter, open‐label, single‐arm trial, irbesartan/HCTZ provided effective and well‐tolerated BP lowering in subgroups of patients with type 2 diabetes or the metabolic syndrome, 23 obesity, 27 and isolated systolic hypertension (S.G. Chyrsant, MD, unpublished data, 2007) and in different racial/ethnic groups. 22 The present analysis confirms that irbesartan/HCTZ is consistently effective and well tolerated in patients who are elderly or obese, or have type 2 diabetes. Importantly, the pooled studies were registrational trials designed to establish and support irbesartan/HCTZ as a first‐step treatment for patients with stage 1 and 2 hypertension.
Antihypertensive treatment that includes a renin‐angiotensin‐aldosterone system (RAAS) blocker may be particularly beneficial in obese patients and those with type 2 diabetes. Activation of the RAAS is a common feature of obesity and is also implicated in the development of insulin resistance and type 2 diabetes. 28 , 29 ARB‐based therapies may also provide renoprotective potential and a small potential for adverse metabolic events. They are, therefore, recommended by current guidelines for patients with type 2 diabetes, in particular patients with target organ damage. 3 , 30 , 31
The greater BP‐lowering efficacy with ARB/HCTZ in patients with high vs low CV risk is consistent with previous antihypertensive studies. In a meta‐analysis of 354 randomized double‐blind placebo‐controlled trials, 5 classes of antihypertensive agents (thiazides, β‐blockers, ACE inhibitors, ARBs, and calcium channel blockers) produced greater BP reductions in patients who had higher pretreatment BP levels than those with lower pretreatment BP levels. 32 Efficacy in patients at risk of CV disease is important given their relatively high pretreatment BP values.
Rates of BP control (<140/90 mm Hg) with irbesartan/HCTZ 150/12.5 mg force‐titrated to 300/25 mg in the present analysis ranged from 35% to 57% in the patient subgroups. The better rates of DBP control and poorer rates of SBP/DBP control in the elderly patients may be explained by their higher SBP and lower DBP levels at baseline and the frequent occurrence of isolated systolic hypertension in the elderly. Patients with low CV risk had better BP control rates than high‐risk patients, and this is explained by the lower BP values at baseline in the low‐risk group. The low rate of reaching the stringent goal of <130/80 mm Hg in patients with and without type 2 diabetes is consistent with American Diabetes Association guidelines suggesting that at least 3 antihypertensive medications may be required to reach such a target. 3
Initial use of combination antihypertensive therapy is recommended in most patients with stage 2 and severe hypertension for maximal CV protection. 1 , 7 This is particularly relevant in patients with severely elevated BP levels that are far from their recommended BP goals. In addition to effective BP lowering, a primary concern of hypertension management is the safety of treatment (ie, that lowering BP too quickly may lead to hypotension, dizziness, and syncope). Treatment with irbesartan/HCTZ in previous studies of up to 1 year have demonstrated the tolerability of treatment. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 33 The present analysis shows that the tolerability of fixed‐dose irbesartan/HCTZ treatment is consistent across patient subgroups divided according to age, obesity, type 2 diabetes, and CV risk. Hypotension did not occur in the elderly or in patients with type 2 diabetes, 2 populations considered to be at risk of this event. 1 Importantly, the pooled analysis has demonstrated that there was no hypotension in either the elderly or in patients with type 2 diabetes, populations in which the propensity for such events may be of special concern. These findings support recommendations by the JNC 7 and ESH/ESC for aggressive 2‐drug therapy as initial treatment for patients with stage 2 hypertension and suggest that this approach can be applied across a broad range of patients with hypertension.
CONCLUSIONS
Treatment guidelines encourage assessment of overall CV risk and individualization of antihypertensive therapy. Thus, knowledge about the BP‐lowering efficacy and tolerability of recommended antihypertensive drug combinations in high‐risk subpopulations is important. Antihypertensive treatment with the maximum dose of fixed‐dose irbesartan/HCTZ, 300/25 mg, provided effective BP lowering that was consistent across subgroups of patients with stage 1 or 2 hypertension, regardless of age, obesity, and type 2 diabetes. Patients with WHO‐defined high CV risk had higher baseline BP levels and experienced greater reductions in BP with this ARB/HCTZ treatment than did low‐risk patients. Fixed‐dose combination was also well tolerated across the broad range of patients with hypertension.
Disclosures:
Matthew R. Weir, MD, is an ad hoc consultant and member of the Speakers' Bureaus for MSD, Bristol‐Myers Squibb/Sanofi Pharmaceuticals Partnership, Novartis, and Boehringer‐Ingelheim. Joel M. Neutel, MD, is a member of the Speakers' Bureaus for Novartis, Bristol‐Myers Squibb/Sanofi Pharmaceuticals Partnership, Boehringer Ingelheim, Pfizer, Forest, Biovail, and Sankyo. Amitabha Bhaumik, PhD; Maria Elena De Obaldia; and Pablo Lapuerta, MD, conducted this work as employees of Bristol‐Myers Squibb.
References
- 1. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization, International Society of Hypertension Writing Group . 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–1992. [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes Association . Standards of medical care in diabetes—2007. Diabetes Care. 2007;30(suppl 1):S4–S41. [DOI] [PubMed] [Google Scholar]
- 4. Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. [DOI] [PubMed] [Google Scholar]
- 5. Ong KL, Cheung BM, Man YB, et al. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. [DOI] [PubMed] [Google Scholar]
- 6. Wolf‐Maier K, Cooper RS, Kramer H, et al. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension. 2004;43:10–17. [DOI] [PubMed] [Google Scholar]
- 7. Guidelines Committee . 2003 European Society of Hypertension‐European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–1053. [DOI] [PubMed] [Google Scholar]
- 8. Benedict CR . Safe and effective management of hypertension with fixed‐dose combination therapy: focus on losartan plus hydrochlorothiazide. Int J Clin Pract. 2000;54:48–54. [PubMed] [Google Scholar]
- 9. Chrysant SG. Fixed combination therapy of hypertension: focus on valsartan/hydrochlorothiazide combination (Diovan/HCT). Expert Rev Cardiovasc Ther. 2003;1:335–343. [DOI] [PubMed] [Google Scholar]
- 10. Croom KF, Curran MP, Goa KL, et al. Irbesartan: a review of its use in hypertension and in the management of diabetic nephropathy. Drugs. 2004;64:999–1028. [DOI] [PubMed] [Google Scholar]
- 11. Bohm M, Sachse A. Safety and tolerability of eprosartan in combination with hydrochlorothiazide. Drug Saf. 2002;25:599–611. [DOI] [PubMed] [Google Scholar]
- 12. Maillard M, Burnier M. Telmisartan/hydrochlorothiazide: a new fixed dose combination. Expert Rev Cardiovasc Ther. 2005;3:375–386. [DOI] [PubMed] [Google Scholar]
- 13. Melian EB, Jarvis B. Candesartan cilexetil plus hydrochlorothiazide combination: a review of its use in hypertension. Drugs. 2002;62:787–816. [DOI] [PubMed] [Google Scholar]
- 14. Chrysant SG, Chrysant GS. Antihypertensive efficacy of olmesartan medoxomil alone and in combination with hydrochlorothiazide. Expert Opin Pharmacother. 2004;5:657–667. [DOI] [PubMed] [Google Scholar]
- 15. Waeber B. Combination therapy with ACE inhibitors/angiotensin II receptor antagonists and diuretics in hypertension. Expert Rev Cardiovasc Ther. 2003;1:43–50. [DOI] [PubMed] [Google Scholar]
- 16. Kochar M, Guthrie R, Triscari J, et al. Matrix study of irbesartan with hydrochlorothiazide in mild‐to‐moderate hypertension. Am J Hypertens. 1999;12:797–805. [DOI] [PubMed] [Google Scholar]
- 17. Rosenstock J, Rossi L, Lin CS, et al. The effects of irbesartan added to hydrochlorothiazide for the treatment of hypertension in patients non‐responsive to hydrochlorothiazide alone. J Clin Pharm Ther. 1998;23:433–440. [DOI] [PubMed] [Google Scholar]
- 18. Howe P, Phillips P, Saini R, et al, on behalf of the Irbesartan Multicenter Study Group . The antihypertensive efficacy of the combination of irbesartan and hydrochlorothiazide assessed by 24‐hour ambulatory blood pressure monitoring. Clin Exp Hypertens. 1999;21:1373–1396. [DOI] [PubMed] [Google Scholar]
- 19. Raskin P, Guthrie R, Flack J, et al. The long‐term antihypertensive activity and tolerability of irbesartan with hydrochlorothiazide. J Hum Hypertens. 1999;13:683–687. [DOI] [PubMed] [Google Scholar]
- 20. Coca A, Calvo C, Sobrino J, et al. Once‐daily fixed‐combination irbesartan 300 mg/hydrochlorothiazide 25 mg and circadian blood pressure profile in patients with essential hypertension. Clin Ther. 2003;25:2849–2864. [DOI] [PubMed] [Google Scholar]
- 21. Neutel JM, Saunders E, Bakris GL, et al. The efficacy and safety of low‐ and high‐dose fixed combinations of irbesartan/HCTZ in patients with uncontrolled systolic blood pressure on monotherapy: the INCLUSIVE trial. J Clin Hypertens (Greenwich). 2005;7:578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ofili EO, Ferdinand KC, Saunders E, et al. Irbesartan/HCTZ fixed combinations in patients of different racial/ethnic groups with uncontrolled systolic blood pressure on monotherapy. J Natl Med Assoc. 2006;98:618–626. [PMC free article] [PubMed] [Google Scholar]
- 23. Sowers JR, Neutel JM, Saunders E, et al. Antihypertensive efficacy of Irbesartan/HCTZ in men and women with the metabolic syndrome and type 2 diabetes. J Clin Hypertens (Greenwich). 2006;8:470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Neutel JM, Franklin SS, Oparil S, et al. Efficacy and safety of irbesartan/HCTZ combination therapy as initial treatment for rapid control of severe hypertension. J Clin Hypertens (Greenwich). 2006;8:850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lapuerta PBA, Ptaszynska A. Irbesartan/HCTZ as initial treatment in patients with moderate hypertension. Poster #493 presented at: 16th Meeting of the European Society of Hypertension; June 1216, 2006; Madrid, Spain. [Google Scholar]
- 26. Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. [DOI] [PubMed] [Google Scholar]
- 27. Lewin AJ, Cable G, Weir MR. Antihypertensive efficacy and safety of irbesartan/HCTZ fixed‐dose combination in obese patients with or without diabetes in the INCLUSIVE trial. Presented at: 67th Scientific Sessions of the American Diabetes Association; June 2226, 2007; Chicago, IL. Abstract #2744‐PO. [Google Scholar]
- 28. Rahmouni K, Correia ML, Haynes WG, et al. Obesity‐associated hypertension: new insights into mechanisms. Hypertension. 2005;45:9–14. [DOI] [PubMed] [Google Scholar]
- 29. Sharma AM. Is there a rationale for angiotensin blockade in the management of obesity hypertension? Hypertension. 2004;44:12–19. [DOI] [PubMed] [Google Scholar]
- 30. Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitations. J Am Soc Nephrol. 2005;16:3027–3037. [DOI] [PubMed] [Google Scholar]
- 31. Kidney Disease Outcomes Quality Initiative (K/DOQI) . K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 1):S1–S290. [PubMed] [Google Scholar]
- 32. Law MR, Wald NJ, Morris JK, et al. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meredith PA. Angiotensin II receptor antagonists alone and combined with hydrochlorothiazide: potential benefits beyond the antihypertensive effect. Am J Cardiovasc Drugs. 2005;5:171–183. [DOI] [PubMed] [Google Scholar]


