Abstract
Hypertension frequently coexists with obesity, diabetes, hyperlipidemia, or the metabolic syndrome; their association with cardiovascular disease is well established. The identification and management of these risk factors is an important part of the overall management of hypertensive patients. Because patients in these special populations are more predisposed to target organ damage (TOD), stringent targets for blood pressure (BP) control have been set in clinical guidelines. However, clinical trial and real‐life evidence suggest that these targets are difficult to achieve. Patients with these comorbidities are more likely to require combination therapy, yet physicians are often reluctant to adjust the number and doses of medications to achieve target BP. There is a particular need for effective 24‐hour BP control in these patients, due to the increased likelihood of nondipping status, which is a risk factor for TOD and mortality. Not all available antihypertensives are equally effective in controlling BP over 24 hours, and some may exacerbate underlying metabolic abnormalities.
Hypertension (HTN) frequently coexists with other cardiovascular disease (CVD) risk factors. In a Canadian study of hypertensive patients aged 35 years or older who were free from clinical evidence of CVD, in a large managed‐care organization, 56% were obese (body mass index [BMI] ≥30 mg/m2) or had diabetes or hyperlipidemia (Figure 1). 1 The rate of risk factor clustering is 4 times that which would be expected by chance alone, suggesting a possible metabolic connection between HTN and other CVD risk factors. Closely related combinations of risk factors, such as the metabolic syndrome, are well recognized. Because the presence of additional risk factors (particularly diabetes) in hypertensive patients substantially increases the risk of CVD and medical care costs, their identification and management is an important part of the overall management of hypertensive patients.
Figure 1.
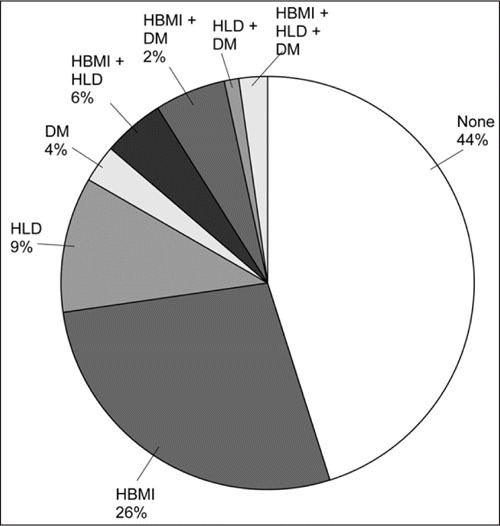
Prevalence of other risk factors for cardiovascular disease among 57,573 hypertensive patients (in a large managed‐care organization and aged 35 years or older) with no history of cardiovascular disease. 1 DM indicates diabetes mellitus; HBMI, high body mass index (≥30 kg/m2); HLD, hyperlipidemia. From the Weycker et al, 2007. 1
The purpose of this review is to discuss the challenges of achieving optimal blood pressure (BP) control in patients with coexisting obesity, diabetes, and/or hyperlipidemia and what contribution sub‐optimal control of BP makes to the increasing tendency toward target organ damage (TOD) in these patients. We also briefly consider how trial data relevant to this special population can be usefully modeled to examine health economic outcomes relating to current and future antihypertensive strategies.
THE METABOLIC SYNDROME
The metabolic syndrome is characterized by a combination of elevated BP, dyslipidemia, insulin resistance, and abdominal obesity. These patients are at high risk for diabetes and CVD. Various groups have produced different diagnostic criteria for the syndrome 2 , 3 , 4 : Table I summarizes the International Diabetes Federation's definition (2005); Table II provides an overview of the US National Cholesterol Education Panel diagnostic criteria (2002).
Table I.
International Diabetes Federation Definition of the Metabolic Syndrome 2
| Central obesity |
| Waist circumference:a ethnicity‐specific values (eg, Europid: ≥94 cm for men, ≥80 cm for women; South Asian: ≥90 cm for men, ≥80 cm for women) |
| Refer also to Wahrenberg et al, 2005 3 |
| Plus any two of the following: |
| Raised triglyceride level |
| >150 mg/dL (1.7 mmol/L) OR treatment for this lipid abnormality |
| Reduced high‐density lipoprotein cholesterol level |
| <40 mg/dL (<1.03 mmol/L) in men /<50 mg/dL (<1.29 mmol/L) in women OR specific treatment for this lipid abnormality |
| Raised blood pressure |
| Systolic >130 mm Hg/diastolic >85 mm Hg OR treatment of previously diagnosed hypertension |
| Raised fasting plasma glucose levelb |
| Fasting plasma glucose level >100 mg/dL (>5.6 mmol/L) OR previously diagnosed type 2 diabetes |
| If >5.6 mmol/L or >100 mg/dL, oral glucose tolerance test is strongly recommended but is not necessary to define presence of syndrome |
| aIf body mass index is >30 kg/m2, central obesity can be assumed and waist circumference need not be measured. bIn clinical practice, impaired glucose tolerance is also acceptable, but all reports of prevalence of metabolic syndrome should use only fasting plasma glucose and presence of previously diagnosed diabetes to define hyperglycemia. Prevalence incorporating 2‐hour glucose results can also be added as supplementary findings. Reproduced with permission from Alberti et al, 2005. 2 |
Table II.
Clinical Identification of the Metabolic Syndrome Using NCEP ATP III Criteria 4
| A diagnosis of the metabolic syndrome is made when ≥3 of the risk determinants are present. | |
| Risk Factor | Defining Level |
| Fasting glucose | ≥110 mg/dL |
| Blood pressure | ≥130/85 mm Hg |
| Triglycerides | ≥150 mg/dL |
| High‐density lipoprotein cholesterol | <40 mg/dL (men) |
| <50 mg/dL (women) | |
| Abdominal obesity | Waist circumferencea |
| >102 cm (>40 in) (men) | |
| >88 cm (>35 in) (women) | |
| aSome males can develop multiple risk factors when the waist circumference is only marginally increased (eg, 94–102 cm, 37–39 in). Such persons may have a strong genetic contribution to insulin resistance. Abbreviation: NCEP ATP III, Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Reprinted with permission from Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), 2002. 5 | |
The prevalence of the metabolic syndrome depends on age and ethnic background and is associated with several potentially modifiable lifestyle factors. Prevalence, as defined by the Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (NCEP ATP III), is estimated at about 22% of US adults; it rises from <10% in persons aged 29 years or younger to >40% in those older than 60 years. 5 The underlying mechanisms of the syndrome are not fully known; however, the core factors appear to be an excess of body fat combined with metabolic susceptibility. 6 Many factors may predispose to this metabolic susceptibility, which often manifests as insulin resistance. The relation between insulin resistance and HTN is well established and relates to several different mechanisms. Elevated pulse pressure levels observed in hypertensive patients with the metabolic syndrome may reflect increased stiffness of the large arteries and may partly explain the associated enhanced cardiovascular (CV) risk. 7 There is no specific treatment for the metabolic syndrome; individual components must be managed appropriately to reduce the risk of TOD. Data from a retrospective cohort study indicate that BP control is poor in a substantial proportion of patients with coexisting HTN and dyslipidemia and that multiple drugs are required to control BP. 8 Poor BP control contributes to the high CV risk occurring in patients with the metabolic syndrome. 9
OBESITY
BP Control in Obesity
Obesity affects one‐third of adults in the United States (32.9% in the 2003–2004 US National Health and Nutrition Examination Survey [NHANES]) 10 and is a major risk factor for the development of HTN, CVD, and chronic renal disease. In addition to elevated clinic BP, body weight is positively correlated with increased ambulatory BP and a higher incidence of elevated nighttime BP (nondipper status). 11 Obese patients have higher levels of renin system (RS) activity, autonomic nervous system activity, and volume expansion than lean patients. 12 Hemodynamically, obese hypertensive patients also have a higher cardiac output and lower systemic vascular resistance. 13 In one study of hypertensive patients aged 35 years or older who were free from CVD, 37% also had a BMI ≥30 kg/m2. 1
At present, HTN guidelines provide no explicit recommendations, such as a specific choice of an antihypertensive drug for the management of obese hypertensives. 9 , 14 Unfortunately, both losing weight and BP control are difficult to achieve in clinical practice. The shortage of large, prospective, comparative trials in obesity and HTN explains the lack of clear guidelines on how to treat this population.
There is some evidence that HTN in obese patients is more difficult to treat and that this population received suboptimal treatment in the real world. 15 , 16 In a Spanish population survey of BP control in hospital‐based HTN units (no specific treatment protocol was followed), the physicians' target was to reduce BP in accordance with international guidelines. A BP level <140/90 mm Hg was achieved in 46% of participants with a BMI <30 mg/m2 compared with only 34% of individuals with a BMI ≥30 mg/m2. 16 In contrast, more recent NHANES data indicate that BP control rates are improving in obese persons. 17
Evidence That Coexisting Obesity Is Related to Poorer Outcomes
There is some evidence that obesity per se is associated with end organ damage. The risk of death from all causes, including CVD, is higher for moderately and severely obese men and women in all age groups. 18 The risk of left ventricular hypertrophy (LVH) and concomitant heart failure increases with increasing BMI. 19 Increased RS activity may be implicated in the association between obesity and LVH. 20
There is controversy, however, as to the extent of CVD risk in obese hypertensive patients compared with lean hypertensive patients. While some studies have suggested that obesity might actually exert protective effects on the cardiovascular system, others disagree with this concept. 13 In a study by Weycker and colleagues', 1 there was no significant increased risk among hypertensive persons whose only additional risk was obesity.
The effect of obesity on CVD risk may be because of its impact on the risk of associated metabolic abnormalities, such as diabetes and hyperlipidemia, which are themselves risk factors for CVD. The higher incidence of elevated nighttime BP (nondipper status) seen in obesity is also a risk factor for TOD and mortality. 11 Obese hypertensive patients, therefore, have a particular need for nighttime BP control.
Choice of Antihypertensive Medication in Patients With Coexisting Obesity
Obesity has an influence on the hemodynamic changes associated with HTN, 13 and this may impact the efficacy of different classes of antihypertensive drugs. Obese patients often have metabolic abnormalities, such as glucose intolerance and increased cholesterol concentrations, that can be exacerbated by certain antihypertensive agents, in particular β‐blockers; β‐blockers can also cause weight gain or make it more difficult to lose weight. 21 Clinical trials and real‐life studies indicate that obese hypertensive patients are more difficult to treat and are more likely to have a nondipping pattern than nonobese hypertensive patients. Despite all these factors, obesity‐associated HTN has been neglected both in clinical studies and therapeutic guidelines. The role of RS activation in the etiology of obesity suggests that drugs acting on the RS may have beneficial effects in this special population, but this has yet to be proven in larger clinical trials.
DIABETES
BP Control in Diabetes
There is a strong association between HTN and diabetes; at least 15% of hypertensive patients have concomitant diabetes. 1 Also, hypertensive individuals develop diabetes more frequently than do nonhypertensives, regardless of any specific treatment. HTN occurs approximately twice as frequently in individuals with diabetes than in those without 22 (prevalence is as high as 70%–80% in patients with type 2 diabetes 9 ). This strong association, together with their cumulative risk for renal damage and CVD, makes aggressive control of BP important in diabetic hypertensive patients. This is reflected in treatment guidelines, which recommend aggressive targets of 130/80 mm Hg. 9 , 14 BP is, however, difficult to control in this patient group, and these targets are often not achieved in everyday practice. 16 , 23 , 24 , 25 , 26 Large trials such as the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood pressure Lowering Arm (ASCOT‐BPLA; Figure 2), 27 the Coronary Artery Calcification in Type 1 Diabetes (CACTI), 28 the Candesartan and Lisinopril in hypertensive patients With Diabetes Study ii (CALM II), 29 and the international Verapamil SR‐Trandolapril Study (INVEST) 30 confirm that BP control is often suboptimal in diabetic hypertensive patients. The results of NHANES 2003–2004 found that BP control (<130/80 mm Hg) was achieved in only 33.2% of treated hypertensive diabetic individuals, a percentage substantially lower than the BP control rate for all treated hypertensive individuals. 17 This low rate of control may be related to the fact that goal BP levels were lowered in recent years. Diabetics require multiple therapies more frequently than nondiabetics to reach treatment goals, but physicians often seem reluctant to adjust the number and doses of medications to achieve the strict BP targets. 16 , 23
Figure 2.
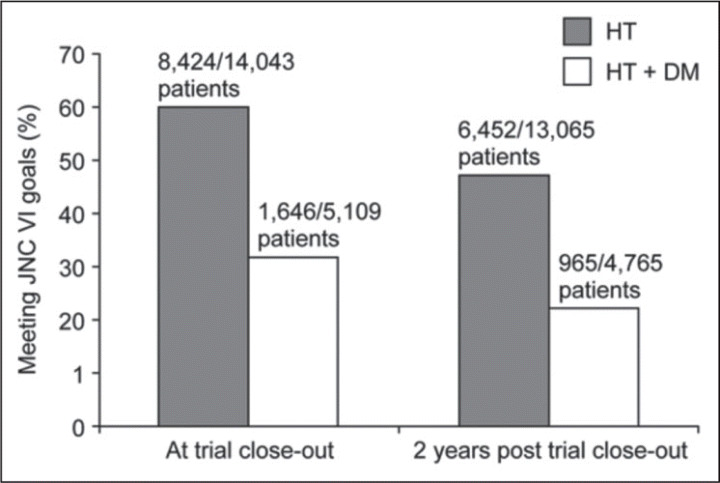
Blood pressure (BP) control in diabetic and nondiabetic patients participating in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA). The target BP was stricter for diabetic patients (<130/80 mm Hg) than for nondiabetic patients (<140/90 mm Hg). 27 HTN indicates hypertension; DM, diabetes; JNC VI, The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.
Evidence That Coexisting Diabetes Is Related to CV Outcomes
Persons with coexisting HTN and diabetes have a greater prevalence of microalbuminuria; left ventricular structural and functional abnormalities (including LVH); other CV risk factors, such as hyperlipidemia, hyperuricemia, and thrombotic tendency; and an increased incidence of stroke, coronary artery disease, congestive heart failure, peripheral artery disease, and CV mortality at every level of BP. 31 , 32 in the study by Weycker and associates 1 , the unadjusted risk of a CV event after 6 years ranged from 18.4% to 22.0% for hypertensives with coexisting diabetes compared with 7.5% to 12.0% for patients without diabetes.
The higher incidence of elevated nighttime BP (nondipper status) occurring in diabetic patients may also be associated with increased TOD and mortality. 33 , 34 HTN control in early diabetes seems to prevent the development of abnormal left ventricular geometry 35 and, together with tight glucose control, reduces the risk of microalbuminuria and the rate of progression to end‐stage renal disease. 36 Clinical trial data from the hypertension Optimal Treatment (HOT; Figure 3), 37 heart Outcomes prevention evaluation (HOPE), 38 and UK prevention Diabetes (UKPDS) 36 trials indicate that intensive BP lowering to lower targets is beneficial in reducing the rate of CV events; small decreases in BP are accompanied by substantial reductions in CV risk.
Figure 3.
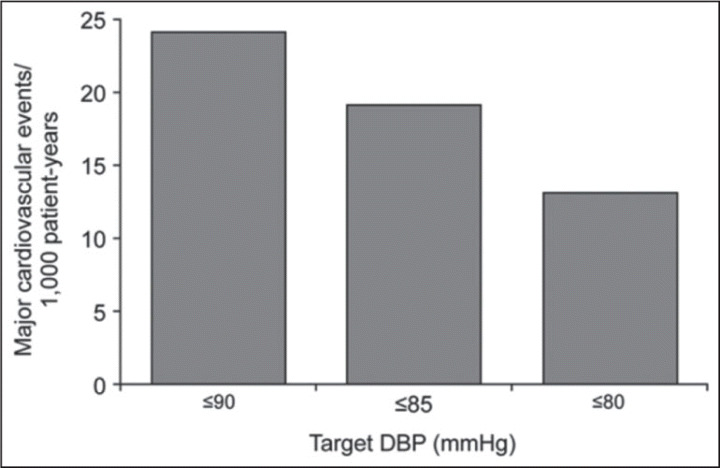
Major cardiovascular events in relation to target diastolic blood pressure (DBP) in patients with hypertension and type 2 diabetes (Hypertension Optimal Treatment [HOT] study; P for trend =.005). 37, 39 Reprinted from McInnes, 2004. 39
Choice of Antihypertensive Agent in Patients With Coexisting Diabetes
Achieving the recommended BP control target of 130/80 mm Hg is difficult in clinical practice. 39 Most patients will require multiple drugs to reach this goal, and this may lead to side effect and compliance issues. Diabetic patients have a particular need for effective 24‐hour BP control. In addition to providing effective 24‐hour BP control, the antihypertensive medications used should not exacerbate underlying metabolic abnormalities. β‐Blockers and thiazide/thiazide‐like diuretics should not generally be used as first‐step therapy because they may worsen insulin resistance. 9 In the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), a diuretic‐based treatment regimen with a β‐blocker added achieved the same coronary heart disease outcome as a calcium channel blocker‐or angiotensin‐converting enzyme inhibitor‐based program in diabetics or patients with the metabolic syndrome, but BP differences occurred, and the rate of new‐onset diabetes was increased overall with diuretics (diuretics, 11.6%; amlodipine, 9.8%; and lisinopril, 8.1%). RS blockers, such as angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers, are recommended due to their superiority in slowing diabetic nephropathy. 9 In studies, these drugs were almost always given with a diuretic for BP control, such as in the large Action in Diabetes and Vascular Diseases, Modified Release Controlled Evaluation (ADVANCE) trial. 40 The new direct renin inhibitor aliskiren offers an alternative way of blocking the RS and providing 24‐hour BP control in combination with a diuretic. Results from the 6‐month Aliskiren in the Evaluation of Proteinuria in Diabetes (AVOID) study in 599 hypertensive type 2 diabetics with nephropathy indicate that aliskiren may offer additional renoprotective effects when added to recommended treatment with losartan and optimal antihypertensive therapy. 41
HYPERLIPIDEMIA
BP Control in Hyperlipidemia
Various population studies suggest that more than 50% of hypertensive patients also have dyslipidemia; these two conditions may be present in 35% of Western European adults. 42 In a study of hypertensive persons aged 35 years or older who are free from CVD, 24% of persons also had hyperlipidemia. 1 Prevalence rates vary between studies due to differing definitions of dyslipidemia. Guidelines recommend aggressive treatment of coexisting HTN and dyslipidemia, especially for patients who already have CVD, 4 , 9 and there is evidence that HTN and hyperlipidemia are often suboptimally managed and available medications are underused. 8 , 42 , 43 In fact, US epidemiologic data suggest that <10% of patients with concomitant HTN and dyslipidemia are at treatment target for both conditions. 44 Poor BP control in hyperlipidemic patients may be due in part to low patient adherence and persistence with prescribed drug therapy or physician inertia. 45
Evidence That Coexisting Hyperlipidemia Is Related to Poorer Morbidity and Mortality Outcomes
Studies indicate that patients with concomitant HTN and hyperlipidemia have a greater additive risk of CVD compared with patients who have either condition in isolation (Figure 4). 46 Even relatively small reductions in BP and cholesterol levels can lead to reductions in the risk of CV events. An evaluation of strategies aimed at the prevention of CVD indicated that pharmacologic treatment of both HTN and dyslipidemia, resulting in 10% reductions in mean BP and cholesterol, could reduce the incidence of CVD by 45%. 47
Figure 4.
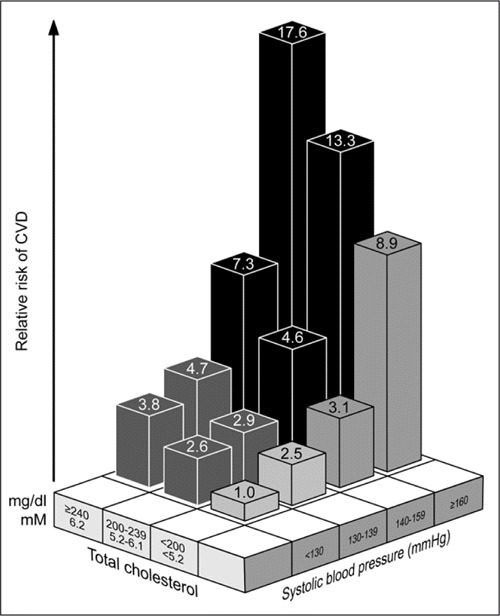
Relationship between relative risk of cardiovascular disease (CVD) and rising cholesterol and systolic blood pressure (SBP) levels among 108,879 French men younger than 55 years. Patients with SBP ≥140 mm Hg and total cholesterol ≥200 mg/dL were classified as having hypertension and dyslipidemia, respectively. 42, 46 Reprinted from Cowie, 2005. 42
Choice of Antihypertensive Agents in Patients With Coexisting Hyperlipidemia
Antihypertensive strategies should not exacerbate any underlying metabolic abnormalities. β‐Blockers are reported to increase triglyceride levels and have been associated with new onset of type 2 diabetes. 48 Recent guidelines, therefore, recommend that unless required by specific indications, β‐blockers should be avoided in patients with the metabolic syndrome due to their potential adverse effects on the incidence of new‐onset diabetes, body weight, insulin sensitivity, and lipid profile. 9 Several trials have shown that the frequency of new‐onset diabetes may be lower with angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers than with other medications, 40 , 49 , 50 but the long‐term impact of this is still unclear. 51 Most recently, analysis of the Valsartan Antihypertensive Long‐Term Use Evaluation (VALUE) supports the view that new‐onset diabetes is associated with increased incidence of congestive heart failure. 52 Angiotensin‐converting enzyme inhibitor therapy is also reported to reduce the increased lipolysis in adipose tissue associated with insulin resistance in hypertensive patients with central obesity. 53
ECONOMIC PERSPECTIVES
Analyses by the US Centers for Disease Control and Prevention suggest that intensive management of glycemic control and dyslipidemia is generally less cost‐effective than intensive HTN control, which is cost‐saving. 54 The authors concluded that intensified HTN control reduces costs and improves health outcomes relative to moderate HTN control.
Gilmer's group 55 used medical claim data from over 1600 diabetic patients in the United States to assess predictors of health care costs. Their analysis indicates that 3‐year costs in patients with HTN and CV end organ damage were 300% greater than those with diabetes alone. This analysis could be used as a basis for calculating hypothetical cost‐savings of specific antihypertensive strategies. Sesso's group 56 developed a CVD event Markov model to estimate the life expectancy benefits of BP reduction. Their model incorporated both systolic and diastolic BP as continuous variables, thus allowing for comparison of the relative impacts of different levels of BP reduction. Using prospective data from more than 57,000 middle‐aged and older persons, they provided evidence that BP lowering reduces primary and secondary CVD events, thereby achieving substantial gains in life expectancy. This model could be adapted to provide estimates of the cost‐effectiveness of different BP‐lowering strategies, using cost per life‐year saved as outcome measures.
CONCLUSIONS
Obesity, diabetes, and hyperlipidemia are common comorbidities in HTN. Patients with these conditions are at increased CV risk, making aggressive BP control important. It is also believed that effective BP control throughout the 24‐hour period is an important goal. However, various studies suggest that hypertensive patients with these additional risk factors are more difficult to treat and more likely to require treatment with a combination of antihypertensive agents. Control of BP at goal levels is often made more difficult to achieve because guidelines have set more stringent BP targets in these populations. Thus, there is a need for effective and safe strategies to provide 24‐hour control of BP in hypertensive patients with coexisting risk factors. Based on this evidence, guidelines now recommend that all patients with high and very high cardiovascular risk, such as patients with diabetes or comorbidities, should primarily receive combination treatment with a 2‐drug combination such as an angiotensin‐converting enzyme inhibitor and a diuretic or calcium channel blocker or an angiotensin receptor blocker and a diuretic or calcium channel blocker. Nevertheless, β‐blockers should be part of the combination therapy in patients with coronary heart disease, angina, and congestive heart failure. Most intriguing, in the Avoiding Cardiovascular Events Through Combination Therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial (released at the American College of Cardiology meeting on March 31, 2008) in whom 60% of the patients had diabetes, the combination of an angiotensin‐converting enzyme inhibitor and calcium channel blocker had a 20% risk reduction of the primary cardiovascular mortality and morbidity end point. Thus, tailored medication according to the comorbidities of each individual patient should be applied for BP control, but medication should not exacerbate underlying metabolic abnormalities and will need to demonstrate cost‐savings in economic analyses.
References
- 1. Weycker D, Nichols GA, O'Keefe‐Rosetti M, et al. Risk‐factor clustering and cardiovascular disease risk in hypertensive patients. Am J Hypertens. 2007;20:599–607. [DOI] [PubMed] [Google Scholar]
- 2. Alberti KG, Zimmett P, Shaw J. The metabolic syndrome: a new worldwide definition. Lancet. 2005;366:1059–1062. [DOI] [PubMed] [Google Scholar]
- 3. Wahrenberg H, Hertel K, Leijonhufvud BM, et al. Use of waist circumference to predict insulin resistance: retrospective study. BMJ. 2005;330:1363–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II) . Final Report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 5. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. [DOI] [PubMed] [Google Scholar]
- 6. Grundy SM. Metabolic syndrome: a multiplex cardiovascular risk factor. J Clin Endocrinol Metab. 2007;92:399–404. [DOI] [PubMed] [Google Scholar]
- 7. Mulè G, Nardi E, Cottone S, et al. Relationship of metabolic syndrome with pulse pressure in patients with essential hypertension. Am J Hypertens. 2007;20:197–203. [DOI] [PubMed] [Google Scholar]
- 8. Rodondi N, Peng T, Karter AJ, et al. Therapy modifications in response to poorly controlled hypertension, dyslipidemia, and diabetes mellitus. Ann Intern Med. 2006;144:475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007;28(12):1462–1536. [DOI] [PubMed] [Google Scholar]
- 10. Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. [DOI] [PubMed] [Google Scholar]
- 11. Kotsis V, Stabouli S, Bouldin M, et al. Impact of obesity on 24‐hour ambulatory blood pressure and hypertension. Hypertension. 2005;45:602–607. [DOI] [PubMed] [Google Scholar]
- 12. Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. [DOI] [PubMed] [Google Scholar]
- 13. Schmieder RE, Messerli FH. Does obesity influence early target organ damage in hypertensive patients? Circulation. 1993;87:1482–1488. [DOI] [PubMed] [Google Scholar]
- 14. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 15. Goodfriend TL, Calhoun DA. Resistant hypertension obesity, sleep apnea and aldosterone: theory and therapy. Hypertension. 2004;43:518–524. [DOI] [PubMed] [Google Scholar]
- 16. Banegas JR, Segura J, Ruilope L, et al. Blood pressure control and physician management of hypertension in hospital hypertension units in Spain. Hypertension. 2004;43:1338–1344. [DOI] [PubMed] [Google Scholar]
- 17. Ong KL, Cheung BMY, Man YB, et al. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. [DOI] [PubMed] [Google Scholar]
- 18. Calle EE, Thun M, Petrelli J, et al. Body‐mass index and mortality in a prospective cohort. N Engl J Med. 1999;341:1097–1105. [DOI] [PubMed] [Google Scholar]
- 19. Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. [DOI] [PubMed] [Google Scholar]
- 20. Gates PE, Gentile CL, Seals DR, et al. Adiposity contributes to differences in left ventricular structure and diastolic function with age in healthy men. J Clin Endocrinol Metab. 2003;88:4884–4890. [DOI] [PubMed] [Google Scholar]
- 21. Sharma AM, Engeli S. Managing big issues on lean evidence: treating obesity hypertension. Nephrol Dial Transplant. 2002;17:353–355. [DOI] [PubMed] [Google Scholar]
- 22. Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension and cardiovascular disease: an update. Hypertension. 2001;37:1053–1059. [DOI] [PubMed] [Google Scholar]
- 23. Berlowitz DR, Ash A, Hickey E, et al. Hypertension management in patients with diabetes: the need for more aggressive therapy. Diabetes Care. 2003;26:355–359. [DOI] [PubMed] [Google Scholar]
- 24. Zgibor JC, Wilson RR, Orchard TJ, et al. Has control of hypercholesterolemia and hypertension in type 1 diabetes improved over time? Diabetes Care. 2005;28:521–526. [DOI] [PubMed] [Google Scholar]
- 25. McFarlane SI, Jacober S, Winer N, et al. Control of cardiovascular risk factors in patients with diabetes and hypertension at urban academic medical centers. Diabetes Care. 2002;25:718–723. [DOI] [PubMed] [Google Scholar]
- 26. Schaars CF, Denig P, Kasje WN, et al. Physician, organizational, and patient factors associated with suboptimal blood pressure management in type 2 diabetic patients in primary care. Diabetes Care. 2004;27:123–127. [DOI] [PubMed] [Google Scholar]
- 27. Dahlöf B, Sever P, Poulter N, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA): a multicenter randomized controlled trial. Lancet. 2005;366:895–906. [DOI] [PubMed] [Google Scholar]
- 28. Maahs DM, Kinney G, Wadwa P, et al. Hypertension prevalence, awareness, treatment, and control in an adult type 1 diabetes population and a comparable general population. Diabetes Care. 2005;28:301–306. [DOI] [PubMed] [Google Scholar]
- 29. Andersen NH, Poulsen P, Knudsen S, et al. Long‐term dual blockade with candesartan and lisinopril in hypertensive patients with diabetes. The CALM II study. Diabetes Care. 2005;28:273–277. [DOI] [PubMed] [Google Scholar]
- 30. Bakris GL, Gaxiola E, Messerli F, et al. Clinical outcomes in the diabetes cohort of the international verapamil SR‐trandolapril study. Hypertension. 2004;44:637–642. [DOI] [PubMed] [Google Scholar]
- 31. Nelson RG, Knowler WC, Pettit DJ, et al. Kidney diseases in diabetes. In: Diabetes in America, National Institutes of Health Publication No. 95–1468. 2nd ed. 2. Bethesda, MD: National Institutes of Health; 1995:349–400. [Google Scholar]
- 32. Zanchetti A, Hansson L, Dahlöf B, et al. Effects of individual risk factors on the incidence of cardiovascular events in the treated hypertensive patients in the Hypertension Optimal Treatment study. J Hypertens. 2001;19:1149–1159. [DOI] [PubMed] [Google Scholar]
- 33. Nakano S, Fukuda M, Hotta F, et al. Reversed circadian blood pressure rhythm is associated with occurrence of both fatal and nonfatal vascular events in NIDDM subjects. Diabetes. 1998;47:1501–1506. [DOI] [PubMed] [Google Scholar]
- 34. Fogari R, Zoppi A, Malamani GD, et al. Ambulatory blood pressure monitoring in normotensive and hypertensive type 2 diabetes. Prevalence of impaired diurnal blood pressure patterns. Am J Hypertens. 1993;6:1–7. [DOI] [PubMed] [Google Scholar]
- 35. Kuch B, Von Scheidt W, Peter W, et al. Influence of antihypertensive therapy and blood pressure control in left ventricular geometry and function in subjects with type II diabetes: the Augsburg Diabetes Family Study. J Hum Hypertens. 2006;20:757–764. [DOI] [PubMed] [Google Scholar]
- 36. UK Prospective Diabetes Study group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 37. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351:1755–1762. [DOI] [PubMed] [Google Scholar]
- 38. Heart Outcomes Prevention Evaluation (HOPE) Study Investigators Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO‐HOPE substudy. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- 39. McInnes GT. How important is optimal blood pressure control? Clin Ther. 2004;26(suppl A):A3–A11. [DOI] [PubMed] [Google Scholar]
- 40. Patel A; ADVANCE Collaborative Group . Effects of a fixed combination of preindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomized controlled trial. Lancet. 2007;370:829–840. [DOI] [PubMed] [Google Scholar]
- 41. Parving HH, Lewis JB, Lewis EJ, et al. Aliskiren in the evaluation of proteinuria in diabetes (AVOID). N Engl J Med. 2008;358:2433–2446. [DOI] [PubMed] [Google Scholar]
- 42. Cowie MR. Simultaneous treatment of hypertension and dyslipidemia may help to reduce overall cardiovascular risk: focus on amlodipine/atorvastatin single‐pill therapy. Int J Clin Pract. 2005;59:839–846. [DOI] [PubMed] [Google Scholar]
- 43. Laffer CL, Elijovich F. Suboptimal outcome of management of metabolic cardiovascular risk factors in Hispanic patients with essential hypertension. Hypertension. 1995;26:1079–1084. [DOI] [PubMed] [Google Scholar]
- 44. Battleman DS, Peterson ED. Estimated prevalence of comorbid hypertension and dyslipidemia and therapeutic goal attainment among US adults in 2000, utilizing data from the National Health and Nutrition Examination Survey (NHANES II). J Manag Care Pharm. 2004;10:186. [Google Scholar]
- 45. Chapman RH, Benner JS, Petrilla AA, et al. Predictors of adherence with antihypertensive and lipid‐lowering therapy. Arch Intern Med. 2005;165:1147–1152. [DOI] [PubMed] [Google Scholar]
- 46. Thomas F, Bean K, Guize L, et al. Combined effects of systolic blood pressure and serum cholesterol on cardiovascular mortality in young (<55 years) men and women. Eur Heart J. 2002;23:528–535. [DOI] [PubMed] [Google Scholar]
- 47. Emberson J, Whincup P, Morris R, et al. Evaluating the impact of population and high‐risk strategies for the primary prevention of cardiovascular disease. Eur Heart J. 2004;25:484–491. [DOI] [PubMed] [Google Scholar]
- 48. Bangalore S, Parkar S, Grossman E, et al. A meta‐analysis of 94,492 patients with hypertension treated with beta blockers to determine the risk of new‐onset diabetes mellitus. Am J Cardiol. 2007;100:1254–1262. [DOI] [PubMed] [Google Scholar]
- 49. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . Major outcomes in high risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker versus diuretic: The Antihypertensive and Lipid Lowering treatment to prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 50. Julius S, Kjeldsen SE, Weber MA, et al.; VALUE Trial Group . Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine; the VALUE randomized trial. Lancet. 2004;363:2049–2051. [DOI] [PubMed] [Google Scholar]
- 51. Moser M. New‐onset diabetes in the hypertension treatment trials: a point of view. J Clin Hypertens (Greenwich). 2004;6:610–613. [DOI] [PubMed] [Google Scholar]
- 52. Aksnes TA, Schmieder RE, Kjeldsen SE, et al. Impact of new‐onset diabetes mellitus on development of atrial fibrillation and heart failure in high‐risk hypertension (from the VALUE Trial). Am J Cardiol. 2008;101(5):634–638. [DOI] [PubMed] [Google Scholar]
- 53. Hennes MMI, O'Shaughnessy IM, Kelly TM, et al. Insulin‐resistant lipolysis in abdominally obese hypertensive individuals: role of the renin‐angiotensin system. Hypertension. 1996;28:120–126. [DOI] [PubMed] [Google Scholar]
- 54. CDC Diabetes Cost‐Effectiveness Group . Cost‐effectiveness of intensive glycemic control, intensified hypertension control, and serum cholesterol level reduction for type 2 diabetes. JAMA. 2002;287:2542–2551. [DOI] [PubMed] [Google Scholar]
- 55. Gilmer TP, O'Connor P, Rush W, et al. Predictors of health care costs in adults with diabetes. Diabetes Care. 2005;28:59–64. [DOI] [PubMed] [Google Scholar]
- 56. Sesso HD, Chen RS, L'Italien GJ, et al. Blood pressure lowering and life expectancy based on a Markov model of cardiovascular events. Hypertension. 2003;42:885–890. [DOI] [PubMed] [Google Scholar]


