Abstract
This study examined patients' perceptions of their providers' participatory decision making (PDM) style and hypertension self‐care behaviors and outcomes. Five hundred fifty‐four veterans with hypertension enrolled in the Veterans' Study to Improve the Control of Hypertension rated providers' PDM styles using a validated 3‐item instrument. Behaviors assessed included presence of a home blood pressure monitor, monitoring frequency, and self‐reported antihypertensive medication adherence. Overall, veterans with hypertension rated providers as highly participatory. In adjusted analyses, a lower PDM score was associated with decreased odds of having a home monitor (odds ratio, 0.90 per 10‐point decrement in PDM score; 95% confidence interval, 0.83–0.98) but not with monitoring frequency, adherence, or blood pressure control. Providers' involvement of patients in decision making, reflected in ratings of PDM style, may be important to securing patients' participation in their own care, but alone this factor seems insufficient. No relationship between PDM score and blood pressure control was observed.
In the United States, hypertension is the most commonly coded primary diagnosis for an office visit, affecting more than 60 million Americans. 1 , 2 The consequences of uncontrolled hypertension are well documented and include increased risk of myocardial infarction, heart failure, stroke, and kidney disease. Yet, control of high blood pressure is still below 40% nationally, far less than the Healthy People 2010 goal of 50%. 3
Participatory decision making (PDM), also known as shared decision making, refers to a patient‐centered style of making health care decisions in which providers present patients with the best available evidence; explicitly consider patients' own values, goals, and capabilities; offer options; and negotiate with patients to arrive at mutually agreed‐upon treatment plans. 4 Having a greater degree of PDM in a patient‐provider relationship has been shown to improve health‐related outcomes in a number of chronic illnesses, including hypertension. 5 , 6 , 7 , 8 , 9 , 10 In one study in which hypertensive patients were asked to rate the level of PDM that occurred at a given visit, higher PDM scores were correlated with lower blood pressures at the next follow‐up visit. 10
It is not known precisely how PDM style improves chronic illness outcomes. 11 Involving patients in their own care may make them more vigilant about their health and potentially improve adherence to medications and other self‐care behaviors. 12 The goal of this study was to explore the relationship between provider PDM style and patients' actual participation in chronic illness care by identifying possible connections between patients' global PDM ratings of providers and specific patient practices in hypertension.
At the start of an ongoing trial of a multifaceted intervention to improve hypertension outcomes in the US Department of Veterans Affairs (VA) Veterans Health Administration, participants were asked to rate their primary care providers in terms of PDM style. They were also asked about a range of behaviors relevant to hypertension, including whether they had home blood pressure monitors, how frequently they used these monitors, and whether they adhered to prescribed antihypertensive medication regimens. We then tested for associations between participants' PDM ratings and these behaviors, which themselves may be associated with improved blood pressure control. 13 , 14 We also examined whether PDM scores were related to blood pressure control at baseline.
To our knowledge, this study is the first to examine the relationship between provider PDM and hypertension among veterans receiving care in the VA health care system. Veterans represent an important population in the United States for studying the effects of patient‐provider relationships because potential confounders related to unequal access to care and lack of coverage for medications and equipment are greatly mitigated. 15
METHODS
Study Design
Data for this cross‐sectional study were obtained from the Veterans' Study to Improve the Control of Hypertension (V‐STITCH), a 4‐year randomized controlled trial of a health services intervention to improve blood pressure control. Overall study design and recruitment practices have been described elsewhere. 15 , 16 The study population for V‐STITCH consisted of 4017 potential participants identified using the outpatient database at the VA Medical Center (VAMC) Durham (Figure). These individuals had a diagnosis of hypertension, were assigned a primary care provider in one of the VAMC Durham ambulatory care clinics and had a prescription for 1 or more antihypertensive medications in the previous 12 months. Research assistants approached 816 randomly selected patients who were eligible to participate in the study: 190 refused, 38 were excluded because of concurrent illness or serious functional impairment, and 588 were enrolled (76% recruitment rate). These 588 participants were cared for by 30 different primary care providers: 22 attending‐level physicians, 2 advanced practice nurses, and 6 physician assistants. 15
Figure.
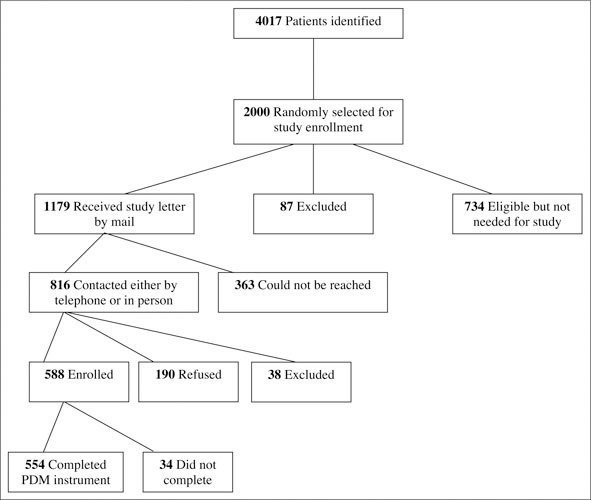
Enrollment in the Veterans' Study to Improve the Control of Hypertension (V‐STITCH). PDM indicates participatory decision making.
After written consent was received, participants completed a survey covering demographics, characteristics and behaviors, social and medical environment, and relationship with their primary care providers. This was administered by trained research assistants not otherwise involved in the care of participating veterans at an already scheduled primary care visit. Participants were told that their responses would not be shared with their providers. The sample used for this study consists of the 554 veterans who answered all 3 PDM questions. This study was approved by the VAMC Durham institutional review board.
Measures
Participants' perceptions of their providers' PDM styles were assessed using a validated 3‐item measure developed by Kaplan and Greenfield. 4 , 5 , 6 , 7 , 8 , 9 , 10 The 3 items are: (1) How likely is your physician to involve you in treatment decisions?, (2) How likely is your physician to give you a sense of control over your medical care?, and (3) How likely is your physician to ask you to take some responsibility for your care? Respondents were instructed to answer with their primary care provider in mind; their responses were scored on a scale of 1 to 10. The scores for each of the 3 items were then added together and this raw score linearly transformed to a 100‐point scale. 4 The internal consistency of the measure was α=0.73.
Adherence to antihypertensive medication regimens was determined using a 4‐item self‐report instrument, which has been found to have good concurrent and predictive validity for measuring actual adherence. 17 Participants were asked to respond to 4 statements: (1) I sometimes forget to take my blood pressure medicine, (2) I am sometimes careless about taking my blood pressure medicine, (3) When I feel better, I sometimes stop taking my blood pressure medicine, and (4) If I feel worse when I take the blood pressure medicine, sometimes I stop taking it. The 5 response options ranged from “strongly agree” to “strongly disagree.” To maximize sensitivity, participants were considered nonadherent if they responded “strongly agree” or “agree” to any of the 4 questions; otherwise, they were considered adherent.
Opportunity for home monitoring of blood pressure was assessed by a single question asking whether or not patients had a home blood pressure monitor. Frequency of home monitoring was reported as daily, weekly, or monthly. Baseline blood pressure control was assessed using blood pressure measurements obtained in clinic on the same day as enrollment in the study; participants being classified as “in” or “out” of control according to the Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 6) guidelines (in control being blood pressure <140/90 mm Hg for patients without diabetes and <130/85 mm Hg for patients with diabetes). 18 This study began before the JNC 7 report was issued.
Analyses
Logistic regression was used to perform unadjusted analyses of the relationship between PDM and each outcome of interest. Given concerns about ceiling effects, a number of different ways of examining the PDM ratings other than as a scaled, continuous variable were considered, including dichotomizing above and below some threshold value and using quartiles or deciles. In the end, the former approach was rejected as being too arbitrary, because it was felt that there was no basis for setting such a threshold in a clinically meaningful way. And the latter approach returned results similar to those obtained when using PDM ratings as a continuous variable. Thus, only the data from analyses using the PDM ratings as a continuous variable are presented.
In addition, because of the large number of participants who reported not having a home blood pressure monitor, our analyses of the frequency of home monitoring question were performed in 2 ways: first, using those without home monitors or patients who did not answer the question as the reference group (ie, set equal to no monitoring at all) and second, excluding this group entirely (ie, only veterans with a home monitor were analyzed, with those reporting monitoring monthly being the reference group). In this second analysis, sample size was 334 participants.
Next, multivariable logistic regression models were estimated to examine the relationship between PDM score and blood pressure control, self‐reported medication adherence, and presence of a home blood pressure monitor. Multinomial logistic regression was used for frequency of monitoring, with people who did not have home monitors as the reference group.
Age, race (white compared with nonwhite), marital status (married or not), Rapid Estimate of Adult Literacy in Medicine (REALM) score, 19 educational attainment (some college vs none), presence of diabetes, and 12‐month average blood pressure before study entry were included as covariates in all models. These are all factors that have also been found to be related to the various outcome measures examined in this study. 20 , 21 , 22 Charlson comorbidity scores were also calculated. The max‐rescaled R2 and the c statistic were calculated for each model to assess the proportion of variance explained and the model fit, respectively. In addition, variance inflation factor tests and analyses of structure were performed to look for evidence of collinearity; none was found. All analyses were performed with SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
Demographic and clinical characteristics of the study sample are presented in Table I. Mean age ± SD was 63.1±11.2 years; nearly all participants were men. Mean PDM score ± SD was 85.4±20.7. Median PDM score was 96.3; 46% gave their providers the highest score possible. Thirty‐four of the 588 original V‐STITCH participants (6%) did not answer all 3 PDM questions; the remaining 554 constituted our study sample. Incomplete responders were several years older (mean age ± SD 68.0±13.2 years vs 63.1±11.2 years; P=.02) and had slightly lower REALM scores (52.6±12.1 compared with 57.9±11.3 SD, of a possible 66 total points; P=.02). They were also less likely to have attended college (odds ratio [OR], 2.9; 95% confidence interval [CI], 1.3–6.3; P=.006). Charlson comorbidity scores between the 2 groups were not significantly different (P=.10), however.
Table I.
General Characteristics of the V‐STITCH Sample
| Participants, No. | 554 |
| Mean age, (SD), y | 63.1 (11.2) |
| Male, % | 98 |
| Race, % | |
| White | 57 |
| Nonwhite | 43 |
| Married, % | 68 |
| Mean REALM score* | 58 |
| Some college, % | 51 |
| Diabetes, % | 36 |
| 12‐mo average blood pressure before study, (SD), mm Hg† | |
| Mean systolic | 140.6 (14.7) |
| Mean diastolic | 76.9 (9.9) |
| Have home blood pressure monitor, % | 60 |
| Frequency of home monitoring, % | |
| Daily | 14 |
| Weekly | 26 |
| Monthly | 10 |
| Do not have monitor/don't know/no response | 50 |
| Adherent to antihypertensive medications, %‡ | 65 |
| Baseline blood pressure, (SD), mm Hg § | |
| Mean systolic | 138.0 (17.6) |
| Mean diastolic | 75.6 (11.3) |
| Blood pressure “not in control,”%| | 55 |
| PDM score | |
| Mean (SD) | 85.4 (20.7) |
| Median | 96.3 |
| V‐STITCH indicates the Veterans' Study to Improve the Control of Hypertension; REALM, Rapid Estimate of Adult Literacy in Medicine; and PDM, participatory decision making. *Scored out of 66 total possible points. Nineteen participants (3%) did not complete the REALM measure. †These data were not available for 3 participants (1%). ‡Assessed by validated 4‐question self‐report instrument. 19 Participants were considered nonadherent if they responded “agree” or “strongly agree” to any of the 4 statements. §Twenty participants (4%) did not have blood pressure measured at initial study visit. |As defined by the Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure guidelines as <140/90 mm Hg for nondiabetic, <130/85 mm Hg for diabetic patients. | |
In unadjusted analyses (Table II), a lower PDM score was significantly associated with decreased odds of having a home blood pressure monitor and an increased likelihood of having controlled blood pressure. PDM score, however, was not associated with frequency of home monitoring (when those without monitors were included as the reference group and when they were excluded entirely) or with self‐reported medication adherence.
Table II.
Unadjusted Relationship Between PDM and Hypertension Care Outcomes*
| Dependent Variable | OR (95% CI)* | P |
|---|---|---|
| Having a home blood pressure monitor | 0.89 (0.82–0.97) | .006 |
| Frequency of home monitoring | 0.94 (0.87–1.02) | .14 |
| Frequency of home monitoring (those without home monitors excluded)† | 0.99 (0.98–1.01) | .28 |
| Adherence to medications | 1.02 (0.94–1.11) | .61 |
| Blood pressure “in control” | 1.09 (1.00–1.19) | .04 |
| PDM indicates participatory decision making; CI, confidence interval. *Odds ratios (OR) were calculated per 10‐unit decrease in PDM score. †Because of the large number of participants who reported not having a home blood pressure monitor, the frequency‐of‐monitoring question was analyzed in 2 ways: (1) Using patients without monitors as the reference group and (2) excluding those without monitors and using the monthly category as the reference. In the latter case, n=334. | ||
The results of adjusted analyses largely mirrored those of unadjusted analyses. A lower PDM score was associated with decreased odds of having a home monitor. For each 10‐point decrease in PDM score, there was a 9% decrease in the odds of having a home blood pressure monitor (OR, 0.91;95% CI, 0.83–0.99; P=.02) (Table III). Of interest, however, was the fact that after taking other factors into account, a lower PDM score was no longer significantly associated with higher odds of blood pressure control (OR, 1.09; 95% CI, 0.99–1.20; P=.09). Finally, as in the unadjusted analyses, PDM style was not significantly associated with either frequency of home monitor use (OR, 1.02; 95% CI, 0.93–1.12; P=.45) or self‐reported adherence to antihypertensive medications (OR, 0.97; 95% CI, 0.89–1.05; P=.64).
Table III.
Multivariable Regression Models of PDM and Hypertension Care Outcomes*
| Dependent Variable | n | Overall P Value (Model) | Max‐Rescaled R2 | C Statistic | OR (95% CI) for PDM† | P Value for PDM |
|---|---|---|---|---|---|---|
| Having a home monitor | 532 | .007 | 0.07 | 0.63 | 0.91 (0.83, 0.99) | .03 |
| Frequency of monitoring | 532 | <.0001 | 0.07 | 0.62 | 0.97 (0.89, 1.05) | .45 |
| Medication adherence | 532 | .11 | 0.03 | 0.60 | 1.02 (0.93, 1.12) | .64 |
| Blood pressure control‡ | 514 | <.0001 | 0.19 | 0.71 | 1.09 (0.99, 1.20) | .09 |
| CI indicates confidence interval. *Age, race, marital status, Rapid Estimate of Adult Literacy in Medicine (REALM) score, level of education, diabetes status, and 12‐month average blood pressure before study entry were included as covariates in all models. Nineteen participants did not complete the REALM measure; 3 did not have blood pressure readings on record before study entry. †Odds ratios (OR) were calculated per 10‐unit decrease in participatory decision making (PDM) score. ‡Twenty participants did not have blood pressure measured at baseline clinic visit. | ||||||
DISCUSSION
Patient self‐management, which includes a spectrum of patient‐directed self‐care activities such as self‐monitoring and adherence to medication regimens, is a key component of effective chronic illness care. 32 PDM style describes the extent to which patients are encouraged by health care providers to be involved in managing their own health care. One might therefore expect that a more highly participatory provider style would be associated with increased patient self‐management, and vice versa. This study, the first to examine PDM and hypertension in veterans, found only limited evidence of such a relationship.
Overall, the veterans in this study rated their providers as highly participatory. Participants who rated their providers lower in terms of PDM also had decreased odds of having blood pressure monitors at home. This finding can be understood as follows: Having a monitor at home is a behavior that is “provider‐controlled,” because in the VA, monitors are dispensed via a provider's prescription. Thus, provider characteristics such as PDM are quite influential. On the other hand, the actual frequency of home monitoring and self‐reported medication adherence, aspects of hypertension care that are more “patient‐controlled,” did not appear to be related to PDM style. These more patient‐controlled behaviors clearly depend on the actions of patients themselves, and intuitively one would expect them to be less influenced by provider characteristics.
Studies of other chronic illnesses such as asthma, diabetes, and HIV have found similar trends. PDM style has been associated with certain provider‐controlled aspects of care, such as the duration of visits and the provision of asthma action plans, but not with other more patient‐controlled activities such as self‐monitoring of blood glucose or adherence to antiretroviral therapy. 7 , 9 , 23 In osteoarthritis, patients with greater PDM scores were slightly less likely to report nonadherence to medications. 24
Unlike a prior study of PDM and hypertension, 5 our study did not find a relationship between PDM style and the key clinical outcome, blood pressure. Lower PDM scores were actually associated with improved blood pressure control in an unadjusted analysis, but not in the final multivariable model that took into account the baseline severity of participants' hypertension.
One could explain these counterintuitive findings if patients with more refractory disease saw their providers more frequently and developed closer and more participatory relationships with them as a result. Patients whose blood pressure was already well controlled, on the other hand, may have required less interaction with their providers and perceive their relationships with them as being less participatory as a result. There is some observational evidence to suggest that this is the case in ambulatory care settings: one study of family practice providers found that they are most likely to facilitate PDM when seeing patients who have complex medical needs. 25 Of course, the possibility of a type II error cannot be overlooked; that is, we did not detect a difference that was actually present due to sample size or other factors.
Key study strengths include the use of a sizable primary care sample. A large proportion of participants were nonwhite and had relatively low incomes, which enhances the generalizability of our findings. In addition, a number of important behaviors and outcomes related to hypertension were evaluated, which gave us the opportunity to gain some insight regarding possible mechanisms for how PDM influences chronic illness care, while being mindful of the limitations discussed below. Finally, potential confounders related to unequal access to care and lack of coverage for medications and home blood pressure monitors were less of an issue in this study because all veterans receiving care in the VAMC have the same basic health insurance coverage.
Limitations of this study include the population studied: US veterans, nearly all men. The sample itself was made up of participants in a study of an intervention to improve hypertension outcomes at the VAMC Durham and may not be representative of hypertensive veterans in general. In addition, many veterans gave their primary care providers the highest PDM rating possible, producing a ceiling effect that has also been observed elsewhere. 23 This skewed distribution may have attenuated the relationships between PDM style and outcomes. Explanations could include high satisfaction with care, because PDM ratings have been found to be correlated with satisfaction. 26 Plus, in the VAMC, satisfaction with care is significantly higher than in private settings. 27 , 28 Older respondents have also been observed to overuse the upper end of response scales, which would further decrease response variability. 29
Other limitations include not having directly observed how participatory the primary care providers rated in this study were in actual practice. The patient‐reported instrument used here, however, has been validated and widely used elsewhere. 4 , 6 , 8 , 9 More important, it captures patients' own perceptions of their relationships with their providers, which are likely to have a greater influence on patients' behavior than would a third‐party assessment of those relationships. We also did not assess patients' preferences for participation, which may interact with PDM to affect health behaviors and outcomes. There is evidence, for example, that the effect of PDM on satisfaction with care is mediated by patient's own participation preferences. 26 , 30 Not all patients want to be active participants. 31 Adherence was also not measured directly; we used a validated self‐report instrument instead. 17
CONCLUSIONS
This study's findings appear to be consistent with those of previous studies of PDM and chronic illness self‐management behaviors. While providers' PDM styles (as reflected in patients' ratings) may produce gains in a provider‐controlled aspect of patients' self‐management practices in hypertension (having home monitors), a highly participatory PDM style alone may not be sufficient to ensure patients' engagement in more patient‐controlled aspects of their care and outcome. In chronic illness care in general, both providers and patients face a variety of obstacles, such as limited provider time and constraints on patient resources, that frustrate translation of provider encouragement of patient participation in a given encounter into actual changes in health behaviors. 25 , 33 Other supportive measures that help activate patients to extend the impact of highly participatory encounters are probably required.
Acknowledgments and disclosures: We thank Tara Dudley for her statistical support. An earlier version of this work was presented as a poster at the Society of General Internal Medicine's 28th Annual Meeting (May 11–14, 2005). Dr Cho is supported by an AHRQ Training Grant 5‐T32‐HS00079. Dr Yancy is supported by VA HSR&D Career Development Award RCD 02–183–1. The Veterans' Study to Improve the Control of Hypertension (V‐STITCH) is supported by an investigator‐initiated research grant (20–034) awarded to Dr Bosworth by the Department of Veterans Affairs, Veterans Health Administration, HSR&D Service (Washington, DC). The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
References
- 1. Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. [DOI] [PubMed] [Google Scholar]
- 2. The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee . The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 3. Fields LE, Burt VL, Cutler JA, et al. The burden of adult hypertension in the United States, 1999 to 2000: a rising tide. Hypertension. 2004;44:398–404. [DOI] [PubMed] [Google Scholar]
- 4. Kaplan SH, Greenfield S, Gandek B, et al. Characteristics of physicians with participatory decision‐making styles. Ann Intern Med. 1996;124:497–504. [DOI] [PubMed] [Google Scholar]
- 5. Greenfield S, Kaplan S, Ware J. Expanding patient involvement in care: effects on patient outcomes. Ann Intern Med. 1985;102:520–528. [DOI] [PubMed] [Google Scholar]
- 6. Greenfield S, Kaplan S, Ware J, et al. Patients' participation in medical care: effects on blood sugar control and quality of life in diabetes. J Gen Intern Med. 1988;3:448–457. [DOI] [PubMed] [Google Scholar]
- 7. Heisler M, Bouknight RR, Hayward RA, et al. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self‐management. J Gen Intern Med. 2002;17:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaplan SH, Greenfield S, Dukes K. Effects of a joint physician patient intervention program on health outcomes and interpersonal care. Clin Res. 1993;41:541A. [Google Scholar]
- 9. Adams RJ, Smith BJ, Ruffin RE. Impact of the physician's participatory style in asthma outcomes and patient satisfaction. Ann Allergy Asthma Immunol. 2001;86:263–271. [DOI] [PubMed] [Google Scholar]
- 10. Kaplan SH, Greenfield S, Ware JE Jr. Assessing the effects of physician‐patient interactions on the outcomes of chronic disease. Med Care. 1989;3(suppl):S110–S127. [DOI] [PubMed] [Google Scholar]
- 11. Lipkin M Jr. Pegasus or Sisyphus? Ann Intern Med. 1996;124:511–513. [DOI] [PubMed] [Google Scholar]
- 12. Boulware LE, Daumit GL, Frick KD, et al. An evidence‐based review of patient‐centered behavioral interventions for hypertension. Am J Prev Med. 2001;21:221–232. [DOI] [PubMed] [Google Scholar]
- 13. Cappuccio FP, Kerry SM, Forbes L, et al. Blood pressure control by home monitoring: meta‐analysis of randomised trials. BMJ. 2004;329:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krousel‐Wood M, Thomas S, Muntner P, et al. Medication adherence: a key factor in achieving blood pressure control and good clinical outcomes in hypertensive patients. Curr Opin Cardiol. 2004;19:357–362. [DOI] [PubMed] [Google Scholar]
- 15. Bosworth HB, Olsen MK, Gentry P, et al. Nurse‐administered telephone intervention for blood pressure control: a patient‐tailored multifactorial intervention. Patient Educ Counsel. 2005;57:5–14. [DOI] [PubMed] [Google Scholar]
- 16. Bosworth HB, Olsen MK, Goldstein MK, et al. The veterans' study to improve the control of hypertension (V‐STITCH): design and methodology. Contemp Clin Trials. 2005;26:155–168. [DOI] [PubMed] [Google Scholar]
- 17. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care. 1986;24:67–74. [DOI] [PubMed] [Google Scholar]
- 18. The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1997;157:2413–2446. [DOI] [PubMed] [Google Scholar]
- 19. Davis TC, Long SW, Jackson RH, et al. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med. 1993;25:391–395. [PubMed] [Google Scholar]
- 20. Baker B, Szalai JP, Paquette M, et al. Marital support, spousal contact, and the course of mild hypertension. J Psychosom Res. 2003;55:229–233. [DOI] [PubMed] [Google Scholar]
- 21. Stamler J, Elliot P, Appel L, et al. Higher blood pressure in middle‐aged American adults with less education—role of multiple dietary factors: the INTERMAP study. J Hum Hypertens. 2003;17:655–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hicks LS, Fairchild DG, Horng MS, et al. Determinants of JNC VI guideline adherence, intensity of drug therapy, and blood pressure control by race and ethnicity. Hypertension. 2004;44:429–434. [DOI] [PubMed] [Google Scholar]
- 23. Schneider J, Kaplan SH, Greenfield S, et al. Better physician patient relationships are associated with higher reported adherence to antiretroviral therapy in patients with HIV infection. J Gen Intern Med. 2004;19:1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dominick KL, Golightly YM, Bosworth HB. Racial differences in analgesic/anti‐inflammatory medication adherence among patients with osteoarthritis. Ethn Dis. 2005;15:116–122. [PubMed] [Google Scholar]
- 25. Gotler RS, Flocke SA, Goodwin MA, et al. Facilitating participatory decision‐making: what happens in real‐world community practice? Med Care. 2000;38:1200–1209. [DOI] [PubMed] [Google Scholar]
- 26. Xu KT. The combined effects of participatory styles of elderly patients and their physicians on satisfaction. Health Serv Res. 2004;39:377–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ross School of Business, University of Michigan. ACSI Scores for US Federal Government . Available at http:www.theacsi.orggovernmentgovt‐05.html. Accessed July 7, 2006.
- 28. Stein R. VA care is rated superior to that in private hospitals. Washington Post. Friday, Jan 20, 2006:A15. [Google Scholar]
- 29. Castle NG, Engberg J. Response formats and satisfaction surveys for elders. Gerontologist. 2004;44:358–367. [DOI] [PubMed] [Google Scholar]
- 30. Harvey RM, Kazis L, Lee AF. Decision‐making preference and opportunity in VA ambulatory care patients: association with patient satisfaction. Res Nurs Health. 1999;22:39–48. [DOI] [PubMed] [Google Scholar]
- 31. Levinson W, Kao A, Kuby A, et al. Not all patients want to participate in decision making: a national study of public preferences. J Gen Intern Med. 2005;20:531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bodenheimer T, Lorig K, Holman H, et al. Patient self‐management of chronic disease in primary care. JAMA. 2002;288:2469–2475. [DOI] [PubMed] [Google Scholar]
- 33. Paterson B. Myth of empowerment in chronic illness. J Adv Nurs. 2001;34:574–581. [DOI] [PubMed] [Google Scholar]


