Abstract
The epidemic of obesity in the United States and around the world is intensifying in severity and scope and has been implicated as an underlying mechanism in systemic hypertension. Obese hypertensive individuals characteristically exhibit volume congestion, relative elevation in heart rate, and high cardiac output with concomitant activation of the renin‐angiotensin‐aldosterone system. When the metabolic syndrome is present, insulin resistance and hyperinsulinemia may contribute to hypertension through diverse mechanisms. Blood pressure can be lowered when weight control measures are successful, using, for example, caloric restriction, aerobic exercise, weight loss drugs, or bariatric surgery. A major clinical challenge resides in converting short‐term weight reduction into a sustained benefit. Pharmacotherapy for the obese hypertensive patient may require multiple agents, with an optimal regimen consisting of inhibitors of the renin‐angiotensin‐aldosterone system, thiazide diuretics, β‐blockers, and calcium channel blockers if needed to attain contemporary blood pressure treatment goals.
Excess body fat has been hypothesized as a major contributing factor to hypertension. 1 In the United States, 122 million adults are now overweight (body mass index [BMI], 25–29 kg/m2) or obese (BMI >30 kg/m2). 2 Sixty million persons in the United States are hypertensive.
Blood pressure and BMI are well correlated, with the International Study of Sodium, Potassium and Blood Pressure (INTERSALT) demonstrating a linear association between weight and both systolic and diastolic blood pressure. 3 The Framingham Study estimated that 78% of cases of hypertension in men and 64% in women were attributable to obesity. 4 Abdominal obesity (central, truncal, or android rather than gynecoid pattern) is more strongly correlated with the development of elevated blood pressure.
The problem of overweight and obesity is increasing in severity in the United States, with the National Health and Nutrition Examination Survey(NHANES)2003–2004 reporting the prevalence of overweight and obesity (BMI >25 kg/m2) rising to 66.3%. 5 The prevalence of obesity (BMI >30 kg/m2) is now estimated at 32.2% (Figure 1). 5 Perhaps even more ominous, the prevalence of overweight in children and adolescents continues to rise, having tripled between the years 1980 and 2002. 6
Figure 1.
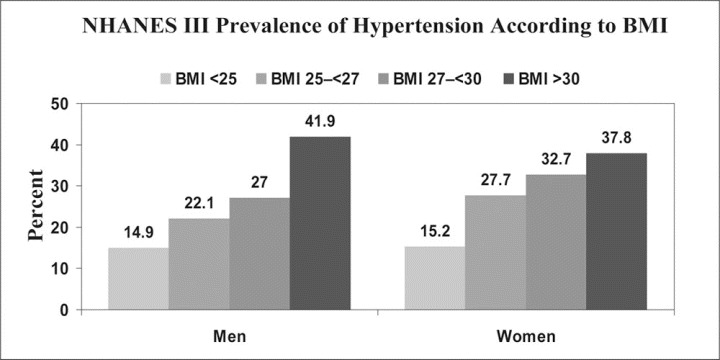
Data from the National Health and Nutrition Examination Survey (NHANES) III 1988–1994 showing prevalence of high blood pressure increasing progressively with higher levels of body mass index (BMI) in men and women. High blood pressure was defined as mean systolic blood pressure ≥140 mm Hg, mean diastolic blood pressure ≥90 mm Hg, or current use of antihypertensive medication. Adapted from Brown et al. 75
In the closely related metabolic syndrome, termed the cardiometabolic syndrome by some authors, there are several abnormalities of note, including high blood pressure, atherogenic dyslipidemia (low high‐density lipoprotein cholesterol and elevated apolipoprotein B, triglycerides, and small, dense low‐density lipoprotein cholesterol particles), insulin resistance with glucose intolerance, as well as proinflammatory and prothrombotic states.
With regard to clinical criteria, the National Cholesterol Education Program defines the metabolic syndrome as the presence of 3 of 5 risk factors: 1) abdominal obesity (waist circumference >102 cm [40 in] in men and >88 cm [35 in] in women), 2) elevated triglycerides (>150 mg/dL), 3) depressed high‐density lipoprotein cholesterol (<40 mg/dL in men and <50 mg/dL in women), 4) elevated blood pressure (>130/85 mm Hg), and 5) elevated fasting glucose (>100 mg/dL). 7 Of significant interest, the mean waist circumference and the prevalence of abdominal obesity continue to rise as well, increasing constantly over 15 years, with more than half of US adults having abdominal obesity in the 2003–2004 NHANES survey. 8 The prevalence of the metabolic syndrome itself is also increasing, at nearly 28% in the 2000 survey, with a progressive increase associated with age. 9
Of special concern is the potential progression from obesity to the metabolic syndrome to frank type 2 diabetes. In the third NHANES report, an average of 80% of patients with the metabolic syndrome had elevated blood pressure. 10 As noted, an increased BMI is independently correlated with higher rates of hypertension. 11 Compared with normal‐weight adults, persons with a BMI >40 kg/m2 have greater than 7 times the chance of being hypertensive (odds ratio, 7.37). 12
A review of serial NHANES papers from 1963 to 2002 has outlined blood pressure trends among children and adolescents aged 8 to 17, examining prevalence of hypertension and the impact of obesity on hypertension incidence. From 1963 to 1988, high blood pressure incidence decreased, yet after 1994 an uptrend was noted. Obesity, on the other hand, has gradually increased from 1963 to 2002 across all racial and ethnic groups. The development of hypertension lagged behind uptrending obesity by 10 years, with the greatest impact in black and Hispanic persons. 13
Among 13,563 healthy, middle‐aged, normotensive men followed in the Physician's Health Study for 15 years, an increased incidence of hypertension was significantly associated with increasing BMI, even among individuals with a BMI within the normal (<25 kg/m2) range. An increase in BMI of more than 5% or 10% in an 8‐year period, regardless of the baseline BMI, was also associated with a 20% or 70% relative risk of developing hypertension, respectively (Figure 2). 14 Thus, there appears to be definite epidemiologic evidence that increasing weight is closely and probably linearly related to blood pressure levels and components of the metabolic syndrome.
Figure 2.
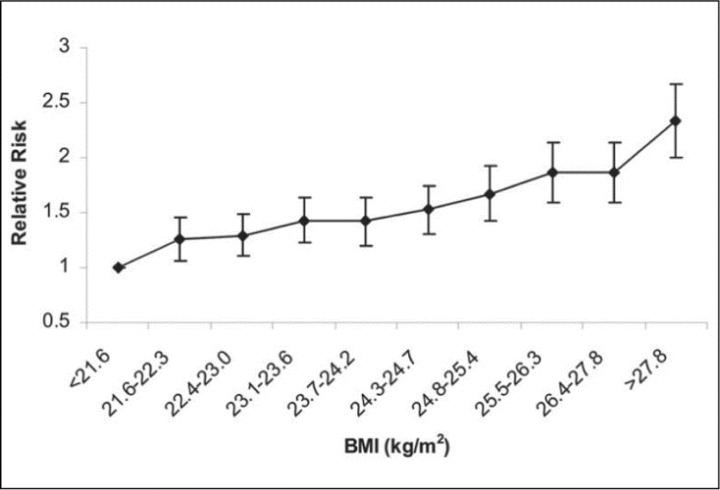
Multivariable‐adjusted relative risk of developing hypertension, according to baseline body mass index decile (BMI) in kg/m2. Adjusted for baseline age, cigarette smoking, alcohol consumption, exercise, parental history of myocardial infarction before the age of 60 years, history of diabetes, and elevated cholesterol. Referent group (relative risk, 1.0): BMI <21.6 kg/m2 at baseline. p for trend <.001. Adapted with permission from Gelber et al. 14
OBESITY AS A FORM OF SECONDARY HYPERTENSION
We propose that obesity represents a form of secondary hypertension because it reflects an imbalance or specific disorder in a bodily system (metabolic or endocrine) and because weight loss by any treatment method may lead to improved blood pressure control.
OBESITY AS A CONTRIBUTOR TO DRUG‐RESISTANT OR DIFFICULT‐TO‐CONTROL HYPERTENSION
Resistant hypertension (blood pressure not at goal level despite administration of 3 drugs [including a diuretic] at full doses) is frequently encountered in obese patients, with an estimated 6.5‐mm Hg increase in systolic blood pressure for every 10% increase in body weight; oftentimes, blood pressure is less responsive to antihypertensive medication because of weight gain. Occasionally, the problem of so‐called difficult‐to‐treat or resistant hypertension can be solved by using a proper‐sized cuff for BP measurement (ie, “pseudoresistance” can be avoided by employing an adequately sized [large] cuff in obese persons). In several series, >40% of patients with resistant hypertension were obese, 15 , 16 implicating obesity as one determining factor in resistance. At a university referral center, 43% of patients with resistant hypertension were discovered to be obese, 17 a tendency that has also been noted in the primary care setting. 18 Among 141 patients with resistant hypertension in another series, the average BMI was in the obese range (mean, 32 kg/m 2 ). 19
Subgroups of patients with resistant hypertension who are also obese often have glucose intolerance and hyperinsulinemia 20 and require more intensive therapy, irrespective of BMI. 21 Obesity is linked to a higher rate of uncontrolled blood pressure and the use of larger doses of medication to achieve blood pressure control when compared with lean patients, even when adjusting for variables such as sex, age, and upper arm circumference. 21
PATHOPHYSIOLOGY
Several pathophysiologic factors explain why patients who are obese or who have the metabolic syndrome may become hypertensive or may develop resistant or difficult‐to‐manage hypertension. 22 Frequently, individuals with obesity or the metabolic syndrome may have high‐normal blood pressure or prehypertension (120–139/80–89 mm Hg) as defined by the Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7). 23 The JNC 7 also suggested that obesity and physical inactivity, as well as dietary factors, may underlie prehypertension. An association between prehypertension and insulin resistance was recently defined. 24
Although causes have not been clarified, obesity and associated metabolic changes may exert a significant influence.
Multiple mechanisms, many still speculative, have been invoked to explain the association between obesity and hypertension. Elevated circulating volume, vasoconstriction, enhanced cardiac output, and activation of the renin‐angiotensin system all may play a part, in addition to potential, yet poorly defined effects of the effluent of adipose tissue on blood pressure. These elements will be discussed in greater detail below.
HEMODYNAMIC CHANGES IN OBESITY AND THE METABOLIC SYNDROME
Physiologically, obese individuals have elevations in heart rate, cardiac output, and intravascular volume. Obesity has been associated with microvascular dysfunction. 25 Animal and small human studies have demonstrated blunting of endothelium‐dependent vasodilation in obese models (ie, endothelial dysfunction). Further, microvascular recruitment (the ability of small vessels to participate in reactive hyperemia under conditions of increased flow in large vascular beds) and insulin‐induced vasodilation of small resistance vessels are impaired in obese individuals with insulin resistance. 26 Obese models have also shown decreased capillary and small resistance vessel proliferation. As obesity increases, there is progressive dysfunction of larger resistance as well as capillary vessels. 25 Visceral adipose tissue cells elaborate substances known as adipokines, such as free fatty acids, adiponectin, leptin, resistin, angiotensinogen, and tumor necrosis factor a. These elements may act to inhibit microvascular dilatation and capillary recruitment, with both hypertensive and insulin resistive effects. 25 Later, such small vessel effects may translate into stiffness in large arterial conduits, with both hypertensive and arteriosclerotic consequences. 27 Although the specific mechanism has not been delineated, obesity, insulin resistance, and hypertension indeed may be linked at the microvascular level. 26
INFLUENCE OF THE SYMPATHETIC NERVOUS SYSTEM
Psychological stress has been implicated in the pathogenesis of the metabolic syndrome. 28 High circulating insulin levels also have sympathomimetic effects, although interestingly patients with insulinoma tend not to exhibit hypertension. Fasting tends to inhibit sympathetic nervous system activity, while overfeeding enhances it. Some earlier studies have found plasma norepinephrine levels to be elevated in obese persons.
INFLUENCE OF LEPTIN
Understanding of the complex effects of leptin and other signaling pathways continues to evolve. Leptin is a hormonal product of the obesity gene, secreted by adipocytes, and is now understood to be one of the most important central and peripheral signals in regulating energy homeostasis. 29 , 30 Elevated levels of leptin have been appreciated in obese models, suggesting a state of leptin resistance. Abnormal levels of leptin may lead to dysregulation of appetite and thermal homeostasis. The effect of increased leptin secretion on upstream signaling pathways may be to increase plasma norepinephrine and epinephrine concentrations via stimulus of the ventral hypothalamus, including interaction with melanocortin‐4 receptors. This elevated adrenergic tone contributes to, among other cardiovascular effects, endothelial dysfunction and decreased arterial responsiveness to vasodilation with overall increased vascular tone. 29 , 30 In an experimental model, long‐term leptin infusion raised blood pressure. 31
ROLE OF SLEEP APNEA
The role of sleep apnea in promoting hypertension in obese patients has recently excited interest. In the past, it has been difficult to determine the relative contribution of sleep apnea to hypertension because obesity may underlie both conditions. A link between sleep apnea and aldosterone excess has been hypothesized, supported by the successful use of aldosterone inhibitors in patients with sleep apnea and hypertension. 32
INSULIN RESISTANCE, HYPERINSULINEMIA, AND THEIR PRESSOR EFFECTS
The presence of obesity, insulin resistance and hyperinsulinemia may promote hypertension through diverse mechanisms, including vasoconstriction and vascular hypertrophy based on the progrowth effects of insulin, with abdominal adiposity predicting vascular endothelial dysfunction. 20 , 33 Insulin resistance attenuates endothelial release of nitric oxide, a potent vasodilator, creating another potential hypertensive stimulus. 34 A recent study in a large national cohort showed that prehypertension was associated with insulin resistance. 24 Although there is a strong association between hypertension and insulin resistance, specific mechanisms have not been well clarified. 35
RENAL SODIUM HANDLING ABNORMALITIES
Obese patients may demonstrate enhanced renal sodium absorption, likely based on elevated glomerular filtration rate and renal blood flow, 36 as well as possible extrinsic compression of the kidney by adipose tissue. 37 High circulating insulin levels also exert sodium retentive effects. Atrial natriuretic peptide levels have been found to be low in obese hypertensive patients. 38
ACTIVATION OF THE RENIN‐ANGIOTENSIN‐ALDOSTERONE SYSTEM IN OBESITY AND HYPERTENSION
Activation of the renin‐angiotensin‐aldosterone system increases sodium retention and peripheral resistance, thereby contributing to hypertension. Angiotensinogen gene expression has been discovered in adipose tissue associated with abdominal fat distribution in obesity. 39 Locally produced angiotensin II, arising from adipose tissue itself, may also play a significant etiologic role. Plasma renin activity is elevated in obese persons and decreases with weight loss. 40 In a recent report, an angiotensin receptor blocker given to patients with hypertensive metabolic syndrome showed equal reduction in blood pressure, yet significant improvement in glucose tolerance testing, insulin levels, and visceral fat content when compared with a calcium channel blocker. 41 Several trials have shown fewer cases of new‐onset diabetes when angiotensin receptor blockers or angiotensin‐converting enzyme inhibitors are used in hypertensive patients. These results are potentially related to angiotensin II effects on insulin signaling pathways, although precise mechanisms are still not clearly defined. 42
TARGET ORGAN EFFECTS
Elevated cardiac output and intravascular volume eventually can contribute to end‐organ damage, such as ventricular hypertrophy, heart failure, and glomerular hyperfiltration with consequent microalbuminuria. 43 When elevated blood pressure and elevated blood glucose are both present, even to a mild degree, adverse cardiovascular outcomes increase markedly. 44
TREATMENT
Optimal treatment for obese patients with hypertension is not well defined, and there is little trial evidence or guideline support concerning therapy. 45
Therapeutic Lifestyle Modification
Weight reduction obviously can be a valuable adjunct to drug therapy, as are other lifestyle modifications, although weight reduction is often difficult to achieve and sustain. 46 , 47 , 48 In the Trials of Hypertension Prevention series (TOHP I and II), there were significant reductions in blood pressure as well as a lower incidence of new hypertension, even with only modest weight loss. 49 Mean weight loss in the intervention group ranged from 4.4 kg at 6 months to 0.2 kg at 36 months, although blood pressure was significantly lower than in the control group at 6, 18, and 36 months. as one might anticipate, individuals who lost at least 4.5 kg at 6 months and maintained this weight reduction throughout the study had the greatest reduction in blood pressure. 49
A pooled analysis of controlled trials of weight loss (caloric restriction plus exercise) reported a 4.4/3.6‐mm Hg decrease in blood pressure for each 5 kg of weight lost, 50 with longer‐term observations reporting some attenuation in effect (a 6.0/4.6‐mm Hg decrease for a 10‐kg weight loss). 51 a dose‐response curve relating weight loss to blood pressure is observed in most series. in obese hypertensive patients, physical exercise with diet exerts a beneficial effect on blood pressure, even in the absence of weight loss. 52 , 53
Role of Weight Loss Drugs
Orlistat and sibutramine are available for weight loss indications in the United states. Orlistat inhibits pancreatic lipase, with several trials reporting success in weight loss as well as simultaneous significant improvement in blood pressure control. 54 , 55 sibutramine, a norepinephrine and serotonin uptake inhibitor, can assist in weight loss, but with only modest blood pressure effects. some patients receiving sibutramine, however, manifest elevations in heart rate and blood pressure. the new class of endocannabinoid receptor inhibitors is currently being evaluated for long‐term weight loss and potential alteration in cardiovascular risk factors such as hypertension.
Response to Bariatric Surgery
The development of bariatric surgery, including gastric bypass and several other variations, has raised the prospect of improving cardiovascular risk factors through surgical weight loss. The Swedish Obese Subjects (SOS) study evaluated 4047 matched obese patients who received either bariatric surgery or nonsurgical therapy. A 4.4/5.5‐mm Hg blood pressure reduction was observed in surgically treated patients at 2 years; this difference did not persist at 10 years. 56
Reviews of other, smaller surgical series reveal that normotensive or only mildly hypertensive individuals do not achieve significant blood pressure reduction post‐surgical intervention when compared with persons with significantly elevated blood pressure. 51 however, both cardiovascular and all‐cause mortality benefits were observed in the surgery group, perhaps underscoring the multifactorial contribution of obesity to cardiovascular risk. 57
Differential Effects of Pharmacologic Classes of Hypertension Drugs
Current guidelines do not offer a specific pharmacologic approach to the treatment of obesity and hypertension. No major trials have addressed this question, and choices must be made on the basis of clinical experience, minimal data, and available knowledge of the mechanistic origins of obesity hypertension. 58
β‐Blockers reduce cardiac output while also inhibiting renin and have been shown in limited studies to have greater antihypertensive efficacy in obese compared with lean individuals. 59 adverse effects on glucose metabolism tend to be minimal, with some modest weight gain reported in limited series.
Angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, renin inhibitors, and calcium channel blockers are metabolically neutral. inhibitors of the renin‐angiotensin system may prevent progressive nephropathy and the onset of new type 2 diabetes as well as improve insulin sensitivity, 60 , 61 , 62 and calcium channel blockers may aid natriuresis. however, volume expansion and elevated sodium intake and retention 63 suggest a major role for diuretics, which are well tolerated metabolically when used in low to moderate doses 64 , 65 ; the use of these agents has been necessary in almost all of the angiotensin‐converting enzyme inhibitor and angiotensin receptor blocker studies to reduce blood pressure. In a trial of obese hypertensive patients, diuretics were as effective in lowering blood pressure as angiotensin‐converting enzyme inhibitors. 66 However, when fixed‐dose combinations of angiotensin‐converting enzyme inhibitor/verapamil were tested against losartan/thiazide diuretic in a study group with impaired fasting glucose, the use of the former combination resulted in fewer cases of new‐onset diabetes. 67 Nonetheless, with lower blood pressure treatment goals in high‐risk populations, multidrug therapy, even including diuretics and β‐blockers, may be obligatory. When β‐blockers must be used to attain control, a combined β‐ and α‐blocking agent such as carvedilol may be optimal, given more favorable effects on glucose metabolism compared with other β‐blockers, 68 potentially relating to better peripheral glucose disposal effects permitted by the vasodilatory component of this drug. When diabetes is present in the obese hypertensive patient, even lower blood pressure goals are sought (130/80 mm Hg); making the use of multidrug therapy is usually necessary. 23
A reasonable initial approach to treating the obese hypertensive patient might include beginning with a specific inhibitor of the renin‐angiotensin system, perhaps with a low‐dose thiazide diuretic, and then adding a β‐blocker or calcium channel blocker if blood pressure control is not achieved. Four or more drugs may be needed in some cases to attain blood pressure goals.
Recently, the effects of metabolic syndrome in contributing to arterial stiffness have been reviewed comprehensively. 69 Of emerging interest, therefore, is the finding that the endothelin‐1 inhibitor darusentan has been shown to be effective in reducing blood pressure in patients with drug‐resistant patients who at baseline were receiving 3 drugs at maximal doses, including a diuretic. 70 In this study the prevalence of obesity (BMI >30 kg/m2) was >50%, with a mean body mass index of 31.4 (range, 20.0–42.5). These findings suggest a potential future role for inhibition of endothelin‐1 in addressing the vasoconstrictive, endothelial dysfunction, and vascular smooth muscle hypertrophic components of obesity‐related hypertension.
Recent data suggest that adipose tissue may elaborate substances that stimulate aldosterone secretion, thus potentially explaining the efficacy of aldosterone inhibitors in a wide range of patients with multidrug‐resistant hypertension, many of whom are obese. 71 , 72
CONCLUSIONS
A major societal challenge resides in stemming the US and worldwide epidemic of obesity with its associated cardiovascular morbidities.
Persistence of weight loss, whether achieved by behavioral, pharmacologic, or surgical methods, also has been an ongoing challenge. Understanding treatment effects on comorbidities of obesity has been limited by the short‐term nature of most studies. Blood pressure substantially decreases initially with moderate weight loss. Yet these results are attenuated after a few years, even with surgical treatment. In the SOS trial noted above, a 4.4/5.5‐mm Hg reduction in blood pressure was observed in the bariatric surgical cohort at 2 years, but this reduction was not maintained at 10 years. 56
Another major challenge lies in addressing the multiple avenues of risk that exist in addressing the metabolic syndrome in obese patients. An ideal program to prevent cardiovascular and renal injury would include weight loss, diet, exercise, insulin‐sensitizing hypoglycemic agents, lipid modification, anti‐inflammatory therapies, and blood pressure control, first with inhibitors of the renin‐angiotensin‐aldosterone system and next with agents exhibiting diverse mechanisms of action to achieve blood pressure goals. 73
While blood pressure lowering and weight loss medications may partly address the multifactorial problem of obesity and hypertension and have probably helped to reduce morbidity and mortality, the ultimate resolution awaits the arrival of a practical and easily applied set of dietary and lifestyle changes that can be adopted by the public at large. 74 Until then, the scope of the clinical challenge of obesity and hypertension will continue to expand.
References
- 1. Stamler J. Epidemiologic findings on body mass and blood pressure in adults. Ann Epidemiol. 1991;1:347–362. [DOI] [PubMed] [Google Scholar]
- 2. Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults 1999–2000. JAMA. 2002;288:1723–1727. [DOI] [PubMed] [Google Scholar]
- 3. Dyer AR, Elliott P, Shipley M. Body mass index versus height and weight in relation to blood pressure. Finding for the 10,079 persons in the INTERSALT Study. Am J Epidemiol. 1990;131:589–596. [DOI] [PubMed] [Google Scholar]
- 4. Garrison RJ, Kannel WB, Stokes J, et al. Incidence and precursors of hypertension in young adults: The Framingham Offspring Study. Prev Med. 1987;16:235–251. [DOI] [PubMed] [Google Scholar]
- 5. Rosamond W, Flegal K, Friday G, et al. Heart Disease and Stroke Statistics–2007 Update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–e171. [DOI] [PubMed] [Google Scholar]
- 6. Ogden CL, Carroll MD, Curin LR, et al. Prevalence of overweight and obesity in the United States 1999–2004. JAMA. 2006;295:1549–1555. [DOI] [PubMed] [Google Scholar]
- 7. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, Third Report (Adult Treatment Panel II, Final Report). Circulation. 2002;106:3142–3421. [PubMed] [Google Scholar]
- 8. Li C, Ford ES, McGuire LC, et al. Increasing trends in waist circumference and abdominal obesity among US adults. Obesity (Silver Spring). 2007;15:216–224. [DOI] [PubMed] [Google Scholar]
- 9. Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among US adults. Diabetes Care. 2004;27:2444–2449. [DOI] [PubMed] [Google Scholar]
- 10. Wong ND, Pio JR, Franklin SS, et al. Preventing coronary events by optimal control of blood pressure and lipids in patients with the metabolic syndrome. Am J Cardiol. 2003;91:1421–1426. [DOI] [PubMed] [Google Scholar]
- 11. Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. [DOI] [PubMed] [Google Scholar]
- 12. Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity‐related health risk factors, 2001. JAMA. 2003;289:76–79. [DOI] [PubMed] [Google Scholar]
- 13. Din‐Dzietham R, Liu Y, Bielo MV, et al. High blood pressure trends in children and adolescents in national surveys 1963–2002. Circulation. 2007;116:1488–1496. [DOI] [PubMed] [Google Scholar]
- 14. Gelber RP, Gaziano JM, Manson JE, et al. A prospective study of body mass index and the risk of developing hypertension in men. Am J Hypertens. 2007;20:370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Isaksson H, Cederholm T, Jansson E, et al. Therapy resistant hypertension associated with central obesity, insulin resistance, and large muscle fibre area. Blood Press. 1993;2:46–52. [DOI] [PubMed] [Google Scholar]
- 16. Hall WD. Resistant hypertension, secondary hypertension, and hypertensive crises. Cardiol Clin. 2002;20:281–289. [DOI] [PubMed] [Google Scholar]
- 17. Hernández‐delRey R, Armario P, Martin‐Baranera M, et al. Target‐organ damage and cardiovascular risk profile in resistant hypertension: influence of the white‐coat effect. Blood Press Monit. 1998;3:331–337. [PubMed] [Google Scholar]
- 18. Bramlage P, Pittrow D, Wittchen HU, et al. Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled. Am J Hypertens. 2004;17:904–910. [DOI] [PubMed] [Google Scholar]
- 19. Garg JP, Elliott WJ, Folker A, et al. Resistant hypertension revisited: a comparison of two university‐based cohorts. Am J Hypertens. 2005;18:619–626. [DOI] [PubMed] [Google Scholar]
- 20. Martell N, Rodgriguez‐Cerillo M, Grobbee DE, et al. High prevalence of secondary hypertension and insulin resistance in patients with refractory hypertension. Blood Press. 2003;12:149–154. [DOI] [PubMed] [Google Scholar]
- 21. Modan M, Almog S, Fuchs Z, et al. Obesity, glucose intolerance, hyperinsulinemia, and response to antihypertensive drugs. Hypertension. 1991;17:565–573. [DOI] [PubMed] [Google Scholar]
- 22. Moser M, Setaro JF. Resistant or difficult to control hypertension. N Engl J Med. 2006;355:385–392. [DOI] [PubMed] [Google Scholar]
- 23. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The JNC 7 Report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 24. Player MS, Mainous AG 3rd, Diaz VA, et al. Prehypertension and insulin resistance in a nationally representative adult population. J Clin Hypertens (Greenwich). 2007;9:424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Serné EH, DeJongh RT, Eringa EC, et al. Microvascular dysfunction: A potential pathophysiological role in the metabolic syndrome. Hypertension. 2007;50:204–211. [DOI] [PubMed] [Google Scholar]
- 26. Jonk AM, Houben AJHM, DeJongh RT, et al. Microvascular function in obesity: A potential mechanism in the pathogenesis of obesity‐associated insulin resistance and hypertension. Physiology (Bethesda). 2007;22:252–260. [DOI] [PubMed] [Google Scholar]
- 27. Struijker‐Boudier HA, Cohuet GM, Baumann M, et al. The heart, macrocirculation, and microcirculation in hypertension: A unifying hypothesis. J Hypertens Suppl. 2003;21:S19–S23. [DOI] [PubMed] [Google Scholar]
- 28. Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 2005;30:1–10. [DOI] [PubMed] [Google Scholar]
- 29. Hall JE, Hildebrandt DA, Kuo J. Obesity hypertension. Role of leptin and sympathetic nervous system. Am J Hypertens. 2001;14:103S–115S. [DOI] [PubMed] [Google Scholar]
- 30. Yang R, Barouch LI. Leptin signaling and obesity: cardiovascular consequences. Circ Res. 2007;101:545–559. [DOI] [PubMed] [Google Scholar]
- 31. Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31:409–414. [DOI] [PubMed] [Google Scholar]
- 32. Goodfriend TL, Calhoun DA. Resistant hypertension, obesity, sleep apnea, and aldosterone: theory and therapy. Hypertension. 2004;43:518–524. [DOI] [PubMed] [Google Scholar]
- 33. Brook RD, Bard RL, Rubenfire M, et al. Usefulness of visceral obesity (waist/hip ratio) in predicting vascular endothelial function in healthy overweight adults. Am J Cardiol. 2001;88:1264–1269. [DOI] [PubMed] [Google Scholar]
- 34. Black PH. The inflammatory response is an integral part of the stress response. Implications for atherosclerosis, insulin resistance, type 2 diabetes, and metabolic syndrome X. Brain Behav Immun. 2003;17:350–364. [DOI] [PubMed] [Google Scholar]
- 35. Reaven GM. Insulin resistance, hypertension, and coronary heart disease. J Clin Hypertens (Greenwich). 2003;5:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. [DOI] [PubMed] [Google Scholar]
- 37. Hall JE. Mechanisms of abnormal renal sodium handling in obesity hypertension. Am J Hypertens. 1997;10:49S–55S. [PubMed] [Google Scholar]
- 38. Dessi‐Fulgheri P, Sarzani R, Tamburrini P, et al. Plasma atrial natriuretic peptide and natriuretic peptide receptor gene expression in adipose tissue of normotensive and hypertensive obese patients. J Hypertens. 1997;15:1695–1699. [DOI] [PubMed] [Google Scholar]
- 39. Van Harmelen V, Elizalde M, Ariapart P, et al. The association of human adipose angiotensinogen gene expression with abdominal fat distribution in obesity. Int J Obes. 2000;24:673–678. [DOI] [PubMed] [Google Scholar]
- 40. Hall JE, Brands MW, Dixon WN, et al. Obesity‐induced hypertension: renal function and systemic hemodynamics. Hypertension. 1993;22:292–299. [DOI] [PubMed] [Google Scholar]
- 41. Shimabukuro M, Tanaka H, Shimabukuro T. Effects of telmisartan on fat distribution in individuals with the metabolic syndrome. J Hypertens. 2007;25:841–848. [DOI] [PubMed] [Google Scholar]
- 42. Fonseca VA. Insulin resistance, diabetes, hypertension, and renin‐angiotensin system inhibition: Reducing risk for cardiovascular disease. J Clin Hypertens (Greenwich). 2006;8:713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Valensi P, Assayag M, Busby M, et al. Microalbuminuria in obese patients with or without hypertension. Int J Obes Relat Metab Disord. 1996;20:574–579. [PubMed] [Google Scholar]
- 44. Sowers JR, Epstein M, Frolich ED. Diabetes, hypertension, and cardiovascular disease: An update. Hypertension. 2001;37:1053–1059. [DOI] [PubMed] [Google Scholar]
- 45. Sharma AM, Engeli S. Managing big issues on lean evidence: treating obesity hypertension. Nephrol Dial Transplant. 2002;17:353–355. [DOI] [PubMed] [Google Scholar]
- 46. Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 47. Appel LJ, Champagne CM, Harsha DW, et al; PREMIER Collaborative Research Group . Effects of comprehensive lifestyle modification on blood pressure control: Main results of the PREMIER Clinical Trial. JAMA. 2003;289:2083–2093. [DOI] [PubMed] [Google Scholar]
- 48. Whelton SP, Chin A, Xin X, et al. Effect of aerobic exercise on blood pressure:A meta‐analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. [DOI] [PubMed] [Google Scholar]
- 49. Stevens VJ, Obarzanek E, Cook NR, et al; Trials for the Hypertension Prevention Research Group . Long‐term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention Phase II. Ann Intern Med. 2001;134(1):1–11. [DOI] [PubMed] [Google Scholar]
- 50. Neter JE, Stam BE, Kok FJ, et al. Influence of weight reduction on blood pressure: A meta‐analysis of randomized controlled trials. Hypertension. 2003;42:878–884. [DOI] [PubMed] [Google Scholar]
- 51. Aucott L, Poobalan A, Smith WCS, et al. Effects of weight loss in overweight/obese individuals and long‐term hypertension outcomes: a systematic review. Hypertension. 2005;45:1035–1041. [DOI] [PubMed] [Google Scholar]
- 52. Blumenthal JA, Sherwood A, Gullette EC, et al. Exercise and weight loss reduce blood pressure in men and women with mild hypertension: effects on cardiovascular, metabolic, and hemodynamic functioning. Arch Intern Med. 2000;160:1947–1958. [DOI] [PubMed] [Google Scholar]
- 53. Carroll JF, Kyser CK. Exercise training in obesity lowers blood pressure independent of weight change. Med Sci Sports Exerc. 2002;34:596–601. [DOI] [PubMed] [Google Scholar]
- 54. Bakris G, Calhoun D, Egan B, et al; Orlistat and Resistant Hypertension Investigators . Orlistat improves blood pressure control in obese subjects with treated but inadequately controlled hypertension. J Hypertens. 2002;20(11):2257–2267. [DOI] [PubMed] [Google Scholar]
- 55. Sharma AM, Golay A. Effect of orlistat‐induced weight loss on blood pressure and heart rate in obese patients with hypertension. J Hypertens. 2002;20:1873–1878. [DOI] [PubMed] [Google Scholar]
- 56. Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. [DOI] [PubMed] [Google Scholar]
- 57. Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. [DOI] [PubMed] [Google Scholar]
- 58. Jordan J, Engell S, Redon J, et al. European Society of Hypertension Working Group on Obesity: background, aims, and perspectives. J Hypertens. 2007;25:897–900. [DOI] [PubMed] [Google Scholar]
- 59. Wofford MR, Anderson DC, Brown CA, et al. Antihypertensive effect of alpha‐ and beta‐adrenergic blockade in lean and obese hypertensive subjects. Am J Hypertens. 2001;14:694–698. [DOI] [PubMed] [Google Scholar]
- 60. Dahlöf B, Devereux RB, Kjeldsen S, et al. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint Reduction in Hypertension study (LIFE): a randomized trial against atenolol. Lancet. 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- 61. Heart Outcome Prevention Evaluation (HOPE) Study Investigators . Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE Study and the MICRO HOPE Substudy. Lancet. 2000;255:253–259. [PubMed] [Google Scholar]
- 62. Brenner BM, Cooper ME, DeZeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy (RENAAL). N Engl J Med. 2001;345:861–869. [DOI] [PubMed] [Google Scholar]
- 63. He J, Ogden LG, Vupputuri S, et al. Dietary sodium intake and subsequent risk of cardiovascular disease in overweight adults. JAMA. 1999;282:2027–2034. [DOI] [PubMed] [Google Scholar]
- 64. Setaro JF. Resistant hypertension. In: Black HR, Elliott W, eds. Clinical Hypertension: A Companion to Braunwald's Heart Disease. Philadelphia, PA: Elsevier/WB Saunders; 2006:498–511. [Google Scholar]
- 65. Reaven GM, Clinkingbeard C, Jeppesen J, et al. Comparing the hemodynamic and metabolic effects of low‐dose hydrochlorothiazide and lisinopril treatment in obese patients with high blood pressure. Am J Hypertens. 1995;8:461–466. [DOI] [PubMed] [Google Scholar]
- 66. Reisin E, Weir MR, Falkner B, et al. Lisinopril versus hydrochlorothiazide in obese hypertensive patients: a multicenter placebo‐controlled trial. Treatment of Obese Patients with Hypertension (TROPHY) Study Group . Hypertension. 1997;30:140–145. [DOI] [PubMed] [Google Scholar]
- 67. Bakris G, Molitch M, Hewkin A, et al. Differences in glucose tolerance between fixed‐dose antihypertensive drug combinations in people with metabolic syndrome. Diabetes Care. 2006;29:2592–2597. [DOI] [PubMed] [Google Scholar]
- 68. Bakris GL, Fonseca V, Katholi RE, et al. Metabolic effects of cardvedilol versus metoprolol in patients with Type 2 diabetes mellitus and hypertension. A randomized controlled trial. JAMA. 2004;292:2227–2236. [DOI] [PubMed] [Google Scholar]
- 69. Sarafidis PA. Metabolic syndrome and arterial stiffness. Evidence for gender disparity and early effects of nontraditional risk factors? J Hypertens. 2007;25:935–938. [DOI] [PubMed] [Google Scholar]
- 70. Black HR, Bakris GL, Weber M, et al. Efficacy and safety of darusentan in patients with resistant hypertension: Results from a randomized, double‐blind, placebo‐controlled dose‐ranging study. J Clin Hypertens (Greenwich). 2007;9:760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schinner S, Willenberg HS, Krause D, et al. Adipocytederived products induce the transcription of the StAR promoter and stimulate aldosterone and cortisol secretion from adrenocortical cells through the Wnt‐signaling pathway. Int J Obes (Lond). 2007;31:864–870. [DOI] [PubMed] [Google Scholar]
- 72. Stas S, Whaley‐Connell AT, Sowers JR. Aldosterone and hypertension in the cardiometabolic syndrome. J Clin Hypertens (Greenwich). 2008;10:94–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sarafidis PA, Whaley‐Connell AT, Sowers JR, et al. Cardiometabolic syndrome and chronic kidney disease: what is the link? J Cardiometab Syndr. 2006;1:58–65. [DOI] [PubMed] [Google Scholar]
- 74. Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome. Circulation. 2008;117:754–761. [DOI] [PubMed] [Google Scholar]
- 75. Brown CD, Higgins M, Donato KA, et al. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000;8:605–619. [DOI] [PubMed] [Google Scholar]


