Abstract
In this randomized, double‐blind, multicenter study, patients whose blood pressure (BP) was uncontrolled by monotherapy were switched directly to amlodipine/valsartan 5/160 mg (n=443) or 10/160 mg (n=451). After 16 weeks, BP control (levels <140/90 mm Hg or <130/80 mm Hg for diabetics) was achieved in 72.7% (95% confidence interval [CI], 68.6–76.9) of patients receiving amlodipine/valsartan 5/160 mg and in 74.8% (95% CI, 70.8–78.9) receiving amlodipine/valsartan 10/160 mg. Incremental reductions from baseline in mean sitting systolic and diastolic BP were significantly greater with the higher dose (20.0±0.7 vs 17.5±0.7 mm Hg; P=.0003 and 11.6±0.4 vs 10.4±0.4 mm Hg; P=.0046). Incremental BP reductions were also achieved with both regimens irrespective of previous monotherapy, hypertension severity, diabetic status, body mass index, and age. Peripheral edema was the most frequent adverse event. These results provide support for the BP‐lowering benefits of complementary antihypertensive therapy with amlodipine and valsartan in patients with hypertension uncontrolled by previous monotherapy.
Worldwide hypertension control remains sub‐optimal despite the availability of a range of effective antihypertensive medications. 1 , 2 , 3 , 4 , 5 A majority of patients with hypertension, especially those at high risk, require ≥2 agents for blood pressure (BP) control to be achieved. 6 , 7 In these individuals, multiple medications may be administered separately or in fixed‐dose combinations, the latter reducing treatment complexity and potentially leading to improved compliance. 8 , 9 , 10
Treatment guidelines note that the combination of an angiotensin receptor blocker (ARB) and a calcium channel blocker (CCB), similar to the combination of an angiotensin‐converting enzyme inhibitor (ACEI) or an ARB plus a diuretic, provides an effective option for patients with hypertension. 7 ACEI/CCB and ARB/CCB combinations incorporate monotherapy components that act via complementary mechanisms of action 11 , 12 and therefore achieve greater sustained BP reductions than when the respective monocomponents are administered alone. 13 , 14 , 15 Tolerability benefits may also be gained from rational drug combinations, such as edema reduction when an ACEI or an ARB is added to a CCB. 16 , 17
This study was designed to evaluate improvement in BP control after switching to one of two doses of a CCB/ARB combination (amlodipine/valsartan) in patients with hypertension not controlled with prior monotherapy.
PATIENTS AND METHODS
Study Design and Patients
This was a randomized, double‐blind, multinational, parallel‐group study conducted at 132 centers in 8 countries (France, Spain, Belgium, Norway, Switzerland, Slovakia, Canada, and the United States). Patients were screened at visit 1 (week −2 to −1) to assess eligibility for participation in the study. At visit 2 (day 1), patients discontinued their previous antihypertensive regimens and without washout were randomized in a 1:1 ratio to 16 weeks' treatment with either amlodipine/valsartan 5/160 mg or amlodipine/valsartan 10/160 mg. If BP was uncontrolled at visit 4 (week 8) (ie, BP was ≥140/90 mm Hg in nondiabetics or ≥130/80 mm Hg in diabetics), open‐label hydrochlorothiazide (HCTZ) 12.5 mg was added. No HCTZ was added if BP was controlled. If BP remained uncontrolled at visit 5 (week 12), the HCTZ dose was increased to 25 mg (patients with controlled BP at visit 4 but not at visit 5 received HCTZ 12.5 mg). All study medications were administered in the morning.
Male and female adult outpatients were eligible for inclusion in the treatment phase of the study if they had uncontrolled hypertension at both screening and randomization despite current antihypertensive monotherapy (defined as treatment with a single antihypertensive agent). All patients were required to be treated with monotherapy for a minimum of 2 months before screening. Patients were excluded if they had a systolic BP (SBP) level ≥180 mm Hg (≥160 mm Hg in diabetics) or a diastolic BP (DBP) level ≥110 mm Hg (≥100 mm Hg in diabetics) at any time between screening and randomization. Enrollment of patients with type 1 diabetes or poorly controlled type 2 diabetes (glycosylated hemoglobin >8.0%) was not permitted. Patients were also excluded if they had evidence of hepatic disease (alanine aminotransferase or aspartate aminotransferase >3 times the upper limit of normal at visit 1 or history of hepatic encephalopathy, esophageal varices, or portocaval shunt) or renal impairment (serum creatinine >1.5 times the upper limit of normal at visit 1 or history of dialysis or nephrotic syndrome). Other exclusion criteria included evidence of secondary hypertension; history of cerebrovascular accident, myocardial infarction, or New York Heart Association grade II to IV heart failure; transient ischemic attack or percutaneous coronary intervention or coronary artery bypass graft surgery 12 months before screening; second‐ or third‐degree heart block without a pacemaker; concomitant unstable angina pectoris or potentially life‐threatening arrhythmia or symptomatic arrhythmia; or clinically significant valvular heart disease. Women who were pregnant, nursing, or of childbearing potential and not using acceptable contraception were also excluded. After randomization, patients were not permitted to use any antihypertensive drugs other than study medication or to take any chronic treatment that may have interfered with BP control. The study was performed with institutional review board/independent ethics committee/research ethics board approval and was conducted in accordance with the International Conference on Harmonization Harmonized Tripartite Guidelines for Good Clinical Practice (with applicable local regulations) and the Declaration of Helsinki. All patients gave written informed consent.
BP Measurement
Sitting and standing BP values were measured using a standard calibrated mercury or aneroid sphygmomanometer. At screening, BP was measured in both arms, and the arm with the higher reading was used for all subsequent BP measurements. Three consecutive sitting SBP/DBP measurements were taken at 1‐ to 2‐minute intervals at each study visit (day 1 and weeks 4, 8, 12, and 16). The mean of these measurements was used as the average sitting office BP. A single measure of standing SBP/DBP was also recorded within 2 minutes after the last sitting BP measurement. All readings were taken at drug trough (ie, before taking the morning dose of medication).
Efficacy Variables
The primary efficacy variable was the proportion of patients in whom BP control was achieved at the study end point (week 16). BP control was defined as mean sitting BP <140/90 mm Hg in nondiabetic patients and <130/80 mm Hg in diabetic patients. The study end point (week 16) was defined as the week 16 value or the last nonmissing postbaseline value (last observation carried forward [LOCF]).
The main secondary efficacy variable was the proportion of patients in whom BP control was reached before the addition of HCTZ (ie, at the pre‐HCTZ end point [week 8], defined as the week 8 value or the LOCF at week 8). Other secondary efficacy variables included overall BP control rates at all weeks and DBP control rates (<90 mm Hg in nondiabetic patients and <80 mm Hg in patients with diabetes) as well as the change from baseline in mean sitting SBP and DBP (MSSBP and MSDBP, respectively) at all weeks/end points.
Prospectively planned analyses were also conducted to evaluate BP control rates and changes from baseline BP in patient subgroups divided according to class of prior antihypertensive therapy; age group; diabetic status; severity of hypertension (defined by 2 categories according to BP levels with monotherapy before switch: “more severe” if baseline mean sitting BP ≥160 and <180 mm Hg/≥100 and <110 mm Hg; “less severe” if baseline mean sitting BP ≥140 and <160 mm Hg/≥90 and <100 mm Hg); body mass index (BMI); and race. Safety and tolerability were assessed by monitoring and recording all adverse events, serious adverse events, laboratory safety evaluation results, physical examination findings, electrocardiographic data, and vital signs.
Statistical Analysis
A sample size of 926 patients, randomized in a 1:1 ratio, was required to ensure that the study had 90% power to detect a between‐treatment group difference in the main secondary efficacy variable using a 2‐sided 5% significance level. For this calculation, it was assumed that BP control rates at the pre‐HCTZ end point (week 8) would be 46.0%in patients receiving amlodipine/valsartan 5/160 mg and 56.6% in those receiving amlodipine/valsartan 10/160 mg, corresponding to an odds ratio of 1.533.
Efficacy analyses were conducted for the intent‐to‐treat population, which consisted of all randomized patients with a baseline and at least 1 post‐baseline efficacy assessment. The safety population consisted of all patients who had received at least 1 dose of double‐blind study medication.
For the primary efficacy outcome (ie, the proportion of patients in whom BP control was achieved at the study end point [week 16]), unadjusted control rates and corresponding asymptotic 2‐sided 95% confidence intervals (CIs) were calculated for each treatment group. The proportion of patients with controlled BP at end point weeks 16 and week 8 and at weeks 4, 8, 12, and 16 (LOCF not applied) were compared using a logistic regression model with treatment strategy and diabetic status as factors and baseline MSSBP and MSDBP as covariates. The odds of having controlled BP, with corresponding 2‐sided 95% CIs, were also assessed.
Changes from baseline in MSSBP and MSDBP at weeks 4, 8, 12, and 16 were analyzed using analysis of covariance (ANCOVA) with treatment strategy, country, and diabetic status as factors and baseline MSSBP or MSDBP, respectively, as a covariate. Differences between the least squares means and associated 95% CIs were calculated. For the proportion of patients in whom DBP control was achieved, unadjusted control rates and corresponding asymptotic 2‐sided 95% CIs were calculated. For the subgroup analyses, descriptive statistics for changes in MSSBP and MSDBP and unadjusted BP control rates with asymptomatic 2‐sided 95% CIs were determined.
RESULTS
Patient Disposition and Baseline Characteristics
A total of 894 patients were enrolled and randomized to treatment with amlodipine/valsartan 5/160 mg (n=443) or amlodipine/valsartan 10/160 mg (n=451) (Figure 1). Baseline demographics and clinical characteristics were well balanced between the 2 treatment groups (Table I). The study was completed by 781 patients: 407/443 (91.9%) received the lower dose (5/160 mg) and 374/451 (82.9%) received the higher dose (10/160 mg). Among patients treated with amlodipine/valsartan 5/160 mg, 24.8% received additional treatment with HCTZ 12.5 mg (n=94 at week 8; n=16 at week 12), of whom 11.5% required HCTZ 25 mg at week 12. For patients given amlodipine/valsartan 10/160 mg, 19.1% required HCTZ 12.5 mg (n=65 at week 8; n=21 at week 12), 6.4% of whom received HCTZ 25 mg at week 12.
Figure 1.
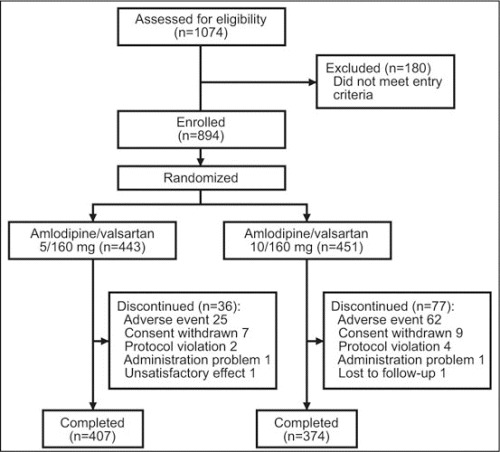
Patient disposition.
Table I.
Baseline Demographic and Clinical Characteristics in the Randomized Population
| Amlodipine/Valsartan 5/160 mg (n=443) | Amlodipine/Valsartan 10/160 mg (n=451) | Total Study Population (N=894) | |
|---|---|---|---|
| Age, ya | 58.5±12.3 | 58.6±12.0 | 58.5±12.2 |
| Age category | |||
| <65 y | 309 (69.8) | 311 (69.0) | 620 (69.4) |
| ≥65 y | 134 (30.2) | 140 (31.0) | 274 (30.6) |
| ≥70 y | 93 (21.0) | 91 (20.2) | 184 (20.6) |
| ≥75 y | 53 (12.0) | 40 (8.9) | 93 (10.4) |
| Sex | |||
| Male | 232 (52.4) | 211 (46.8) | 443 (49.6) |
| Female | 211 (47.6) | 240 (53.2) | 451 (50.4) |
| Race | |||
| White | 405 (91.4) | 418 (92.7) | 832 (92.1) |
| Black | 19 (4.3) | 17 (3.8) | 36 (4.0) |
| Other | 19 (4.3) | 16 (3.5) | 35 (3.9) |
| MSSBP, mm Hga | 149.8±9.7 | 150.4±10.5 | 150.1±10.1 |
| MSDBP, mm Hga | 90.8±7.7 | 90.6±7.6 | 90.7±7.7 |
| Sitting pulse, bpma | 71.2±9.3 | 71.6±9.7 | 71.4±9.5 |
| Prior treatment | |||
| ARB | 176 (39.7) | 176 (39.0) | 352 (39.4) |
| ACEI | 92 (20.8) | 106 (23.5) | 198 (22.1) |
| β‐Blocker | 77 (17.4) | 55 (12.2) | 132 (14.8) |
| CCB | 54 (12.2) | 70 (15.5) | 124 (13.9) |
| Diuretic | 41 (9.3) | 39 (8.6) | 80 (8.9) |
| Other | 3 (0.7) | 5 (1.1) | 8 (0.9) |
| Severity of hypertensionb | |||
| More severe | 97 (22.8) | 101 (22.9) | 198 (22.8) |
| Less severe | 329 (77.2) | 341 (77.1) | 670 (77.2) |
| Diabetic status | |||
| Diabetic | 72 (16.3) | 74 (16.4) | 146 (16.3) |
| Nondiabetic | 371 (83.7) | 377 (83.6) | 748 (83.7) |
| Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; bpm, beats per minute; CCB, calcium channel blocker; MSDBP, mean sitting diastolic blood pressure; MSSBP, mean sitting systolic blood pressure. Values are No. (%) unless otherwise indicated. aMean ± SD. bMore severe, ≥160 and <180 mm Hg/≥100 and <110 mm Hg; less severe, ≥140 and <160 mm Hg/≥90 and <100 mm Hg. Blood pressure at baseline is with monotherapy (ie, before switch to amlodipine/valsartan therapy). | |||
Primary and Secondary Efficacy Outcomes
At the study end point (week 16), the primary outcome of BP control was achieved in 320 patients (72.7%; 95% CI, 68.6–76.9) in the amlodipine/valsartan 5/160 mg (± HCTZ) group and 336 (74.8%; 95% CI, 70.8–78.9) treated with amlodipine/valsartan 10/160 mg (± HCTZ). The corresponding estimated odds for BP control were 1.35 (95% CI, 1.04–1.75) and 1.64 (95% CI, 1.27–2.13), respectively, giving an odds ratio of 0.82 (95% CI, 0.59–1.14). At the pre‐HCTZ end point (week 8), the proportion of patients in whom BP control was achieved was greater with the higher dose compared with the lower dose (76.4%vs 71.1%; P<.03 [logistic regression]). DBP control at the study end point (week 16) was achieved in 85.7% of patients in the amlodipine/valsartan 5/160 mg treatment group (95% CI, 82.4–88.9) and in 87.3% of those treated with amlodipine/valsartan 10/160 mg (95% CI, 84.2–90.4). Figure 2 shows the change from baseline in MSSBP and MSDBP over time for both treatments. At the study end point (week 16), incremental reductions from baseline in mean sitting BP were significantly greater in patients receiving the higher dose than in those treated with amlodipine/valsartan 5/160 mg (MSSBP, 20.0±0.7 mm Hg vs 17.5±0.7 mm Hg, respectively [P=.0003, ANCOVA]; MSDBP, 11.6±0.4 mm Hg vs 10.4±0.4 mm Hg [P=.0046, ANCOVA]; Table II).
Figure 2.
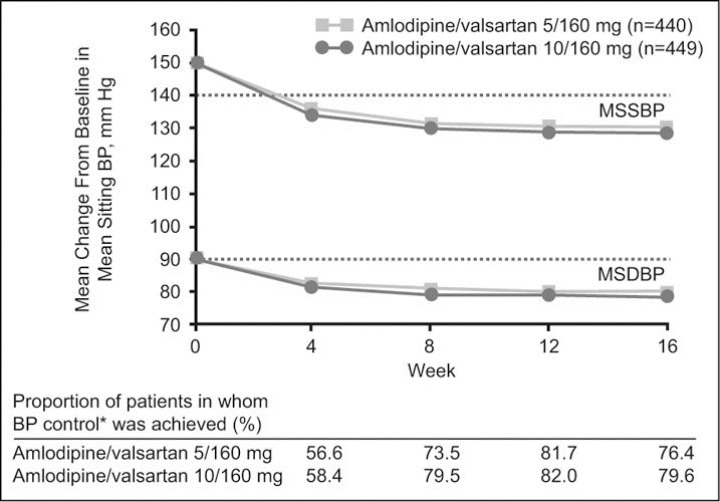
Incremental antihypertensive effects following a direct switch to amlodipine/valsartan 5/160 mg and 10/160 mg in patients with hypertension previously uncontrolled with monotherapy. Mean change from baseline in mean sitting systolic and diastolic blood pressure (MSSBP and MSDBP, respectively) and the proportion of patients in whom blood pressure (BP) control* was achieved over time (intent‐to‐treat population).*BP control is defined as BP <140/90 mm Hg for nondiabetic patients and <130/80 mm Hg for diabetic patients.
Table II.
Incremental Blood Pressure‐Lowering Effects of Amlodipine/Valsartan 5/160 mg and 10/160 mg in the Intent‐to‐Treat Population and According to the Severity of Hypertension at Baseline
| Week and Disease Severity | Amlodipine/Valsartan 5/160 mg MSSBP/MSDBP | Amlodipine/Valsartan 10/160 mg MSSBP/MSDBP | Amlodipine/Valsartan 5/160 vs 10/160 mg MSSBP/MSDBP | |||||
|---|---|---|---|---|---|---|---|---|
| No.a | Baseline, mg Hg | Change From Baseline, mg Hg (SEM) | No.a | Baseline, mg Hg | Change From Baseline, mg Hg (SEM) | Difference in Change (95% CI) | P Value | |
| Week 4 | ||||||||
| Intent‐to‐treatb | 440 | 149.8/90.8 | −13.9 (0.7)/−8.4 (0.4) | 449 | 150.4/90.6 | −16.1 (0.7)1−9.7 (0.4) | 2.2 (0.8–3.5)/1.3 (0.4–2.2) | .0016/.0038 |
| Less severec | 329 | 147.3/89.8 | −13.1/−8.2 | 341 | 147.1/89.3 | −15.0/−9.3 | ||
| More severed | 97 | 160.7/95.9 | −20.7/−10.9 | 101 | 162.7/95.5 | −23.6/−12.0 | ||
| Week 8 | ||||||||
| Intent‐to‐treatb | 423 | 149.6/90.9 | −18.0 (0.7)/−9.9 (0.5) | 410 | 150.8/90.9 | −20.8 (0.7)/−11.9 (0.5) | 2.8 (1.4–4.1)/2.0 (1.1–2.9) | <.0001/<.0001 |
| Less severec | 319 | 147.2/89.8 | −16.1/−93 | 310 | 147.4/89.7 | −18.5/−11.2 | ||
| More severed | 91 | 160.3/96.0 | −24.8/−13.2 | 93 | 163.4/95.7 | −29.7/−15.1 | ||
| End point week 8e | ||||||||
| Intent‐to‐treatb | 440 | 149.8/90.8 | −16.5 (0.7)/−9.3 (0.5) | 449 | 150.4/90.6 | −19.2 (0.7)/−11.3 (0.5) | 2.7 (1.3–4.1)/2.1 (1.2–3.0) | .0001/<.0001 |
| Less severec | 329 | 147.3/89.8 | −15.5/−9.0 | 341 | 147.1/89.3 | −17.5/−10.8 | ||
| More severed | 97 | 160.7/95.9 | −23.9/−12.5 | 101 | 162.7/95.5 | −28.8/−14.7 | ||
| Week 12 | ||||||||
| Intent‐to‐treatb | 410 | 149.9/91.0 | −19.2 (0.6)/−10.8 (0.4) | 388 | 150.7/91.0 | −21.8 (0.6)/−12.2 (0.4) | 2.6 (1.4–3.9)/1.4 (0.5–2.2) | <.0001/.0016 |
| Less severec | 309 | 147.3/89.8 | −17.5/−10.8 | 296 | 147.4/89.7 | −19.7/−12.1 | ||
| More severed | 91 | 160.3/96.0 | −25.9/−14.1 | 87 | 163.1/95.5 | −31.0/−15.4 | ||
| Week 16 | ||||||||
| Intent‐to‐treatb | 406 | 149.9/90.9 | −19.4 (0.7)/−11.3 (0.4) | 378 | 150.7/91.0 | −22.1 (0.7)/−12.4 (0.4) | 2.7 (1.4–4.0)/1.1 (0.2–1.9) | <.0001/.0121 |
| Less severec | 307 | 147.4/89.8 | −17.3/−10.9 | 289 | 147.3/89.7 | −19.9/−12.3 | ||
| More severed | 89 | 160.4/95.9 | −25.8/−14.6 | 85 | 163.1/95.9 | −30.6/−14.8 | ||
| Endpoint Week 16f | ||||||||
| Intent‐to‐treatb | 440 | 149.8/90.8 | −17.5 (0.7)/−10.4 (0.4) | 449 | 150.4/90.6 | −20.0 (0.7)/−11.6 (0.4) | 2.5 (1.1–3.8)/1.2 (0.4–2.1) | .0003/.0046 |
| Less severec | 329 | 147.3/89.8 | −16.3/−10.5 | 341 | 147.1/89.3 | −18.3/−11.7 | ||
| More severed | 97 | 160.7/95.9 | −24.7/−13.9 | 101 | 162.7/95.5 | −29.7/−14.5 | ||
| Abbreviations: CI, confidence interval; MSDBP, mean sitting diastolic blood pressure; MSSBP, mean sitting systolic blood pressure; SEM, standard error of the mean. aNumber of patients with nonmissing measurements at respective week. bThe least squares mean change from baseline is shown at each week for the intent‐to‐treat population. cPatients with MSSBP at baseline of ≥140 and <160 mm Hg or MSDBP of ≥90 and <100 mm Hg. dPatients with MSSBP at baseline of ≥160 and <180 mm Hg or MSDBP of ≥100 and <110 mm Hg. ePre‐hydrochlorothiazide end point (ie, week 8 or last observation carried forward [LOCF] value. fEnd point week 16 is the value at week 16 or LOCF value. The intent‐to‐treat population is based on a cutoff of 140/90 mm Hg; diabetic patients with a blood pressure value of 130–140 and 80–90 mm Hg were not included in the severity subgroup population. | ||||||||
Subgroup Evaluations
Prior Antihypertensive Therapy. BP control rates in the individual treatment groups did not differ over time by previous antihypertensive monotherapies. The proportion of patients in whom BP control was reached at week 8 ranged from 66.7% to 77.1% (range includes “other” prior monotherapy) for amlodipine/valsartan 5/160 mg and 74.6% to 83.0% for amlodipine/valsartan 10/160 mg (Figure 3A). The 2 treatment groups were also similar with respect to the change from baseline in mean sitting BP across all prior medications, and by week 4 MSSBP was <140 mm Hg for all previous monotherapy groups (Figure 3B). Incremental MSSBP reductions at week 8 were in the range of 17.4 to 18.7 mm Hg (which includes “other” prior monotherapy) in the amlodipine/valsartan 5/160 mg group and 19.5 to 24.3 mm Hg for the 10/160 mg treatment arm (Figure 3A). In those patients who had previously received amlodipine 10 mg monotherapy, changes in MSS BP at week 8 were −9.6 mm Hg and −16.3 mm Hg, respectively, with amlodipine/valsartan 5/160 mg and 10/160 mg. The corresponding changes in those previously treated with valsartan 160 mg monotherapy were −14.9 mm Hg and −18.5 mm Hg.
Figure 3.
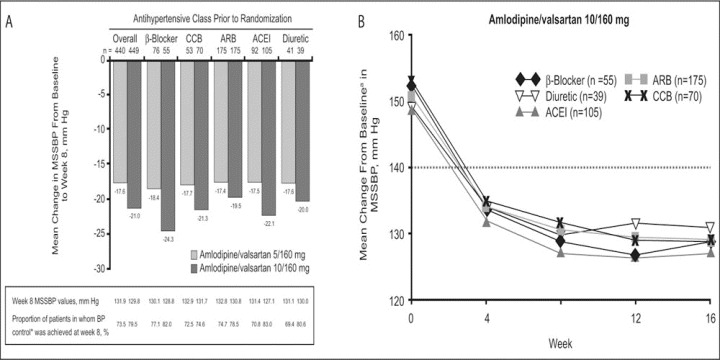
The impact of prior antihypertensive monotherapy in patients switched directly to amlodipine/valsartan on mean change in mean sitting systolic blood pressure (MSSBP) from baseline to week 8 and the proportion of patients in whom blood pressure (BP) control* is achieved at week 8 (A) and mean change from baseline in MSSBP over time with amlodipine/valsartan 10/160 mg according to previous antihypertensive monotherapy (B). *BP control is defined as BP <140/90 mm Hg for nondiabetic patients and BP <130/80 mm Hg for diabetic patients. CCB indicates calcium channel blocker; ARB, angiotensin receptor blocker; ACEI, angiotensin‐converting enzyme inhibitor. aBaseline MSSBP values (mm Hg): β‐blocker, 152.3; ARB, 150.3; ACEI, 148.8; diuretic, 149.2; and CCB, 152.7.
Severity of Hypertension. At baseline, 198 patients had more severe hypertension (Table I). In both treatment groups, changes in mean sitting BP were numerically greater the more severe the hypertension. The change from baseline in MSSBP according to severity of hypertension is shown in Figure 4 for amlodipine/valsartan 10/160 mg. The incremental MSSBP reductions in the more severe hypertension subgroup were −23.9 mm Hg for the lower dose and −28.8 mm Hg for the higher dose at the pre‐HCTZ end point week 8 and −24.7 mm Hg and −29.7 mm Hg, respectively, at study end point week 16 (Table II).
Figure 4.
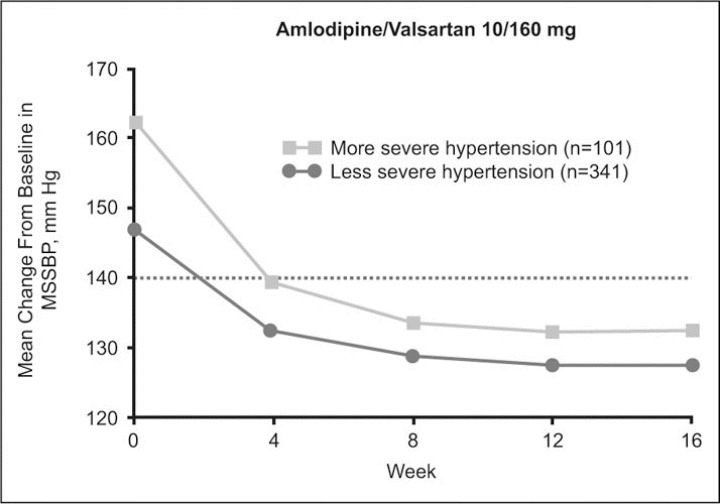
Incremental antihypertensive effects following a direct switch to amlodipine/valsartan 5/160 mg and 10/160 mg in patients with hypertension previously uncontrolled with monotherapy. Mean change from baseline in mean sitting systolic blood pressure (MSSBP) over time with amlodipine/valsartan 10/160 mg according to severity of hypertension (less severe hypertension defined as a baseline blood pressure [BP] value of ≥140 and <160 mm Hg/≥90 and <100 mm Hg; more severe hypertension defined as a baseline BP value of ≥160 and <180 mm Hg/≥100 and <110 mm Hg).
Diabetic Status. Among patients with diabetes (n=145), control of BP to values <140/90 mm Hg at weeks 8 and 16 were comparable to the rates of BP control observed in nondiabetic patients (Figure 5). In the amlodipine/valsartan 5/160 mg treatment arm, BP control at week 16 was achieved in 48 of 61 diabetic patients (78.7%; 95% CI, 68.4–89.0), while in the amlodipine/valsartan 10/160 mg group a BP level of <140/90 mm Hg was achieved in 54 of 59 patients with diabetes (91.5%; 95% CI, 84.4–98.6). The respective values at week 8 were 69.7% and 80.0%. The proportions of diabetic patients in whom the lower BP target of <130/80 mm Hg was reached at week 16 were 45.9% and 40.7% for the lower and higher doses, respectively. Reductions in MSSBP/MSDBP from baseline to week 16 in the diabetic subgroup were similar for the 2 regimens: 16.8/9.5 mm Hg for amlodipine/valsartan 5/160 mg and 17.7/11.5 mm Hg for amlodipine/valsartan 10/160 mg.
Figure 5.
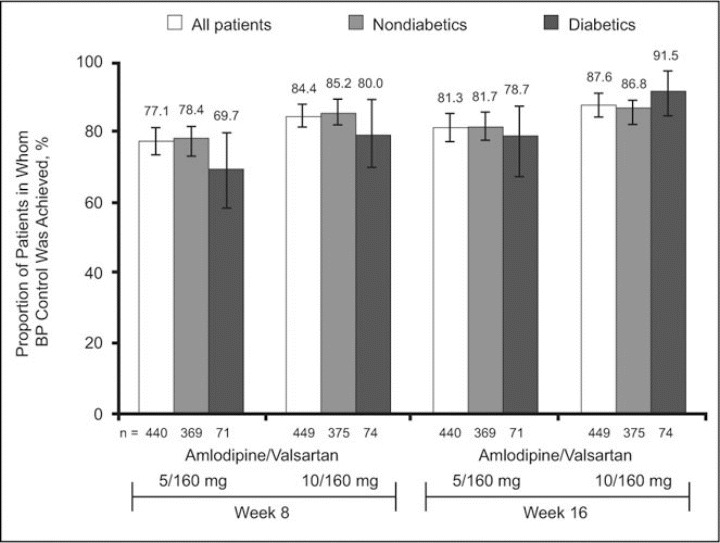
Proportion of diabetic and nondiabetic patients in whom a blood pressure (BP) goal of <140/90 mm Hg was reached with amlodipine/valsartan 5/160 and 10/160 mg at week 8 and week 16 (error bars show 95% confidence intervals).
Age. At the study end point (week 16), BP control rates for the low and high dose amlodipine/valsartan groups were 73.4% and 78.6%, respectively, for patients younger than 65 years, compared with 71.2% and 66.4%, respectively, for patients aged 65 years or older. Within the 2 groups, mean incremental reductions in MSSBP did not differ greatly with respect to age, although minor differences were seen for MSDBP. In the amlodipine/valsartan 5/160 mg arm, the mean change in mean sitting BP at the end point (week 16) was −17.9/−11.6 mm Hg in patients younger than 65 years and −18.2/−9.8 mm Hg in patients 65 years or older. The corresponding reductions in MSSBP/MSDBP with amlodipine/valsartan 10/160 mg were 20.6/12.6 and 21.2/11.3 mm Hg, respectively.
BMI, Sex, and Race. In the subgroup of patients with a BMI ≥30 kg/m2, BP control at the study end point (week 16) was achieved in 115 of 180 (63.9%) patients receiving the lower dose of the combination and by 118 of 170 (69.4%) patients treated with the higher dose. Corresponding reductions in mean sitting BP were 17.2/10.8 mm Hg and 18.6/11.2 mm Hg, respectively. There were no major differences between treatment groups with respect to sex or race.
Safety and Tolerability
There were no deaths during the study. Overall, 113 patients discontinued therapy: 36 of 443 patients (8.1%) in the amlodipine/valsartan 5/160 mg group and 77 of 451 (17.1%) in the amlodipine/valsartan 10/160 mg arm. The most common reasons for discontinuation were adverse events, involving 25 patients (5.6%) receiving the lower dose and 62 (13.7%) treated with the higher dose (Figure 1). Adverse events were not severe; the most frequent were peripheral edema, headache, back pain, dizziness, and muscle spasms (Table III). Peripheral edema was the most frequent drug‐related adverse event and led to discontinuation in 10 patients (2.3%) in the amlodipine/valsartan 5/160 mg group and in 41 patients (9.1%) treated with amlodipine/valsartan 10/160 mg. The majority of reported cases of peripheral edema occurred between the time of randomization and week 8 (5.6% with amlodipine/valsartan 5/160 mg and 20.4% with amlodipine/valsartan 10/160 mg; P<.0001). Serious adverse events occurred in 3 patients (0.7%) receiving the lower dose and 13 (2.9%) patients in the other group. Only 1 serious adverse event, a case of nephrotic syndrome in 1 patient (0.2%) in the amlodipine/valsartan 5/160 mg group, was considered drug‐related by the treating physician. Orthostatic BP changes (ie, decreases of at least 20 mm Hg in SBP or at least 10 mm Hg in DBP when a patient moves from a sitting to a standing position) were observed in 21 patients (4.7%) in the 5/160 mg treatment regimen and 30 patients (6.7%) in the 10/160 mg treatment group. Orthostatic hypotension was reported as an adverse event in 3 patients (0.7%) receiving amlodipine/valsartan 5/160 mg and in 1 patient (0.2%) treated with amlodipine/valsartan 10/160 mg. There were no cases of serious orthostatic hypotension: no patients discontinued due to orthostatic hypotension.
Table III.
Most Frequent Adverse Events by Treatment Group
| Amlodipine/Valsartan 5/160 mg (n=443) | Amlodipine/Valsartan 10/160 mg (n=450) | |
|---|---|---|
| Patients | ||
| Reported an adverse event | 187 (42.2) | 253 (56.2) |
| Reported a treatment‐related adverse event | 60 (13.5) | 140 (31.1) |
| Reported a serious adverse event | 3 (0.7) | 13 (2.9) |
| Adverse event | ||
| Peripheral edema | 36 (8.1) | 113 (25.1) |
| Headache | 10 (2.3) | 17 (3.8) |
| Back pain | 10 (2.3) | 15 (3.3) |
| Dizziness | 9 (2.0) | 10 (2.2) |
| Muscle spasms | 7 (1.6) | 9 (2.0) |
| Treatment‐related adverse event | ||
| Peripheral edema | 30 (6.8) | 100 (22.2) |
| Headache | 0 (0.0) | 10 (2.2) |
| Dizziness | 0 (0.0) | 9 (2.0) |
| Values are No. (%). Adverse events at 16 weeks were considered frequent if they occurred in ≥2% of the safety population for any regimen. | ||
DISCUSSION
In patients with hypertension uncontrolled with previous antihypertensive monotherapy, switching to a dual‐mechanism treatment strategy with a CCB (amlodipine) and an ARB (valsartan) resulted in BP control rates >70% at study end (week 16); BP control was somewhat greater with amlodipine/valsartan 10/160 mg (74.8%) than with amlodipine/valsartan 5/160 mg (72.7%). Control rates in both treatment groups were better than those reported in another study involving low‐dose CCB/ARB combination therapies in patients whose hypertension was uncontrolled with high‐dose monotherapy, 14 perhaps due in part to a carry‐over effect from monotherapy or a lack of a prespecified run‐in period.
Incremental BP‐lowering effects were achieved with both amlodipine/valsartan regimens; however, reductions from baseline in mean sitting BP levels were significantly greater with the higher dose compared with the lower dose (20.0 vs 17.5 mm Hg; P=.0003 for MSSBP [difference of 2.5 mm Hg] and 11.6 vs 10.4 mm Hg; P=.0046 for MSDBP [difference of 1.2 mm Hg], respectively). Similar reductions in BP values have been reported recently in patients with hypertension treated with amlodipine plus valsartan. 16 These responses are expected when one effective agent is added to another. After 4 weeks of treatment, the mean BP values reached in both groups were <140/90 mm Hg (Figures 2 and 4). This may be important because inappropriate delays in BP control or failure to maintain adequate BP control may increase cardiovascular event rates. 18 , 19
Patients with uncontrolled BP at weeks 8 or 12 were obliged to have HCTZ added to their treatment regimen, but this add‐on therapy was not necessary for the majority of patients (>70% in each treatment group at week 8). Those requiring HCTZ were mainly receiving amlodipine/valsartan 5/160 mg. Of note, the addition of HCTZ to amlodipine/valsartan after week 8 appeared to have little additional effect on BP in either treatment group; however, it must be remembered that only the patients with the most resistant hypertension received this add‐on therapy.
Subgroup analysis demonstrated that the additional antihypertensive efficacy of the combination at either dose was well maintained regardless of previous antihypertensive monotherapy, hypertension severity, diabetic status, BMI, or age. As expected, when analyzed by severity of hypertension, the magnitude of reduction in MSSBP was proportional to baseline BP. These findings are consistent with results reported in other studies evaluating the BP‐lowering efficacy of antihypertensive agents. 17 , 20 BP control rates (<140/90 mmHg) in patients with diabetes were similar to those in nondiabetic patients.
A limitation of the present study is that results obtained after week 8 must be interpreted with caution because some patients were receiving HCTZ add‐on therapy after this time point.
Adverse events occurred in 42.2% of patients in the amlodipine/valsartan 5/160 mg treatment group and in 56.2% of patients treated with the higher‐dose combination. The proportions of patients who discontinued treatment were 8.1% and 17.1%, respectively, in the low‐ and high‐dose groups, while the proportion of patients reporting headache, back pain, dizziness, and muscle spasms was comparable between the 2 regimens. The number of patients reporting peripheral edema in the amlodipine/valsartan 10/160 mg group was more than 3‐fold greater than in the amlodipine/valsartan 5/160 mg group (25.1% vs 8.1%). The edema rates reported in this study are higher than those reported in other studies involving similar doses of these agents. 15 , 20 Edema remains the most frequent adverse event associated with amlodipine and other long‐acting dihydropyridine CCBs. 21 Frequencies of peripheral edema as high as 70% have been reported in various studies with CCBs. 22 Other studies involving amlodipine 10 mg monotherapy 18 , 23 , 24 have reported higher frequencies of peripheral edema than were found with amlodipine/valsartan 10/160 mg in this study, suggesting that ARBs may attenuate, but not eliminate, amlodipine‐induced peripheral vasodilatory edema. 16 , 17 , 21
CONCLUSIONS
These results provide additional support for the rationale of combining antihypertensive drugs with complementary mechanisms of action for the treatment of patients with hypertension. These data add to the literature indicating that combination therapy lowers BP to a greater degree than monotherapy. Amlodipine/valsartan was found to be an effective and well‐tolerated strategy for BP control in a wide range of patients with hypertension not previously controlled by use of a single antihypertensive agent.
Acknowledgments and disclosures:
The authors acknowledge all investigators and study coordinators at the participating centers and all patients for their commitment to the study, which was supported by Novartis Pharma AG, Basel, Switzerland. The authors were assisted in the preparation of this text by professional medical writers Sharon Smalley and Joanne Bentley (ACUMED, Tytherington, Macclesfield, UK); this support was funded by Novartis Pharma AG. Dr Izzo has received research and educational grants, consultant fees, and honoraria from Novartis and has served on Novartis scientific advisory boards. Dr Allemann has given talks, attended conferences, and participated in this and other trials and advisory boards sponsored by Novartis. Dr Fraile has participated in other clinical trials of hypertension and diabetes mellitus sponsored by Novartis. Dr Lambert has participated in this and other trials sponsored by Novartis. Drs Barbier and Ferber are employees of Novartis Pharma AG.
References
- 1. Antikainen RL, Moltchanov VA, Chukwuma C Sr, et al. Trends in the prevalence, awareness, treatment and control of hypertension: the WHO MONICA Project. Eur J Cardiovasc Prev Rehabil. 2006;13:13–29. [DOI] [PubMed] [Google Scholar]
- 2. Cushman WC. The burden of uncontrolled hypertension: morbidity and mortality associated with disease progression. J Clin Hypertens (Greenwich). 2003;5(3 suppl 2):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaplan NM, Opie LH. Controversies in hypertension. Lancet. 2006;367:168–176. [DOI] [PubMed] [Google Scholar]
- 4. Wolf‐Maier K, Cooper RS, Kramer H, et al. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension. 2004;43:10–17. [DOI] [PubMed] [Google Scholar]
- 5. Wang YR, Alexander GC, Stafford RS. Outpatient hypertension treatment, treatment intensification, and control in Western Europe and the United States. Arch Intern Med. 2007;167:141–147. [DOI] [PubMed] [Google Scholar]
- 6. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 7. Mancia G, De Backer G, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension. The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 8. Dezii CM. A retrospective study of persistence with single‐pill combination therapy vs. concurrent two‐pill therapy in patients with hypertension. Manag Care. 2000;9(suppl 9):2–6. [PubMed] [Google Scholar]
- 9. Taylor AA, Shoheiber O. Adherence to antihypertensive therapy with fixed‐dose amlodipine besylate/benazepril HCl versus comparable component‐based therapy. Congest Heart Fail. 2003;9:324–332. [DOI] [PubMed] [Google Scholar]
- 10. Bramley TJ, Gerbino PP, Nightengale BS, et al. Relationship of blood pressure control to adherence with antihypertensive monotherapy in 13 managed care organizations. J Manag Care Pharm. 2006;12:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kjeldsen SE, Aksnes TA, De La Sierra A, et al. Amlodipine and valsartan: calcium channel blockers/angiotensin II receptor blockers combination for hypertension. Therapy. 2007;4:31–40. [Google Scholar]
- 12. Sica DA. Rationale for fixed‐dose combinations in the treatment of hypertension: the cycle repeats. Drugs. 2002;62:443–462. [DOI] [PubMed] [Google Scholar]
- 13. Morgan T, Anderson A. A comparison of candesartan, felodipine, and their combination in the treatment of elderly patients with systolic hypertension. Am J Hypertens. 2002;15:544–549. [DOI] [PubMed] [Google Scholar]
- 14. Andreadis EA, Tsourous GI, Marakomichelakis GE, et al. High‐dose monotherapy vs low‐dose combination therapy of calcium channel blockers and angiotensin receptor blockers in mild to moderate hypertension. J Hum Hypertens. 2005;19:491–496. [DOI] [PubMed] [Google Scholar]
- 15. Smith TR, Philipp T, Vaisse B, et al. Amlodipine and valsartan combined and as monotherapy in stage 2, elderly, and black hypertensive patients: subgroup analysis of 2 randomized, placebo‐controlled studies. J Clin Hypertens (Greenwich). 2007;9:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fogari R, Zoppi A, Derosa G, et al. Effect of valsartan addition to amlodipine on ankle oedema and subcutaneous tissue pressure in hypertensive patients. J Hum Hypertens. 2007;21:220–224. [DOI] [PubMed] [Google Scholar]
- 17. Philipp T, Smith TR, Glazer R, et al. Two multicenter, 8‐week, randomized, double‐blind placebo controlled, parallel‐group studies evaluating the efficacy and tolerability of amlodipine and valsartan in combination and as monotherapy in adult patients with mild to moderate essential hypertension. Clin Ther. 2007;29:563–580. [DOI] [PubMed] [Google Scholar]
- 18. Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. [DOI] [PubMed] [Google Scholar]
- 19. Leenen FH, Nwachuku CE, Black HR, et al. Clinical events in high‐risk hypertensive patients randomly assigned to calcium channel blocker versus angiotensin‐converting enzyme inhibitor in the antihypertensive and lipid‐lowering treatment to prevent heart attack trial. Hypertension. 2006;48:374–384. [DOI] [PubMed] [Google Scholar]
- 20. Poldermans D, Glazer R, Karagiannis S, et al. Tolerability and blood pressure‐lowering efficacy of the combination of amlodipine plus valsartan compared with lisinopril plus hydrochlorothiazide in adult patients with stage 2 hypertension. Clin Ther. 2007;29:279–289. [DOI] [PubMed] [Google Scholar]
- 21. Messerli FH . Vasodilatory edema: a common side effect of antihypertensive therapy. Am J Hypertens. 2001;14:978–979. [DOI] [PubMed] [Google Scholar]
- 22. Sica DA. Calcium channel blocker‐related peripheral edema: can it be resolved? J Clin Hypertens (Greenwich). 2003;5:291–294, 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jamerson KA, Nwose O, Jean‐Louis L, et al. Initial angiotensin‐converting enzyme inhibitor/calcium channel blocker combination therapy achieves superior blood pressure control compared with calcium channel blocker monotherapy in patients with stage 2 hypertension. Am J Hypertens. 2004;17:495–501. [DOI] [PubMed] [Google Scholar]
- 24. Ruilope LM, Malacco E, Khder Y, et al. Efficacy and tolerability of combination therapy with valsartan plus hydrochlorothiazide compared with amlodipine monotherapy in hypertensive patients with other cardiovascular risk factors: the VAST study. Clin Ther. 2005;27:578–587. [DOI] [PubMed] [Google Scholar]


