Abstract
Background:
The Monetary Incentive Delay task (MID) has been used extensively to probe anticipatory reward processes. However, individual differences evident during this task may relate to other constructs such as general arousal or valence processing (i.e., anticipation of negative versus positive outcomes). This investigation used a latent variable approach to parse activation patterns during the MID within a transdiagnostic clinical sample.
Methods:
Participants were drawn from the first 500 individuals recruited for the Tulsa-1000 (T1000), a naturalistic longitudinal study of 1000 participants aged 18-55 (n = 476 with MID data). We employed a multiview latent analysis method, group factor analysis, to characterize factors within and across variable sets consisting of: (1) region of interest (ROI)-based blood oxygenation level-dependent (BOLD) contrasts during reward and loss anticipation; and (2) self-report measures of positive and negative valence and related constructs.
Results:
Three factors comprised of ROI indicators emerged to accounted for >43% of variance and loaded on variables representing: (1) general arousal or general activation; (2) valence, with dissociable responses to anticipation of win versus loss; and (3) region-specific activation, with dissociable activation in salience versus perceptual brain networks. Two additional factors were comprised of self-report variables, which appeared to represent arousal and valence.
Conclusions:
Results indicate that multiview techniques to identify latent variables offer a novel approach for differentiating brain activation patterns during task engagement. Such approaches may offer insight into neural processing patterns through dimension reduction, be useful for probing individual differences, and aid in the development of optimal explanatory or predictive frameworks.
1. Introduction
Responding to motivationally salient appetitive and aversive stimuli is considered a fundamental process guiding human behavior. As such, much empirical work has been conducted on measuring reward and loss processing as well as positive and negative affect in both subjective experience (i.e., self-report)1,2 and in objective metrics (e.g., behavior, neuroimaging)3-7. This field of measurement has been instrumental in informing our understanding of human behavior in normative conditions5 as well as in the context of cognitive and emotional disorders8,9. In particular, the study of reward and loss processing has been widely used in studying addiction10, depression11, and anxiety12-14. Thus, refining metrics of reward and loss processing has wide ranging applications from understanding basic functioning to developing disease models and informing intervention and prevention efforts.
The Monetary Incentive Delay task (MID)4 is among the most common paradigms employed in neuroimaging research to examine reward and loss processing. Research using the task has had a large impact on our understanding of motivationally salient stimuli processing, decision making, and goal directed behavior. A key advantage of this paradigm is the ability to probe both anticipation and receipt of reward and loss5. The inclusion of gain and loss conditions enables direct comparison of opposing affective valance or reinforcement processing. Early MID research, in conjunction with functional magnetic resonance imaging (fMRI), focused particularly on reward anticipation and processing demonstrating that the nucleus accumbens (NAcc) is recruited during reward anticipation (even more so than during receipt of reward)4 and is sensitive to degree of reward15. Meta-analytic work indicates that the striatum more broadly (both ventral and dorsal) is active during anticipation of reward and loss7 and whole-brain analyses have provided a more holistic understanding of the regions involved in reward and loss anticipation6. These findings suggest that a broader network of regions is involved in reward anticipation (i.e., right NAcc, middle frontal gyrus [MFG], inferior frontal gyrus [IFG], paracentral lobule, left occipital gyrus, and left parahippocampal gyrus; superior temporal gyrus [STG] and left angular gyrus) versus loss anticipation (i.e., parieto-occipital transition, right supramarginal gyrus, left hippocampus [l-HIPP], right fusiform gyrus, and left MFG), and some regions are involved in both (i.e., superior frontal gyrus. [SFG], caudate, putamen)6.
The simplicity of the MID makes this task a viable candidate to examine neural anticipatory reward processing impairments as a function of psychopathology as well as a useful tool to determine whether these differences can be used to predict clinically meaningful outcomes. The MID also demonstrates promising clinical utility in mental health research and has been especially informative in addiction10 and depression11, and with burgeoning research in anxiety12-14. Addiction has been associated with modulated (often attenuated) ventral striatum (VS) activation in anticipation of reward; however, the consistency and nature of the VS modulation is less clear due to variability in examination of state vs. trait drug influences, task design, and analytic differences (e.g., gain vs. loss contrast calculations)10. Major depressive disorder has also been associated with attenuated VS activation during reward anticipation, though this is not consistent across studies9. Instead, meta-analytic analyses of monetary gain anticipation in depression suggests attenuated caudal response and heightened responding of the MFG11.
Theoretical models of emotional motivation posit that emotion is characterized by valence and arousal3 and numerous self-report measures have attempted to account for these factors2,16. It is less known if brain responses occur in terms of such dimensions or may be represented by different factors. It unclear from previous work whether the various regions recruited during MID anticipation reflect similar processing distinctions (e.g., arousal or general task activation) or if different regions contribute to distinct cognitive-emotional processing (e.g., valence processing, gain/loss discrimination). Multiview machine learning analytic frameworks provide an avenue for examining the influence of different modes of data in concert, so that each compliments the other and improves learning17. Such exploratory techniques (e.g., group factor analysis; GFA) may be helpful in delineating these relevant neural dimensions. Identifying such dimensions of neural response may be important for (1) enhancing our basic understanding of how the brain processes information; and (2) characterizing when functioning is awry in the case of neurological or psychiatric conditions. The current study aims to extend the utility of the MID through (1) identifying latent variables of regional brain activation during anticipation of reward and loss; and (2) determining whether neural factors relate to self-report factors of positive and negative valence. This will be accomplished using group factor analytic approaches of the MID and several relevant self-report measures within the first 500 individuals recruited for the Tulsa-1000 (T1000), a naturalistic longitudinal study of 1000 people, the majority treatment-seekers for psychiatric disorders18. Factor analytic techniques offer a novel approach to indexing regional blood oxygenation level-dependent (BOLD) fMRI responses and disentangling latent variables of neural processing. Specifically, GFA is an unsupervised exploratory analysis extends traditional factor analytic techniques to reveal latent dependencies in data within and between groups in the data structure19. The current study examined activations corresponding to recent meta-analytic findings of MID anticipation6 using Brainnetome atlas regions of interest (ROIs)20. Furthermore, we examined the potential for latent variables to explain variability across brain derived and self-report variables as well as their relationship to symptoms and/or diagnosis of substance use, depression, and anxiety disorders.
2. Materials and Methods
2.1. Participants
Participants included the first 500 participants to complete the baseline assessment in the Tulsa-1000 (T1000) study18, which recruited treatment seeking individuals with mood, anxiety, substance use and eating disorders, as well as healthy controls, aiming to identify brain, behavioral, self-report, physiology, and blood-based variables that can be linked to clinically useful treatment predictions. This parent project included participants 18-55 years screened for inclusion on the basis of the following scores: (1) Patient Health Questionnaire (PHQ-9)21 ≥10; (2) Overall Anxiety Symptom and Impairment Scale (OASIS)22 ≥8; (3) Drug Abuse Screening Test (DAST-10)23 ≥3 and/or (4) Eating Disorder Screening (SCOFF) ≥2. Additionally, healthy controls with no psychiatric diagnoses or elevation in symptoms were included. Exclusion criteria were positive urine drug screen; lifetime bipolar, schizophrenia spectrum, antisocial personality, or obsessive compulsive disorders; active suicidal ideation with intent or plan; moderate to severe traumatic brain injury; severe and or unstable medical concerns; changes in psychiatric medication dose in the last 6 weeks; and fMRI contraindications (e.g., metal in body). Full exclusion criteria can be found in the parent project protocol paper18. The protocol was approved by the Western Institutional Review Board. All participants provided written informed consent prior to participation, in accordance with the Declaration of Helsinki, and were compensated for participation. ClinicalTrials.gov identifier: #NCT02450240.
After exclusion criteria, the current investigation included 476 participants after removing those with unusable fMRI data (e.g., excessive motion, i.e., participants with average Euclidian norm values across all repetition time intervals (TRs) >0.3; or no MID data available). All participants were administered the MINI International Neuropsychiatric Interview (6.0 or 7.0)24,25 by study staff to evaluate lifetime mental disorders, defined by the Diagnostic and Statistical Manual of Mental Disorders (4th or 5th Edition)26,27. Sample demographic information, and screening measure scores are described in Table 1.
Table 1.
Descriptive statistics (n = 476) for all demographic and self-report variables.
| Mean (SD) | Range | Skew | Kurtosis | |
|---|---|---|---|---|
| Age | 34.42(10.57) | 18-55 | 0.58 | −1.67 |
| Education | 5.94(1.88) | 1-11 | 0.39 | −0.96 |
| PHQ-9 | 9.27(6.57) | 0-27 | −0.42 | −0.17 |
| OASIS | 7.50(4.75) | 0-20 | 0.26 | −0.80 |
| DAST-10 | 2.75(3.63) | 0-10 | −0.07 | −0.95 |
| BAS-Drive | 10.98(2.71) | 4-16 | 0.90 | −0.87 |
| BAS-Fun seeking | 11.68(2.54) | 5-16 | −0.16 | −0.41 |
| BAS-Reward | 17.19(2.16) | 9-20 | −0.38 | −0.49 |
| PANASX-Negative | 20.66(7.90) | 10-45 | −0.82 | 0.80 |
| PANASX-Positive | 28.64(8.63) | 10-50 | 0.59 | −0.28 |
| PROMIS/Neuro-QOL PAW | 49.81(7.46) | 32-68 | 0.12 | −0.57 |
| TAS- Difficulty | 16.87(3.55) | 7-33 | 0.36 | −0.11 |
| TAS- External Thinking | 26.09(10.35) | 8-40 | 0.21 | −0.74 |
| TAS- total | 57.44(10.35) | 20-96 | −0.40 | 1.89 |
| TEPS-Anticipatory | 43.17(8.59) | 14-60 | 0.01 | 0.27 |
| TEPS-Consummatory | 38.47(6.38) | 16-48 | −0.55 | 0.06 |
| UPPSP-Negative | 30.24(7.71) | 12-47 | −0.78 | 0.26 |
| UPPSP-Positive | 26.70(9.81) | 14-56 | −0.26 | −0.59 |
| Sex: Female (n = 304; 63.9%); Male (n = 172; 36.1%) | ||||
| Diagnosis: HC (n = 58), ANX( n = 17), MDD (n = 70), MDD+ANX (n = 164), SUD (n = 149), ED (n = 18) | ||||
Note: PHQ-9 = Patient Health Questionnaire; DAST-10 = ; OASIS = ; BAS = Behavioral Activation Scale; PANAS-X = Positive and Negative Affect Scales, Expanded Form; PROMIS/Neuro-QOL-PAW = Patient Reported Outcomes Measurement System/ Neurology Quality of Life Neuro positive affect and wellbeing; TAS= Toronto Alexithymia Scale; TEPS = Temporal Experiences of Pleasure Scale; UPPSP = Urgency, Premeditation, Perseverance, Sensation seeking, and Positive Urgency; HC = Healthy Control, ANX = anxiety only, MDD = depression only, MDD+ANX = comorbid anxiety and depression, SUD = substance use disorder, ED = eating disorder
2.2. Procedure
General procedures comprised a clinical interview session and a neuroimaging session completed within two weeks on average18. Although the parent project (i.e., T100018) consisted of a broader range of protocols, only details relevant to the current study are presented here.
2.2.1. Clinical interview and measures.
Study staff administered the MINI clinical interview. During this session, participants also provided self-reported information on demographics (i.e., age, education, income, ethnicity, and race), anxiety, approach and avoidance motivation, depression, emotional processing, impulsivity, pleasure, substance use, and trauma. We identified the following measures as relevant to positive and negative affect (1) Toronto Alexithymia Scale (TAS)28; (2) Temporal Experiences of Pleasure Scale (TEPS)29; (3) Behavioral Activation (BAS) Scale30; (4) Positive and Negative Affect Scales, Expanded Form (PANAS-X)2; (5) UPPSP impulsive behavior scale31 (6) Patient Reported Outcomes Measurement System/Neurology Quality of Life Neuro positive affect and wellbeing (PROMIS/Neuro-QOL-PAW)1. We used the following scales as indices for relevant symptomatology: PHQ-921; DAST-1022; OASIS23.
2.2.2. Neuroimaging session.
Participants engaged in 90 trials of the MID4 for approximately 19 minutes (i.e., two runs: 45 trials/run, 568s/run). Trials consisted of three visual stimuli: cue stimulus (2s) indicating potential gain (circle), loss (square), or neither (circle or square); a 2.25-3s delay; target (approximately 250ms, white triangle) requiring a button press; a response window (approximately 1.25s); and feedback (2s) indicating the outcome (i.e., gain, loss, or no change). Magnitude of potential gains and losses was indicated using the placement of a line presented in the cue: a line toward the bottom of the cue = no win or loss; a line in the middle of the cue = low win or loss (+/− $1); a line at the top of the cue = high win or loss (+/− $5). Text below the cue also indicated potential gains/losses (−5/−1/−0/+0/+1/+5). In order to obtain a gain or avoid a loss, participants were required to press a button as fast as possible following the target stimulus (Figure S1). Time range of responses needed for success were individually calibrated to each participant’s reaction time (RT) during a pre-scan practice session to a hit rate of 66%. On average participants earned $30 during the task, paid as a bonus in addition to their standard compensation.
fMRI images during the MID were acquired with two identical GE MR750 3T scanners consisting of contiguous echo-planar imaging (EPI) volumes (39 axial slices, TR/TE = 2000/27ms, FOV/slice = 240/2.9mm, 128 x 128 matrix). Additionally, high-resolution structural images were obtained through a 3D axial T1-weighted magnetization-prepared rapid acquisition with gradient echo sequence (TR/TE = 5/2.0 12 ms, FOV/slice = 240 × 192/0.9mm, 186 axial slices).
2.3. Neuroimaging Data Processing
Processing and analyses of neuroimaging data were conducted using Analysis of Functional Neuroimaging (AFNI, http://anfi.nimh.nih.gov) software32. The first three EPI volumes for each run were discarded to account for signal stabilization and noise adaptation. Subsequently, data were despiked, corrected for slice timing, co-registered to anatomical volumes, corrected for motion, smoothed (4 × 4 × 4 mm3 full width at half maximum), and normalized to Montreal Neurological Institute (MNI) standard space (resampled voxel size 2 × 2 × 2 mm3). A general linear model was used to apply boxcar regressors, defined for single participant data, to the BOLD response during the anticipation phase of the MID. The boxcar regressors were 4s duration, made by convolving AFNI’s BLOCK function of width 4s with the event times. The first 4 polynomial baseline terms were included, along with 6 motion parameters (roll/pitch/yaw/x/y/z translation), and task conditions loss, gain, no loss, no gain. Model fits resulting from single subject general linear models (i.e., beta coefficients for condition contrasts gain minus no gain and loss minus no loss) were extracted for the GFA analyses (Figure S2). Data were then extracted for ROIs corresponding to recent meta analytic work on the anticipation phase of the MID (basal ganglia[BG], cingulate gyrus[CG], fusiform gyrus[FuG], hippocampus[Hipp],insula[INS], inferior parietal lobule[IPL], inferior temporal gyrus[ITG], lateral occipital cortex[LOcC], middle frontal gyrus[MFG], paracentral lobule[PCL], parahippocampal gyrus[PhG], superior frontal gyrus [SFG], and superior temporal gyrus [STG])6 using the Brainnetome Atlas (Fan et al., 2016; specific Brainnetome labels ROIs are reported in supplemental materials; Table S1) all regions were examined bilaterally for use in GFA. When extracting contrast for an ROI, only voxels with a temporal signal to noise ratio of greater than 50 were included.
2.4. Analytic Strategy
Analyses were conducted in R 3.6.133 using RStudio 1.146334 and RMarkdown35,36, employing the following packages: chemometrics37, DMwR38, GFA39, ggplot240, psych41 , and stats33. Missing questionnaire data were imputed using K nearest neighbor imputation for GFA. All R and R Markdown scripts are available as html documents in the supplemental materials and can be found on Open Science Framework (https://osf.io/32kpn/).
2.5. Group Factor Analysis
GFA19 was used as a multiview method to identify patterns of activation across different groups (i.e., views) of measures. Two groups were defined: 1) BOLD ROI contrasts (i.e., gain/loss anticipation); and 2)self-report measures. GFA employs Bayesian inference to assign automatic relevance determination priors on the factor solution, assuming low-rank representation of factor loadings42. Estimation in the current analysis employed a full rank approach19,39. The model included 248 ROI and 13 self-report predictors (subject to predictor ratio of 1.82). Previous simulation research utilizing Bayesian inference to prevent overfitting has demonstrated the adequate performance of GFA in subject to predictor ratios from 30:28 (1.07) up to 30:700 (0.04); thus the current ratio of 1.8 (476:261) is appropriate19. Furthermore, GFA results could depend on initial values of parameters so we replicated estimation 10 times and extracted robust factors that cohere across 10 replicates of the factor analysis. The specific matching procedure (the robustComponents function in the GFA package) was described as follows: (1) factor loadings of a factor from a replicate were correlated to loadings of all factors in another replicate, (2) the factor showing the strongest correlation across replicates, and for which the correlation exceeded a threshold (corThr) was considered “matched”; (3) repeat Steps (1) and (2) for all replicates and claim a factor “robust” if the proportion of “matched” across replicates exceeded a threshold (matchThr). With this matching procedure, we obtained a number of robust factors for a given pair of threshold parameters. However, different threshold parameters might lead to different numbers of robust factors. We thus conducted a 7 x 7 grid search on values of 0.1, 0.3, 0.5, 0.6, 0.7, 0.8, and 0.9 for the (corTh, matchThr) pairs. For each parameter pair, we reconstructed the data by multiplying the robust factor loadings and scores, which were compared to the observed data by mean-square-error (MSE). The optimal threshold parameter pair was then chosen by the one that gave the fewest robust factors with MSE within 1 standard error of the minimal MSE across all pairs. GFA enables the examination of variance within a group of variables, but also covariance between the sets. Thus, the advantages for this particular study are two-fold: (1) characterizing factors related to both BOLD signal contrasts and self-report measures of interest; and (2) determining if factors have relationships to both neural activation and self-report measures. Factors presented here were chosen based on either accounting for at least 3% percent of overall model variance or 10% of variance within a set of variables (i.e., BOLD ROIs, self-report).
2.6. General Linear Models (GLM)
We conducted follow-up GLM analyses to explore the potential clinical significance of identified factors. Participants were grouped by diagnosis as determined by the MINI24,25, including major depressive disorder only (‘MDD’), anxiety disorders only (‘ANX’; social anxiety, generalized anxiety, panic, or posttraumatic stress disorder), comorbid MDD and anxiety disorders (‘MDD+ANX’), substance use disorders (‘SUD’; recreational drugs, excluding alcohol or nicotine; with or without comorbid ANX/MDD), eating disorders (‘ED’; with or without comorbid MDD, ANX, or SUDs), and healthy comparisons with no psychiatric diagnoses (‘HC’) as in43. We conducted separate one-way ANOVAs to examine mean differences on each of the five factor scores extracted (criteria detailed above) according to diagnosis (6 levels; HC, ANX, MDD, MDD+ANX, SUD, ED). Bonferroni correction performed for multiple comparison in post-hoc analyses when relevant. Similarly, regression models were employed to examine relationships between symptoms of depression (PHQ-9), anxiety (OASIS), and substance use (DAST-10) and each of the factor scores.
2.7. Behavioral and demographic analyses.
We also examined Spearman’s correlations between factor scores and MID RT by conditions (high magnitude gains/losses, neutral outcome). Furthermore, we examined the relationship of the factor scores with sex, age, and education level using Welch’s independent sample t-test, linear regression, and one-way ANOVA respectively.
3. Results
GFA output from R along with full resolution images of the factor loading plots for latent variables of interest can be found on Open Science Framework https://osf.io/32kpn/.
3.1. Demographics and sample characteristics
Table 1 presents the demographic information of the current sample as well as descriptive statistics regarding self-report measures of symptoms and positive and negative valence.
3.2. GFA examining BOLD signal response and self-reported positive/negative valence
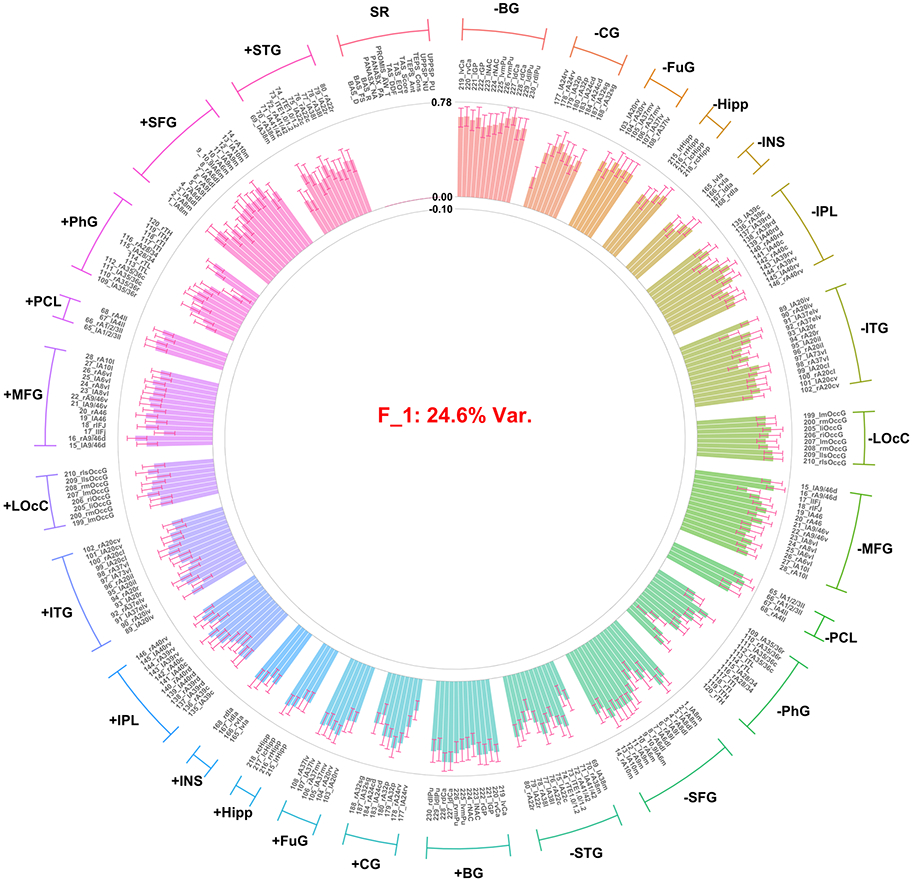
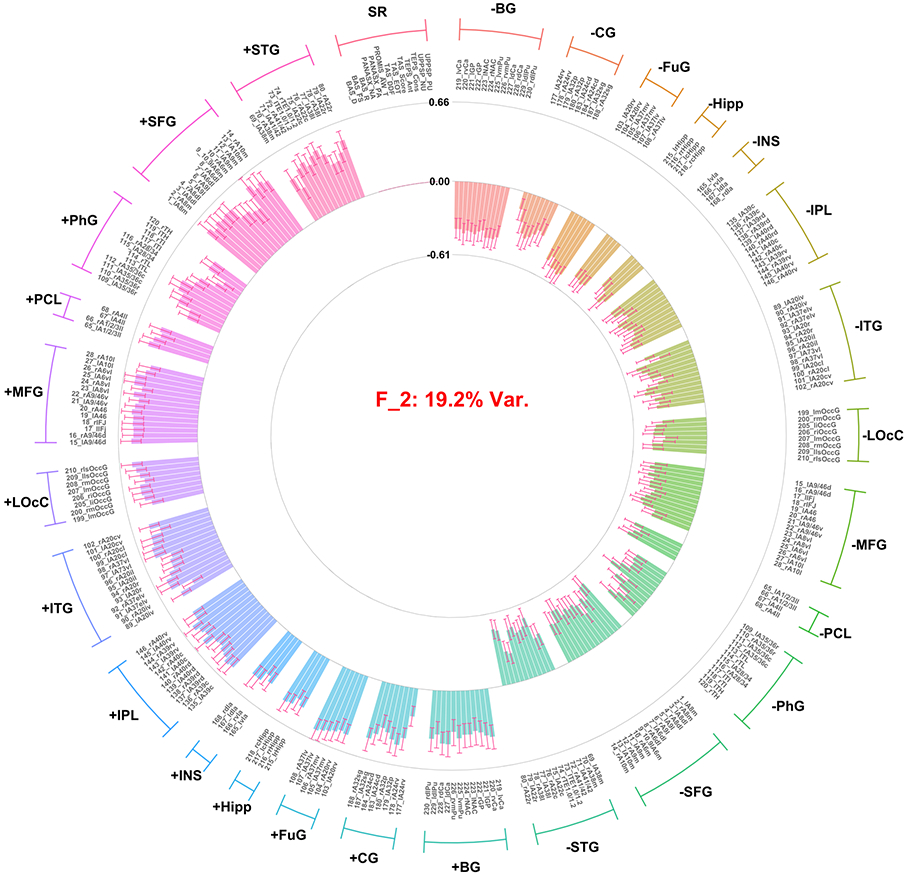
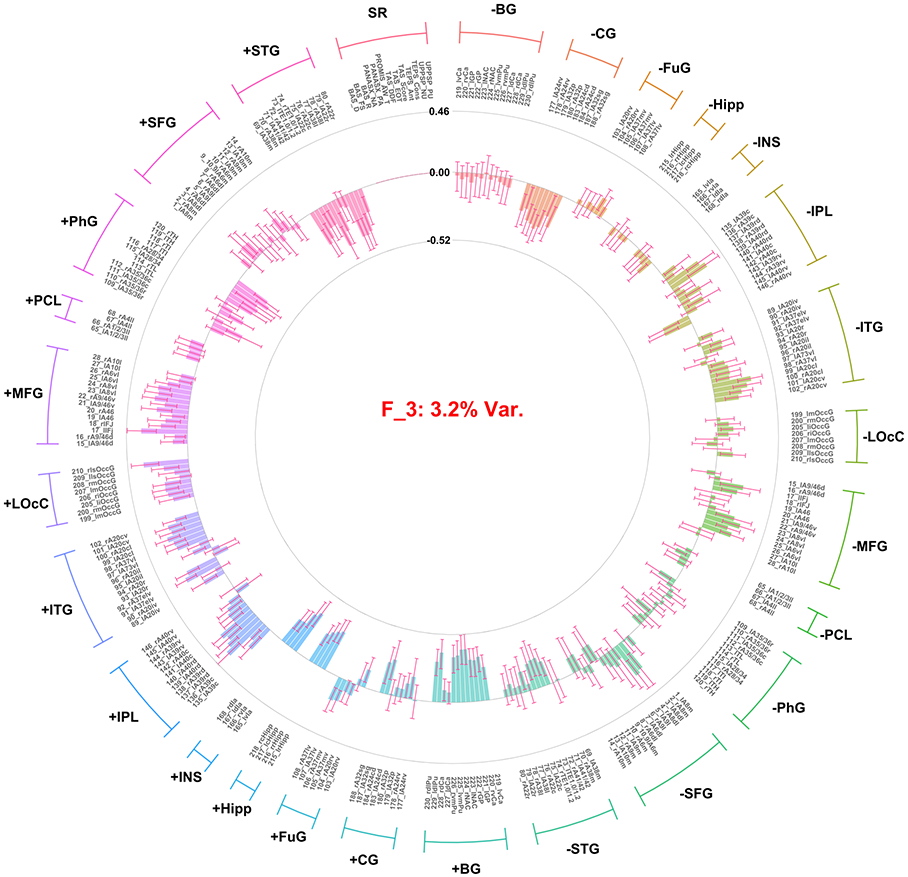
Distribution and correlations of factor scores can be found in the supplement demonstrating the assumption of orthogonality was met (Figures S3 and S4). Twenty-nine factors were extracted, together accounting for 63.6% of the total variance. This included three factors accounting for >3% of the total variance across both the brain imaging and self-report variable sets; and two additional factors that explained >10% variance within the self-report variable set (no additional factors were identified that accounted for >10% in the brain imaging variable set). These five factors accounted for 49.42% of the overall variance. Notably the two factors specific to the self-report variables accounted for only 2.37% of the variance in the total model due to there being a much larger number of ROI contrast variables (n = 248) in the model relative to self-report variables (n = 13). Combined the two self-report factors extracted accounted for 42.65% of variance within the self-report variables. Thus, these five factors were interpreted and used for further analysis. Median strongest correlation values of the robust factors across the 10 replicates conducted in the GFA indicated that the robust factors were reliable (all r’s > 0.8; Figure 1). The first three factors identified only included ROI contrast variables, with no significant loadings observed for self-report variables. The first factor (F1; arousal/general task activation factor) accounted for 24.61% of the overall variance in the model. All loadings were positive across conditions (i.e., gain, loss), indicating that F1 represented an overall activation, or valence-independent general activation (Figure 2). The second (F2; valence/condition discrimination factor) accounted for 19.24% of the overall variance. Indicators from the gain anticipation condition loaded positively and the loss anticipation condition negatively, indicating that F2 may represent differentiation of activation according to valence (Figure 3). The third factor (F3; region specific factor) accounted for 3.2% of the overall variance. The loadings on this factor seemed to differentiate according to brain region, with higher loadings for gain conditions. Strong positive loadings were observed for subcortical (i.e., striatum, insula, hippocampus, basal ganglia), cingulate, and superior temporal regions, whereas strong negative loadings were observed for inferior parietal, inferior temporal, middle frontal, and occipital cortical regions (Figure 4).
Figure 1.
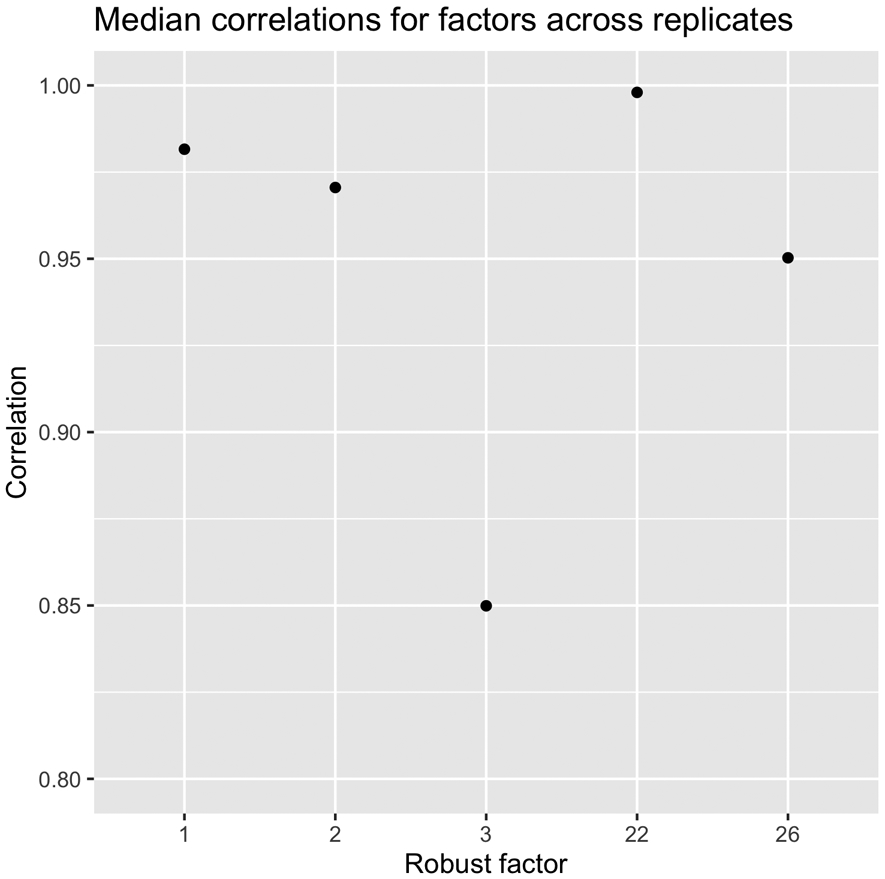
Median correlation values of the robust factors across all 10 replicates of the GFA. Values indicate good to excellent reliability.
Figure 2.
Factor loadings for the F1 (i.e., arousal/genera task activation) resulting from GFA. Median factor loadings and 95% confidence interval are presented for each Brainnetome ROI and were drawn from the GFA W matrix. ROIs are grouped into anatomical regions by condition using brackets and color coding. Labels for ROI variables include Brainnetome id and anatomical descriptor (r=right; l=left). Individual ROI predictors are grouped into broader regional descriptors: BG=basal ganglia, CG=cingulate gyrus, FuG=fusiform gyrus, Hip=hippocampus, INS=insula, IPL=inferior parietal lobule, ITG=inferior temporal gyrus, LOcC=lateral occipital cortex, MFG=middle frontal gyrus, PCL=paracentral lobule, PhG=Parahippocampal gyrus, SFG=superior frontal gyrus, STG= superior temporal gyrus. SR = self-report variables. MID conditions are organized such that gain (+) is on the left half of the plot and loss (−) is on the right with corresponding anatomical regions directly across the circle.
Figure 3.
Factor loadings for the second factor (i.e., valence/condition discrimination) resulting from group factor analysis. Factor loadings presentation and labels are consistent with Figure 2.
Figure 4.
Factor loadings for the third factor (i.e., region specific) resulting from group factor analysis. Factor loadings presentation and labels are consistent with Figure 2.
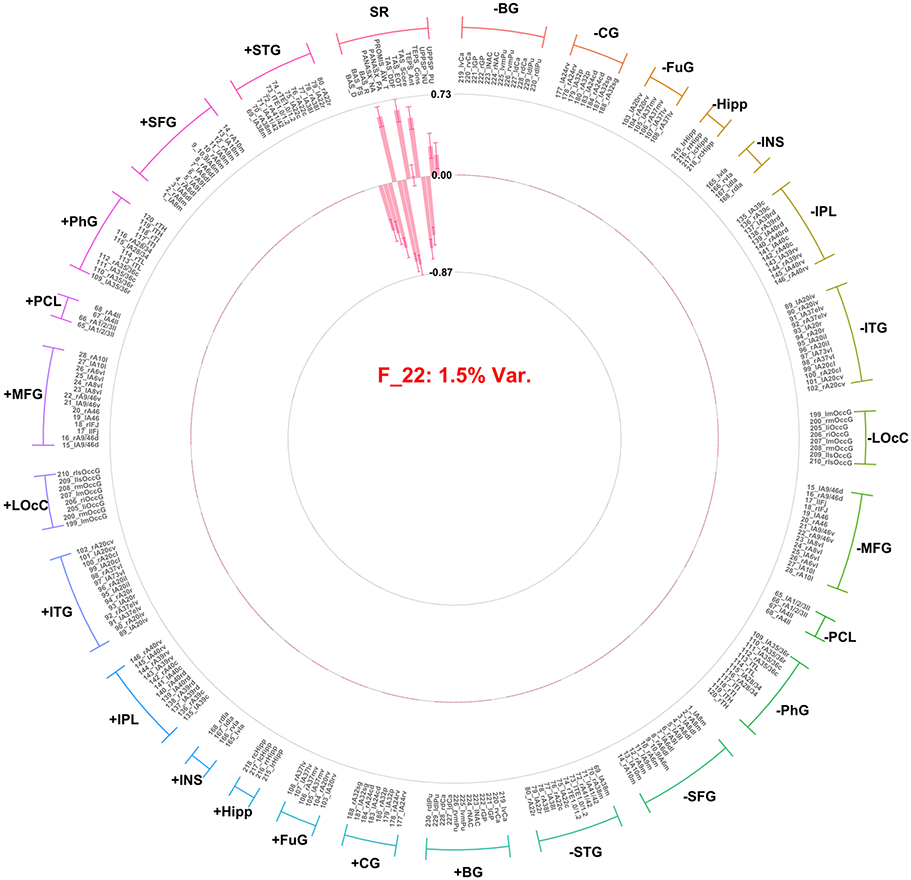
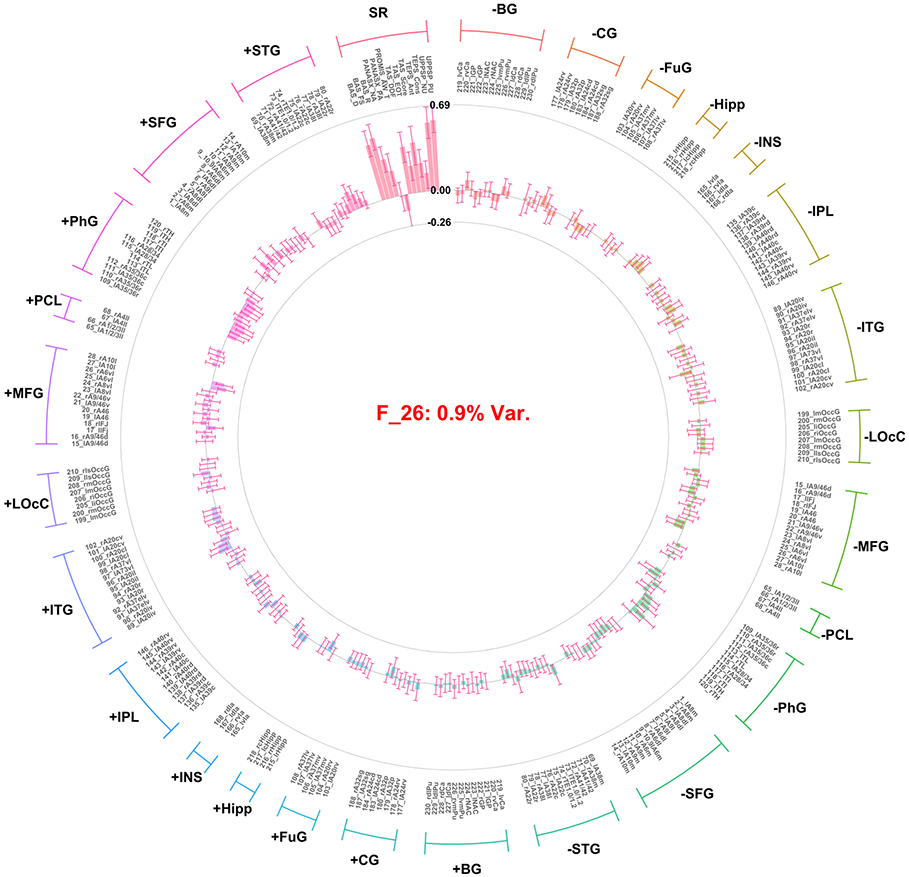
Factor 22 (F22) and factor 26 (F26) are interpreted here given that they accounted for a significant amount of variance in the self-report variable block. F22 (self-report valence factor) accounted for 29.4% of the variance among self-report variables (1.47% overall). Importantly the amount of variance accounted for among all predictors was low likely due to the large number of ROI predictors in the model resulting in high factor numbers (22 and 26). However, they do predict a large amount of variance within their particular group (view; i.e. self-report) and demonstrated high coherence across replicates (see: Figure 1). The pattern of loadings indicated that positive affect, pleasure, and approach motivation variables loaded positively, and negative affect, alexithymia, and impulsivity loaded negatively, (Figure 5). F26 (arousal/response intensity factor) accounted for 13.25% of the variance in the self-report variables (0.9% overall) and all self-report indicators loaded significantly in the same direction, with the exception of PANAS-X positive affect and PROMIS wellbeing, which did not load significantly (Figure 6). In addition to self-report loadings, some ROI indicators loaded onto F26; however, these loadings were not interpreted, as the general pattern of standard errors included zero with the exception of the parahippocampal gyrus in the gain condition. Notably, F26 accounted for only 0.23% of variance among the ROI contrast variable set. Notably, no identified factor is related to dependencies between ROI and self-report variable groups.
Figure 5.
Factor loadings for the fourth factor (i.e., self-report valence) resulting from group factor analysis. Factor loadings presentation and labels are consistent with Figure 2.
Figure 6.
Factor loadings for the fifth factor (i.e., self-report arousal/response intensity) resulting from group factor analysis. Factor loadings presentation and labels are consistent with Figure 2.
3.3. GLMs
ANOVA models indicated that no factor score means differed across diagnostic groups (p’s = 0.16-0.67), with the exception of the self-report valence factor (F(5,460) = 2.35, p = .0402) . Pairwise comparisons indicated that HC had a higher mean score (M = 0.24) relative to individuals with MDD only (M = −0.23; d = 0.53, CI95 [0.18-0.89]), indicative of higher positive and lower negative affect. It is important to note that the omnibus effect would not survive correction for multiple comparisons. Regression analyses were also non-significant for all factors and symptom measure scales (p-values = 0.06-0.95) with the exception of the OASIS score relating negatively to the self-report valence factor (β =−0.09, p = 0.039, R2adj = 0.007).
3.4. Demographic and behavioral data results
Independent samples t-tests indicated that no factor scores differed by sex (p’s = 0.13-0.71) with the exception of the valence/condition discrimination factor, t(375.98) = −1.99, p = 0.046, d = 0.21, such that males (M = 0.12) had higher scores than females (M = −0.06), indicative of greater differential activation during gain anticipation as compared to loss anticipation. Regression models indicated that age did not predict any factor scores (p’s = 0.06-0.94) with the exception of the region specific factor (β =0.11, p = 0.015, R2adj = 0.01) indicating that older participants demonstrated larger differentiation between subcortical, superior temporal, and cingulate regions as compared to inferior parietal, inferior temporal, middle frontal, and occipital cortical regions. Though significant, effect sizes of these findings were small, accounting for approximately 1% of the variance. ANOVA also revealed that education did not predict any of the factor scores (p’s = 0.38-0.97). Correlations indicated that there were no significant associations between RT in any MID condition and factor scores (Figure S3).
4. Discussion
The current study applied a multi-view method, group factor analysis (GFA), to fMRI BOLD signal contrasts elicited by anticipation of high magnitude gain and loss outcomes relative to neutral outcomes during the MID task and relevant self-report indicators of positive and negative affect. Factors of interest were examined for their relationship with psychopathology diagnoses and symptoms. Results indicate that GFA identified factors corresponding to BOLD signal activity indexing: general task activation, discrimination of gain/loss anticipation, and region-specific activity. Separate factors distinguished patterns of self-report responses associated with arousal and valence. No factor significantly predicted variance across brain-derived and self-report indicators.
4.1. fMRI BOLD signal activation contrasts
The factors accounting for the greatest amount of variance in the overall model (i.e., arousal/general task activation, valence/condition discrimination, region specific factors) had significant loadings from the ROI indicators and no loadings from self-report data. The arousal/general task activation factor had loadings with consistent directionality across regions and conditions, suggesting that this factor may represent overall arousal or attentional processes. Alternatively, this factor may reflect a global BOLD signal influenced by physiological differences impacting the BOLD signal. However, if primarily due to physiological differences (i.e., in cerebral vasculature), one might expect the factor to significantly correlate with age – which it did not. It will be useful for future work to identify whether similar factors can be identified across a variety of tasks and whether the factor is modulated by experimental manipulation of blood flow or arousal. For example, many factors influence the BOLD response in addition in neural metabolic processes, including a wide range of autoregulatory processes, making cautious interpretation of BOLD signal responding critical44. Regardless, a factor analytic approach like used here could be useful for controlling variance associated with arousal or global signal to allow for more specific focus on activation relevant to conditions of interest.
Results suggest that the valence/condition discrimination factor demonstrate patters of loadings representing distinctions between positive and negative valence or between positive and negative reinforcement (i.e., avoiding loss vs. obtaining gain). The identification of such a factor provides further evidence that such distinction in neural processing exists across a range of brain regions that have been previously identified as activated during the MID task. Future research could be useful to determine whether additional regions other than those focused on here would also load on a valence-specific factor, and whether similar valence-specific factors could be identified for tasks other than the MID.
The region-specific factor may characterize relative activation in circuits associated with salience processing (e.g., striatum, insula) as compared to executive processing (occipital cortex, inferior parietal lobule, inferior temporal gyrus). The pattern of relative inverse factor loadings of activation in these regions (i.e., salience vs. executive) may indicate that individuals down regulate basic attentional processing regions in favor of increasing activation of salience processing during MID anticipation. Importantly, the direction of factor loading (i.e., positive/negative) is not indicative of brain activation or deactivation; thus, inverse loadings do not imply a particular directionality of brain activity and the alternative hypothesis that these factor loadings represents down regulation in salience processing in favor of executive function regions cannot be ruled out.
Overall these findings are consistent with theoretical assertions that anticipatory affect comprises both arousal and valence5. Importantly, the current results suggest that brain regions active during reward and loss anticipation are involved in both general salience processing and in discerning potential gain versus loss. However, these data do not preclude the interpretation that the arousal/general task activation represent a global signal that may not be specific to the MID. As such future GFA work is needed within other fMRI tasks to determine if region specific factors in other tasks relate to previously reports specific effects. Results also extend recent research suggesting that particular brain regions are sensitive to anticipated outcomes in the MID (i.e., gain/loss)6,15,45.Importantly, this previous empirical work has often been relegated to circumscribed regions such as Nacc15 and VS45, which has been noted as a limitation of this body of literature7. Current results support the idea that numerous brain regions likely respond as part of a broader network during MID anticipation, consistent with recent meta-analytic data,6 and extend this literature demonstrating that factors discern arousal and valence in gain and loss anticipation across brain regions. Moreover, ROIs in the current study loaded on both the arousal factor and valence factor; whereas traditional fMRI analytic approaches may have difficulty discerning these responses, the GFA approach provides an avenue for distinguishing the two.
4.2. Self-report indicators
Two factors examined from the GFA represented a significant amount of variance within self-report indicators (i.e., self-report valence factor, self-report arousal/response intensity; Figures 5 & 6). Notably, these factors did not account for much variance in the overall model due to the relatively fewer predictors as compared to ROI variables. The self-report valence factor displayed pattern of loadings suggesting that it represents discernment of valence across questionnaire indicators. Positive affect variables, pleasure, approach motivation, and well-being loaded positively, whereas impulsivity, alexithymia, and negative affect loaded negatively. The self-report arousal/response intensity factor comprised loadings from self-report variables all in a consistent direction, indicating this factor may represent response intensity (i.e., arousal). The pattern of the self-report factors findings is analogous to the valence and arousal findings in the ROI valence/condition discrimination and arousal/general task activation factors. Furthermore, the distinction between valence and arousal in the self-report factors is consistent with previous self-report literature2,16. However, PANAS-X positive affect did not load significantly and PROMIS/Neuro-QOL-PAW loaded slightly opposite of the rest of the significant loadings on the self-report arousal/response intensity factor. This may indicate that constructs captured on the PANAS-X2 and PROMIS/Neuro-QOL-PAW (items such as: “proud”, “interested, “inspired”; and feeling “at ease”, “relaxed”, “peaceful” respectively), while positive, may not be as intense or arousing as what is measured by other scales.
4.3. Clinical relevance
With respect to psychiatric diagnoses and symptoms, there were no mean differences between diagnostic groups on the arousal/general task activation or valence/condition discrimination factor scores. These factors also did not relate to dimensional psychopathology symptoms. This is inconsistent with research reporting modulated VS activation in addiction10, as well as attenuated VS9, reduced dorsal striatum, and increased MFG activation in depression11. However, these studies focused on specific regional activations, not latent variables of neural activation. The current results indicate that both neural activation during MID and self-report variables were associated with analogous but distinct pairs of factors characterizing general activation (e.g., arousal) and condition discernment (e.g., valence). Based on these findings, some readers may be tempted to call into question the legitimacy of either the MID or self-report measures as legitimate probes of arousal or valence discernment. However, we would argue that both likely measure legitimate aspects of these constructs but tap into very different features of underlying processes. Given the lack of relationships to self-reported affect, we hypothesize that the brain derived factors may represent general neural response functioning which may not involve conscious awareness, whereas self-report derived factors are representative of an individual’s conscious experience of emotions. In other words, if the identified factors are robust ways in which the human brain responds to salient stimuli, they may not be as dynamic as one’s state level of arousal or valence and represent more trait-like measures. Thus, perhaps intermediate or more subtle, region-specific neural responses (not detected via GFA) may drive the affect actually experienced by individuals from one state to another. One possibility to be tested in future work would be that the brain-derived factors identified here may be more likely to relate to more trait-like or “hard-wired” levels of measurement (e.g., genetics) whereas the self-report derived factors may be more likely to relate to functional levels of analyses (i.e., psychiatric symptoms, diagnoses). Accordingly, the only factor which showed any relationship to our psychiatric measures was the self-report derived valence factor. Results indicate that healthy individuals endorsed significantly higher self-report valence discernment scores than individuals with depression. Examination of dimensional symptom measures indicate self-report valence discrimination was only associated with anxiety symptoms, such that higher anxiety related to lower valence discernment. These results are consistent with prior work demonstrating blunted emotional valence responding in depression and anxiety46-49
Results also indicated that men had higher levels of differential brain activation between anticipation of gains as compared to losses. These findings extend literature indicating differential reward processing between males and females50, in which animal models indicate that females exhibit stimulus directed, as opposed to goal-directed reward behavior51. Event-related potential analysis indicated that adolescent males had increased responding to rewards and punishment than adolescent females52. Furthermore, results indicated that there was a significant association of age and F3, which may reflect differential impacts of age on regional brain volume across the ROIs or distinct patterns of network-based activation during gain and loss anticipation. Although the size of these effects were small, this is consistent with recent work effect sizes from more traditional ROI based analyses conducted in large samples designed to reduce false-positive results53.
4.3. Limitations
A key limitation to the current study is the cross-sectional nature of the design, precluding interpretation of causal influences and directionality in identified relationships. The current analysis only considered anticipation during high magnitude trials from the MID. Although this enabled inclusion of a broad range of brain regions implicated in MID anticipation in previous meta analytic work6, it is possible that inclusion of different magnitudes could alter the underlying factor structure. We chose to focus on the set of brain regions and contrasts that we did in order to minimize the number of variables included in the GFA, in a way that was optimally informed by previous work with the MID. We also chose to focus on mean percent signal change across the anticipation phase of the task, within predefined regions of interest, as one way of using factor analytic approaches to identify more parsimonious dimensions of interest. We recognize that there are numerous approaches that can be used to reduce dimensionality of fMRI data, such as using latent class analysis to identify different temporal trajectories of activation throughout the task or the use of voxel-based pattern analysis. The approach taken here provides one strategy that maintains similarity to more traditional, ROI-based approaches to analysis, which thus supports interpretation of findings in relation to that previous literature.
Additionally, the goal of the T1000 study was longitudinal investigation of dimensional characteristics of a transdiagnostic clinical sample consistent with RDoC recommendations18; thus, the clinical categories examined in the current study did not account for comorbidity in the sample or heterogeneity within the SUD group (e.g., alcohol use disorder). Notably, the sample consisted primarily of individuals with mood, anxiety, and substance disorders. This may impact the generalizability of the current findings. Future work clarifying the relationship between the identified factor scores and psychopathology would benefit from nuanced consideration of comorbidity and or explorations of factor structures within various clinical groups. Finally, dimensional indicators of psychopathology symptoms employed in the current study were self-reported, not clinician rated. However, self-report measures have been reported to converge with clinician-administered scales54,55. Moreover, results were consistently null regarding clinician administered interview (MIN) and self-reported symptom variables.
4.4. Conclusions
The current analyses provide novel evidence that neural activity during gain and loss anticipation is associated with factors which differentiate general task activation and condition specificity. An important next step in this line of work is to determine if these factors are reproducible and reliable over time56,57. The current study did not demonstrate strong evidence for the relationship between factors and clinically relevant variables but did demonstrate greater differentiation between gain and loss anticipation among males compared to females. Also, age was associated with greater distinction between salience and executive function regions. Thus, aforementioned reliability work may support the hypothesis that functional circuitry indexed during the MID demonstrates integrity in the context of psychopathology under experimental conditions and clinical disruption may be more nuanced. It would be beneficial for future studies to: 1) examine factors related to various magnitudes of outcomes: and 2) to manipulate the experimental context during MID (e.g., cognitive load, level of arousal, modulation of blood flow). Overall, current results indicate that a factor analytic approach may be informative alongside more traditional fMRI analyses to serve as a general assessment of arousal and valence processing.
Supplementary Material
Acknowledgements
This work has been supported in part by The William K. Warren Foundation, National Institute of Mental Health (K23MH112949 (SSK), K23MH108707 (RLA)), and the National Institute of General Medical Sciences Center Grant Award Number 1P20GM121312. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial Disclosures:
Evan J. White Ph.D.; Rayus Kuplicki, Ph.D. Jennifer L. Stewart, Ph.D., Namik Kirlic, Ph.D., Justin Feinstein, Ph.D., Jonathan Savitz, Ph.D., Robin Aupperle Ph.D., and Martin Paulus M.D. receive funding from the National Institute of General Medical Sciences (NIGMS) center grant P20GM121312; Sahib Kahlsa M.D. Ph.D. has grant funding from the National Institute of Mental Health (NIMH; K23MH112949); Robin Aupperle Ph.D. has additional grant funding from NIMH (K23MH108707; R01MH123691); and Martin Paulus, M.D. has additional grant funding from the National Institute of Drug Abuse (U01DA041089) and Dr. Paulus is an advisor to Spring Care, Inc., a behavioral health startup, he has received royalties for an article about methamphetamine in UpToDate; Hung-Wen Yeh, Ph.D. has no financial disclosures to report Jerzy Bodurka Ph.D. has no financial disclosures to report; Yoon-Hee Cha, M.D. has no financial disclosures to report. Teresa Victor, Ph.D. has no financial disclosures to report.
Appendix
The Tulsa 1000 Investigators include the following contributors: Jerzy Bodurka, Ph.D., Yoon-Hee Cha, M.D., Justin Feinstein, Ph.D., Sahib S. Khalsa, M.D., Ph.D., Jonathan Savitz, Ph.D., Teresa A. Victor, Ph.D.
Footnotes
The ClinicalTrials.gov identifier for the clinical protocol associated with data published in the current paper is NCT02450240, “Latent Structure of Multi-level Assessments and Predictors of Outcomes in Psychiatric Disorders”.
References
- 1.Salsman JM, Victorson D, Choi SW, et al. Development and validation of the positive affect and well-being scale for the neurology quality of life (Neuro-QOL) measurement system. Qual Life Res. 2013;22(9). doi: 10.1007/s11136-013-0382-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson D, Clark LA. The PANAS-X: Manual for the Positive and Negative Affect Schedule - Expanded Form. Department of Psychological & Brain Sciences Publications. Published online August 1, 1999. doi: 10.17077/48vt-m4t2 [DOI] [Google Scholar]
- 3.Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001;1(3):276–298. doi: 10.1037/1528-3542.1.3.276 [DOI] [PubMed] [Google Scholar]
- 4.Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12(17):3683–3687. doi: 10.1097/00001756-200112040-00016 [DOI] [PubMed] [Google Scholar]
- 5.Knutson B, Greer SM. Anticipatory affect: neural correlates and consequences for choice. Phil Trans R Soc B. 2008;363(1511):3771–3786. doi: 10.1098/rstb.2008.0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson RP, Colizzi M, Bossong MG, et al. The Neural Substrate of Reward Anticipation in Health: A Meta-Analysis of fMRI Findings in the Monetary Incentive Delay Task. Neuropsychol Rev. 2018;28(4):496–506. doi: 10.1007/s11065-018-9385-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oldham S, Murawski C, Fornito A, Youssef G, Yücel M, Lorenzetti V. The anticipation and outcome phases of reward and loss processing: A neuroimaging meta-analysis of the monetary incentive delay task. Hum Brain Mapp. 2018;39(8):3398–3418. doi: 10.1002/hbm.24184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knutson B, Heinz A. Probing Psychiatric Symptoms with the Monetary Incentive Delay Task. Biological Psychiatry. 2015;77(5):418–420. doi: 10.1016/j.biopsych.2014.12.022 [DOI] [PubMed] [Google Scholar]
- 10.Balodis IM, Potenza MN. Anticipatory Reward Processing in Addicted Populations: A Focus on the Monetary Incentive Delay Task. Biological Psychiatry. 2015;77(5):434–444. doi: 10.1016/j.biopsych.2014.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang W-N, Chang S-H, Guo L-Y, Zhang K-L, Wang J. The neural correlates of reward-related processing in major depressive disorder: A meta-analysis of functional magnetic resonance imaging studies. Journal of Affective Disorders. 2013;151(2):531–539. doi: 10.1016/j.jad.2013.06.039 [DOI] [PubMed] [Google Scholar]
- 12.Gorka AX, Fuchs B, Grillon C, Ernst M. Impact of induced anxiety on neural responses to monetary incentives. Soc Cogn Affect Neurosci. 2018;13(11):1111–1119. doi: 10.1093/scan/nsy082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maresh EL, Allen JP, Coan JA. Increased default mode network activity in socially anxious individuals during reward processing. Biol Mood Anxiety Disord. 2014;4:7. doi: 10.1186/2045-5380-4-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyer AE, Choate VR, Detloff A, et al. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. Am J Psychiatry. 2012;169(2):205–212. doi: 10.1176/appi.ajp.2011.11010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of Increasing Monetary Reward Selectively Recruits Nucleus Accumbens. J Neurosci. 2001;21(16):RC159–RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang PJ. International Affective Picture System (IAPS) : Technical Manual and Affective Ratings. In: ; 1995. [Google Scholar]
- 17.Nguyen ND, Wang D. Multiview learning for understanding functional multiomics. PLoS Comput Biol. 2020;16(4):e1007677. doi: 10.1371/journal.pcbi.1007677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Victor TA, Khalsa SS, Simmons WK, et al. Tulsa 1000: a naturalistic study protocol for multilevel assessment and outcome prediction in a large psychiatric sample. BMJ Open. 2018;8(1):e016620. doi: 10.1136/bmjopen-2017-016620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klami A, Virtanen S, Leppäaho E, Kaski S. Group Factor Analysis. IEEE Trans Neural Netw Learn Syst. 2015;26(9):2136–2147. doi: 10.1109/TNNLS.2014.2376974 [DOI] [PubMed] [Google Scholar]
- 20.Fan L, Li H, Zhuo J, et al. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb Cortex. 2016;26(8):3508–3526. doi: 10.1093/cercor/bhw157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skinner HA. The drug abuse screening test. Addictive Behaviors. 1982;7(4):363–371. doi: 10.1016/0306-4603(82)90005-3 [DOI] [PubMed] [Google Scholar]
- 23.Norman SB, Cissell SH, Means-Christensen AJ, Stein MB. Development and validation of an Overall Anxiety Severity And Impairment Scale (OASIS). Depression and Anxiety. 2006;23(4):245–249. doi: 10.1002/da.20182 [DOI] [PubMed] [Google Scholar]
- 24.Sheehan D, Lecrubier Y. The Mini International Neuropsychiatric Interview Version 6.0. MINI 6.0. Medical Outcomes Systems Inc.; 2010. [Google Scholar]
- 25.Sheehan D, Janavs J, Baker R, Sheehan KH, Knapp E, Sheehan M. MINI International Neuropsychiatric Interview-Version 7.0. (MINI 7.0). Medical Outcomes Systems Inc.; 2015. [Google Scholar]
- 26.American Psychiatric Association. Diagnostic and Statistical Mannual of Mental Disorders. 4th Edition, Text Revision. American Psychiatric Association; 2000. [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. American Psychiatric Association; 2013. [Google Scholar]
- 28.Bagby RM, Taylor GJ, Parker JD. The Twenty-item Toronto Alexithymia Scale--II. Convergent, discriminant, and concurrent validity. J Psychosom Res. 1994;38(1):33–40. doi: 10.1016/0022-3999(94)90006-x [DOI] [PubMed] [Google Scholar]
- 29.Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: A scale development study. Journal of Research in Personality. 2006;40(6):1086–1102. doi: 10.1016/j.jrp.2005.11.001 [DOI] [Google Scholar]
- 30.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology. 1994;67(2):319–333. doi: 10.1037/0022-3514.67.2.319 [DOI] [Google Scholar]
- 31.Whiteside SP, Lynam DR, Miller JD, Reynolds SK. Validation of the UPPS impulsive behaviour scale: a four-factor model of impulsivity. Eur J Pers. 2005;19(7):559–574. doi: 10.1002/per.556 [DOI] [Google Scholar]
- 32.Cox RW. AFNI: What a long strange trip it’s been. NeuroImage. 2012;62(2):743–747. doi: 10.1016/j.neuroimage.2011.08.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2019. https://www.R-project.org/ [Google Scholar]
- 34.RStudio Team. RStudio: Integrated Development Environment for R. RStudio, Inc.; 2016. http://www.rstudio.com/ [Google Scholar]
- 35.Allaire JJ, Xie Y, McPherson J, et al. Rmarkdown: Dynamic Documents for R.; 2020. https://github.com/rstudio/rmarkdown [Google Scholar]
- 36.Xie Y, Allaire JJ, Grolemund G. R Markdown: The Definitive Guide. Chapman and Hall/CRC; 2018. https://bookdown.org/yihui/rmarkdown [Google Scholar]
- 37.Filzmoser P, Varmuza K. Chemometrics: Multivariate Statistical Analysis in Chemometrics.; 2017. https://CRAN.R-project.org/package=chemometrics [Google Scholar]
- 38.Torgo L Data Mining with R, Learning with Case Studies. Chapman and Hall/CRC; 2010. http://www.dcc.fc.up.pt/ ltorgo/DataMiningWithR [Google Scholar]
- 39.Virtanen S, Klami A, Khan SA, Kaski S. Bayesian Group Factor Analysis. In: Proceedings of the Fifteenth International Conference on Artificial Intelligence and Statistics (AISTATS). Vol 22. ; 2012:1269–1277. http://research.ics.aalto.fi/mi/online-papers/virtanen12.pdf [Google Scholar]
- 40.Wickham H Ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag; New York; 2016. https://ggplot2.tidyverse.org [Google Scholar]
- 41.Revelle W Psych: Procedures for Psychological, Psychometric, and Personality Research. Northwestern University; 2018. https://CRAN.R-project.org/package=psych [Google Scholar]
- 42.Tipping ME. Sparse Bayesian Learning and the Relevance Vector Machine. Journal of Machine Learning Research. 2001;1(Jun):211–244. [Google Scholar]
- 43.Aupperle RL, Paulus MP, Kuplicki R, et al. Web-Based Graphic Representation of the Life Course of Mental Health: Cross-Sectional Study Across the Spectrum of Mood, Anxiety, Eating, and Substance Use Disorders. JMIR Mental Health. 2020;7(1):e16919. doi: 10.2196/16919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nair DG. About being BOLD. Brain Research Reviews. 2005;50(2):229–243. doi: 10.1016/j.brainresrev.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 45.Diekhof EK, Kaps L, Falkai P, Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude - an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia. 2012;50(7):1252–1266. doi: 10.1016/j.neuropsychologia.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 46.Taylor-Clift A, Morris BH, Rottenberg J, Kovacs M. Emotion-modulated startle in anxiety disorders is blunted by co-morbid depressive episodes. Psychol Med. 2011;41(1):129–139. doi: 10.1017/S003329171000036X [DOI] [PubMed] [Google Scholar]
- 47.Sandre A, Bagot RC, Weinberg A. Blunted neural response to appetitive images prospectively predicts symptoms of depression, and not anxiety, during the transition to university. Biological Psychology. 2019;145:31–41. doi: 10.1016/j.biopsycho.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 48.Grant DM, Judah MR, White EJ, Mills AC. Worry and Discrimination of Threat and Safety Cues: An Event-Related Potential Investigation. Behavior Therapy. 2015;46(5):652–660. doi: 10.1016/j.beth.2014.09.015 [DOI] [PubMed] [Google Scholar]
- 49.Dillon DG, Rosso IM, Pechtel P, Killgore WDS, Rauch SL, Pizzagalli DA. Peril and Pleasure: An RDoC-inspired examination of threat responses and reward processing in anxiety and depression. Depress Anxiety. 2014;31(3):233–249. doi: 10.1002/da.22202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fattore L. Reward processing and drug addiction: does sex matter? Front Neurosci. 2015;9. doi: 10.3389/fnins.2015.00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hammerslag LR, Gulley JM. Age and Sex Differences in Reward Behavior in Adolescent and Adult Rats. Dev Psychobiol. 2014;56(4):611–621. doi: 10.1002/dev.21127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greimel E, Bakos S, Landes I, et al. Sex differences in the neural underpinnings of social and monetary incentive processing during adolescence. Cogn Affect Behav Neurosci. 2018;18(2):296–312. doi: 10.3758/s13415-018-0570-z [DOI] [PubMed] [Google Scholar]
- 53.Paulus MP, Thompson WK. The Challenges and Opportunities of Small Effects: The New Normal in Academic Psychiatry. JAMA Psychiatry. 2019;76(4):353–354. doi: 10.1001/jamapsychiatry.2018.4540 [DOI] [PubMed] [Google Scholar]
- 54.Rush AJ, Carmody TJ, Ibrahim HM, et al. Comparison of Self-Report and Clinician Ratings on Two Inventories of Depressive Symptomatology. PS. 2006;57(6):829–837. doi: 10.1176/ps.2006.57.6.829 [DOI] [PubMed] [Google Scholar]
- 55.Löwe B, Kroenke K, Herzog W, Gräfe K. Measuring depression outcome with a brief self-report instrument: sensitivity to change of the Patient Health Questionnaire (PHQ-9). Journal of Affective Disorders. 2004;81(1):61–66. doi: 10.1016/S0165-0327(03)00198-8 [DOI] [PubMed] [Google Scholar]
- 56.Bennett CM, Miller MB. How reliable are the results from functional magnetic resonance imaging? Annals of the New York Academy of Sciences. 2010;1191(1):133–155. doi: 10.1111/j.1749-6632.2010.05446.x [DOI] [PubMed] [Google Scholar]
- 57.Poldrack RA, Baker CI, Durnez J, et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci. 2017;18(2):115–126. doi: 10.1038/nrn.2016.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.