Abstract
Objective:
To understand the basics of sleep quality in a pastoralist population and to explore predictors of this variation.
Design:
Cross-sectional.
Setting:
Northern Namibia, dry seasons of 2016 and 2017.
Participants:
The Himba, a nonindustrial seminomadic agropastoralist population without access to the electrical grid.
Measurements:
Using Actiwatch-2 devices, 75 participants completed 721 days of wear. We calculated sleep duration, efficiency, and activity counts before and after sunset/sunrise and onset/offset. Participants were also interviewed about sleeping arrangements and nighttime disruptions.
Results:
Himba show lower sleep duration and efficiency than other populations studied, and men had substantially lower duration and efficiency than women. Sex differences were not attenuated when napping was included with total sleep time. Age predicted longer sleep duration and lower evening and morning activity levels. Number of adult co-sleepers predicted increased sleep duration and efficiency in women. Livestock disturbance was not a commonly reported cause of nighttime waking.
Conclusions:
These findings support predictions that pastoralist groups may have lower sleep quality than other subsistence populations, but this does not appear to be a consequence of noise from livestock. Instead, lower sleep quality appears to be the result of subsistence and social activities, particularly for men and young adults overall.
Keywords: Pastoralism, Actigraphy, Activity, Cross-cultural comparison, Sleep disturbance, Sleeping arrangement
Introduction
Recent sleep research has focused on defining normal sleep patterns by conducting observational research among diverse populations worldwide, especially those who lack access to electric light. Although electricity’s influence on sleep patterns in postindustrial and urban populations is both pronounced and undisputed,1,2 much of the early research in these populations suggest other environmental factors have similarly impactful influence on sleep duration and quality in nonindustrial small-scale societies. Moreover, although average sleep duration does not appear to differ substantially among these types of populations, nighttime activity, sleep timing, efficiency, and intraindividual nightly consistency vary considerably.3–9 The extant diversity in sleep patterns suggests that sleep exhibits phenotypic plasticity, whereby variation in an individual’s sleep environment and general life conditions elicits a diverse array of sleep patterns, regardless of the presence or absence of electric lighting or other facets of the modern, industrialized lifestyle. One can expect significant variation in sleep across small-scale societies, depending on the type and timing of productive tasks, the opportunity costs of sleep, and the importance of nighttime activities. Furthermore, where men’s and women’s activities differ due to the sexual division of labor or to differential participation in ritual or other social activities, we should see gendered variance in sleep parameters. To establish a more nuanced understanding of human sleep patterns, a broader perspective is required that explores the full range of diversity in human sleep ecology and associated sleep patterns. This study aims to contribute toward this end by providing the first quantitative description of actigraphically measured sleep patterns in a pastoralist population.
In addition to the many industrialized populations where sleep has been extensively investigated, recent studies of adult sleep in nonelectrified villages and small-scale societies include market agriculturalists, food-producing agriculturalists, subsistence horticulturalists, and hunter-gatherers.1,4–6,8–11 To date, sleep patterns in pastoralist groups have not been investigated despite the fact that nomadic, seminomadic, and agropastoralism remain common subsistence strategies across Africa, Asia, and Europe, with an estimated 200 million pastoralists worldwide.12 There are many reasons to expect that pastoralist sleep patterns might differ from small-scale societies that rely on other modes of production. Despite ongoing increases in market integration, loss of grazing lands, and associated increases in sedentarization, many pastoralists are still highly mobile, and high mobility can lead to frequent changes in sleeping conditions.13,14 In particular, transhumance (seasonal mobility) entails frequent camp relocation, especially during the dry season. This results in lower mobility during the wet season, when livestock graze closer to central households, and higher mobility during the dry season, when cattle are grazed farther afield.15 However, nonseasonal factors can also result in short- or long-term mobility. Senegalese Fulani pastoralists report transhumance as a result of heterogeneous rainfall, fire, disease, or loss of water at a borehole.16 Variance in mobility is explained by a wide variety of social and environmental concerns; for example, Turkana pastoralists have been observed to move 15 times per year on average,17 and these patterns of movement intensify in response to raiding concerns.18 The burden of mobility may be particularly taxing for young men, who spend more time herding livestock and are highly mobile, traveling between waterposts, cattleposts, and various grazing areas frequently.16 For example, Gabra boys and young men may shift frequently between main settlements and temporary encampments, sleeping on the ground, whereas girls traditionally only leave the natal household upon marriage.19 Camp relocation can also cause increased work for women; women report concern about workload stress associated with such moves.16,18 Overall, the frequency of changes in sleep structure, co-sleeper composition, and sleeping location is predicted to be associated with higher variance in sleep duration, timing, and consolidation in a subsistence pastoralist population.
Many pastoralists sleep in close proximity to their livestock and may suffer frequent waking and loss of sleep because of disruptions such as noise or an animal escaping the kraal (fenced pen). Moreover, pastoralists may need to maintain vigilance during the night to deter threats to their livestock, such as predators or theft. This may be an important predictor of nighttime activity and nightly variation in sleep patterns among pastoralists. There is some evidence that asynchronous group sleep in small-scale populations may be an evolutionary strategy against predators or hostile persons4, and this may be a strategy used by pastoralists against loss of livestock as well. Indeed, Akpa et al20 report that “throughout the night, the pastoral Fulani must keep vigil on the animals, protecting them from night marauders.” Cattle theft and raiding are common among some pastoralist groups, where they are particularly salient sources of stress.18,21,22 One solution is the keeping of domestic dogs, which can alert people to predators or raiders but can also result in additional sleep disruptions from general barking at night.19 Overall, nighttime pastoralist vigilance against loss of livestock is predicted to result in frequent waking and lower-quality sleep.
In general, sleep trades off against other waking behaviors, including subsistence, social, and reproductive pursuits. Although it had long been assumed that the advent of artificial light was responsible for late onset times, recent work suggests that there are ample nighttime activities that people in small-scale, subsistence societies regularly engage in.3,9,23 These opportunity costs may be particularly relevant for pastoralists, as subsistence tasks, such as milking, watering, or pasturing livestock, often begin early in the morning and can extend late into the evening. For example, in the Maasai, “cows are milked twice daily by women and girls, at sunrise before the cattle leave for pasture and at sunset when they have returned to the kraal.”24 Likewise, Gabra are reported to milk and take livestock to pasture before sunrise, to avoid the hottest period of the day, and again at dusk, resulting in very early wake times and very late bedtimes.19 Therefore, the labor demands of pastoralism are expected to modulate sleep times, but this modulation may be age and sex specific. Pastoralists also often engage in social activities in the evening, including storytelling and sharing news of the day, as in other small-scale groups.23
In this study, we conducted the first direct investigation of pastoralist sleep patterns using wrist-worn actigraphy. We first describe the average sleep onset, offset, and duration for this population. Based on the ecology and labor demands of pastoralism, sleep interruption and reduced sleep efficiency may be relevant dimensions of typical Himba sleep patterns, but these effects may not be the same for men and women due to the sexual division of labor. To investigate this issue, we compare sleep duration, timing, and efficiency between men and women and test for any significant age effects. We predict that pastoralists will have, on average, lower sleep duration and efficiency relative to populations using other subsistence strategies. Next, we examine the circadian pattern of morning and evening activity, focusing on comparing the magnitude of activity in the hour before and the hour after both sunrise and sunset, to understand individual variation and sex differences in sleep parameters. Given the sex-specific labor demands in this population, we predict that activity patterns will map onto sleep parameters, resulting in lower sleep duration for men. Based on these results, we then quantitatively examined men’s and women’s sleep ecologies and test for the predictive power of some of these factors for sleep timing, duration, and efficiency. We further predict that participants will report nighttime livestock disruptions as causal agents of nighttime waking and that co-sleepers—and number of children in particular—will be further linked to sleep disruptions and lower sleep quality.
Methods
Ethnographic context
The Himba are seminomadic agropastoralists living in the Kunene region of northern Namibia.25,26 In the study area, there is no electric light or running water, although some small solar panels power small items like cell phones. Households consist of extended families from 10 to 30 individuals, all living within a fenced compound.25 Compounds in this area are slightly larger than in other areas where Himba reside.21 At the center of the compound (roughly 20–50 ft from the residence structures) is a corral for livestock, with additional smaller corrals used for baby sheep and goats. Other domesticated animals such as chickens, donkeys, and dogs are also all quite common and similarly reside at night within the larger fenced area. Depending on household size, compounds may have a single or, more often, multiple residence structures made from thick mud and cow dung. These rounded structures have a single entrance and are completely open on the inside except for a central support beam. Typically, a fire is lit at the center for warmth and burns throughout the night. The majority of huts do not have a chimney, resulting in thick smoke inside the structure when the fire is lit. The Himba rarely have sleeping pads or mattresses, and instead sleep on mats made from cow hides and use wooden headrests.
Households are frequently polygynous, and most include at least 2–3 generations.27 Co-wives each have their own hut, where they sleep with small children, and their husband rotates between them while keeping a main residence with his first wife. Young children co-sleep with their parents, whereas older children will often sleep together on their own. Women report waking early to begin making butter from sour milk that was left to sit overnight, where as men may rise early to take the livestock to water and pasture. These early morning tasks may result in sex-differentiated shifts in onset and offset times. Age may also be an important factor. Young men and adolescent boys are primarily responsible for the herding and grazing of livestock, whereas older men may stay in the compound until later in the day.26 The family reconvenes in the early evening for a meal and the evening milking. It is common for households to gather around the fire for several hours after dark, singing and telling stories. Men are also known to visit their extramarital lovers after dark, at times traveling substantial distances to do so.28
Data collection
Male and female sleep data were collected in the dry seasons of 2016 and 2017, respectively. Participants were recruited opportunistically at compounds as part of a separate project. We excluded any participants who were visiting or planned to leave the study area within the span of 2–3 weeks. In 2017, in addition to actigraphy, we conducted short, structured interviews with women at the end of the sampling period, asking participants to list the number of children and adults they normally shared a hut with and whether they usually slept with a fire in the hut. We also asked each participant to free-list any disturbances that resulted in nighttime waking. For men, informal notes were recorded opportunistically about their activity, sleep patterns, and sleep locations on 54 nights. For all participants, ages were calculated using a traditional year-name system (see Scelza29 for details). Sleep data were collected using Actiwatch-2 devices worn on the left wrist for up to 2 weeks at a time. Because individuals often move to new locations or travel to visit family in other areas, we asked participants to return the watches early if they were to leave the study area before the end of the study period.
Data processing and analysis
The Actiwatch-2 sleep monitor by Philips Respironics relies on actigraphic measurements of bodily movement to distinguish sleep from wake for each 1-minute epoch recorded. This method has been validated extensively against polysomnography30 and has been used in several recent studies of sleep in nonelectrified villages1,5,6,8,9,11 as well as in industrialized populations.31–33 When worn on the wrist, these devices are prone to high error rates for measuring overall physical activity.34 After raw data actigraphy data were imported into R, sleep intervals were manually identified and scored with the Respironics algorithm (adapted from Cole et al35) according to the protocol detailed in Yetish et al.3 We then calculated sleep onset (time of first sleep initiation for the night), sleep offset (time of final sleep termination for the night), “time in bed” (here referring to the duration of the “sleep period,” which extends from sleep onset to sleep offset), total sleep duration (total time spent in bed minus all minutes spent waking after sleep onset prior to sleep offset), and sleep efficiency (sleep duration divided by time in bed).
Daily sleep was calculated similarly to night sleep but with the modified criteria of using the “high sensitivity” threshold value to distinguish sleep from wake for a given epoch and requiring at least 15 minutes of continuous sleep to be distinguished from rest, as detailed in Samson et al.10 We also implemented additional inclusion and exclusion criteria to reduce false positives for nap identification. If there was more than 1 hour of nonwear that day (identified as 60 continuous minutes with an activity level of 0 recorded), daily sleep data were considered unknown/unmeasured (100/749 person-days). Daily sleep, by definition, must occur between sunrise and sunset, but if sleep offset were later than sunrise, it was not included (11/ 649 measured person-days). Sleep onset never preceded sunset in this database, so this did not produce any false-positive nap identifications. As an added level of quality control, all person-days with more than 60 minutes of daily sleep recorded (85/649) were reviewed graphically. Additional nonwear periods became obvious in this way when 24-hour activity patterns showed near-0 light exposure values and stochastic but consistently patterned non-0 activity levels for at least 14 hours. This likely indicates nonwear of the watch while the watch was kept in a bag or on a vehicle (16/85 data points rescored as unknown/ not recorded). Measures of daily sleep were added to total sleep duration from the preceding night for a measure of true total sleep duration.5
Average values for each subject i were calculated for each sleep parameter μi. In addition, we calculated a measure of intraindividual variation in sleep parameters for each study participant εi, calculating the absolute value of the deviation from individual mean for each night measured and averaging it over the entire period of observation (Yetish et al3).
All individuals were included in the final analysis regardless of number of nights of sampling, resulting in data from 75 participants, for a total of 721 nights of observation, with an average of 9.61 (SD = 2.9) sample nights after data cleaning. Mean age was 35.7 (range = 15–78 years) with 20% of participants age 50 or older. Only 2 participants had fewer than 5 nights of sleep data (range = 4–14). In addition to sleep parameters, we also calculated total activity counts as scored by the actigraphic device 1 hour after sunset and sunrise, and 1 hour before onset and offset for each individual on each day of wear. Sex differences in sleep parameters for individual means were compared using the BayesFactor package with default priors,36 and Bayes factors are reported to reflect these differences, where values can be interpreted as the strength of the evidence for the alternative hypothesis (see Jarosz and Wiley37 for further interpretation). To assess the influence of age and sex on sleep parameters, Bayesian multilevel models with varying intercepts for individual ID, partial pooling, and regularizing priors were used to predict standardized sleep and activity parameters as predicted by sex (where male = 1), and standardized age. Nonlinear effects of age were also evaluated but did not increase mode fit, nor were such effects supported by data visualization. Using female data, we ran similar models to predict sleep parameters using standardized age, number of children in the hut, and number of adults in the hut. Because 1 participant left the area before study completion (leaving the device with a family member), a final interview was not conducted with this woman. For these data, predictors were instead imputed in the model. Effect of predictors was evaluated by calculating the posterior probability of a negative (b[pr<0]) or positive effect (b[prN0]) on the outcome. All models were fit to RStan38 using the rethinking package39 with 3 chains of 5000 iterations per chain, including a warm-up sequence of 2000 iterations. Model fit was assessed via the Gelman-Rubin convergence diagnostic.
Free-listing responses for nighttime disturbances resulting in waking were coded for all women who could remember any cause of nighttime waking (n = 36, individuals who said “no reason” or could not recall any specific cause were excluded). In coding these responses, we separated breastfeeding as a cause for waking from other child disturbances, which included children making noise, moving, or otherwise disturbing the subject.
Results
Sleep parameters
Average sleep parameters by sex, and statistical comparisons by sex, are shown in Table 1 (and see Fig. 1). Average (and standard deviation) individual total sleep time was 5.47 hours (1.09). Average onset time and offset time for all participants were 9:34 PM (0:41) and 5:48 AM (0:41), respectively (Central Africa Time).
Table 1.
Descriptive statistics
| Men (n = 29) | Women (n = 46) | BF | ||
|---|---|---|---|---|
| Age (y) | 36.0 (14.5) | 35.5 (15.4) | ||
| Number of nights | 12.3 (2.1) | 7.9 (1.9) | ||
| Nap duration (min) | 53.2 (22.3) | 46.6 (26.1) | 0.41 | |
| Days napped (%) | 32.9 | 27.7 | 0.33 | |
| Onset | μi | 9:42 pm (0:42) | 9:29 pm (0:40) | 0.53 |
| ε | 0:59 | 0:50 | 0.75 | |
| Offset | μi | 5:37 am (0:19) | 5:56 am (0:24) | 42.92 |
| ε | 0:32 | 0:25 | 4.97 | |
| Time in bed (h) | μi | 7.93 (0.76) | 8.46 (0.73) | 10.18 |
| ε | 1.17 | 0.99 | 1.47 | |
| Total sleep time (h) | μi | 4.76 (0.92) | 5.92 (0.95) | 9161.1 |
| ε | 0.86 | 0.88 | 0.25 | |
| Sleep efficiency (%) | μi | 60.3 (9.0) | 70.2 (8.8) | 1359.5 |
| ε | 6.9 | 8.2 | 1.68 | |
| True total sleep time (h) | μi | 5.06 (0.94) | 6.17 (1.00) | 1855.4 |
| ε | 1.09 | 0.95 | 0.27 | |
Mean μi (standard deviation) and mean intraindividual error ε of sleep parameters by sex. Nap duration refers to average nap length in minutes in any given day where any nap was detected.
BF, Bayes factor for comparison of sex differences by mean of nights per individual.
Fig. 1.

Mean and standard error for 3 sleep parameters. Men in gray; women in black.
Daily sleep was calculated for 88% of the total days of data when there were no significant periods of nonwear, and napping was observed on 206/649 person-days. Average duration was 50 minutes. Twenty-four percent of participants recorded no napping on any day. Comparisons of means find no support for sex differences in percent of days napped or napping duration. Naps were observed on 206/649 person-days, with a median daily sleep value of 45 minutes (48.7) for those that did nap. When daily sleep was included to calculate true total sleep time, average time increased to 5.74 hours (1.11).
Comparison of means indicates very strong support for sex differences in these parameters, with women receiving an average of 1.16 hours more nightly sleep. Additionally, women had longer time in bed. These 2 differences resulted in substantially lower sleep efficiency for men relative to women. Overall, average individual sleep efficiency was 66.4% (10.1%), with 70.2% for women and 60.3% for men. There was little sex difference in mean onset time, but men woke an average of 19 minutes earlier and at more variable times from day to day (Fig. 2). There was no sex difference in the intraindividual nightly variation in sleep duration. Linear models support the sex differences found in the statistical comparison; men have lower sleep efficiency, time in bed, and total sleep time relative to women (b[pr<0] = 100%, 99%, and 100% respectively, Table 2). Including interactions between age and sex did not impact results or increase model fit, and these were not included in final models described here.
Fig. 2.

Sleep onset (circles) and offset times (triangles) for all nights are plotted for each subject, shown in gray. Mean and 89% intervals for posterior predictions shown in black. Each horizontal line represents a different subject (women in the top panel, men in the bottom), sorted by difference in predicted offset and onset times. Dotted lines represent mean sunset and sunrise times during the observation period.
Table 2.
Results from linear models predicting sleep parameters
| Parameter | Sleep efficiency | Time in bed | Total sleep time | True total sleep time |
|---|---|---|---|---|
| Men and women | ||||
| Age | 0.10 (−0.02 to 0.22) | 0.11 (0.03–0.19) | 0.15 (0.04–0.26) | 0.16 (0.06–0.28) |
| Male | −0.69 (−0.94 to −0.45) | −0.34 (−0.50 to −0.17) | −0.74 (−0.96 to −0.52) | −0.73 (−0.95 to −0.51) |
| Women only | ||||
| Age | 0.20 (0.04–0.36) | 0.12 (0.00–0.23) | 0.23 (0.08–0.38) | 0.26 (0.11–0.40) |
| Number of adults | 0.18 (0.02–0.34) | 0.04 (−0.08 to 0.15) | 0.15 (0.00–0.28) | 0.18 (0.03–0.31) |
| Number of kids | −0.09 (−0.26 to 0.09) | −0.02 (−0.14 to 0.10) | −0.08 (−0.23 to 0.08) | −0.06 (−0.23 to 0.09) |
Posterior mean and 89% intervals.
Age was negatively correlated with both mean onset (r = −0.42) and offset (r = −0.30) by individual; older individuals tended to wake earlier and begin sleep earlier. Age had a modest influence on all sleep parameters, where older individuals have higher sleep efficiency, time in bed, and total sleep time (b[pr>0] = 91.5%, 98.1%, and 98.5%, respectively), in line with qualitative predictions.
Activity
Participants in this sample show substantial activity after sunset and before sunrise, and this activity helps explain variance in sleep parameters by sex and age. To compare these parameters between men and women, sex and age were used to predict total activity counts for the hour after sunset, the hour before onset, the hour before sunrise, and the hour after offset (Table 3). Linear models indicate that men had higher activity both after sunset and before sunrise (b[pr>0] = 95.3%, and 94.4%, respectively), but being male has no effect on predicted activity in the hours before onset or after offset (b[pr>0] = 41.7%, and 70.2%, respectively). Linear models also predicted that older individuals have lower activity levels after sunset, before sunrise, and in the first hour after waking (b[pr<0] = 99.8%, 99.9%, and 99.8%, respectively). Similarly, age negatively predicted activity in the hour before sleep onset and after sleep offset in women (b[pr<0] = 96.6% and 96.4%, respectively). Number of adult or child co-sleepers had little effect on any activity parameter (Table 3).
Table 3.
Results from linear models predicting activity parameters
| Men and women | After sunset | Before onset | Before sunrise | After offset |
| Age | −0.15 (−0.23 to −0.07) | −0.04 (−0.10 to 0.03) | −0.20 (−0.31 to −0.10) | −0.18 (−0.27 to −0.08) |
| Male | 0.16 (0.01–0.32) | −0.02 (−0.15 to 0.12) | 0.21 (0.00–0.42) | 0.07 (−0.13 to 0.27) |
| Women only | Before onset | After onset | Before offset | After offset |
| Age | 0.01 (−0.08 to 0.10) | −0.02 (−0.11 to 0.08) | −0.10 (−0.19 to −0.01) | −0.10 (−0.19 to −0.01) |
| Number of adults | 0.08 (−0.02 to 0.17) | 0.08 (−0.02 to 0.18) | −0.06 (−0.14 to 0.03) | −0.06 (−0.15 to 0.03) |
| Number of kids | 0.01 (−0.08 to 0.11) | 0.00 (−0.10 to 0.09) | 0.08 (0.00–0.17) | 0.08 (−0.01 to 0.17) |
Posterior mean and 89% intervals.
Women's sleep ecology
Results from the interviews with women find that women shared a hut with an average of 0.65 adults (range 0–3) and 2.5kids(range 0–6), for an average of 3.1 total co-sleepers (range 1–7). Results of linear models predicting sleep parameters indicate that co-sleeping with other adults was associated with increased total sleep time and sleep efficiency (b[pr>0] = 95.0% and 96.2%, respectively; Table 2) but not time in bed (b[pr>0] = 59%), whereas number of child co-sleepers had little impact on either sleep parameter (b[pr<0] = 80.0%, 79.8%, and 58.7%, respectively). Nearly all women reported sleeping with a fire in the hut (95%), so this variable was not included in any analyses.
Women reported that waking to urinate was the most frequent cause of nighttime waking (36.1% reporting), followed by child disturbances (25%), pain (19.4%), and waking to stoke the fire (16.6%). Contrary to predictions, only 8.3% of respondents reported waking as a result of livestock disturbances (including animal making noise, goat escaping its pen, etc; Fig. 3).
Fig. 3.
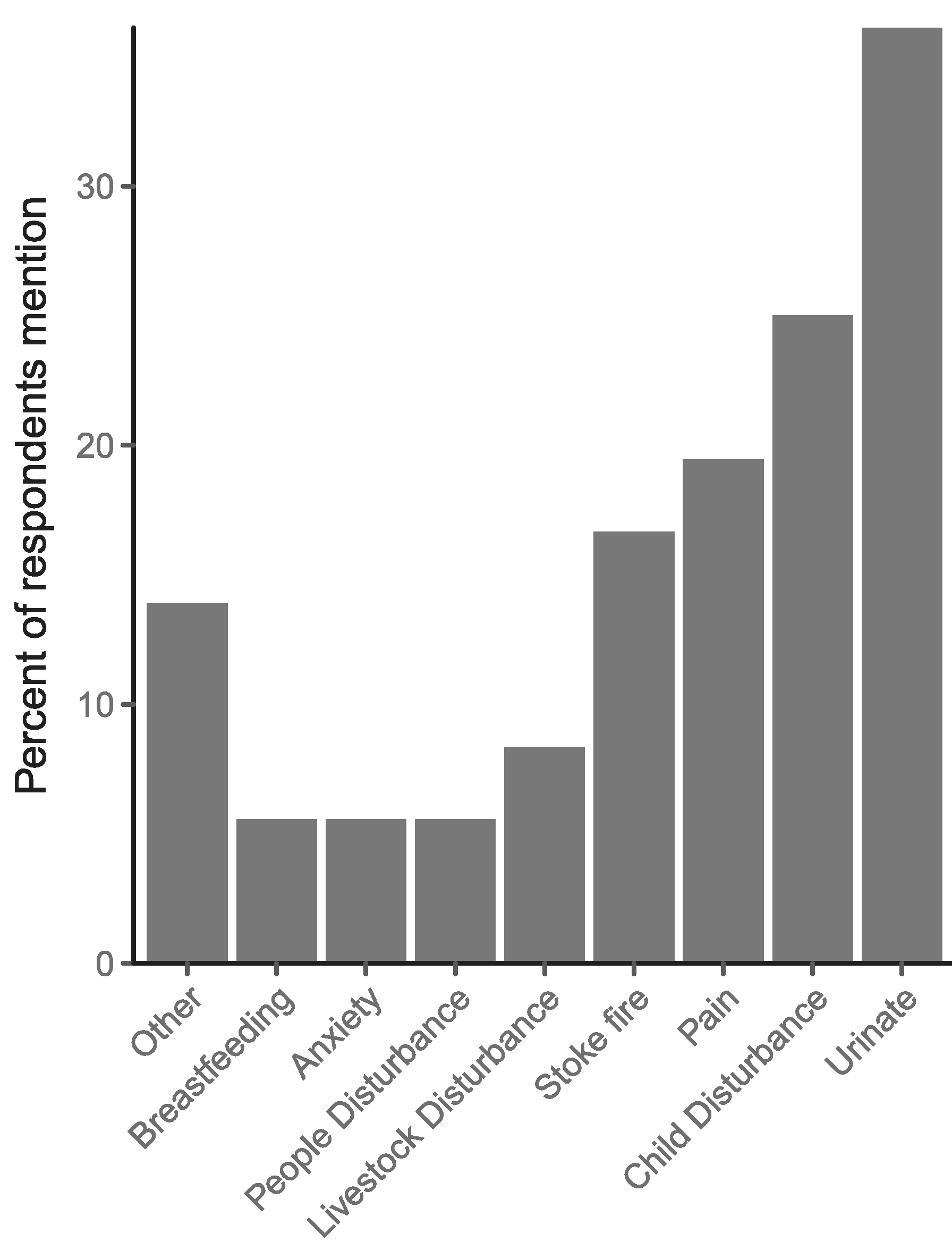
Percentage of respondents (n = 36) who report sleep disturbances by category, excluding participants who could not recall any specific cause of sleep disturbance. “Other” includes sleep walking, dreams, hunger, and socializing. Livestock disturbances were only reported for 8.3% of respondents.
Men's sleep ecology
Opportunistic notes were taken about men’s sleep ecology on 54 nights. These interviews were not as systematic as the women’s interviews, but they do offer some insights into men’s sleep arrangements and possible sources of sleep disruption. Forty-five percent of the men in the sample were currently married to an adult wife (50% of these were polygamous marriages) and reported routinely sharing a hut with one of their wives and young children. The other 55% of men in the sample shared huts mainly with other men or younger children, whereas 3 slept alone. Strikingly, of the 40 nights where men reported where they slept, 24 of those nights (60%) were spent sleeping away from their main residence. This included spending the night at the sites of rituals (a circumcision ceremony) and funerals, visiting other communities, with informal romantic partners, and in the bush tending to livestock activities. It is not known how these various activities might affect sleep patterns, but it is possible that each of them could lead to substantial sleep disturbances or unusual sleep/wake patterns. In addition, on 7 nights, men reported sickness or body pain that affected their sleep.
Discussion
Using data from the first study of sleep patterns in a pastoralist population, we find short mean sleep durations (5.47 hours) and low mean sleep efficiency (66.4%) among Himba pastoralists. Even when napping, which occurs at relatively high rates, is included in the analysis, sleep duration remains the lowest ever recorded for a small-scale society. These findings provide additional evidence to refute the notion that small-scale, nonelectric populations will have longer average sleep durations than industrialized populations. Moreover, it highlights the probability that variability in sleep parameters is related to subsistence strategies and cultural norms. We also found significant sex differences in sleep patterns, where men tended to have shorter sleep, have lower sleep efficiency, and generally be more active between sunset and sunrise. There was no significant difference in napping duration or frequency by sex. Additionally, based on reports of co-sleeping, women who co-slept with other adults exhibited higher sleep duration and efficiency, but number of children has little effect. We examine these results in light of the ethnographic context, highlighting how workload variability and social activities by age and sex may influence sleep quality.
Activity and labor demands modulate sleep patterns
Sleep patterns appear to correspond to the times of day that labor demands are highest in pastoralist populations. Men and boys are expected to rise early to take the livestock to water before beginning herding for the day. Himba women also have early morning tasks, including milking and “shaking the calabash” to make milk fat, but these demands appear to be more variable than that of young men, and milking occurs later in the morning after the cows have been brought to water. Although “shaking the calabash” requires strenuous use of upper body strength, it does not require much walking around. It can also be done with one hand, and we expect that women likely favored their dominant hand (typically right), whereas watches were always worn on the left. Men’s early evening activities are again more standardized, bringing the cows back to the compound, whereas women’s work may be more variable, depending on what is needed to be done that day and how many women there were in the compound to share the work. Wrist-worn actigraphy is a rough measure of physical activity, but results indicate a clear pattern of men being more active in the hours after sunset and before sunrise, which may correspond with these labor demands. These results contrast with observations in industrialized populations, where women report both earlier sleep onset and offset times than men.40,41 Himba men may face steeper trade-offs in sleep as a result of subsistence activities. It should be noted that this can occur even if men’s overall energy expenditure across the day is lower but concentrated at the beginning and end of the day, whereas women’s work is more concentrated in the midday hours.
Using linear models, we found that age was associated with increases in both sleep duration and sleep efficiency. This contrasts with many observations from industrialized contexts, where advanced age is associated with higher wake after sleep onset and lower sleep duration and efficiency.42 However, age is also associated with lower activity before sunrise and after sunset, suggesting that perhaps the trade-off between work and sleep is skewed toward greater sleep duration in older men but toward greater activity in younger men as a function of social and subsistence activities. This corresponds with labor expectations in Himba culture, where young men are primarily responsible for herding activities and spend larger periods of time away from the compound or cattlepost. Conversely, older men, particularly those with male children or kin, can delegate labor to others and spend more time at home. Similarly, although older women are still expected to engage in a variety of subsistence activities, younger women co-residents are relied upon for many burdensome tasks including carrying water and collecting firewood. Younger men and women are also likely to be participating more often or more actively in social and reproductive activities. Activity patterns in this population appear to reverse the usual age-related trend in measured sleep quality and quantity and emphasize the importance of understanding subsistence-based activity when considering cultural and environmental variation in sleep parameters. Additionally, although we were unable to detect a nonlinear effect of age on sleep parameters, sample size in older individuals is relatively limited in this study. Larger sample sizes of elderly individuals may yield more insight, particularly on nonlinear effects of age in predicting sleep duration and quality.
Sleeping arrangements
The majority of Himba women and men report sharing their sleeping quarters with multiple adults and children. Contrary to predictions, number of children was not significantly associated with before onset or after offset activity levels, or any sleep parameters, suggesting that co-sleeping children are not affecting overall sleep quality. However, one-quarter of Himba women report that they wake at night as the result of child disturbances, the second most commonly reported interruption. In laboratory studies, co-sleeping did not impact sleep duration but did increase arousal frequency.43 Alternately, co-sleeping with other adults was associated with increased sleep efficiency and total sleep time. One possible explanation for this pattern is that it enables co-sleeping adults to share responsibilities for nightly tasks, such as maintaining a lit fire, caring for young children, or maintaining general vigilance against danger, thus reducing sleep interruptions and associated sleep loss. Additionally, multiple individuals sharing a hut could increase the temperature inside the hut, which then facilitates higher-quality sleep. Although we did not collect detailed data on the demographics of co-sleepers beyond distinguishing between adults and children, the relationship between adult and child co-sleepers on overall quality is also likely mediated by age and sex.
Cross-cultural comparison in sleep duration and efficiency
The results of this study show that Himba have the lowest average sleep efficiency and sleep duration of any population studied using actigraphy (Fig. 4). Although we are not able to investigate all facets of how pastoralism could affect sleep, these results support predictions that sleep quality in traditionally living pastoralist groups may be on the lower end of the spectrum. Further study in other pastoral groups is necessary to confirm this conclusion. Pastoralists, particularly young men, may show reductions in sleep quality as a result of activity patterns related to herding livestock, frequent mobility, nighttime vigilance, and other nighttime disturbances. Contrary to predictions, self-reports on sleep disturbances suggest that livestock noise at night may not be the cause of low sleep quality. However, livestock and domestic dog disturbances may negatively affect sleep quality without respondents’ ability to report these as causes of waking. Women’s sleep quality, although still on the lower end of the scale cross culturally, is higher than their male counterparts, indicating that the trade-offs between sleep and other activities may be less steep for them.
Fig. 4.

Comparison of Himba sleep parameters with other populations using actigraphy, divided into small-scale, rural and developing, and industrialized populations. Mean and standard deviation for samples from multiple populations which use actigraphy to measure sleep parameters. Reported population mean and standard deviation in black. Where reported, male (light gray) and female (dark gray) means and standard deviations are shown. Values from the CARDIA study report sex differences between white (triangle) and black (square) participants. San and Tsimane values from August sampling, which are directly comparable to Himba values. Small-scale population values from Samson et al5 (Hadza) and Yetish et al9 (Tsimane and San). Rural and developing population values from Samson et al6 (Madagascar), Beale et al8 (Mozambique), and Knutson11 (Haiti). Industrialized population values from Carnethon et al31 (USA Chicago Area Sleep Study), Lauderdale et al32 (USA CARDIA), and Natale et al.33 (Italy).
Although mean sleep values reported here are lower than all others reported, distributions are similar to published values from Hadza foragers and from a rural population of cash-crop agriculturalists in Madagascar.5,6 However, caution must be taken in these comparisons, as the studies presented here vary by season of sampling, temperature, latitude, degree of market integration, and measurement differences like type of actigraphic device, sampling procedure, and data processing and exclusion of error or nonwear values. It is possible that many of the differences seen across populations may be the result of these sampling and methodological considerations. Of particular value, then, is the comparison of sleep patterns in the Himba population, and those reported in the San and Tsimane populations, who live at similar latitudes, were captured in the same season and had data processed and analyzed by the same researchers.9 Comparisons between San and Himba are particularly insightful, as they live approximately 550 miles apart and share a very similar ecology but show stark differences in sleep parameters. These differences lend weight to the argument that pastoralists subsistence strategies result in variation in sleep quality.
Future directions
In this study, we were able to illuminate some of the basic facets of sleep ecology among the Himba and propose explanations for the low level of sleep quantity and quality that we observed. However, this study raised several questions that could be addressed in future research. For example, we hypothesized that nighttime disturbances from animals may be influencing sleep quality, but self-reports presented here suggest that animal disturbances do not cause frequent disruption. Focused measurement of nighttime noise correlated with sleep disruptions would be useful to discern whether animal and livestock noise at night does cause sleep disturbances, or whether Himba are acclimated to nighttime noise. Napping rates were higher than those seen in other nonelectric populations, and future research can investigate demographic-, lifestyle-, and personal condition–related predictors of napping rates. We also found some evidence that sleep duration may also vary as a function of reproductive efforts. Himba culture is somewhat unique in that sexual infidelity and concurrent partnerships are common and protected through cultural norms, which result in a high frequency of extrapair partnerships and a high degree of extrapair paternity.28,44 This infidelity is manifested in “nighttime visits” by the men to their girlfriends’ huts, often sneaking in after dark and rising to leave before dawn. In this case, the men may negotiate beforehand to visit a particular woman or may simply try to sneak into a compound. Himba cultural norms dictate that when the woman is married, the visiting boyfriend should not be seen by the husband or other parties, and effort should be made to be respectful of the husband. This nighttime activity may result in significant sleep loss, particularly in men, who must travel between compounds after dark and before dawn. This pattern of behavior may have contributed to lower sleep efficiency seen in Himba men. Further studies that track sleep, sexual behavior, and nighttime mobility could address this question in more detail.
Although this study was useful in adding a pastoralist group to the suite of sleep studies in nonindustrialized societies, further studies of pastoralist sleep are needed. Given their divergent patterns of activity, propensity for nighttime vigilance, and frequent sleep disruptions, pastoralists may have lower sleep quality than small-scale societies with other modes of production. However, sleep may also vary across pastoralist populations. Environmental factors that co-vary with sleep patterns, such as temperature and light exposure, differ substantially by geography and natural environment. The importance of nighttime vigilance may depend on the prevalence of raiding and predators in that area. Other factors like mobility patterns, housing type, and sleep substrate may also vary substantially, depending on the level of sedentism and market integration. Sleep studies in other pastoralists would help to highlight the factors that are most closely associated with mode of production and which are more a product of living in rural areas with limited exposure to urban light and noise.
Conclusions
We present the first actigraphy-based sleep data on sleep in a pastoralist population, which find that this population has lower sleep duration and efficiency than other small-scale societies. Additionally, we find sex differences in sleep, with men receiving less and lower-quality sleep overall, waking earlier, and having higher after-sunset and before-sunrise activity. Likewise, older individuals tend to have higher sleep quality, likely due to labor demands shifting to younger members of the household. Although future study is necessary to understand the proximate causes of sleep disruption and sleep loss in this population, we suggest that labor demands and nighttime social activities may be driving these results, especially for younger adults.
Acknowledgments
We would like to thank all the individuals who agreed to participate in this study and the families of Omuhonga for their continued support of our work. John Jakurama translated interviews and helped conduct research. In addition, we thank David Samson and 2 anonymous reviewers for their helpful comments on previous versions of this manuscript. We would also like to acknowledge the UCLA Center for Behavior Evolution and Culture for helping to bring these co-authors together. Funding was provided by the National Science Foundation (BCS-1534682).
Footnotes
Disclosure The work described has not been published previously in any other journal, and authors have no additional information to disclose.
References
- 1.la Iglesia de HO, Fernández-Duque E, Golombek DA, et al. Access to electric light is associated with shorter sleep duration in a traditionally hunter-gatherer community. J Biol Rhythm. 2015;30(4):342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chellappa SL, Steiner R, Oelhafen P, et al. Acute exposure to evening blue-enriched light impacts on human sleep. J Sleep Res. 2013;22(5):573–580. [DOI] [PubMed] [Google Scholar]
- 3.Yetish G, Kaplan H, Gurven M. Sleep variability and nighttime activity among Tsimane forager-horticulturalists. Am J Phys Anthropol. 2018;166(3):590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samson DR, Crittenden AN, Mabulla IA, Mabulla AZP, Nunn CL. Chronotype variation drives night-time sentinel-like behaviour in hunter-gatherers. Proc Biol Sci. 2017;284(1858):20170967–20170968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samson DR, Crittenden AN, Mabulla IA, Mabulla AZP, Nunn CL. Hadza sleep biology: evidence for flexible sleep-wake patterns in hunter-gatherers. Am J Phys Anthropol. 2017;162(3):573–582. [DOI] [PubMed] [Google Scholar]
- 6.Samson DR, Manus MB, Krystal AD, Fakir E, Yu JJ, Nunn CL. Segmented sleep in a nonelectric, small-scale agricultural society in Madagascar. Am J Hum Biol. 2017; 39:e22979. [DOI] [PubMed] [Google Scholar]
- 7.Samson DR, Crittenden AN, Mabulla IA, Mabulla AZP. The evolution of human sleep: technological and cultural innovation associated with sleep-wake regulation among Hadza hunter-gatherers. J Hum Evol. 2017;113:91–102. [DOI] [PubMed] [Google Scholar]
- 8.Beale AD, Pedrazzoli M, da Silva B Goncalves B, et al. Comparison between an African town and a neighbouring village shows delayed, but not decreased, sleep during the early stages of urbanisation. Sci Rep. 2017:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yetish G, Kaplan H, Gurven M, et al. Natural sleep and its seasonal variations in three pre-industrial societies. Curr Biol. 2015;25(21):2862–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samson DR, Yetish GM, Crittenden AN, Mabulla IA, Mabulla AZP, Nunn CL. What is segmented sleep? Actigraphy field validation for daytime sleep and nighttime wake. Sleep Health. 2016;2(4):341–347. [DOI] [PubMed] [Google Scholar]
- 11.Knutson KL. Sleep duration, quality, and timing and their associations with age in a community without electricity in haiti. Am J Hum Biol. 2013;26(1):80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rota A, Sperandini S. Livestock and Pastoralism. IFAD Livestock Thematic Papers; 2009.
- 13.Fratkin E. East African pastoralism in transition: Maasai, Boran, and Rendille cases. Afr Stud Rev. 2001;44(3):1. [Google Scholar]
- 14.Fratkin E, Roth E, Nathan M. When nomads settle: the effects of commoditization, nutritional change, and formal education on Ariaal and Rendille pastoralists. Curr Anthropol. 1999;40(5):729–735. [PubMed] [Google Scholar]
- 15.Butt B. Seasonal space-time dynamics of cattle behavior and mobility among Maasai pastoralists in semi-arid Kenya. J Arid Environ. 2010;74(3):403–413. [Google Scholar]
- 16.Adriansen HK. Understanding pastoral mobility: the case of Senegalese Fulani. Geogr J. 2008;174(3):207–222. [Google Scholar]
- 17.McCabe JT. Drought and recovery: livestock dynamics among the Ngisonyoka Turkana of Kenya. Hum Ecol. 1987;15(4):371–389. [Google Scholar]
- 18.Pike IL. The biosocial consequences of life on the run: a case study from Turkana District, Kenya. Hum Organ. 2004;63(2):221–235. [Google Scholar]
- 19.Worthman CM, Melby MK. In: Carskadon MA, editor. Toward a Comparative Developmental Ecology of Human Sleep. Cambridge: Cambridge University Press; 2009. p. 69–117. [Google Scholar]
- 20.Akpa GN, Alphonsus C, Abdulkareem A. Evaluation of herd structure of white Fulani cattle holdings in Zaria, Nigeria. Sci Res Essays. 2012;7(42):3605–3608. [Google Scholar]
- 21.Bollig M. Ethnic conflicts in North-West Kenya: Pokot-Turkana Raiding 1969–1984. Anthropos. 1990;115:73–90. [Google Scholar]
- 22.Gray S, Sundal M, Wiebusch B, Little MA, Leslie PW, Pike IL. Cattle raiding, cultural survival, and adaptability of East African pastoralists. Curr Anthropol. 2003;44(S5): S3–S30. [Google Scholar]
- 23.Wiessner PW. Embers of society: firelight talk among the Ju/’hoansi bushmen. Proc Natl Acad Sci U S A. 2014;111(39):14027–14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Århem K. Maasai food symbolism: the cultural connotations of milk, meat, and blood in the pastoral Maasai diet. Anthropos. 1989;84:1–23. [Google Scholar]
- 25.Bollig M. Risk Management in a Hazardous Environment., Vol. 2 Boston, MA: Springer US; 2006. [Google Scholar]
- 26.Malan JS. Peoples of Namibia. Wingate Park, Pretoria: Rhino Publishers; 1995. [Google Scholar]
- 27.Scelza BA. Perceptions of polygyny: the effects of offspring and other kin on co-wife satisfaction. Biodemography Soc Biol. 2015;61(1):98–110. [DOI] [PubMed] [Google Scholar]
- 28.Scelza BA, Prall SP. Partner preferences in the context of concurrency: what Himba want in formal and informal partners. Evol Hum Behav. 2018;39(2):212–219. [Google Scholar]
- 29.Scelza BA. Female mobility and postmarital kin access in a patrilocal society. Hum Nat. 2011;22(4):377–393. [DOI] [PubMed] [Google Scholar]
- 30.de Souza L, Benedito-Silva AA, Pires MLN, Poyares D, Tufik S, Calil HM. Further validation of actigraphy for sleep studies. Sleep. 2003;26(1):81–85. [DOI] [PubMed] [Google Scholar]
- 31.Carnethon MR, De Chavez PJ, Zee PC, et al. Disparities in sleep characteristics by race/ethnicity in a population-based sample: Chicago Area Sleep Study. Sleep Med. 2016;18(C):50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lauderdale DS, Knutson KL, Yan LL, et al. Objectively measured sleep characteristics among early-middle-aged adults. Am J Epidemiol. 2006;164(1):5–16. [DOI] [PubMed] [Google Scholar]
- 33.Natale V, Plazzi G, Martoni M. Actigraphy in the assessment of insomnia: a quantitative approach. Sleep. 2009;32(6):767–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lambiase MJ, Gabriel KP, Chang Y-F, Kuller LH, Matthews KA. Utility of Actiwatch sleep monitor to assess waking movement behavior in older women. Med Sci Sports Exerc. 2014;46(12):2301–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15(5):461–469. [DOI] [PubMed] [Google Scholar]
- 36.Morey RD, Rouder JN. BayesFactor: computation of Bayes factors for common designs. http://CRAN.R-project.org/package=BayesFactor;2015. [Google Scholar]
- 37.Jarosz AF, Wiley J. What are the odds? A practical guide to computing and reporting bayes Factors. J Probl Solving. 2014;7(1):2–9. [Google Scholar]
- 38.Stan Development Team. RStan: the R interface to Stan.mc-stan.org;2017.
- 39.McElreath R. rethinking: Statistical Rethinking Book Package; 2017.
- 40.Tonetti L, Fabbri M, Natale V. Sex difference in sleep-time preference and sleep need: a cross-sectional survey among Italian pre-adolescents, adolescents, and adults. Chronobiol Int. 2009;25(5):745–759. [DOI] [PubMed] [Google Scholar]
- 41.Adan A, Natale V. Gender differences in morningness-evenness preference. Chronobiol Int. 2002;19(4):709–720. [DOI] [PubMed] [Google Scholar]
- 42.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. [DOI] [PubMed] [Google Scholar]
- 43.Mosko S, Richard C, McKenna J. Maternal sleep and arousals during bedsharing with infants. Sleep. 1997;20(2):142–150. [DOI] [PubMed] [Google Scholar]
- 44.Scelza BA. Female choice and extra-pair paternity in a traditional human population. Biol Lett. 2011;7(6):889–891. [DOI] [PMC free article] [PubMed] [Google Scholar]


