Abstract
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease that has become a leading cause of death in recent years. Impairments in spatial learning and memory are an important clinical feature of AD. Melatonin (MLT), the main product secreted by the pineal gland, showed multiple antioxidant, anti-inflammatory, and neuroprotective properties.
Purpose
The present study aimed to explore the possible prophylactic effects of MLT against spatial memory deficits in a sporadic mouse model of AD induced by D-galactose and aluminium chloride (AlCl3).
Methods
Four groups of mice (n = 10 per group) were prepared: control, AD (the D-galactose and AlCl3 AD model group), AD+MLT (AD mice treated with 80 mg/kg MLT), and AD+DON (AD mice treated with 3 mg/kg donepezil). We then used the object location and Y-maze tests to assess spatial memory in the four groups. Gene expression levels of brain-derived neurotrophic factor (Bdnf) and cAMP-responsive element-binding protein (Creb1) were measured using real-time polymerase chain reaction.
Results
We found that MLT improved spatial memory in the sporadic AD mice. MLT ameliorated Creb1 gene expression and significantly increased Bdnf gene expression in the hippocampus of AD model mice compared with the AD group.
Conclusion
MLT could have a substantial potential to alleviate memory impairment in sporadic AD if introduced at early stages.
Keywords: melatonin, Alzheimer’s disease, spatial memory, CREB, BDNF
Introduction
Alzheimer’s disease (AD) is an irreversible, progressive neurodegenerative disorder that is characterized by cognitive decline, memory loss, and behavioural abnormalities.1 Impairments in spatial learning and memory are an important clinical feature of this disease.2 The accumulation of extracellular β-amyloid (Aβ) plaques and neurofibrillary tangles in the brain are a major pathological feature of AD.3 Moreover, oxidative stress, neuroinflammation, and cholinergic and mitochondrial dysfunction play crucial roles in the pathophysiology of AD.3–7
Melatonin (MLT; N-acetyl-5-methoxytryptamine) is the main product secreted by the pineal gland.8 It is involved in many physiological functions including the control of circadian rhythms and the regulation of body temperature.9–11 Many studies have reported that MLT has strong antioxidant and anti-inflammatory effects.12–14 MLT stimulates certain antioxidant enzymes, including superoxide dismutase 1 and glutathione peroxidase, in the brains of AD mice.15 Moreover, MLT can protect these antioxidant enzymes from oxidative stress.16 Chronic MLT administration can enhance synaptogenesis and synaptic function, and can also preserve glial cell structure in the AD rat brain.17,18 In addition, MLT efficiently prevents the formation and accumulation of Aβ and neurofibrillary tangles in the brains of AD model animals.19,20 MLT also improves performance in novel object recognition and passive avoidance tasks in an adult ICR AD mouse model,21 and spatial memory assessed by Morris water maze and Y-maze tasks in young and aged AD model mice.10,22–24 Furthermore, MLT metabolites improve long‐term recognition task performance in young, middle aged and old MLT‐deficient mice.25 Protective effects of MLT against learning and memory deficits have also been observed in several other animal studies such as vascular dementia and tau hyperphosphorylation animal models induced by wortmannin, isoproterenol, and calyculin A.26,27
Reduction of brain-derived neurotrophic factor (BDNF) has a crucial role in learning, memory, and synaptic plasticity.28 It is expressed mostly in the hippocampus as well as in the cortex and stimulates long-term potentiation of hippocampal synapses and enhance spatial memory.28,29 Reduction of BDNF expression has been associated with AD pathogenesis and is reduced in early stages of AD.30,31 Another study found that BDNF expression level is decreased in the hippocampus and cortex of AD patients.32 In animal studies, the expression of BDNF was measured in two transgenic AD mouse models and indicated that mRNA is considerably downregulated in cortical tissue when compared to the control.33 In other studies, the levels of BDNF were significantly reduced in the hippocampus of Aβ-induced AD model and was considerably decreased in the scopolamine-induced AD model when compared to the control.23,34
Cyclic AMP response element binding protein (CREB) is a nuclear binding protein that binds to specific DNA sequences, act as a transcription factor for several genes including BDNF, and has an important role in the memory formation and retention.35,36,38 In addition, CREB is a component of intracellular signalling that control the circadian rhythms of long-term memory effectors in the hippocampus.36 Previous studies showed that the CREB level is reduced with aging as well as in AD.36,37 Hence, it is suggested that a correlation exists between the role of BDNF gene expression and its regulation via CREB in reversing cognitive impairments.38 It has been reported that Aβ and scopolamine induced neurotoxicity down regulates BDNF and its main regulatory molecule CREB in the cortex and hippocampus of AD model.23,39
Mouse models of AD induced by D-galactose and/or aluminium chloride (AlCl3) have been used extensively to study the mechanisms of AD and to investigate the effects of drugs.10,40,41 D-galactose, a widely used to induce brain damage in rodents.42 It is a reducing sugar that is naturally present in the body in small amounts, however when present in excess quantities, D-galactose lead to increased production of reactive oxygen species and decreased levels of antioxidant enzymes.43 Other mechanisms have been reported in D-galactose-induced aging in the animal brain, including inflammation, apoptosis, and mammalian target of rapamycin upregulation.42 AlCl3 is a neurotoxic agent that causes oxidative damage and enhances inflammatory responses in the rodent brain.41 Chronic administration of AlCl3 promotes the overproduction of extracellular Aβ plaques and intracellular neurofibrillary tangles formation.44 AlCl3 toxicity disrupts calcium homeostasis, thereby inducing neurodegeneration.45 The co-administration of D-galactose and AlCl3 has been used to induce a sporadic AD animal model that shows several pathological features of AD.40,46 In fact, a 10-week co-administration of AlCl3 and D-galactose produced memory impairment, cholinergic system defect, and brain pathology.40
No previous studies have investigated the effects of MLT treatment on the BDNF/CREB pathway in the sporadic AD model induced by D-galactose/AlCl3. Therefore, the purpose of this study was to investigate whether treatment with MLT can ameliorate spatial memory impairments induced by D-galactose and AlCl3 compared to Donepezil, a brain-selective acetylcholine esterase inhibitor, which had shown to improve learning and memory tasks in Alzheimer animal models.
Materials and Methods
Animals
Forty adult (4–8 weeks old) male Swiss mice (SWR/J) weighing 18–22 g were obtained from the animal facilities of King Fahd Medical Research Center, King Abdulaziz University, Jeddah. The mice were kept in the experimental area for 2 weeks (4–6 weeks old) for habituation phase. The experiment started by administration of prophylactic doses of MLT at age of 6–8 weeks old for two weeks followed by AD induction at age of 8–10 weeks old for 8 weeks. The mice were housed at five mice per cage at an appropriate temperature (22 ± 2 °C) and humidity (60% ± 10%), with a standard 12-hour light/dark cycle and free access to water and standard food. The mice were not mixed between procedures and all experiments were performed with one batch of mice. All mice were cared for following the guidelines of the Animal Care and Use Committee at King Fahd Medical Research Center All experiments were performed according to the guidelines of the biomedical ethics research committee (reference no. 102-19) of King Abdulaziz University, which comply with the guidelines of the “System of ethics of research on living creatures”, prepared by the King Abdulaziz City for Science and Technology, approved by Royal Decree No. M/59 on 24 August 2010.
Treatment Preparation
D-galactose extra pure powder (Loba Chemie Pvt Ltd, Mumbai, India) was dissolved in normal saline. AlCl3 (Techno PharmChem, Haryana, India) was dissolved in distilled water. D-galactose and AlCl3 were freshly prepared twice per week and stored at 4 °C. MLT crystalline powder (M5250; Sigma-Aldrich, St Louis, MO, USA) was dissolved in 20% ethanol and then diluted with saline; the final ethanol concentration was 1%. MLT was freshly prepared before administration in dark bottles to avoid oxidation of the solution by light. Donepezil hydrochloride, tablets (5 mg) were purchased from a local pharmacy, ground to a fine powder then dissolved in distilled water. All treatments were given to mice in volumes of 0.2 mL. Doses were adjusted weekly according to body weight.
Treatment Groups
Forty mice were randomly divided into the following four groups (n = 10 per group): control (treated with the drug vehicles; mice received saline subcutaneously, distilled water orally, and 1% ethanol in saline intraperitoneally (IP), AD (the AD model; mice received 120 mg/kg D-galactose subcutaneously, 20 mg/kg AlCl3 orally, and 1% ethanol in saline IP), AD+MLT (the MLT treatment group; mice received 120 mg/kg D-galactose subcutaneously, 20 mg/kg AlCl3 orally, and 80 mg/kg MLT IP), and AD+DON (a positive control group; mice received 120 mg/kg D-galactose subcutaneously, 20 mg/kg AlCl3 and 3 mg/kg donepezil orally, and 1% ethanol in saline IP). We used the highest prophylactic dose of MLT (80 mg/kg), which does not produce any signs of toxicity in mice.14,18,47
Experimental Design
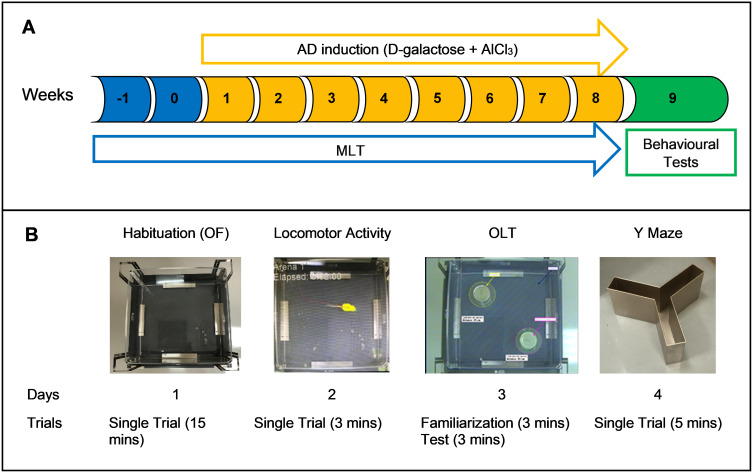
The drug administration regimen lasted for 10 weeks. MLT administration started on week –1, whereas D-galactose, AlCl3, and donepezil administration was started on week 1. Each treatment was given once per day. The animals received MLT doses from 8:00 to 9:00 a.m. and received D-galactose and AlCl3 doses from 10:00 to 12:00 p.m. The behavioural tests were performed during week 9 (Figure 1).
Figure 1.
(A) Timeline of the experiment. (B) Sequence of the behavioural tests.
Abbreviations:AD, Alzheimer’s disease; AlCl3, aluminium chloride; MLT, melatonin; OF, open field; OLT, object location test.
Open Field Test
To assess the locomotor activity of mice, the OF test was used to measure velocity and total distance moved (TDM). The locomotor activity of mice was monitored using an EthoVision XT8A system (Noldus Information Technology, Wageningen, Netherlands). Mice were placed individually in the arena, which was a transparent square box (45 × 45 cm) and allowed to move freely for 3 minutes.48 The EthoVision XT8A system was used to calculate velocity and TDM.
Object Location Test (OLT)
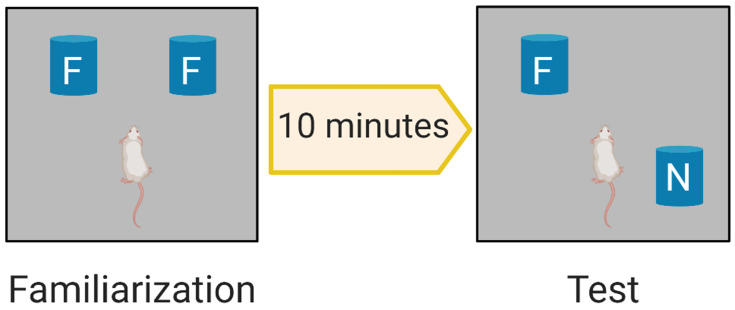
The OLT is a widely used behavioural test for assessing spatial memory deficits in mice.49 Each mouse was habituated for 15 minutes the day before the test. The aim of habituation was to introduce the mice to the arena (without objects) to minimize confounding anxiety and novelty factors, which can negatively affect interaction with and discrimination of objects. On the day of the OLT, we first conducted a familiarisation trial (Figure 2), in which two identical objects (eg, two metal cylinders) were placed in a particular location within the arena and the animals were allowed to explore these two objects for 3 minutes (Supplementary Figure S1). The zones are defined as 1 inch around each object to ensure detection of exploration behaviour around each object. The objects used in this experiment were selected according to particular properties (ie, cleanable and not easily moved).50 After 10 minutes, we conducted the test trial, in which the location of one of the familiar objects was changed (Figure 2) and object exploration was recorded for 3 minutes (Supplementary Figure S2). Parameters were recorded and analysed using a video tracking system (EthoVision XT8A). The frequency of bringing the nose close to both objects (sniffing) was assessed in both the familiarisation and test trials. Mouse memory was also evaluated by the time spent in the familiar and novel zones during the test trial. The discrimination index (DI) was calculated according to the following equation: DI = (TN–TF)/(TN+TF) where TN is the time spent in the novel (displaced object in the new location) zone and TF is the time spent in the familiar zone.48
Figure 2.
OLT Phases. Familiarization phase allows the rodent to explore two identical familiar objects F. The test phase evaluates memory by investigating the ability of the rodent to discriminate the displaced new location; N object from the previously explored F object.
Spontaneous Alternation Test (Y-Maze)
Spontaneous alternation is another test of spatial memory and was performed using a Y-maze with symmetrical arms. Each arm of the maze was 60 cm long, 10 cm wide, and 15 cm high.51 This test consisted of only one 5-minute trial and was conducted in a sound-insulated room. The mice were individually placed at the end of one arm and allowed to explore all arms of the maze freely. Between each trial, the maze arms were wiped with 70% alcohol to avoid odour cues.52 The maze arms were labelled A, B, and C. The spontaneous alternation score was defined as sequential entry into the three different arms (ie, ABC, CBA, or BAC). Re-entry into the same arm such as ABA or ACA was considered an entry error. The spontaneous alternation percentage (SAP) was calculated by SAP = number of alternations/(total arm entries – 2) × 100.51
Brain Tissue Collection
After behavioural testing, mice were anaesthetized with isoflurane and euthanized by decapitation following the rules and regulations of King Fahd Medical Research Center. Whole brains were carefully removed and washed in saline. The hippocampus of each brain was dissected, and the left hippocampus was submerged in RNAlater solution (Invitrogen) to be used for gene expression analysis.
Gene Expression
Expression levels of the genes encoding cAMP-responsive element-binding protein (Creb1) and brain-derived neurotrophic factor (Bdnf) in the hippocampus of mice were measured using real-time polymerase chain reaction (PCR) analysis.
RNA Extraction and Real-Time PCR Analysis
Total pure RNA was extracted from hippocampal tissue using a PureLink™ RNA Mini Kit (Ambion, Austin, TX, USA) according to the manufacturer’s protocol. Complementary DNA synthesis was performed using a SuperScript™ IV VILO™ Master Mix Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Real-time PCR was performed using SsoAdvanced™ Universal SYBR® Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s protocol. Glyceraldehyde 3-phosphate dehydrogenase gene (Gapdh) was used as a housekeeping gene. Details of the primers used are shown in Table 1.
Table 1.
Sequences of PCR Primers
| Gene | Accession Number | Oligo | Sequence | Amplicon Length |
|---|---|---|---|---|
| Gapdh | NM_001289726.1 | Forward primer | 5′-GTGAACGGATTTGGCCGTATT-3′ | 70 |
| Reverse primer | 5′-CAATCTCCACTTTGCCACTGC-3′ | |||
| Creb1 | NM_001037726.1 | Forward primer | 5′-ACCCACGAGCACCATTGC-3′ | 120 |
| Reverse primer | 5′-TGCCTCCCTGTTCTTCATTAG-3′A | |||
| Bdnf | NM_001048139.1 | Forward primer | 5′-AAGGGCCAGGTCTGTTAATCG-3′ | 70 |
| Reverse primer | 5′-ATGGCTCTATGAAACTGTTCTGGT-3′ |
Abbreviations: Bdnf, brain-derived neurotrophic factor; Creb1, cAMP-responsive element-binding protein; Gapdh, glyceraldehyde 3-phosphate dehydrogenase.
Statistical Analysis
All data are presented as the mean ± SEM. The D’Agostino-Pearson normality test and Q-Q plots were used to test the normality assumption of parametric statistics. One-way analysis of variance (ANOVA) followed by post hoc Tukey’s test was used to analyse locomotor activity, DI, Y-maze, and gene expression data. In addition, we used two-way ANOVA followed by post hoc Bonferroni multiple comparison test to compare differences within groups of percentages of mice sniffing and exploration time spent in familiar and novel objects during the familiarization and test phases of the experiment. Differences between groups were considered statistically significant if P was less than 0.05. GraphPad Prism 8.3.8 was used for the statistical analysis.
Results
Effects of MLT on Locomotor Activity
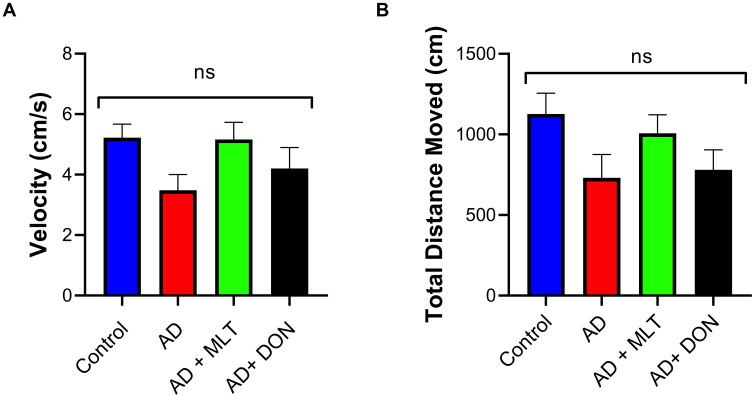
There was no significant difference in velocity between study groups [F (3, 36) = 2.19, P = 0.106; Figure 3A]. Moreover, there was no significant difference in TDM among groups [F (3, 36) = 2.12, P = 0.114; Figure 3B].
Figure 3.
Locomotor activity. Data are presented as the mean ± SEM and one-way ANOVA was used to compare differences between groups (P ˃ 0.05). (A) There were no significant differences in mouse velocity among any of the treatment groups. (B) There were no significant differences in the TDM among groups. N=10. Non-significant (ns).
Effects of MLT on the OLT
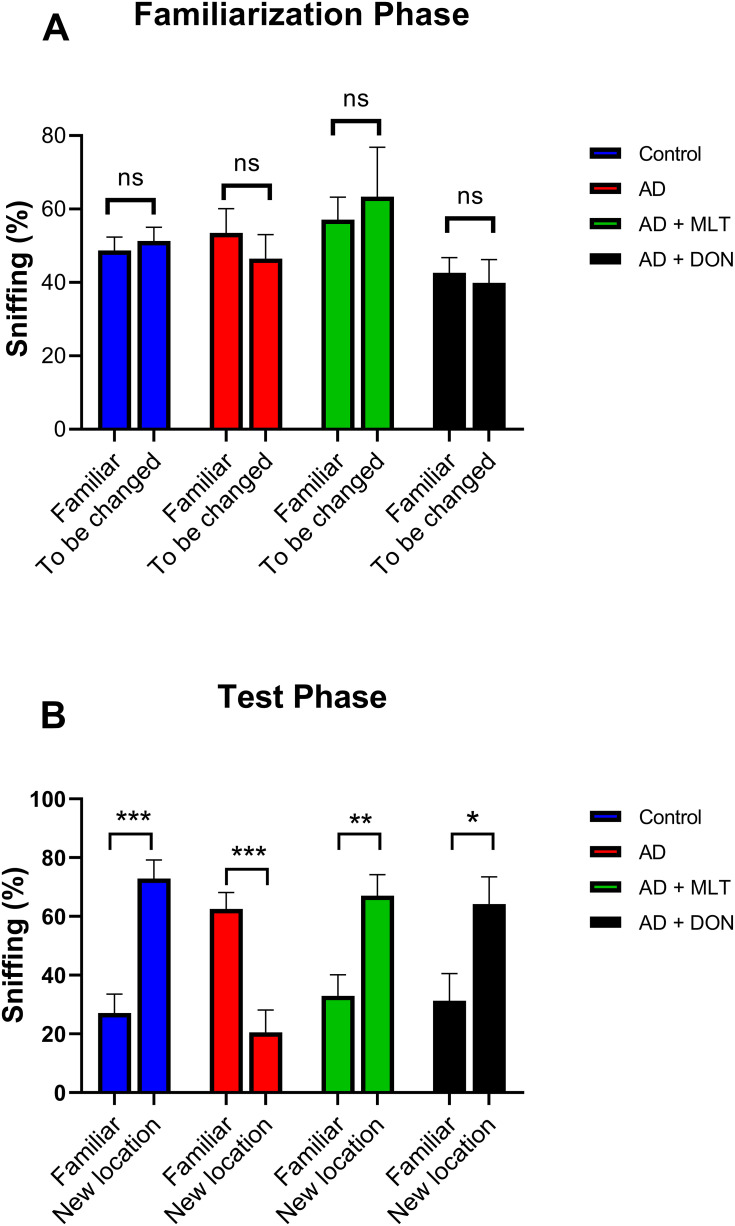
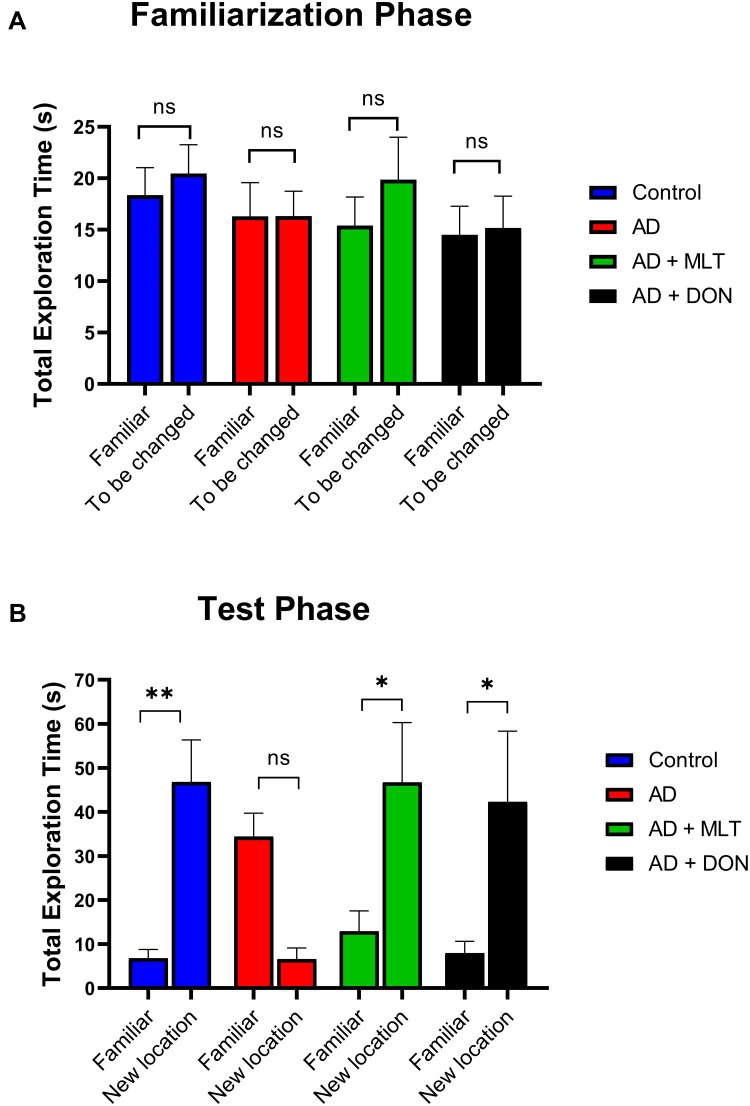
There was no significant difference between the percentage of sniffs of each object (familiar vs to be changed) in all groups in the familiarisation phase [F (1, 72) = 0.002, P = 0.963; Figure 4A]. However, in the test phase, the percentage of sniffs of the familiar object was significantly different from that of the novel object in all groups [F (1, 72) = 11.27, P = 0.001; Figure 4B]. For instance, there was a significantly higher percentage of sniffs of the novel object than of the familiar object in the control group (P = 0.0002). In contrast, in the AD group, the percentage of sniffs was significantly lower for the novel object than for the familiar object (P = 0.0006). The AD+MLT and AD+DON groups both had a significantly higher percentage of sniffs of the novel object than of the familiar object (P = 0.007 and P = 0.010, respectively).
Figure 4.
Percentages of sniffs in both phases of the object location test. Data are presented as the mean ± SEM. Two-way ANOVA was used, followed by the Bonferroni multiple comparisons test. (A) There were no significant differences among the groups in the percentages of sniffs of each object (familiar vs to be changed) in the familiarisation phase. (B) There were significant differences among groups in the percentages of sniffs of each object (familiar vs novel) in the test phase. N=10. Non-significant (ns). *P < 0.05, **P < 0.01, ***P < 0.001.
There was no significant difference between the total exploration time of each object zone (familiar vs to be changed) in all groups in the familiarisation phase [F (1, 72) = 0.7168, P = 0.400; Figure 5A]. However, in the test phase, the time spent in the familiar object zone was significantly different from that of the novel object zone in all groups [F (1, 72) = 10.75, P=0.001; Figure 5B]. For instance, there was a significantly higher exploration time to the novel object than of the familiar object in the control group (P = 0.006). However, in the AD group, the total exploration time was not significantly different between objects (P = 0.101). The AD+MLT and AD+DON groups both had a significantly higher exploration time in the novel object zone than of the familiar object (P = 0.029 and P = 0.025, respectively).
Figure 5.
Total exploration time in both phases of the object location test. Data are presented as the mean ± SEM. Two-way ANOVA was used, followed by the Bonferroni multiple comparisons test. (A) There were no significant differences among the groups in the total exploration time spent in each object zone (familiar vs to be changed) in the familiarisation phase. (B) There were significant differences among groups in the total exploration time spent in each object zone (familiar vs novel) in the test phase. N=10. Non-significant (ns). *P < 0.05, **P < 0.01.
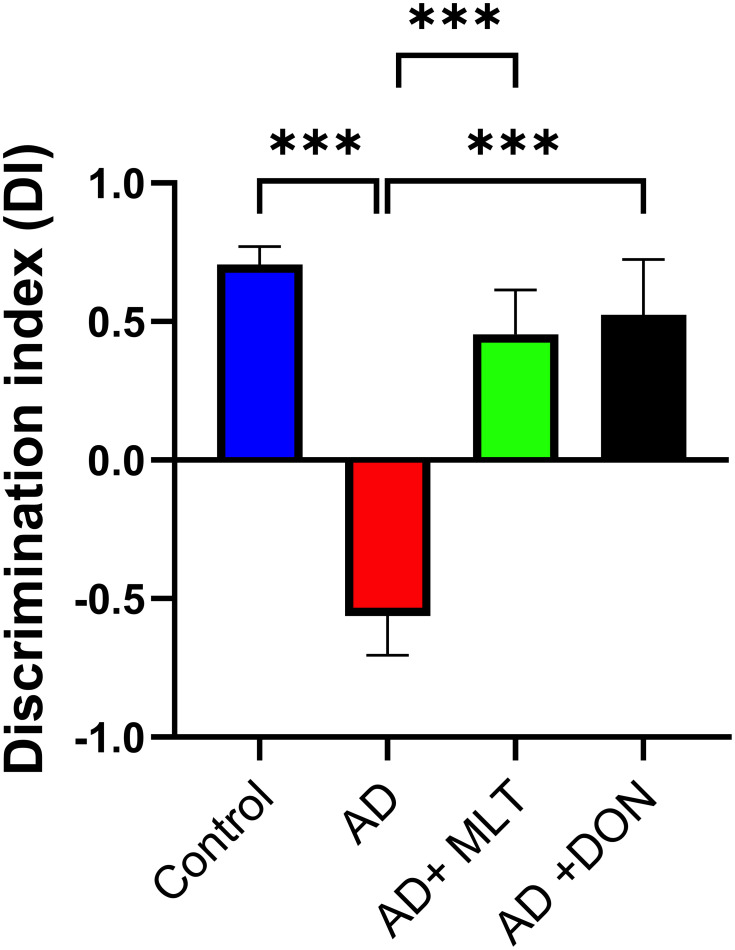
In the OLT, the DIs for all groups were significantly greater than the chance level of zero [control: t(9) = 11.05, P < 0.0001; AD: t(9) = 3.96 P = 0.003; AD+MLT: t(9) = 2.83, P = 0.019; AD+DON t(9) =2.62, P = 0.027]. Also, there were statistically significant differences in the DI between study groups [F (3, 36) = 14.54, P < 0.0001; Figure 6]. For instance, the post hoc analysis showed that the control (P < 0.0001), AD+MLT (P = 0.0002) and AD+DON (P < 0.0001) groups had significantly higher DIs than the AD group. However, there was no statistically significant difference between the control and AD+MLT or AD+DON groups (P = 0.636 and P = 0.827, respectively).
Figure 6.
Discrimination index in the object location test. Data are presented as the mean ± SEM. One-way ANOVA was used, followed by Tukey’s multiple comparisons test. The DI was significantly lower in the AD group vs the control group. DIs were significantly higher in both the AD+MLT and AD+DON groups vs the AD group. N=10. ***P < 0.001.
Effects of MLT on the Y-Maze Test
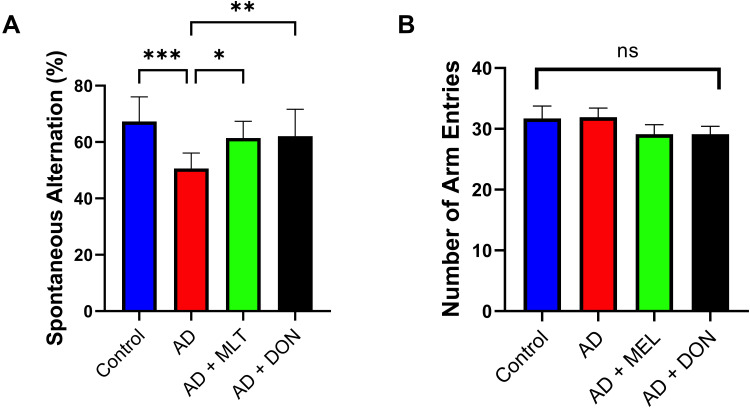
The SAP results of all study groups are shown in Figure 7A. There were statistically significant differences between the study groups [F (3, 36) = 8.427, P = 0.0002]. For instance, there was a lower SAP in the AD group compared with the control group (P = 0.0001). In contrast, the SAPs in the AD+MLT and AD+DON groups were not significantly different compared with the control group (P = 0.328 and P = 0.432, respectively). The SAPs in the AD+MLT and AD+DON groups were significantly higher than that of the AD group (P = 0.015 and P = 0.009, respectively). Additionally, there was no significant difference in the number of arm entries between groups [F (3, 36) = 0.91, P = 0.447; Figure 7B].
Figure 7.
Spontaneous alternation and number of arm entries in the Y-maze task. Data are presented as the mean ± SEM. One-way ANOVA was used, followed by Tukey’s multiple comparisons test. (A) SAP was significantly lower in the AD group vs the control group. SAPs were significantly higher in the AD+MLT and AD+DON groups vs the AD group. (B) There were no significant differences in the number of arm entries among the groups. N=10. Non-significant (ns). *P < 0.05, **P < 0.01, ***P < 0.001.
Effects of MLT on Bdnf and Creb1 Expression in the Hippocampus of AD Mice
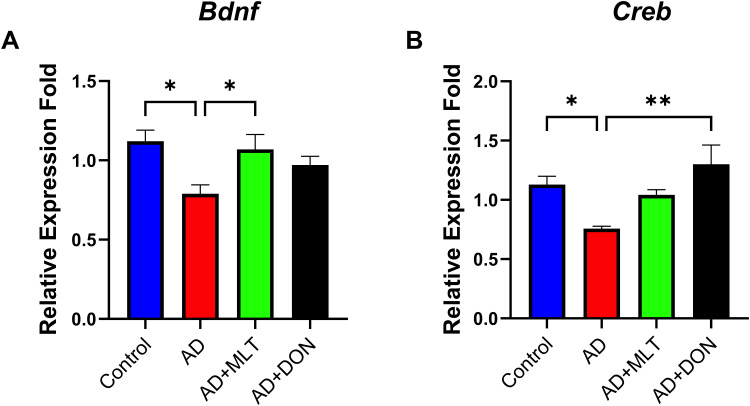
There were statistically significant differences in Bdnf expression among groups [F (3, 35) = 4.221, P = 0.014; Figure 8A]. For instance, Tukey’s post hoc analysis showed significantly lower Bdnf expression in the AD group (M = 0.78, SEM = 0.05) compared with the control group (M =1.12, SEM= 0.07, P =0.013). In addition, Bdnf expression was significantly higher in the AD+MLT group (M = 1.07, SEM = 0.09) compared with the AD group (P = 0.045). Furthermore, there was no statistically significant difference between the control group and AD+MLT and AD+DON groups (P = 0.958 and P = 0.447, respectively). There were significant differences in Creb1 expression among groups [F (3, 28) = 4.40, P = 0.003; Figure 8B]. Post hoc analysis showed a significant reduction in Creb1 expression in the AD group (M = 0.76, SEM = 0.02) compared with the control group (M = 1.13, SEM = 0.07; P = 0.041). There was also a significant increase in Creb1 expression between AD and AD+DON groups (M =1.3, SEM=0.16, P = 0.008). However, there was only a marginal increase in the expression of Creb1 in the AD+MLT group (M = 1.043, SEM = 0.04) compared with the AD group.
Figure 8.
Expression of Bdnf and Creb1 in the hippocampus. Data are presented as the mean ± SEM. One-way ANOVA was used, followed by Tukey’s multiple comparisons test. (A) Bdnf expression was significantly lower in the AD group vs the control group. Bdnf expression was significantly higher in the AD+MLT group vs the AD group. (B) Creb1 expression was significantly lower in the AD group vs the control group. Creb1 expression was significantly higher in the AD+DON group vs the AD group. N=10. *P < 0.05, **P < 0.01.
Discussion
In the present study, we aimed to investigate the possible prophylactic effects of MLT against memory deficits in the D-galactose/AlCl3-induced sporadic AD mouse model, focusing on the impacts of MLT on spatial memory.
Several animal models of AD have been used to investigate the effectiveness of different treatments targeting AD.53,54 These models vary in their cognitive and behavioural performances, and these differences in exploratory, activity, and anxiety behaviours are reflected in several learning and memory tests.55,56 It has been noted that the D-galactose/AlCl3-induced mouse model mimics the cognitive deficits and pathological alterations of clinical AD.57,58 Although many studies have been performed using different sporadic AD models, cognitive and behavioural data from the D-galactose/AlCl3 mouse model are limited.54 In the present study, we therefore used a combined administration of D-galactose and AlCl3 to induce an AD mouse model. We first assessed the motor activity of mice to ensure that there were no motor limitations that would affect memory test outcomes.59 The open field test demonstrated that there were no significant differences in motor activity between the AD group and any other group. This is consistent with previous studies that also reported no significant differences in motor activity between the D-galactose/AlCl3 AD model and control groups.57,60 Based on this finding, any learning and memory improvements caused by the treatments in this study are likely to be mnemonic, rather than sensorimotor.
In many AD models, spatial memory is severely affected because of neuronal damage, especially in hippocampal areas.61–63 Consistent with previous studies, our results revealed that spatial memory was affected in D-galactose/AlCl3 AD mice.51,65 We assessed spatial memory using the OLT and the Y-maze place recognition tasks. A decline in spatial memory is associated with disrupted hippocampus-dependent functions, and these features are a primary characteristic of the cognitive impairments that are observed in AD patients.64
Several studies have indicated that MLT prevents the progression of AD and improves the cognitive impairment associated with the disease via several mechanisms.10,26,27 For these effects, MLT doses need to be approximately twice those required to influence sleep and circadian rhythmicity.19 Clinical studies that use MLT in the range of 50–100 mg/day are needed to investigate the therapeutic validity of MLT treatment in AD.19 Interestingly, no previous studies have used high doses of MLT (eg, 80 mg/kg) to ameliorate memory decline in AD animal models.47 In addition, no previous studies have investigated the potential prophylactic effects of MLT on an AD model induced by the co-administration of D-galactose and AlCl3. In our study, we thus used the highest prophylactic dose of MLT (80 mg/kg), which does not produce any signs of toxicity in mice.14,18,66 This high dose of MLT is found to be a potent antioxidant and anti-inflammatory which improved memory outcomes and locomotor activity in multiple sclerosis animal model.14,18 Moreover, this high dose of MLT altered the T effector/regulatory balance which therefore protect against experimental autoimmune encephalomyelitis animal model.66
In the current study, spatial memory-dependent tasks (the OLT and Y-maze) revealed an improvement in spatial memory performance by MLT-treated mice compared with the AD model mice. In the OLT, we measured the DI, exploring time, and sniff frequency for each group. By calculating the frequency that each object was sniffed and the exploration time for each object we found that each group took similar chances to explore both objects in the familiarisation phase. However, there were significant differences in the frequency of sniffing and the exploration time between the familiarisation and test phases among the groups. Our results indicate that MLT and DON treatment improved the spatial memory outcomes measured by the OLT and Y-maze tasks, compared with the AD group, which is consistent with previous studies.10,23,24
BDNF is a crucial molecule involved in synaptic plasticity, learning and memory, and neurogenesis.28 BDNF signalling can stimulate long-term potentiation of hippocampal synapses and enhance spatial memory.28 Reduction in BDNF expression levels have been reported in AD, especially in brain areas important for learning and memory, such as the hippocampus.30 BDNF levels are also reduced in the early stages of AD.31 In our study, Bdnf expression was significantly depleted in the hippocampus of the AD model, as has been previously reported.31,36,66 However, MLT increased Bdnf expression levels and DON ameliorated Bdnf expression levels in the AD model. This finding is consistent with those of previous studies, which indicated that MLT treatment can reverse the decreases in BDNF protein levels in the hippocampus of AD models.24,67,68 This beneficial effect of MLT in reversing BDNF level in AD hippocampus is reflected by improving the spatial memory in AD model in our study.
CREB is a crucial nuclear transcription factor that plays a key role in BDNF transcription.35 A reduction in CREB phosphorylation leads to the downregulation of BDNF levels, which ultimately results in working memory dysfunction.69 In addition, CREB is a component of the intracellular signalling that controls the circadian rhythms of long-term memory effectors in the hippocampus.36 However, CREB expression levels decrease with age as well as in some neurodegenerative diseases such as AD.36,37 In the present study, Creb1 expression levels were markedly reduced in the hippocampus of D-galactose/AlCl3 AD model mice. This finding is consistent with the results of previous studies, which reported that CREB expression levels are significantly reduced in the hippocampus of AD animal models.17,35,70 In addition, DON increases Creb1 expression levels and MLT ameliorated the reduction in Creb1 expression in the present study, similar to the results of previous reports.71–73
Conclusions
To the best of our knowledge, no previous studies have investigated the effects of MLT treatment on the BDNF/CREB pathway in the D-galactose/AlCl3 AD model. Our results indicate that MLT reversed the spatial memory decline in a sporadic D-galactose/AlCl3 AD model. This could be due to its role in improving Bdnf and Creb1 gene expression in the AD mouse hippocampus. Therefore, MLT could have a substantial potential to alleviate memory impairment in AD if introduced at early stages. However, further biochemical, genetic, and immunohistochemical analyses are needed to provide more information about the mechanisms underlying the effects of MLT in a sporadic AD model.
Acknowledgment
We thank Amal Binsalman for her assistance with gene selection.
Funding Statement
King Abdulaziz University, Jeddah, Saudi Arabia.
Abbreviations
AD, Alzheimer’s disease; AlCl3, aluminium chloride; MLT, melatonin; OLT, object location test; TDM, total distance moved; DI, discrimination index; SAP, spontaneous alternation percentage; DON, donepezil; ELISA, enzyme-linked immunosorbent assay; PCR, polymerase chain reaction; BDNF, brain-derived neurotrophic factor; CREB, cAMP-responsive element-binding protein; Aβ, β-amyloid; ANOVA, analysis of variance.
Data Sharing Statement
The datasets used and/or analysed are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
All experimental protocols and procedures were approved by the Animal Care and Use Committee of KFMRC and performed according to the guidelines of the biomedical ethics research committee (reference no. 102-19) of King Abdulaziz University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
- 1.Salcudean A, Todoran AM. Pathophysiology of Alzheimer’s disease. Rom J Psychopharmacol. 2010;10:1–8. [Google Scholar]
- 2.Sun C, Qiu X, Wang Y, et al. Long-term oral melatonin alleviates memory deficits, reduces amyloid-β deposition associated with downregulation of BACE1 and mitophagy in APP/PS1 transgenic mice. Neurosci Lett. 2020;735:135192. doi: 10.1016/j.neulet.2020.135192 [DOI] [PubMed] [Google Scholar]
- 3.Imbimbo BP, Lombard J, Pomara N. Pathophysiology of Alzheimer’s disease. Neuroimaging Clin N Am. 2005;15(4):727–753. doi: 10.1016/j.nic.2005.09.009 [DOI] [PubMed] [Google Scholar]
- 4.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81(2):741–766. doi: 10.1152/physrev.2001.81.2.741 [DOI] [PubMed] [Google Scholar]
- 5.Feng Z, Chang Y, Cheng Y, et al. Melatonin alleviates behavioral deficits associated with apoptosis and cholinergic system dysfunction in the APP 695 transgenic mouse model of Alzheimer’s disease. J Pineal Res. 2004;37(2):129–136. doi: 10.1111/j.1600-079X.2004.00144.x [DOI] [PubMed] [Google Scholar]
- 6.Asl ZR, Sepehri G, Salami M. Probiotic treatment improves the impaired spatial cognitive performance and restores synaptic plasticity in an animal model of Alzheimer’s diseas. Behav Brain Res. 2019;376:112183. doi: 10.1016/j.bbr.2019.112183 [DOI] [PubMed] [Google Scholar]
- 7.Pj M, L B, Ag G, Rodríguez-cruz A. The etiology of alzheimer’s disease; 2016:1–14. DOI: 10.1016/j.bbr.2014.12.012 [DOI]
- 8.Bonmati-Carrion MA, Arguelles-Prieto R, Martinez-Madrid MJ, et al. Protecting the melatonin rhythm through circadian healthy light exposure. Int J Mol Sci. 2014;15(12):23448–23500. doi: 10.3390/ijms151223448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon KJ, Kim HJ, Shin CY, Han SH. Melatonin potentiates the neuroprotective properties of resveratrol against beta-amyloid-induced neurodegeneration by modulating AMP-activated protein kinase pathways. J Clin Neurol. 2010;6(3):127–137. doi: 10.3988/jcn.2010.6.3.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali T, Badshah H, Kim TH, Kim MO. Melatonin attenuates D-galactose-induced memory impairment, neuroinflammation and neurodegeneration via RAGE/NF-KB/JNK signaling pathway in aging mouse model. J Pineal Res. 2015;58(1):71–85. doi: 10.1111/jpi.12194 [DOI] [PubMed] [Google Scholar]
- 11.Alghamdi BS. The neuroprotective role of melatonin in neurological disorders. J Neuro Res. 2018;96(7):1136–1149. doi: 10.1002/jnr.24220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esposito E, Cuzzocrea S. Antiinflammatory activity of melatonin in central nervous system. Curr Neuropharmacol. 2010;8(3):228–242. doi: 10.2174/157015910792246155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonnefont-Rousselot D, Collin F. Melatonin: action as antioxidant and potential applications in human disease and aging. Toxicology. 2010;278(1):55–67. doi: 10.1016/j.tox.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 14.Abo Taleb HA, Alghamdi BS. Neuroprotective effects of melatonin during demyelination and remyelination stages in a mouse model of multiple sclerosis. J Mol Neurosci. 2020;70(3):386–402. doi: 10.1007/s12031-019-01425-6 [DOI] [PubMed] [Google Scholar]
- 15.Olcese JM, Cao C, Mori T, et al. Protection against cognitive deficits and markers of neurodegeneration by long‐term oral administration of melatonin in a transgenic model of Alzheimer disease. J Pineal Res. 2009;47(1):82–96. doi: 10.1111/j.1600-079X.2009.00692.x [DOI] [PubMed] [Google Scholar]
- 16.Mayo JC, Tan D-X, Sainz RM, Lopez-Burillo S, Reiter RJ. Oxidative damage to catalase induced by peroxyl radicals: functional protection by melatonin and other antioxidants. Free Radic Res. 2003;37(5):543–553. doi: 10.1080/1071576031000083206 [DOI] [PubMed] [Google Scholar]
- 17.Rudnitskaya EA, Maksimova KY, Muraleva NA, et al. Beneficial effects of melatonin in a rat model of sporadic Alzheimer’s disease. Biogerontology. 2015;16(3):303–316. doi: 10.1007/s10522-014-9547-7 [DOI] [PubMed] [Google Scholar]
- 18.Alghamdi BS, Abo Taleb HA. Melatonin improves memory defects in a mouse model of multiple sclerosis by up-regulating cAMP-response element-binding protein and synapse-associated proteins in the prefrontal cortex. J Integr Neurosci. 2020;19:229–237. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y, Swaab DF. The human pineal gland and melatonin in aging and Alzheimer’s disease. J Pineal Res. 2005;38(3):145–152. doi: 10.1111/j.1600-079X.2004.00196.x [DOI] [PubMed] [Google Scholar]
- 20.Cardinali DP, Vigo DE, Olivar N, Vidal MF, Brusco LI. Melatonin therapy in patients with Alzheimer’s disease. Antioxidants. 2014;3(2):245–277. doi: 10.3390/antiox3020245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong Y, Hua N, Zang X, Huang T, He L. Melatonin ameliorates Aβ1‐42‐induced Alzheimer’s cognitive deficits in mouse model. J Pharm Pharmacol. 2018;70(1):70–80. doi: 10.1111/jphp.12830 [DOI] [PubMed] [Google Scholar]
- 22.Ali T, Kim MO. Melatonin ameliorates amyloid beta‐induced memory deficits, tau hyperphosphorylation and neurodegeneration via PI 3/Akt/GS k3β pathway in the mouse hippocampus. J Pineal Res. 2015;59(1):47–59. doi: 10.1111/jpi.12238 [DOI] [PubMed] [Google Scholar]
- 23.Muhammad T, Ali T, Ikram M, Khan A, Alam SI, Kim MO. Melatonin rescue oxidative stress-mediated neuroinflammation/neurodegeneration and memory impairment in scopolamine-induced amnesia mice model. J Neuroimmune Pharmacol. 2019;14(2):278–294. [DOI] [PubMed] [Google Scholar]
- 24.Ozdemir D, Tugyan K, Uysal N, et al. Protective effect of melatonin against head trauma-induced hippocampal damage and spatial memory deficits in immature rats. Neurosci Lett. 2005;385(3):234–239. doi: 10.1016/j.neulet.2005.05.055 [DOI] [PubMed] [Google Scholar]
- 25.Iwashita H, Matsumoto Y, Maruyama Y, Watanabe K, Chiba A, Hattori A. The melatonin metabolite N1‐acetyl‐5‐methoxykynuramine facilitates long‐term object memory in young and aging mice. J Pineal Res. 2020;e12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin L, Huang Q-X, Yang -S-S, Chu J, Wang J-Z, Tian Q. Melatonin in Alzheimer’s disease. Int J Mol Sci. 2013;14(7):14575–14593. doi: 10.3390/ijms140714575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen D, Tian X, Sang W, Song R. Effect of melatonin and resveratrol against memory impairment and hippocampal damage in a rat model of vascular dementia. Neuroimmunomodulation. 2016;23(5–6):318–331. doi: 10.1159/000454681 [DOI] [PubMed] [Google Scholar]
- 28.Tanila H. The role of BDNF in Alzheimer’s disease. Neurobiol Dis. 2017;97(Pt B):114–118. doi: 10.1016/j.nbd.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 29.Psotta L, Rockahr C, Gruss M, et al. Impact of an additional chronic BDNF reduction on learning performance in an Alzheimer mouse model. Front Behav Neurosci. 2015;9:58. doi: 10.3389/fnbeh.2015.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bali P, K Lahiri D, Banik A, Nehru B, Anand A. Potential for stem cells therapy in Alzheimer’s disease: do neurotrophic factors play critical role? Curr Alzheimer Res. 2017;14(2):208–220. doi: 10.2174/1567205013666160314145347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker MP, LaFerla FM, Oddo SS, Brewer GJ. Reversible epigenetic histone modifications and Bdnf expression in neurons with aging and from a mouse model of Alzheimer’s disease. Age. 2013;35(3):519–531. doi: 10.1007/s11357-011-9375-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belrose JC, Masoudi R, Michalski B, Fahnestock M. Increased pro–nerve growth factor and decreased brain-derived neurotrophic factor in non–Alzheimer’s disease tauopathies. Neurobiol Aging. 2014;35(4):926–933. doi: 10.1016/j.neurobiolaging.2013.08.029 [DOI] [PubMed] [Google Scholar]
- 33.Rosa E, Mahendram S, Ke YD, Ittner LM, Ginsberg SD, Fahnestock M. Tau downregulates BDNF expression in animal and cellular models of Alzheimer’s disease. Neurobiol Aging. 2016;48:135–142. doi: 10.1016/j.neurobiolaging.2016.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prakash A, Kumar A. Role of nuclear receptor on regulation of BDNF and neuroinflammation in hippocampus of β-amyloid animal model of Alzheimer’s disease. Neurotox Res. 2014;25(4):335–347. doi: 10.1007/s12640-013-9437-9 [DOI] [PubMed] [Google Scholar]
- 35.Pugazhenthi S, Wang M, Pham S, Sze C-I, Eckman CB. Downregulation of CREB expression in Alzheimer’s brain and in Aβ-treated rat hippocampal neurons. Mol Neurodegener. 2011;6(1):60. doi: 10.1186/1750-1326-6-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sung J-Y, Bae J-H, Lee J-H, Kim Y-N, Kim D-K. The melatonin signaling pathway in a long-term memory in vitro study. Molecules. 2018;23(4):737. doi: 10.3390/molecules23040737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saura CA, Valero J. The role of CREB signaling in Alzheimer’s disease and other cognitive disorders. Rev Neurosci. 2011;22(2):153–169. doi: 10.1515/rns.2011.018 [DOI] [PubMed] [Google Scholar]
- 38.Lee Y, Kim J, Jang S, Oh S. Administration of phytoceramide enhances memory and upregulates the expression of pCREB and BDNF in hippocampus of mice. Biomol Ther (Seoul). 2013;21(3):229. doi: 10.4062/biomolther.2013.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hota SK, Barhwal K, Baitharu I, Prasad D, Singh SB, Ilavazhagan G. Bacopa monniera leaf extract ameliorates hypobaric hypoxia induced spatial memory impairment. Neurobiol Dis. 2009;34(1):23–39. doi: 10.1016/j.nbd.2008.12.006 [DOI] [PubMed] [Google Scholar]
- 40.Xiao F, Li X-G, Zhang X-Y, et al. Combined administration of D-galactose and aluminium induces Alzheimer-like lesions in brain. Neurosci Bull. 2011;27(3):143–155. doi: 10.1007/s12264-011-1028-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang W, Shi L, Chen L, et al. Protective effects of perindopril on d-galactose and aluminum trichloride induced neurotoxicity via the apoptosis of mitochondria-mediated intrinsic pathway in the hippocampus of mice. Brain Res Bull. 2014;109:46–53. doi: 10.1016/j.brainresbull.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 42.Azman KF, Zakaria R. D-Galactose-induced accelerated aging model: an overview. Biogerontology. 2019;20(6):763–782. doi: 10.1007/s10522-019-09837-y [DOI] [PubMed] [Google Scholar]
- 43.Gao J, He H, Jiang W, et al. Salidroside ameliorates cognitive impairment in a d-galactose-induced rat model of Alzheimer’s disease. Behav Brain Res. 2015;293:27–33. doi: 10.1016/j.bbr.2015.06.045 [DOI] [PubMed] [Google Scholar]
- 44.Vučetić-Arsić S, Radonjić NV, Jovanović M, et al. Oxidative stress precedes mitochondrial dysfunction in gerbil brain after aluminum ingestion. Environ Toxicol Pharmacol. 2013;36(3):1242–1252. doi: 10.1016/j.etap.2013.10.008 [DOI] [PubMed] [Google Scholar]
- 45.Singh S, Singh R, Kushwah AS, Gupta G. Neuroprotective role of antioxidant and pyranocarboxylic acid derivative against AlCl3 induced Alzheimer’s disease in rats. J Coast Life Med. 2014;2:547–554. [Google Scholar]
- 46.Wei Y, Liu D, Zheng Y, Li H, Hao C, Ouyang W. Protective effects of kinetin against aluminum chloride and D-galactose induced cognitive impairment and oxidative damage in mouse. Brain Res Bull. 2017;134:262–272. doi: 10.1016/j.brainresbull.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 47.Álvarez-sánchez N, Cruz-Chamorro I, López-González A, et al. Melatonin controls experimental autoimmune encephalomyelitis by altering the T effector/regulatory balance. Brain Behav Immun. 2015;50:101–114. [DOI] [PubMed] [Google Scholar]
- 48.Alghamdi BSA. Possible prophylactic anti-excitotoxic and anti-oxidant effects of virgin coconut oil on aluminium chloride-induced Alzheimer’s in rat models. J Integr Neurosci. 2018;17(3–4):593–607. doi: 10.3233/JIN-180089 [DOI] [PubMed] [Google Scholar]
- 49.Ameen-Ali KE, Easton A, Eacott MJ. Moving beyond standard procedures to assess spontaneous recognition memory. Neurosci Biobehav Rev. 2015;53:37–51. doi: 10.1016/j.neubiorev.2015.03.013 [DOI] [PubMed] [Google Scholar]
- 50.Lueptow LM. Novel object recognition test for the investigation of learning and memory in mice. J Vis Exp. 2017;126:e55718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xing Z, He Z, Wang S, et al. Ameliorative effects and possible molecular mechanisms of action of fibrauretine from fibraurea recisa pierre on d-galactose/AlCl3-mediated Alzheimer’s disease. RSC Adv. 2018;8(55):31646–31657. doi: 10.1039/C8RA05356A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolf A, Bauer B, Abner EL, Ashkenazy-Frolinger T, Hartz A. A comprehensive behavioral test battery to assess learning and memory in 129S6/Tg2576 mice. PLoS One. 2016;11(1):e0147733. doi: 10.1371/journal.pone.0147733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goodarzi P, Payab M, Alavi-Moghadam S, et al. Development and validation of Alzheimer’s disease animal model for the purpose of regenerative medicine. Cell Tissue Bank. 2019;20(2):141–151. doi: 10.1007/s10561-019-09773-8 [DOI] [PubMed] [Google Scholar]
- 54.Zhang L, Chen C, Mak MSH, et al. Advance of sporadic Alzheimer’s disease animal models. Med Res Rev. 2020;40(1):431–458. doi: 10.1002/med.21624 [DOI] [PubMed] [Google Scholar]
- 55.Esquerda-Canals G, Montoliu-Gaya L, Güell-Bosch J, Villegas S. Mouse models of Alzheimer’s disease. J Alzheimers Dis. 2017;57(4):1171–1183. doi: 10.3233/JAD-170045 [DOI] [PubMed] [Google Scholar]
- 56.Elder GA, Gama Sosa MA, De Gasperi R. Transgenic mouse models of Alzheimer’s disease. Mt Sinai J Med. 2010;77(1):69–81. doi: 10.1002/msj.20159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang C, Cai X, Hu W, et al. Investigation of the neuroprotective effects of crocin via antioxidant activities in HT22 cells and in mice with Alzheimer’s disease. Int J Mol Med. 2019;43(2):956–966. doi: 10.3892/ijmm.2018.4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang W-N, Hu X-D, Han H, et al. The effects of valsartan on cognitive deficits induced by aluminum trichloride and d-galactose in mice. Neurol Res. 2014;36(7):651–658. doi: 10.1179/1743132813Y.0000000295 [DOI] [PubMed] [Google Scholar]
- 59.Dang H, Chen Y, Liu X, et al. Antidepressant effects of ginseng total saponins in the forced swimming test and chronic mild stress models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1417–1424. doi: 10.1016/j.pnpbp.2009.07.020 [DOI] [PubMed] [Google Scholar]
- 60.Xu P, Wang K, Lu C, et al. The protective effect of lavender essential oil and its main component linalool against the cognitive deficits induced by D-galactose and aluminum trichloride in mice. Evid Based Complement Altern Med. 2017;2017:7426538. doi: 10.1155/2017/7426538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fu H, Rodriguez GA, Herman M, et al. Tau pathology induces excitatory neuron loss, grid cell dysfunction, and spatial memory deficits reminiscent of early Alzheimer’s disease. Neuron. 2017;93:533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu H, Yan H, Tang NA, et al. Impairments of spatial memory in an Alzheimer’s disease model via degeneration of hippocampal cholinergic synapses. Nat Commun. 2017;8(1):1–13. doi: 10.1038/s41467-017-01943-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bassani TB, Bonato JM, Machado MMF, et al. Decrease in adult neurogenesis and neuroinflammation are involved in spatial memory impairment in the streptozotocin-induced model of sporadic Alzheimer’s disease in rats. Mol Neurobiol. 2018;55(5):4280–4296. doi: 10.1007/s12035-017-0645-9 [DOI] [PubMed] [Google Scholar]
- 64.Tu S, Wong S, Hodges JR, Irish M, Piguet O, Hornberger M. Lost in spatial translation–a novel tool to objectively assess spatial disorientation in Alzheimer’s disease and frontotemporal dementia. Cortex. 2015;67:83–94. doi: 10.1016/j.cortex.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 65.Feng L, Wang X, Peng F, et al. Walnut protein hydrolysates play a protective role on neurotoxicity induced by D-galactose and aluminum chloride in mice. Molecules. 2018;23(9):2308. doi: 10.3390/molecules23092308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peng S, Garzon DJ, Marchese M, et al. Decreased brain-derived neurotrophic factor depends on amyloid aggregation state in transgenic mouse models of Alzheimer’s disease. J Neurosci. 2009;29(29):9321–9329. doi: 10.1523/JNEUROSCI.4736-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmed HH, Estefan SF, Mohamd EM, Farrag AE-RH, Salah RS. Does melatonin ameliorate neurological changes associated with Alzheimer’s disease in ovariectomized rat model? Indian J Clin Biochem. 2013;28(4):381–389. doi: 10.1007/s12291-012-0284-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen BH, Park JH, Lee T-K, et al. Melatonin attenuates scopolamine-induced cognitive impairment via protecting against demyelination through BDNF-TrkB signaling in the mouse dentate gyrus. Chem Biol Interact. 2018;285:8–13. doi: 10.1016/j.cbi.2018.02.023 [DOI] [PubMed] [Google Scholar]
- 69.Bavithra S, Priya ES, Selvakumar K, Krishnamoorthy G, Arunakaran J. Effect of melatonin on glutamate: BDNF signaling in the cerebral cortex of polychlorinated biphenyls (PCBs)-exposed adult male rats. Neurochem Res. 2015;40(9):1858–1869. doi: 10.1007/s11064-015-1677-z [DOI] [PubMed] [Google Scholar]
- 70.Matsuzaki K, Yamakuni T, Hashimoto M, et al. Nobiletin restoring β-amyloid-impaired CREB phosphorylation rescues memory deterioration in Alzheimer’s disease model rats. Neurosci Lett. 2006;400(3):230–234. doi: 10.1016/j.neulet.2006.02.077 [DOI] [PubMed] [Google Scholar]
- 71.Yiu AP, Rashid AJ, Josselyn SA. Increasing CREB function in the CA1 region of dorsal hippocampus rescues the spatial memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2011;36(11):2169–2186. doi: 10.1038/npp.2011.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peng C, Hong X, Chen W, et al. Melatonin ameliorates amygdala-dependent emotional memory deficits in Tg2576 mice by up-regulating the CREB/c-Fos pathway. Neurosci Lett. 2017;638:76–82. doi: 10.1016/j.neulet.2016.11.066 [DOI] [PubMed] [Google Scholar]
- 73.Yoo DY, Kim W, Lee CH, et al. Melatonin improves D‐galactose‐induced aging effects on behavior, neurogenesis, and lipid peroxidation in the mouse dentate gyrus via increasing pCREB expression. J Pineal Res. 2012;52(1):21–28. doi: 10.1111/j.1600-079X.2011.00912.x [DOI] [PubMed] [Google Scholar]