Abstract
New neurons are generated in the dentate gyrus of the adult brain throughout life. They incorporate in the granular cell layer of the dentate gyrus and integrate in the hippocampal circuitry. Increasing evidence suggests that new neurons play a role in learning and memory. In turn, a large body of evidence suggests that neurogenesis is impaired in Alzheimer’s disease, contributing to memory deficits characterizing the disease. We out-line here current knowledge about the biology of adult hippocampal neurogenesis and its function in learning and memory. In addition, we discuss evidence that neurogenesis is dysfunctional in Alzheimer’s disease, address the controversy in the literature concerning the persistence of hippocampal neurogenesis in the adult and aging human brain, and evaluate the therapeutic potential of neurogenesis-based drug development for the treatment of cognitive deficits in Alzheimer’s disease.
1. Introduction
1.1. Alzheimer’s disease
Alzheimer’s disease (AD) is an age-related neurodegenerative disease characterized by progressive loss of memory and cognitive impairments. These impairments can include difficulties in learning, problem solving, deficient spatial recognition and slow cognitive performance.1 The pathological hall-marks of AD include amyloid β-protein deposits and neurofibrillary tangles. Neuronal degeneration takes place in specific brain areas, such as the hippocampus and cortex.2 Early onset familial Alzheimer’s disease represents 5% of patients and is caused by mutations in either the presenilin 1,2 (PSEN1, PSEN2) or amyloid precursor protein (APP) genes.3 Late onset sporadic Alzheimer’s disease accounts for most of the AD cases and has no known cause. However, many genetic and environmental factors, such as aging, APOE ε4, and lifestyle modulate the risk for the development of AD.3 APP processing via the amyloidogenic pathway contributes to the formation of amyloid deposits. In brief, sequential processing of APP by the enzymes β-secretase (BACE-1) and γ-secretase leads to the formation of Aβ. Under pathological conditions, this process leads to excessive formation and accumulation of neurotoxic oligomeric Aβ and senile plaques.3 Presenilin (PS1,2) is the catalytic core of the γ-secretase complex. Mutations in PSEN facilitate amyloidosis.3 Amyloid deposition and neurofibrillary tangles appear first in the entorhinal cortex and hippocampus.2
1.2. Adult hippocampal neurogenesis (AHN)
Neurogenesis is the process by which new neuron are formed from neural stem cells and incorporate in neural circuits.4 Traditionally, neurogenesis was thought to take place in the mammalian brain during embryogenesis. However, numerous studies in the last three decades provided substantial evidence establishing the formation of new neurons in specific brain areas, including the dentate gyrus (DG) of the hippocampus and the subventricular zone (SVZ) of the lateral ventricle in rodents.4 Neural stem cells (NSCs) pass through different developmental stages before getting incorporated into the hippocampal circuitry and becoming mature neurons.5
In brief, NSCs are Type I radial glia-like cells (RGLs) present in the subgranular zone of the DG. They can remain quiescent, self-renew, or proliferate, giving rise to proliferating intermediate progenitor cells (IPCs, type II cells) that possess a transient amplifying characteristic. Type II cells can be further subdivided into two subpopulations: (1) type IIa cells, which continue to express the NSC marker Sox2; and (2) type IIb cells, which are early committed NPCs that express the transcription factors Prox1, NeuroD1, and DCX. Type 2 cells give rise to neuroblasts (type III) that subsequently differentiate into mature dentate granule neurons6 (Scheme 1). These newly formed neurons project axons into the CA3 region of the hippocampus and dendrites into the outer molecular layer of the DG, forming synapses with neurons in layer II of the entorhinal cortex.7 Thus, new neurons integrate into the hippocampal circuitry and contribute to learning and memory.8,9
Scheme 1.
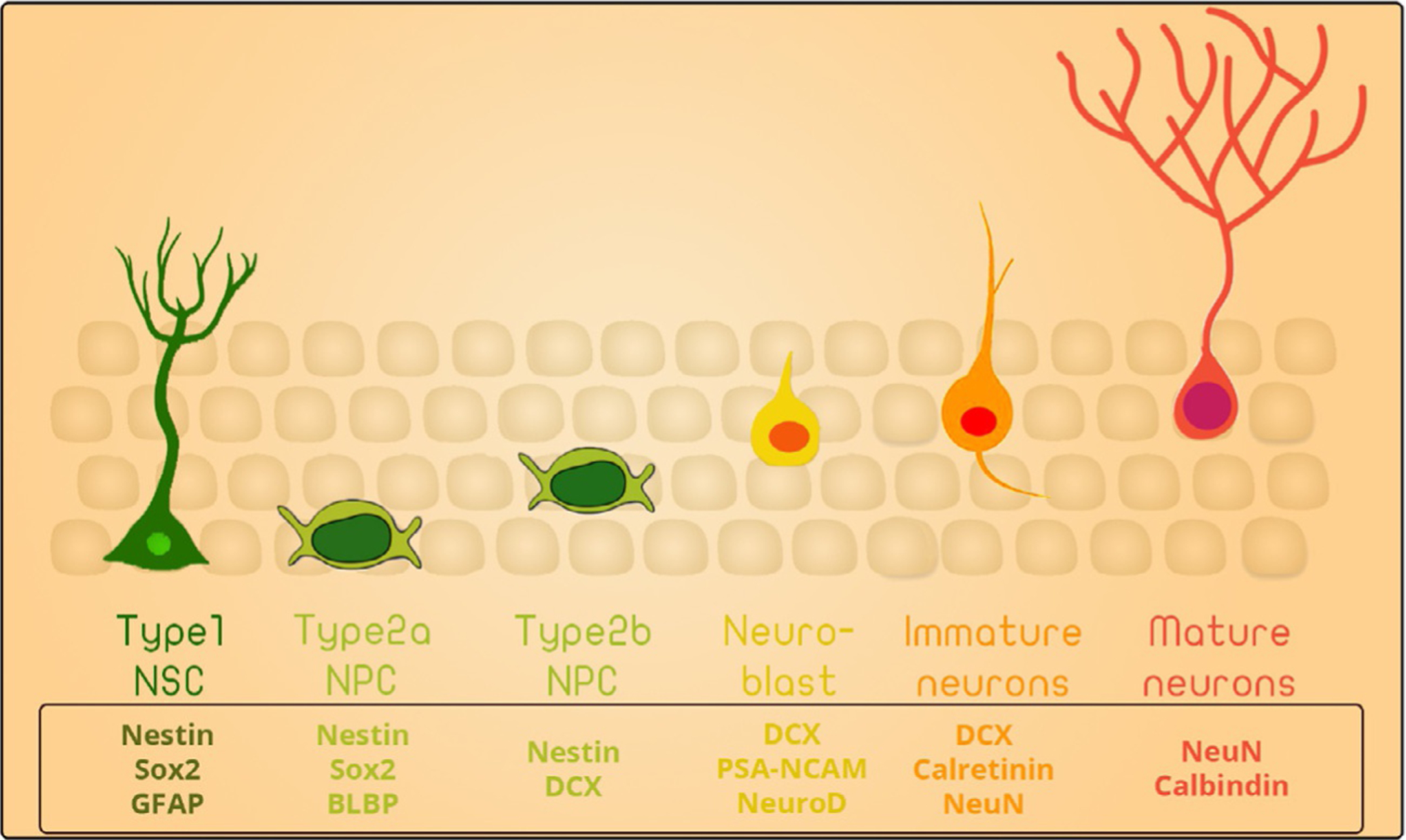
Stages of hippocampal neurogenesis.
2. Adult hippocampal neurogenesis in learning and memory
During the first half of the twentieth century, occasional reports of postnatal neurogenesis were recorded. However, it was not until the early 1960s that the first evidence of postnatal neurogenesis in adult rats was published.10 Neurogenesis was reported in several parts of the brain including the olfactory bulb, the neocortex, and most relevant to our discussion here, the dentate gyrus. The DG has demonstrated a significant role in decreasing memory inference and promoting pattern separation. Its role has been investigated in numerous studies in rodents, non-human primates, and humans. The DG transforms similar inputs into more dissimilar outputs, decreasing inference between memories and minimizing overlap between similar patterns of neurological activities.11
The presence of AHN in a crucial area of memory acquisition and consolidation suggested a role in learning and memory. Indeed, numerous studies established a role of adult-born hippocampal neurons in pattern separation.11 Nakashiba et al. showed that older neurons are mainly involved in pattern completion as they recognize relatively distinct situation. However, younger neurons are essential for fine discrimination of close contexts. This observation suggests age-related functional changes of neurons and that continuous neurogenesis in adults is crucial for memory discrimination.12 In support of this observation, Shors et al. tested the impact of ablating neurogenesis using an antimitotic agent, called methyl-azoxymethanol (MAM), on learning and memory. Administration of MAM caused spatial memory impairments that were resolved 3 weeks after its cessation.13,14 This finding suggests that newly born neurons are essential, and may be solely responsible, for trace memory acquisition.13 A follow up study using the same treatment, found that blocking AHN using MAM treatment impairs remote spatial memory performance in the Morris Water Maze (MWM) task.15 Treatment with several other antimitotic drugs that are known to ablate neurogenesis caused impaired performance of mice in hippocampus-dependent memory tasks, such as spatial recognition. Some examples of these agents include 5-fluorouracil,16 cyclophosphamide,17 and temozolomide.18 Recent studies using X-ray irradiation to impair hippocampal neurogenesis in mice have revealed impairments in space separation behavioral tasks involving discrimination between two similar targets.13 Another study reported that radiation-induced impairment of AHN in adult C57Bl/6 mice compromises short term memory and impairs the animals’ performance in contextual fear conditioning tasks.19 Rats receiving irradiation showed compromised long-term memory in MWM tasks, as well as short term memory impairments in contextual fear conditioning.11 Similarly, halting neurogenesis using chemicals like temozolomide reduced the ability of mice to memorize new positions in MWM and perform the newly introduced tasks. Instead, these rats preferred to perform the tasks that were previously taught, which indicated an elongated old memory retention.18 Nonetheless, these techniques had numerous side effects, which necessitated better approaches to address this question.
The generation of transgenic mice showing conditional genetic ablation of neurogenesis produced higher specificity and reduced undesired side effects.18a Garcia et al. developed an inducible glial fibrillary acidic protein (GFAP)-thymidine kinase (TK) mouse model that eliminated neurogenesis upon the administration of ganciclovir.20 Studies done using this mouse model showed impaired performance on contextual fear conditioning task.20 Deng et al. generated another nestin-thymidine kinase mouse model eliminating dividing neural progenitors using ganciclovir-mediated toxicity. These mice displayed defects in spatial preference following ablation of AHN by overexpressing a Bax gene specifically in neural progenitor cells using a Dox-on system.21 Using this model, a compromised acquisition of spatial reference memory in the MWM task was detected.21 Nevertheless, no impairment was found in cue guidance or orientation.21 Tronel et al. showed that mice with impaired neurogenesis had deficient performance in the pattern separation task despite prolonged training, while maintaining intact associative memory, thus supporting the role of nascent neurons in memory discrimination.22 The NSEDTA/Nes-CreERT2 transgenic mouse was developed by abolishing the NPCs in the DG using diphtheria toxin following tamoxifen administration. Utilizing this mouse model, impaired contextual fear conditioning was detected.23
A study by Niibori et al. suggested that new neurons play a role in memory encoding within the DG. In this study, the investigators recorded clear defects in contextual discrimination following the ablation of hippocampal neurogenesis. In addition, the authors reported that chemical and genetic halting of neurogenesis impaired population-based coding of similar contexts in CA3.24 This suggests a role for adult neurogenesis in pattern separation, and given the correlation between efficiency of memory acquisition and feedforward of DG-CA3, it is possible that the mossy fibers’ back projection to newly forming neurons in the DG may have a role in memory coding within the DG.25
Recent studies revealed an interesting role of AHN in forgetting existing memories.26 Despite the fact that this role appears controversial in light of prior studies, it may be essential for maintaining hippocampal plasticity. Animals need to delete or unlearn certain tasks so new memories can be processed and stored. Researchers have suggested that neurogenesis may enable the hippocampal network to develop and store new memories, which in turn requires deleting some old memories to optimize learning capacity (Scheme 2).
Scheme 2.
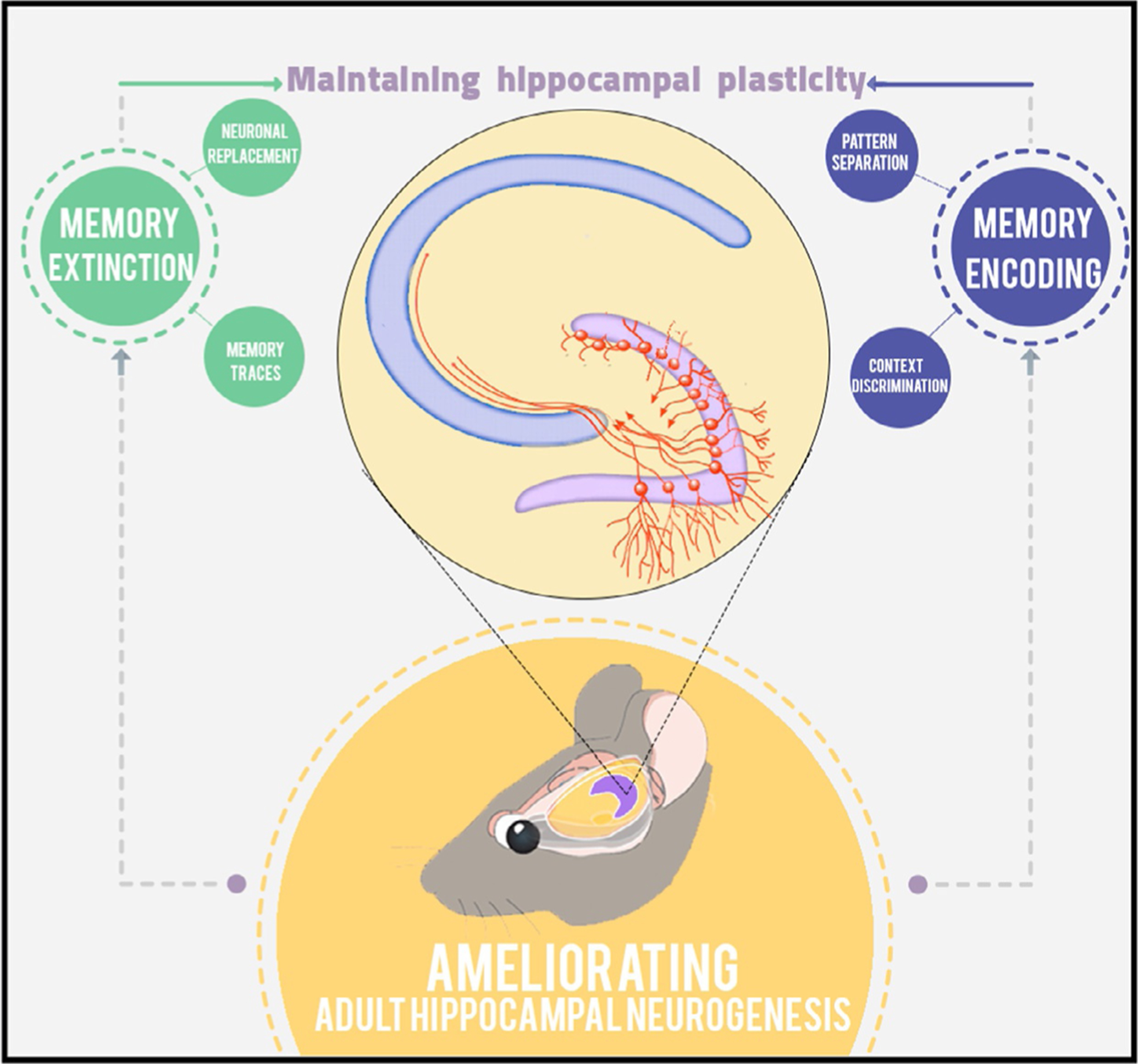
A model for the role of AHN in learning and memory.
A study done by Feng et al., using forebrain-specific presenilin-1 knockout (PS1-KO) mice that exhibits neurogenesis impairment, found that an enriched environment provided before the learning task enhanced neurogenesis and improved the animals’ performance in contextual and cued fear conditioning tests. Interestingly, introducing environmentally enriched conditions for 2 weeks following the learning task improved the PS1-KO animals’ performance in the contextual retention test while not affecting the wildtype mice. This observation highlights the importance of hippocampal neurogenesis and suggests that while impaired hippocampal neurogenesis may induce defective memory acquisition, it can prevent clearing traces of contextual memories.27 Deisseroth et al. found that Cav1.2/1.3 (l-type) Ca2+ channels and NMDA receptors induce an activity sensing property on neural precursor cells within the hippocampus, suggesting that the activity of local neural networks surrounding these cells may modulate the neurogenic process. The response to this neuronal activity during the formation of new neurons may play a role in the formation of new memories and forgetting old memories. Moreover, the authors demonstrated that ameliorating neurogenesis can lead to a faster clearance of old memories, and at the same time, neurogenesis is crucial to acquire new memories. A regulated and appropriate level of neurogenesis thus is needed for the hippocampus to balance old memory storage and new memory formation. Additionally, a timely clearance of old memories will improve the efficiency in developing and storing recent memories within the already existing hippocampal network.28
Another study done by Akers and colleagues investigated the correlation between memory retention and hippocampal neurogenesis.26 The authors compared the behavioral performance in memory retention between adult mice with decreased neurogenesis as a result of aging and ~17 day old postnatal pups that are reported to have relatively high neurogenetic activity. Fear-conditioning training was performed on the mice in day 1 testing for 6 weeks. The authors then measured the differences in the freezing activity between the two groups and noticed more stable memory retention in the adult mice throughout the 6 weeks while a similar memory retention at the beginning, followed by a fast decline within a week, was observed in the pups. Interestingly, enhancement of neurogenesis in adult mice using either a running wheel (a form of exercise) or an antidepressant (fluoxetine) facilitated forgetfulness while reducing neurogenesis in infant mice after fear conditioning enhanced memory retention.26 This study provided evidence regarding the role of neurogenesis in eliminating memories from the DG and suggested a new model in which neurogenesis may facilitate the development and storage of new memories in the hippocampal network, which in term requires deleting some old ones to optimize the learning capacity.
Taken together, these studies clearly support a role for hippocampal neurogenesis in learning and memory in the adult rodent brain, as well as in memory discrimination, encoding, and consolidation. Moreover, a well-established body of literature shows the impact of impairing AHN on learning and memory, suggesting a role for AHN in pathological conditions, such as Alzheimer’s disease.
3. Deficits in neurogenesis in Alzheimer’s disease mouse models
Altered and dysfunctional neurogenesis in the SGZ of the dentate gyrus have been reported in numerous experiments using transgenic mouse models of familial Alzheimer’s disease (FAD). The majority of the mouse models overexpress FAD-linked mutant genes amyloid precursor protein (APP), presenilin1 (PSEN1), and Presenilin 2 (PSEN2). The differentiating elements between these mouse models is whether they bear single or multiple mutations, the promoter driving the transgene expression, and the location of the transgene in the genome. These differences, accompanied with mouse age and gender, significantly affect the rate of AD pathology progression and its extent. Many studies have attributed the impairments in neurogenesis to Aβ accumulation. For instance, Haughey et al. used a transgenic mouse model overexpressing the Swedish mutation of APP. Using this model, the authors found that the proliferation of NPCs and their survival, as well as the number of new neurons, are impaired in 12–14-month-old mice when accumulation of amyloid deposition is abundant. However, this impairment was not detected in 3-month old mice prior to amyloid deposition. Additionally, exogenous treatment with Aβ42 altered the proliferation and differentiation of cultured humans and rodents’ neurons in vitro.29 Another study used 8-month-old APP/PS1 mice, a mouse line that expresses the Swedish mutation in APP and the L166P mutated human presenilin (PS1) under the Thy-1 promoter element. This study showed a decrease in quiescent nestin-positive glial-like neural stem cells with preserved numbers of transient amplifying progenitor cells. Both cell populations manifested morphological abnormalities during amyloid deposition.30 These findings showed that amyloid deposition may cause NPC dysfunction and alters AHN in mouse models. In concordance with the previous results, aged APPswe/PS1ΔE9 mice were reported to show a reduction in the numbers of BrdU- or Doublecortin (DCX)-positive cells at 9 months of age, while these differences were not observed in 5 months old mice.31 In PDAPP mice, age-dependent decreases in SGZ proliferation were seen in AHN homozygotes. Abnormal maturation of new neurons, in addition to fewer dying neurons compared to the control group, were observed at 1 year of age, suggesting abnormal turnover in the DG.32
Other studies have found that the impairments in neurogenesis precedes the development of AD pathology. Demars et al. using the APPswe/PS1ΔE9 mouse model, reported that these FAD mice, at 2 months of age, show severe reduction in NPC proliferation as well as neuronal differentiation. This early impairment in neurogenesis was accompanied by an increase in the levels of phosphorylated tau within the neurogenic niche. These results suggest that impaired neurogenesis is an early event that precedes AD pathology in the course of the disease and is correlated with the early memory impairments seen in these mice.33 Wang et al. studied neurogenesis using the PS1M146V knock-in mice in which the M146V mutation was integrated into the endogenous mouse PS1 gene. This study observed that, at 3 months of age, these mice exhibit impaired hippocampus-dependent learning, as assessed by contextual fear conditioning. This impairment was correlated with a reduction in the number of proliferating cells (BrdU+) as well as with newly formed mature neurons (NeuN+ BrdU+). These results support a correlation between impairments in neurogenesis and memory defects early in the disease course of AD.34 Rodríguez et al. used a triple-transgenic mouse model (3xTg-AD) expressing the Swedish mutation of APP and the human P301L tau mutation in conjunction with a homozygous presenilin (1PS1M146V) knock-in background. A significant reduction in neurogenesis was recorded at 4 months of age, with intra-neuronal Aβ accumulation recorded in the CA1 region of the hippocampus. At 12 months of age, and in correlation with the increase in the extracellular deposition of amyloid proteins within the DG neurons, these mice showed severer reduction in neurogenesis. These results suggest that while the impairment in neurogenesis precedes Aβ deposition in the DG, the severity of this impairment is directly correlated with the extent of β-amyloid accumulation within hippocampal neurons.35 Another mouse model was developed expressing the human Swedish APP mutation on M233T and L235P PSEN1 mutations knock-in background with an early degenerative and behavioral phenotype called (APP/PS1KI). These mice showed a significantly lower number of mitotic Ki67-immunoreactive cells in the DG, and almost complete absence of DCX-positive cells at the age of 6 months.36 This observation suggests that the loss of DCX-positive cells partially reflects alterations in multipotent progenitor cell proliferation.36 Lastly, Tg2576 transgenic mice showed reduced capacity of cell proliferation as early as 3 months of age. This impairment may underlie behavioral changes in contextual but not in cued memory. The results suggest early impairments in neurogenesis in the dentate gyrus of FAD mice that may play a role or exacerbate defective discrimination memory.37
In contrast to these studies, a few reports using double or triple mutations of APP (APP751swe, APPswe) observed increased proliferation and differentiation of newborn neurons.38–40 This discrepancy could be a result of a transgene-induced off-target effect, a compensatory mechanism as a result of progressive pathology, or enhanced gliogenesis rather than neurogenesis.38 Examination of neurogenesis in the human brain, as described below, and future studies examining the effect of these mutations in knock-in mice may alleviate this discrepancy.
It should be noted that neurogenic impairments in FAD mice were observed in multiple checkpoints and processes, including reduced proliferation of NPCs, reduced survival of newborn neurons in the DG, distortion in the balance between GABAergic and glutamatergic inputs synapsing onto the newly born neurons,41 defective neuronal maturation, e.g., impaired dendritic trees of new neurons,42 and loss of γ-secretase function in the NPCs and the newborn neurons.42 In the SVZ, studies reported increased expression of the less potent proliferation factor sAPPβ metabolite.43,44
To further investigate the implication of impaired hippocampal neurogenesis for cognitive defects in AD, a few studies have manipulated AHN in AD mouse models and assessed the impact on cognitive performance. Hollands et al. crossed an APPswe/PS1ΔE9 mice with mice expressing nestin-driven thymidine kinase (δ-HSV-TK) to investigate the association between cognitive defects in AD and adult neurogenesis.45 The authors reported that ablating AHN in the hippocampus of these mice exacerbated cognitive deficits.45 On the other hand, Dong et al. have reported that administration of fluoxetine, a drug known to increase AHN, to an AD mouse models overexpressing the Swedish mutation (Tg2576) resulted in enhancement of AHN and improved contextual memory performance of the Tg2576 mice.37 Together these two studies suggest a role for AHN in learning and memory in AD. Moreover, these studies imply that enhancing AHN may be a novel therapeutic intervention for the amelioration of cognitive deficits in AD.
Molecular signals that are linked to AD have been found to play a role in regulation of hippocampal neurogenesis. For example, several studies have highlighted the role of APP in regulating neurogenesis. Demars et al. showed that the soluble form of APP (sAPPα) plays an important role in regulating the proliferation and survival of neural progenitor cells. Moreover, the authors found that injecting the soluble form of APP (sAPPα) into the SVZ of aging mice enhanced neurogenesis.43 In concordance with these results, Caillé et al. found that sAPPα binds specifically to the stem cell pool within the SVZ and participates in EGF-promoted neural stem cell proliferation.46 Moreover, this study showed that blocking the secretion of APP by α-secretase inhibits the proliferation of the NSCs.46 Zhang et al. found that APP modulates neural progenitor cells through the modulation of the microRNA miR-574–5P.47 Overexpressing miR-574–5P reduced the neural progenitor pool, while suppressing miR-574–5P expression stimulated NPC proliferation both in vitro and in utero. Moreover, the authors show that inhibiting the expression of miR-574–5P in an APP-knockout mouse rescued impaired neurogenesis.47 Another important FAD-linked signal is PS1, the catalytic core of γ-secretase. Mutations in PS1 impair γ-secretase function and cause FAD.48 It is essential for the metabolism and processing of numerous neurogenic signals, such as APP and Notch. Bonds et al. found that PS1 regulates NPC differentiation and maturation of neurons in adult brains.42 The authors specifically downregulated PS1 in the DG of C57BL/6 mice using a PS1 knockdown shRNA. Knocking down PS1 in neural progenitor cells in these mice resulted in impaired pattern separation and novel object recognition tasks.42 Moreover, they reported a lower survivability rate for these NPCs as well as a decline in dendritic branching and spines, which may contribute to defective learning and memory in these mice. This might suggest that PS1-mediated neurogenesis impairment may lead to cognitive deficits in mice. The author also highlighted that this effect of PS1 on neural progenitor cells is notch- and β-catenin-mediated.42 Gadadhar et al. found similar results. They observed that knocking down PS1 in hippocampal NPCs led to a reduced population of proliferating NPCs as a result of premature neuronal differentiation. Choi et al. found that FAD mice harboring wild-type human PS1 (PS1hWT) mutation did not exhibit enhanced proliferation nor the enhanced differentiation of NPCs associated with environmental enrichment (EE), a behavioral manipulation known to enhance neurogenesis and NPC proliferation in particular.49 This clearly highlights the importance of PS1 signaling in regulating neurogenesis. Tau maintains the stability of microtubules and its hyper-phosphorylation and aggregation play an essential role in AD pathophysiology. The tau-3R isoform is expressed in adult neurons during development and it overlaps with DCX and NeuN co-expression in the DG.50 Moreover, the phosphorylation of Tau was found to be linked to the neurogenic proxies DCX and NeuroD both spatially and temporally.51 Komuro et al. used the hTau mouse model that employs the MAPT human transgene on a Mapt−/− knockout background and reported a detectable early reduction in neurogenesis and neuronal proliferation at 2 months of age.52 This reduction occurred before significant tau pathology, and this suggests a link between tau pathology and AD and may suggest that impaired neurogenesis is an early indication of tau pathology in AD.
4. Adult hippocampal neurogenesis in primates
Non-human primates are advantageous for the study of neurogenesis because of the greater neuroanatomical and functional similarity of their brains to those of humans. Primates exhibit a decline in adult neurogenesis following the juvenile stage accompanied with longer neuronal turn over cycle and elongated maturation compared with other mammalian species.53 Using a combination of BrdU labeling and immunohistochemistry, Gould et al. reported life-long existence of AHN in old world monkeys54 as well as new world monkeys.55 Several studies suggest that AHN plays an important role in learning and memory in primates. Ngwenya et al. studied the effect of neurogenesis on cognitive function in 42 adult rhesus male monkeys (Macaca mulatta), aged 6.1–31.5 years. Evidence was obtained of the existence of AHN throughout the Monkeys’ life. Moreover, the authors reported a decline of AHN with aging. This decline was correlated with a decrease in the learning and memory capabilities of the old monkeys, thus implying an essential role for hippocampal neurogenesis in primates’ learning and memory.56 In concordance with these results, Aizawa et al. studied the correlation between hippocampal neurogenesis and cognitive performance in seven cynomolgus monkeys aged 5–23 years.57 Using a visual pattern discrimination task (VDP), they reported a decline in the cognitive performance in these monkeys as a result of aging. Interestingly, the decline in learning abilities of these monkey was correlated with a lower extent of neurogenesis in their DG, suggesting an interdependent relation between the extent of neurogenesis and cognitive performance in primates.57 These results show evidence for the existence of AHN across multiple species, and specifically in non-human primates, a model of special biological relevance to humans. Moreover, given the anatomical brain similarities between humans and non-human primates, it provides us with a proximate estimation of the timeline of neurogenesis within the brains of higher primates. Lastly, it highlights the positive correlation between AHN and learning and memory in higher primates.
5. Adult hippocampal neurogenesis in aging humans and AD patients
It was not until 1998 that the first evidence of hippocampal neurogenesis in the adult human brain was reported. In a pioneer study, Eriksson and colleagues examined postmortem brain sections of patients treated with BrdU (5-bromo-2′-deoxyuridine), a thymidine analog that incorporates in the DNA of proliferating cells.58 Similar to rodents,59 colocalization between BrdU and neuronal proxies, like NeuN and calbindin, was observed, indicating that these are new neurons. Moreover, new neurons were found in the hippocampus only in granular and subgranular layers of the dentate gyrus (DG).58 Two years later, Roy et al. isolated NPCs from the hippocampus of adult humans and successfully maintained them in culture conditions. These NPCs were able to differentiate into mature granular neurons. This suggested the presence of NPCs in the DG and provided evidence for active neurogenesis in this region.60 However, this method did not allow accurate quantification of the number of new neurons in vivo. In 2013, Spalding et al. provided quantitative evidence for the existence of hippocampal neurogenesis in the adult human.61 Using 14C birth dating by quantifying the isotope concentration in the genomic DNA of neurons, Spalding et al. estimated the rate of new neurons as 700/day. Moreover, they determined that 35% of the DG neurons undergo turnover with renewal rate of 1.75% per year. In light of that, they concluded that a full renewal of the neuronal population of the dentate gyrus takes place in the course of a lifetime.61
Using immunohistochemistry, Dennis et al. investigated the extent of neurogenesis in 23 postmortem hippocampal samples from human subjects or age 1–59 years.62 The authors were not able to detect hippocampal neurogenesis in the adult human dentate gyrus. Moreover, they reported that the only proliferating cellular population that they were able to detect in the DG or its adjacent parenchyma were microglia.62 Sorrells et al. investigated the extent of neurogenesis over the life span of humans using 19 pre- and postnatal samples of brain tissue and 18 samples of adult postmortem hippocampal tissue.63 The authors reported that while the pre- and postnatal dentate gyrus up to a year old contained a great number of immature neurons, they were not able to detect any newly born neurons in the DG of the adult samples, suggesting that hippocampal neurogenesis comes to a halt during adulthood.63 In contrast, three recent reports have shown evidence for the persistence of hippocampal neurogenesis during adulthood. Using similar proxy markers, Boldrini et al. investigated postmortem hippocampal samples obtained from 28 individual aged 14 to 79 years.64 The authors were able to detect thousands of NPCs and immature neurons in the DG of healthy adult humans.64 Tobin et al. investigated the extent of AHN in postmortem hippocampal tissue of 18 individuals aged 79–99 years and categorized them into three categories based on their cognitive performance; healthy aging, mild cognitive impaired (MCI), and AD.65 The authors not only provided evidence for the existence of AHN up to the 10th decade of life in healthy humans, but were also able to detect NSCs, NPCs and new neurons in MCI and AD patients.65 Moreover, they reported that the number of neuroblasts was lower in MCI and AD patients and correlated with their cognitive diagnosis, thus indicating that AHN is impaired early in the AD disease course and has the potential of being a therapeutic target of early intervention.65 Lastly, Moreno-Jiménez et al. investigated AHN in postmortem tissue obtained from 13 healthy aging individuals aged 43–87 years and from 45 AD patients aged 43–87 years.66 In this study, the authors provided evidence for the existence of AHN until 90 years of age, while seeing a moderate reduction in AHN during aging. Moreover, using stereological counting, the authors reported that AHN declines drastically in AD patients and is correlated with the patients’ Braak stage.66
This controversy could have a number of reasons, including the differences in experimental condition among different reports. Investigating postmortem tissue should be done very carefully and diligently as postmortem interval and time of fixation can have a great impact on protein degradation. Moreno-Jiménez et al. have reported that over-fixation of the tissue postmortem can degrade essential neurogenic markers.66 Moreover, antigen retrieval has been found to be crucial in immunolabeling of neurogenic markers and inappropriate antigen retrieval might result in unsuccessful immunostaining. Thus, using a standardized protocol may help alleviate the controversy. Another possible reason is the use of postmortem tissue from patients with comorbidities that can affect AHN. For instance, the majority of the tissue used by Sorrells et al. came from patients with epilepsy. Thus, detailed medical records of the participating patients should be in place and uniform criteria should be applied across research labs. Lastly, this controversy clearly highlights the limitations of using proxy biomarkers in studying AHN in human. Thus, future studies should aim at developing advanced methodologies for the detection of AHN in the human brain.
6. Modulation of neurogenesis: A potential therapeutic approach for the treatment of AD
A large body of evidence suggests that exercise, learning and environmental enrichment, each enhances neurogenesis and has profound beneficial effect on cognition.67 In humans, physical exercise training for 6 weeks has resulted in significantly better performance in hippocampus-dependent visual pattern separation task in participants.68 However, these interventions affect numerous targets. Thus, producing direct evidence for the effect of augmented neurogenesis on cognition is warranted. Sahay et al. have reported that increasing the number of newly born neurons in mice, by augmenting the survival of these neurons following genetic ablation of Bax, significantly enhances the ability to discriminate between two similar contexts, i.e., pattern separation.69 Moreover, combining genetic augmentation of AHN with exercise results in vigorous enhancement in exploratory behavior.69 In another study, Wang and colleagues developed a gain-of-function knock-in mouse model of enhanced AHN by activating of endogenous ERK5 MAP kinase specifically in the dentate gyrus.55 The authors reported that in these mice, increasing neurogenesis was sufficient for the enhancement of spatial learning, even upon vigorous challenging conditions.70 Moreover, enhancing AHN significantly prolonged the duration of the memory, thus supporting long-term memory.70 Choi et al. found that enhancing AHN, in combination with overexpressing BDNF, mimicked exercise-induced beneficial effects and rescued cognitive impairments in the 5xFAD mouse model.71 However, it was previously reported that hippocampal infusion of BDNF enhances cognitive performance,72 thus the beneficial outcome in this study might not be specific to AHN. Moreover, diminishing neurogenesis early in life can aggravate neuronal vulnerability.71 These finding imply that enhancing neurogenesis may be a promising therapeutic intervention for the amelioration of cognitive deficits in AD. The authors suggest that impairments in AHN precedes AD pathology and may be a primary event in the disease course that may engender neurodegeneration later on in AD, indicating that enhancing AHN in the earlier stages may halt neuronal loss and increase brain reserves, which could be a potential therapeutic strategy for AD treatment.
7. Remaining questions and concluding remarks
Over the last decade, the AHN field has flourished. Our knowledge about the characteristic of neurogenic niches, the potential function of the newly formed neurons, and impacts on hippocampal plasticity under physiological and pathological conditions has expanded significantly. However, fundamental challenges remain unresolved. First, whether specifically ameliorating AHN in AD mouse model can rescue cognitive defects remains unclear. AHN has been found to be impaired in AD mouse models and correlated with cognitive defects in these mice. The use of more sophisticated techniques, such as Designer Receptor Exclusively Activated by Designer Drugs (DREADs) or optogenetics, to specifically ameliorate AHN in AD mice model can provide better understanding of the role of neurogenesis in AD. Second, the timeline of the formation of new neurons across-species is not fully elucidated. This process is species-specific and can vary with age, gender or genetic background. While in rodents this process is estimated to take 4–6 weeks, it is thought to take months in humans and non-human primates. Moreover, whether neurogenesis in the human brain culminates at a specific time point or takes place at a constant rate throughout life is also unclear. Understanding the developmental timeline of neurogenesis and its activity rate throughout the lifespan will enhance our understanding of its function in the human brain. Third, new advanced methodologies for the imaging, detection and characterization of neurogenesis in live organisms are warranted. Spatial transcriptomics, expression profiles and scRNAseq would each allow the characterization of NSCs, NPCs and newly born neurons within the dentate gyrus and enhance our understanding of the mechanisms regulating the neurogenic niche. Fourth, despite the recent reports of the drastic decline of neurogenesis in AD65,66 and its correlation with cognitive function,65 molecular characterization of defective processes in AD patients would help us develop effective therapeutic approaches.
Funding
This work was supported by NIA AG033570, AG060238, AG062251, AG061628.
Footnotes
Competing interests
The authors declare no competing financial interests.
References
- 1.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. [DOI] [PubMed] [Google Scholar]
- 3.Lazarov O, Demars MP. All in the family: how the APPs regulate neurogenesis. Front Neurosci. 2012;6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazarov O, Mattson MP, Peterson DA, Pimplikar SW, van Praag H. When neurogenesis encounters aging and disease. Trends Neurosci. 2010;33(12):569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao C, Teng EM, Summers RG, Ming G-l, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006; 26(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tobin MK, Bonds JA, Minshall RD, Pelligrino DA, Testai FD, Lazarov O. Neurogenesis and inflammation after ischemic stroke: what is known and where we go from here. J Cereb Blood Flow Metab. 2014;34(10):1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke L, van der Kooy D. The adult mouse dentate gyrus contains populations of committed progenitor cells that are distinct from subependymal zone neural stem cells. Stem Cells. 2011;29(9):1448–1458. [DOI] [PubMed] [Google Scholar]
- 8.Zhao X, Ueba T, Christie BR, et al. Mice lacking methyl-CpG binding protein 1 have deficits in adult neurogenesis and hippocampal function. Proc Natl Acad Sci U S A. 2003;100(11):6777–6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altman J Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anat Rec. 1963;145(4):573–591. [DOI] [PubMed] [Google Scholar]
- 11.Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70(4):589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakashiba T, Cushman JD, Pelkey KA, et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012; 149(1):188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–376. [DOI] [PubMed] [Google Scholar]
- 14.Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002; 12(5):578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodman T, Trouche S, Massou I, et al. Young hippocampal neurons are critical for recent and remote spatial memory in adult mice. Neuroscience. 2010;171(3):769–778. [DOI] [PubMed] [Google Scholar]
- 16.Mustafa S, Walker A, Bennett G, Wigmore PM. 5-Fluorouracil chemotherapy affects spatial working memory and newborn neurons in the adult rat hippocampus. Eur J Neurosci. 2008;28(2):323–330. [DOI] [PubMed] [Google Scholar]
- 17.Yang M, Kim J-S, Song M-S, et al. Cyclophosphamide impairs hippocampus-dependent learning and memory in adult mice: possible involvement of hippocampal neurogenesis in chemotherapy-induced memory deficits. Neurobiol Learn Mem. 2010;93(4):487–494. [DOI] [PubMed] [Google Scholar]
- 18.Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One. 2009;4(5):e5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009; 29(43):13532–13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko H-G, Jang D-J, Son J, et al. Effect of ablated hippocampal neurogenesis on the formation and extinction of contextual fear memory. Mol Brain. 2009;2(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia A, Denise R, Ngan B, Doan TI, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7(11):1233–1241. [DOI] [PubMed] [Google Scholar]
- 21.Dupret D, Revest J-M, Koehl M, et al. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3(4):e1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tronel S, Belnoue L, Grosjean N, et al. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus. 2012;22(2):292–298. [DOI] [PubMed] [Google Scholar]
- 23.Imayoshi I, Sakamoto M, Ohtsuka T, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11(10):1153. [DOI] [PubMed] [Google Scholar]
- 24.Niibori Y, Yu T-S, Epp JR, Akers KG, Josselyn SA, Frankland PW. Suppression of adult neurogenesis impairs population coding of similar contexts in hippocampal CA3 region. Nat Commun. 2012;3(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myers CE, Scharfman HE. Pattern separation in the dentate gyrus: a role for the CA3 backprojection. Hippocampus. 2011;21(11):1190–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akers KG, Martinez-Canabal A, Restivo L, et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344(6184):598–602. [DOI] [PubMed] [Google Scholar]
- 27.Feng R, Rampon C, Tang Y-P, et al. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron. 2001;32(5):911–926. [DOI] [PubMed] [Google Scholar]
- 28.Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42(4): 535–552. [DOI] [PubMed] [Google Scholar]
- 29.Haughey NJ, et al. Disruption of neurogenesis by amyloid β-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer’s disease. J Neurochem. 2002; 83(6):1509–1524. [DOI] [PubMed] [Google Scholar]
- 30.Ermini FV, Grathwohl S, Radde R, et al. Neurogenesis and alterations of neural stem cells in mouse models of cerebral amyloidosis. Am J Pathol. 2008;172(6):1520–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taniuchi N, Niidome T, Goto Y, Akaike A, Kihara T, Sugimoto H. Decreased proliferation of hippocampal progenitor cells in APPswe/PS1dE9 transgenic mice. Neuroreport. 2007;18(17):1801–1805. [DOI] [PubMed] [Google Scholar]
- 32.Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J Comp Neurol. 2006;495(1):70–83. [DOI] [PubMed] [Google Scholar]
- 33.Demars M, Hu Y-S, Gadadhar A, Lazarov O. Impaired neurogenesis is an early event in the etiology of familial Alzheimer’s disease in transgenic mice. J Neurosci Res. 2010; 88(10):2103–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang R, Dineley KT, Sweatt JD, Zheng H. Presenilin 1 familial Alzheimer’s disease mutation leads to defective associative learning and impaired adult neurogenesis. Neuroscience. 2004;126(2):305–312. [DOI] [PubMed] [Google Scholar]
- 35.Rodríguez JJ, Jones VC, Tabuchi M, et al. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer’s disease. PLoS One. 2008;3(8): e2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faure A, Verret L, Bozon B, et al. Impaired neurogenesis, neuronal loss, and brain functional deficits in the APPxPS1-Ki mouse model of Alzheimer’s disease. Neurobiol Aging. 2011;32(3):407–418. [DOI] [PubMed] [Google Scholar]
- 37.Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;127(3):601–609. [DOI] [PubMed] [Google Scholar]
- 38.Jin K, Peel AL, Mao XO, et al. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101(1):343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.López-Toledano MA, Shelanski ML. Increased neurogenesis in young transgenic mice overexpressing human APP Sw, Ind. J Alzheimers Dis. 2007;12(3):229–240. [DOI] [PubMed] [Google Scholar]
- 40.Gan L, Qiao S, Lan X, et al. Neurogenic responses to amyloid-beta plaques in the brain of Alzheimer’s disease-like transgenic (pPDGF-APPSw,Ind) mice. Neurobiol Dis. 2008;29:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun B, Halabisky B, Zhou Y, et al. Imbalance between GABAergic and glutamatergic transmission impairs adult neurogenesis in an animal model of Alzheimer’s disease. Cell Stem Cell. 2009;5(6):624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonds JA, Kuttner-Hirshler Y, Bartolotti N, et al. Presenilin-1 dependent neurogenesis regulates hippocampal learning and memory. PLoS One. 2015;10(6):e0131266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demars MP, Hollands C, Zhao KDT, Lazarov O. Soluble amyloid precursor protein-α rescues age-linked decline in neural progenitor cell proliferation. Neurobiol Aging. 2013;34(10):2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demars MP, Bartholomew A, Strakova Z, Lazarov O. Soluble amyloid precursor protein: a novel proliferation factor of adult progenitor cells of ectodermal and mesodermal origin. Stem Cell Res Ther. 2011;2(4):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hollands C, Tobin MK, Hsu M, et al. Depletion of adult neurogenesis exacerbates cognitive deficits in Alzheimer’s disease by compromising hippocampal inhibition. Mol Neurodegener. 2017;12(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caillé I, Allinquant B, Dupont E, et al. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development. 2004;131(9):2173–2181. [DOI] [PubMed] [Google Scholar]
- 47.Zhang W, Thevapriya S, Kim PJ, et al. Amyloid precursor protein regulates neurogenesis by antagonizing miR-574–5p in the developing cerebral cortex. Nat Commun. 2014; 5(1):1–14. [DOI] [PubMed] [Google Scholar]
- 48.Xia D, Watanabe H, Wu B, et al. Presenilin-1 knockin mice reveal loss-of-function mechanism for familial Alzheimer’s disease. Neuron. 2015;85(5):967–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi SH, Veeraraghavalu K, Lazarov O, et al. Non-cell-autonomous effects of presenilin 1 variants on enrichment-mediated hippocampal progenitor cell proliferation and differentiation. Neuron. 2008;59(4):568–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Llorens-Martin M, Teixeira CM, Fuster-Matanzo A, et al. Tau isoform with three microtubule binding domains is a marker of new axons generated from the subgranular zone in the hippocampal dentate gyrus: implications for Alzheimer’s disease. J Alzheimers Dis. 2012;29(4):921–930. [DOI] [PubMed] [Google Scholar]
- 51.Fuster-Matanzo A, Llorens-Martín M, Jurado-Arjona J, Avila J, Hernández F. Tau protein and adult hippocampal neurogenesis. Front Neurosci. 2012;6:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Komuro Y, Xu G, Bhaskar K, Lamb BT. Human tau expression reduces adult neurogenesis in a mouse model of tauopathy. Neurobiol Aging. 2015;36(6):2034–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan T-F, Li J, Ding F, Arias-Carrion O. Evidence of adult neurogenesis in non-human primates and human. Cell Tissue Res. 2014;358(1):17–23. [DOI] [PubMed] [Google Scholar]
- 54.Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult old-world primates. Proc Natl Acad Sci U S A. 1999;96(9):5263–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A. 1999;96(10):5768–5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ngwenya LB, Heyworth NC, Shwe Y, Moore TL, Rosene DL. Age-related changes in dentate gyrus cell numbers, neurogenesis, and associations with cognitive impairments in the rhesus monkey. Front Syst Neurosci. 2015;9:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aizawa K, Ageyama N, Yokoyama C, Hisatsune T. Age-dependent alteration in hippocampal neurogenesis correlates with learning performance of macaque monkeys. Exp Anim. 2009;58(4):403–407. [DOI] [PubMed] [Google Scholar]
- 58.Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. [DOI] [PubMed] [Google Scholar]
- 59.Kempermann G New neurons for’survival of the fittest’. Nat Rev Neurosci. 2012; 13(10):727–736. [DOI] [PubMed] [Google Scholar]
- 60.Roy NS, Benraiss A, Wang S, et al. Promoter-targeted selection and isolation of neural progenitor cells from the adult human ventricular zone. J Neurosci Res. 2000; 59(3):321–331. [DOI] [PubMed] [Google Scholar]
- 61.Spalding KL, Bergmann O, Alkass K, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dennis CV, Suh LS, Rodriguez ML, Kril JJ, Sutherland GT. Human adult neurogenesis across the ages: an immunohistochemical study. Neuropathol Appl Neurobiol. 2016; 42(7):621–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sorrells SF, Paredes MF, Cebrian-Silla A, et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555(7696):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boldrini M, Fulmore CA, Tartt AN, et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22(4):589–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tobin MK, Musaraca K, Disouky A, et al. Human hippocampal neurogenesis persists in aged adults and Alzheimer’s disease patients. Cell Stem Cell. 2019;24(6):974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med. 2019;25(4):554–560. [DOI] [PubMed] [Google Scholar]
- 67.Van Praag H, Kempermann G, Gage FH. Neural consequences of enviromental enrichment. Nat Rev Neurosci. 2000;1(3):191–198. [DOI] [PubMed] [Google Scholar]
- 68.Déry N, Pilgrim M, Gibala M, et al. Adult hippocampal neurogenesis reduces memory interference in humans: opposing effects of aerobic exercise and depression. Front Neurosci. 2013;7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sahay A, Scobie KN, Hill AS, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang W, Pan Y-W, Zou J, et al. Genetic activation of ERK5 MAP kinase enhances adult neurogenesis and extends hippocampus-dependent long-term memory. J Neurosci. 2014;34(6):2130–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choi SH, Bylykbashi E, Chatila ZK, et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science. 2018; 361(6406), eaan8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang L, Fang Y, Lian Y, et al. Brain-derived neurotrophic factor ameliorates learning deficits in a rat model of Alzheimer’s disease induced by aβ1–42. PLoS One. 2015;10(4): e0122415. [DOI] [PMC free article] [PubMed] [Google Scholar]


