Abstract
Recent developments in chemogenetic approaches to the investigation of brain function have ushered in a paradigm change in the strategy for drug and behavior research and clinical drug-based medications. As the nature of the drug action is based on humoral regulation, it is a challenge to identify the neuronal mechanisms responsible for the expression of certain targeted behavior induced by drug application. The development of chemogenetic approaches has allowed researchers to control neural activities in targeted neurons through a toolbox, including engineered G protein-coupled receptors or ligand-gated ion channels together with exogenously inert synthetic ligands. This review provides a brief overview of the chemogenetics toolbox with an emphasis on the DREADDs (Designer Receptors Exclusively Activated by Designer Drugs) technique used in rodent models, which is applicable to the investigation of how specific neural circuits regulate behavioral processes. The use of chemogenetics has had a significant impact on basic neuroscience for a better understanding of the relationships between brain activity and the expression of behaviors with cell- and circuit-specific orders. Furthermore, chemogenetics is potentially a useful tool to deconstruct the neuropathological mechanisms of mental diseases and its regulation by drug, and provide us with transformative therapeutics with medication. We also review recent findings in the use of chemogenetic techniques to uncover functional circuit connections of serotonergic neurons in rodent models.
Keywords: Behavioral Pharmacology, Chemogenetics, Drug treatment, Neural circuits, Animal models, Research strategies, DREADDs
1. Conventional behavioral pharmacology
1.1. Introduction
Decades of behavioral pharmacological studies have given rise to a framework for the majority of studies of drug effects on behavior, particularly in the domain of drug-driven behavior, such as substance use and drug-behavior control and in the control of maladaptive behaviors associated with mental diseases (Johanson, 1990). A number of laboratory studies have also focused on improving the understanding of the brain mechanisms on behavioral regulations that are relevant to drug control in clinical psychopathology through systemic or site-specific receptor-ligands (e.g., neuromodulators) treatment (Zimmermann and Poling, 2016). Recent technical developments in the genetics of model organisms and genetic modulators such as chemogenetics and optogenetics have stimulated renewed interest in laboratory methodologies for the evaluation of the behavioral and central nervous system effects of drug administration. More significantly, these newly developed techniques have ushered in reforms of the conceptual framework of conventional behavioral pharmacology (Navabpour et al., 2020). In this review article, we have presented an overview of the conceptual and practical problems in conventional behavioral pharmacology (e.g., psychopharmacology) and discuss how the current genetic approaches impact the frameworks of studies of the interaction between drug treatment and its cognitive behavioral expression. With this understanding, genetic approaches will surely provide next-stage methodologies and advanced strategies for the study of drug effects on behavior in animal models as well as human clinical problems.
1.2. The framework for conventional behavioral pharmacology
Valuable information on how drugs affect behavior has contributed to our understanding of the behavioral and pharmacological mechanisms in the central nervous system, and consequently provided effective strategies for the treatment of drug and for health professionals seeking to appropriately control and administer drugs (Johanson, 1990; Willner et al., 2019). The majority of research into conventional behavioral pharmacology has yielded a shift in focus from the effect of the drug itself on the cognate neural networks to the dynamic molecular-behavioral interaction between the individual and environmental stimuli in the controlling drug effects on behavior. In the field of research into behavioral effects of drugs over the last two decades, the major trend has been for studies that mainly assess drug-induced and drug-related behaviors, and the behavioral and environmental determinants that control behavior (Gerak et al., 2019). Laboratory studies also often aim to achieve a greater understanding of the antecedents and consequences of drug use to develop better behavioral therapies with drug treatment. In this context, researchers have considered various mechanisms to improve the quantitative precision of measurements of reinforcing efficacy and developing a strategy for the efficient investigation of the myriad of environmental variables that may influence behavior. Conditioning techniques and schematic analyses of the stimulus-response interactions in drug effects on behavior have also provided numerous strategies to control drug-induced behaviors and valuable assessment methods (Quisenberry et al., 2016; van Haaren, 2016).
At the same time, the history of experimental work in this area is a testament to the substantial difficulties in the selective definition of specific drug activity at the neural and behavioral levels (Stenson and Roth, 2014; Willner et al., 2019). Although the promise of more precise experimental control and operational definition has been shown, a vast majority of psychopharmacological studies have technical limitations that complicate their interpretation. One practical reason to be emphasized is that experimental efforts on animals, along with clinical applications, have, in most instances, resorted to the systemic administration of drugs; therefore, it is difficult to identify a primary target or structure (e.g., even central or peripheral) responsible for drug effects on a particular behavior. This is an aggravating issue facing spatial resolution in the assessment of drug effects, since drug treatment fundamentally possesses humoral diverse feature of its effectiveness (e.g., Shields et al., 2017). Administration of drugs even brain site-specifically produces distributed molecules throughout tissues and neurons, which induces multitude reactions in several neuronal and synaptic sites. Moreover, the restrictions imposed by the analysis of the sequence of certain behaviors that the drug treatment is targeted are another problem. The effectiveness of drugs is usually over several hours to days, which significantly hampers the identification of specific behaviors targeted by drug administration. For example, several types of behaviors representing a certain element of psychological states including sensory process, general activity, learning, and convulsive behavior, as well as motivational and emotional responses, are simultaneously influenced during the time-course of drug effectiveness. These issues, including spatial resolution in the assessment of drug targets and temporal resolution in the evaluation of behavioral effects, have remained over laboratory research and clinical applications to interpret how the drug effects interact with specific behavioral expression (Farrell and Roth, 2013).
1.3. Controversial effects of drug treatment
The development of prescription treatment medications, mainly oral pills, is the major commercial demand for controlling problematic or pathological behaviors associated with psychiatric diseases and drug-related maladaptive behaviors (Shields et al., 2017; Gerak et al., 2019). In the experimental investigations of the drug effects on behavior, trials for systemic treatment combined with site-specific infusion complicate the elucidation of neural mechanisms in drug action, and the results have been controversial.
Serotonin (5-HT) is a major neurotransmitter, implicated in the processing of various behavioral functions, such as sleep rhythms, perception, emotion, and cognition (Berger et al., 2009; Artigas, 2015). The serotonergic cell bodies in the brain reside mainly in the dorsal and median raphe nuclei, but distribute their axons almost to the entire brain (Charnay and Léger, 2010), along with a family of 14 (or more) 5-HT receptor subtypes (Hoyer et al., 1994), mediating the multitudinous behavioral effects of 5-HT. The 5-HT1A receptor is implicated in the regulation of anxiety-like behaviors along with depression-like behaviors (Heisler et al., 1998; Toth, 2003). A traditional anxiolytic, buspirone, is a partial 5-HT1A agonist (Toth, 2003), and common antidepressants, the SSRIs, target the 5-HT1A receptors (Artigas, 2015). 5-HT1A receptors are distributed widely throughout the brain and are present in both the pre- and postsynaptic sites and the dendrite bodies (Riad et al., 2000; Matias et al., 2017). Presynaptic 5-HT1A receptors in the dorsal and median raphe nuclei act as autoreceptors forming a negative feedback loop to tightly regulate 5-HT neuronal activity (Toth, 2003; Stiedl et al., 2015). Thus, major anxiolytic treatments acutely stimulate several 5-HT receptors and then induce a reduction in total brain 5-HT level via 5-HT1A receptor stimulation, whereas extra-synaptic 5-HT levels are increased (Berger et al., 2009; Stiedl et al., 2015), which results in both hyper and hypo 5-HT levels depending on brain regions and sequence following treatment (Stiedl et al., 2015). SSRIs are effective in the treatment of both anxiety and depression, to maintain heightened brain 5-HT levels, while an acute SSRIs treatment stimulate 5-HT1A receptors leads to lower 5-HT availability in a time-course and brain-sites dependent manner (Jennings et al., 2010). These examples involving 5-HT implicate the need for functional investigation with cell- or circuit-type specific manipulation to uncover the indubitable neural mechanisms of target neurotransmitters/molecules that are associated with drug effects. In the following chapters, we will discuss a next-stage methodology that allows researchers to dissect specific functions discretely at the cell or circuit levels.
2. Use of chemogenetic manipulation
2.1. Advances in chemogenetics
The development of an innovative platform that enable us to manipulate cell (neurons)- or region-specific activity is required to fulfill the demands described above in studies on behavioral pharmacology (Sternson and Roth, 2014; Urban and Roth, 2015). During the last decade, a revolution in neuroscience techniques has resulted in increasingly precise less-invasive methods to manipulate neural systems in awake, behaving animals, which are also applicable to humans’ clinical cases (O’Connor and Boulis, 2015; Santiago-Ortiz and Schaffer, 2016; Grimm and Büning, 2017). Chemogenetic technology provides researchers with a number of powerful advantages, including multiplexed spatiotemporal control of molecularly circumscribed cell types ranging from single synapses to the entire neuronal ensembles. Chemogenetic regulation is based on cellular signaling pathways mediated by combinations of inert chemical actuators (ligands) and genetically engineered receptors that, in theory, can be applied to almost any cell population. (Roth, 2016; Atasoy and Sternson, 2018). Therefore, chemogenetics refers to the application that allows for the reversible remote control of cell populations and neural circuitry through systemic or micro-infusion of an exogeneous activating ligand (Alexander et al., 2009; Armbruster et al., 2007).
Indeed, chemogenetics is used extensively to obtain a better understanding of the specific neural circuits regulating cognate behaviors, which is crucial for the progression of development in both behavioral pharmacology and behavior-targeted mediation. This review highlights the practical approaches in which chemogenetic toolboxes are used in animal models with an emphasis on mouse models, and then covers how these newly developed procedures could amend the direction/strategy of behavioral pharmacology.
2.2. Chemogenetic modulators
Engineered G protein-coupled receptors:
Designer Receptors Exclusively Activated by Designer Drugs (DREADDs)-based chemogenetics, which are the main focus of this review (Table 1), have been used in numerous neuroscience studies to identify the neural circuits and cellular signals regulating desired behavioral outcomes (Roth, 2016). This technique utilizes the cellular signal transduction systems underlying G protein-coupled receptors (GPCR) in order to permit manipulation of targeted neuronal activities. GPCRs mediate extensive cellular responses to various cellular stimuli by exposing a ligand-binding element on the extracellular part of the receptors (Fredriksson et al., 2003). Accordingly, GPCRs have substantial function in influencing the signal transduction pathways, ion channels, and synapses (Fredriksson et al., 2003; McCudden et al., 2005). DREADDs are designed as engineered GPCR tools that have high binding selectivity to an engineered ligand, e.g., clozapine-N-oxide (CNO), with low sensitivity to endogenous ligands (Armbruster et al., 2007). Ideally, CNO as an engineered ligand, is an inactive metabolite of the antipsychotic drug clozapine, which is an agonist of endogenous muscarinic acetylcholine receptors. However, as addressed later, CNO has been known not to be an ideal effective molecule as a DREADD activator for in vivo experiments.
Table 1.
A list of chemogenetic modulators and ligands
| Modulators | Ligands | |
|---|---|---|
| GPCR | hM3Dq, hM1Dq, hM5Dq | Clozapine-N-oxide (CNO) |
| -- activating | Compound 21 (C21) | |
| hM4Di | Deschloroclozapine (DCZ) | |
| -- inhibitory | ||
| rM3Ds | ||
| -- activating | ||
| KORD | Salvinorin B | |
| -- inhibitory | ||
| Ligand-gated ion channels | PSAM-5-HT3 | PSEM 89S |
| -- activating | PSEM 308 | |
| PSAM-GlyR, PSAM-GABAc | ||
| -- inhibitory | ||
| PSAM4-5-HT3 | uPSEM792 | |
| -- activating | uPSEM817 | |
| PSAM4-GlyR | ||
| -- inhibitory | ||
A modified human muscarinic acetylcholine receptor 3 (hM3), which couples to Gq-type G proteins (referred to as hM3Dq), was one of the first GPCR-based DREADDs (Armbruster et al., 2007). The treatment of cells expressing hM3Dq with CNO depolarized neurons, which prove well suited to the activation of neuronal firing in a phospholipase C-dependent manner (Alexander et al., 2009). In many studies, the neural activity markers such as c-Fos are used to confirm neuronal activation mediated by the excitatory DREADD hM3Dq, along with monitor of electrophysiological activity, in order to validate the effectiveness of hM3Dq (Alexander et al., 2009; Krashes et al., 2011).
Moreover, a Gi-coupled inhibitory DREADD was generated by application of the hM3Dq mutations at homologous residues in the hM4 receptor, a Gi-coupled human muscarinic acetylcholine receptor (Armbruster et al., 2007). The expression of hM4Di in neurons renders cells sensitive to CNO-induced hyperpolarization via the reduction of forskolin-induced cyclic AMP production. The CNO-mediated activation of hM4Di results in a reduction of action firing in neurons (Armbruster et al., 2007) and inhibition of synaptic release (Mahler et al., 2014; Stachniak et al., 2014). There is also an hM4Di variant containing a C-terminal intracellular sequence of neurexin-1α, which allows the receptor to target axon and axon terminal for selective axonal silencing (Stachniak et al., 2014). To date, hM3Dq and hM4Di are the most commonly used Gq- and Gi-coupled DREADDs to modulate cellular activity. Both DREADDs are activated by the same ligand. As an alternative choice for hM4Di inhibitory DREADD, a kappa-opioid-based receptor DREADD (KORD) is another type of Gi-coupled inhibitory DREADD that requires salvinorin B as a ligand for its activation (Vardy et al., 2015). As the activation of hM3Dq and KORD requires a distinctive ligand, the use of these receptors together achieves to manipulate the same targeted neuronal population in the same animal in two opposing ways; i.e., hM3Dq-CNO for excitation; KORD-salvinorin B for inhibition (Vardy et al., 2015; Marchant et al., 2016).
A number of studies have also used a GPCR-based chemogenetic modulator coupled with Gs-type G proteins (Gs-DREADD), in which the intracellular regions of rat muscarinic receptor 3 DREADD (ratM3Dq) are substituted by the equivalent parts of turkey erythrocyte β-adrenergic receptor (Farrell et al., 2013; Akhmedov et al., 2017; Mahler et al., 2019). Upon Gs-DREADD activation by CNO, this system recruits the protein kinase A signaling pathway via inducing cyclic AMP production mediated by Gs α-subunit. This receptor also binds to Gαolf, a Gs-like α-subunit expressed in certain brain regions such as the striatum (Zhuang et al., 2000), which also allows researchers to investigate certain behavioral expression regulated by Gs-like signaling in distinct brain regions (Alcacer et al., 2017; Wang and Zhou, 2019; Garr and Delamater, 2020).
Selective β-arrestin signaling is also an important GPCR signaling pathway, which leads to distinct behavioral/physiological expression to well-known balanced canonical GPCR paradigm, and are believed to induce unwanted negative side effects caused by drugs (Lefkowitz and Shenoy, 2005; Raehal et al., 2005; Bruchas and Chavkin, 2010; Allen et al., 2011). DREADDs that selectively recruit the β-arrestin dependent signaling were developed by introducing an additional mutation on ratM3Dq (ratM3Dq(R165L), rM3Darr). CNO selectively activates β-arrestin dependent signaling via ratM3Dq(R165L) in vitro experiments, but in high concentration which is not an appropriate dose for in vivo experiments (Nakajima and Wess, 2012; Nakajima et al., 2016). More recently, Roth and colleagues have also developed GA-PAIR (GPCR/β-arrestin–plant protein and (+)-abscisic acid (ABA)-induced recruitment), in which a plant hormone, ABA, controls the interaction of hM3Dq and β-arrestin via plant proteins (Gotoh et al., 2018). This system induces membrane translocation of the β-arrestin2/hM3Dq complex in the presence of ABA but without the ligand for the receptor. This system has yet to be tested in vivo, but may be a potential toolbox to elucidate the regulatory mechanism of specific behavioral profiles underlying β-arrestin signaling.
Engineered ligand-gated ion channels:
In addition to GPCR-based chemogenetic modulators, engineered ligand-gated ion channels have also been used to manipulate specific neural circuits to understand behavioral expression observed under certain experimental environments (Magnus et al., 2011). Pharmacologically selective actuator modules (PSAMs) are engineered modulators based on the α7 nicotinic acetylcholine receptor, which are activated by brain-penetrating small molecule agonists termed pharmacologically selective effector molecules (PSEMs). In theory, it is possible to construct multiple types of PSAMs through combination with the ion pore domain (IPD) from different types of ion channels. For example, PSAMs attached to the IPD from the glycine receptor form chloride-selective channels that elicit neuronal inhibition in the presence of PSEM agonists. PSAMs, chimeric ion channels contained with GABAc are also inhibitory (Sternson and Roth, 2014). In contrast, PSAMs combined with the serotonin receptor 3 IPD lead to neuronal excitation owing to its cation-selective features in the response to the agonists (Eiselé et al., 1993; Grutter et al., 2005). More recently, Magnus and colleagues have also developed ultrapotent PSAM variants (PSAM4-GlyR and PSAM4-5HT3) that selectively bind to a clinically approved drug varenicline and its variants discussed below. Similar to the kappa-opioid-based DREADDs, these ion channel-based chemogenetic tools can be applied with either hM3D or hM4D DREADDs in order to manipulate neuronal activity within the targeted cells.
2.3. Ligands for chemogenetic modulators
Delivery of the chemical actuator responsible for the activation of engineered chemogenetic modulators but minimally activating any endogenous receptors can achieve ideal invasive chemogenetic agonism. CNO is water soluble and can be administered via drinking water, injected intraperitoneally, or even directly infused into a targeted intracranial tissue region (Burnett and Krashes, 2016; Campbell and Marchant, 2018). Although CNO had been considered little overt effects on physiology and behavior (Smith et al., 2016), an optimal dose of CNO administration (e.g., 1 mg/kg) affects amphetamine-induced dopamine release and behavior (the acoustic startle reflex) in rats without DREADD expression (MacLaren et al., 2016). Recent reports on systemic CNO administration have revealed that CNO is metabolically converted to clozapine in plasma (~2%) (Raper et al., 2017; Manvich et al., 2018). Gomez et al. (2017) also observed no penetration of radiolabeled CNO, [11C]CNO, in the rat brain by autoradiography and positron emission tomography. These studies indicate that metabolically derived clozapine, but not CNO, is the active actuator for in vivo muscarinic-based DREADD experiments in the brain (Gomez et al., 2017; Manvich et al., 2018). As clozapine is known to act on many endogenous receptors and neurotransporters, such as 5-HT2A/2C, dopamine D4, and histamine H1 receptors (Schmid et al., 2014; Williams et al., 2012), the systemic administration of CNO can potentially produce confounding off-targeted side effects that may cause unwanted alterations of the desired behavioral responses.
Alternative DREADD agonists such as compound 21 (C21) and deschloroclozapine (DCZ), were developed recently to diminish the off-target side effects of CNO and clozapine (Chen et al., 2015; Thompson et al., 2018; Nagai et al., 2020; Upright and Baxter, 2020). These agonists have similar selectivity and affinity as CNO, and are brain penetrable. Compared to CNO, C21 selectively and more rapidly induces the activation of muscarinic DREADDs, such as the excitatory hM3Dq, hM1Dq, and inhibitory hM4Di (Jendryka et al., 2019). Typically, the dose of C21 used is 0.5–3 mg/kg, which is generally lower than those of CNO (e.g., 1–10 mg/kg) (Jendryka et al., 2019). However, C21 is also known to show moderate binding affinity for several GPCRs, such as α1A-adrenoreceptor, histamine H1 receptor, dopamine (D1, D2), muscarinic (M1, M2, M3), opioid and serotonin receptors, as a functional antagonist in vivo, assessed by radio-labelled ligands binding enzyme assays (Jendryka et al., 2019). The C21 affinity may result in sedative side effects when high dose of C21 (~10 mg/kg) is used (Chen et al., 2015; Jendryka et al., 2019). Furthermore, a recent study using rats demonstrates that C21 could potentially increase a significant off-target effect with the commonly used doses (e.g., 0.5 to 1 mg/kg) in the substantia nigra, which is potentially through 5-HT2 and histamine H1 receptors (Goutaudier et al., 2020). It is also implied that there is a potential sex difference in the dose used to modulate their targeted behavior (Goutaudier et al., 2020). These indicate that the use of C21 still needs further investigations for optimal dosages applicable for each experimental condition, including sex and species of animal models, and targeted-cell types and -tissues.
DCZ is another alternative DREADD agonist, which has recently been reported as a ligand with approximately 60 to 100-fold greater affinity and potency for muscarinic DREADDs compared to C21 (Nagai et al., 2020). DCZ has displayed minimal affinity to most of other GPCRs, in which low affinities for endogenous receptors, including muscarinic acetylcholine M1 and M5 and serotonin 2A, 2B, 3, and 7 receptors, represents approximately eight- to ten-fold higher binding selectivity of DCZ for muscarinic DREADDs compared to these endogenous targets (Nagai et al., 2020). DCZ has also been used for behavioral experiments in monkeys with substantially lower dose (1–100 ug/kg) compared to the commonly used doses of CNO and C21 (Jendryka et al., 2019). These data suggest that DCZ is a next useful alternative to eliminate the negative off-target features observed with the current existing DREADD ligands. It is noteworthy that, although several attractive features in use of DCZ are found compared to the other DREADD ligands, the effective doses should be carefully determined in each experiment and paradigm, in order to optimize the effectiveness on behavioral/physiological parameters since limited information are available at this stage.
For the PSAM system, PSEMs are required for the activation of PSAMs (Magnus et al., 2011). One of the early developed PSEMs is based on PNU-282987 (Magnus et al., 2011), which affiliates moderately with α2- and β4-adrenoreceptors, 5-HT3, and α7-achechilcoline receptors. Owing to the molecular features of PSEMs, such as a short acting time and variable potency, the PSEM-PSAM system was originally thought to be an insufficient toolbox for in vivo neural manipulation applications. However, recent studies have overcome these limitations of PSEMs by developing highly selective and ultrapotent PSAM agonists, uPSEMs derived from brain-penetrant analogs of the smoking cessation drug varenicline (Magnus et al., 2019). A varenicline variant, uPSEM793 is effective to PSAM4s with dose of 0.03 mg/kg, which is about a 1000-fold improvement on the previously developed PSEMs with no detectable potential off-target behavioral effect for typical varenicline targets (Yizhar and Wiegert, 2019). Interestingly, the varenicline itself can also effectively silence neuronal activities in PSAM4-GlyR-expressing mice for more than 2 weeks by a systemic injection at low dose (0.1 mg/kg) that is considerably lower dose than that of clinical application (Yizhar and Wiegert, 2019). These data imply a potential use of this ion channel-based chemogenetics as a clinically applicable therapeutic tool for certain neurological disorders by manipulating the actions of specific neuronal populations.
2.4. Delivery of chemogenetic modulator genes
The chemogenetics have been applied to several model organisms as well as humans’ gene therapy (Roth, 2016; Jüttner et al., 2019). Here we focus on discussing experimental applications of chemogenetics in rodent models (i.e., with an emphasis on mice). There are two main strategies to deliver chemogenetic transgenes into targeted tissue regions; the use of transgenic mice expressing DREADD, and viral vectors encoding DREADD (Figure 1). The development of transgenic DREADD-expressing mouse lines has led to the current achievement of whole or cell type specific DREADDs expression. One of the early models used the tTA-off system, in which local removal of doxycycline permits hM3D expression at a targeted site from a CamKII promoter (Alexander et al., 2009). There are also Cre-dependent reporter mouse lines for hM3Dq and hM4Di, in which DREADD genes knocked into ROSA26 locus are expressed under the regulation of CAG promoter and a Lox-Stop-Lox cassette (Hausen et al., 2016; Zhu et al., 2016) (Fig. 1C,F). These Cre-dependent DREADD-expressing mouse lines allow researchers to target distinct cell types expressing Cre recombinase under cell type-specific promoters (Fig. 1C). In addition, by administering CNO site-specifically into floxed DREADD mouse lines, high temporal cell type- or spatial-specific neuronal regulation can be obtained by the DREADD technique.
Figure 1.
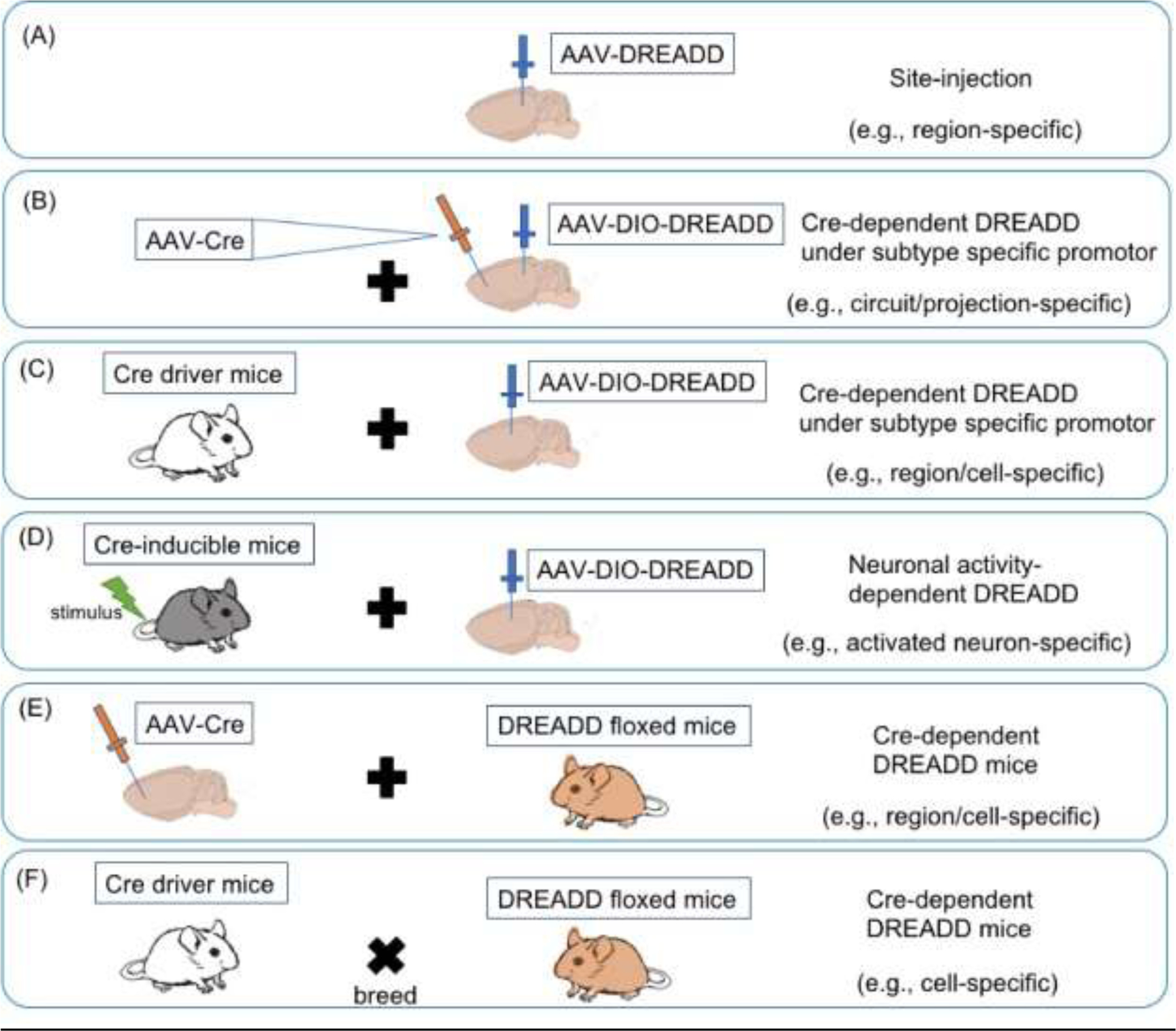
DREADD delivery strategies. (A) Virally injected DREADD into the targeted region in wildtype mice. (B) Virally injected cre-dependent DREADD in combination with virally injected cre driver target in wildtype mice. (C) Virally injected cre-dependent DREADD in cre driver mice. (D) Virally injected cre-dependent DREADD into transgenic mice that can express exogenously (e.g., tamoxifen) inducible cre recombinase from promoter elements (e.g., Fos-TRAP2 mice (Allen et al., 2017)). (E) Virally injected cre driver target in DREADD floxed mice (e.g., hM3Dq-DREADD mice (Zhu et al., 2016)). (F) Breeding cre driver mice with DREADD floxed mice, then F1-hybrid offspring expresses cre-dependent DREADD. Abbreviations: AAV (Adeno-associated virus), DIO (Double-floxed inverted), and DREADD (Designer receptors exclusively activated by designer drugs).
More often, viral vectors injected into the targeted tissue regions are used for the local delivery of chemogenetic transgenes via pre-mapped stereotactic coordinates (Fig. 1A). Recombinant viral vectors encoding a chemogenetic transgene diffused into the target tissue region permit anatomically restricted expression that is dependent on the features of viruses including the polarity, serotype, and tropism (Murlidharan et al., 2014). The most commonly used viral vectors to deliver chemogenetic modulators are the nontoxic adeno-associated virus (AAV), which contains a single-stranded DNA genome of approximately 4.8 kb (Rose et al., 1966).
Recombinant AAV, which lacks a self-amplifiable component, is designed to package genes of interest at any size under 5 kb (Dong et al., 2010). AAV vectors are either anterogradely or retrogradely delivered to target cells (Carter et al., 2013; Tervo et al., 2016: Francois et al., 2017). AAVs are used widely, and there are constructs with numerous promoters and transgenes. In addition, some of these vectors also contain loxP sites with transgenes that result in Cre-dependent expression (Atasoy et al., 2012)(Fig. 1B).
Virally mediated DREADD delivery removes the effort required to generate a novel mouse line. Virally introduced DREADDs expression allows for highly spatial and temporal control of the anatomical location expressing DREADD and avoids interference with any critical developmental process. In addition to regionally limited distribution, selective expression can be achieved using Cre-driver transgenic mouse lines (Fig. 1C) or through the administration of a second virus-driven expression of Cre-recombinase in wildtype mice (Fig.1B). Viruses can drive the expression of DREADDs by a cell-specific promoter, such as CamKII or GFAP (Stamatakis et al., 2013; Tervo et al., 2016; Zingg et al., 2017).
It should be noted that there are certain caveats in the use of AAV delivery strategies. The diffusion of the virus depends on several factors, including titer (viral particle concentration), diffusion rate, and potential tropism limitations in the target cell type of interest; moreover, each batch of virus has different profiles as described below (Murlidharan et al., 2014). The transduction efficiency varies across animals results in an unequal distribution and transduction of cells (Runegaard et al., 2019). Therefore, the actual number of neurons transduced will vary between animals that are treated in an identical manner, which requires precise post hoc histological analyses of viral transduction to determine the exact location of DREADD expression (Atasoy et al., 2012). To this extent, functional validation of DREADDs has been determined by monitoring expression of Fos, a neural activity marker, using immunohistochemistry (Krashes et al., 2011), or by recording neuronal activity in the targeted cells (or tissue regions) such as electrophysiology (Alexander et al., 2009; Krashes et al., 2011), fiber photometry (Steculorum et al., 2016) and calcium imaging (Anacker et al., 2018; Corder et al., 2019).
2.5. Cre-driver mice
Mouse genetic analysis with fluorescent proteins and DNA recombinase systems (Cre/loxP and Flp/FRT) has proven to be a powerful toolset to obtain a better understanding of specific neuronal functions at the molecular and neural circuitry levels. Note that Flp (flippase) is another commonly used site-specific DNA recombinase derived from yeast, which targets 34 bp FRT recognition sites (Raymond and Soriano, 2007). Numerous studies have demonstrated the power of mouse genetic engineering in allowing us to address the functions of particular neural circuits using hundreds of gene-specific promotor-driven Cre mouse lines along with neuroanatomical tracers and genetic modulators including tetracycline on/off effector system, chemogenetics and optogenetics. Researchers also have access to Cre-ER (human estrogen receptor) system, in which the activation of Cre recombinase is regulated by tamoxifen, an estrogen receptor ligand and its derivative. This system enables researchers to temporally regulate the Cre-dependent transgene recombination while the desired behavior is happening (Metzger et al., 1995; Metzger and Chambon, 2001). Recent studies in these mouse lines combined with Cre-dependent genetic tools such as DREADD and optogenetics provide ample opportunities to dissect the functionalities of cell- and molecular-specific circuits in cognitive behaviors.
There are also Cre-driver mouse lines to target stimulus-dependent activation of neurons under immediate early gene promotors, such as Fos and Arc (Guenthner et al., 2013; Sakurai et al., 2016; Allen et al., 2017). These Cre-driver mice allow the genetic preservation of neurons only activated by specific stimuli during a particular time period that can be set up with tamoxifen treatment, and enable us to characterize the neuronal ensembles that are regulating specific behavior by eliminating functional heterogeneity which is caused by the same neuronal types regulating other behaviors in the targeted tissue region. Altogether, neuroanatomical data for a number of Cre-driver mouse lines are readily available in public databases, which enables the determination of appropriate genetically-engineered mouse strains based on our research needs (Gerfen et al., 2013; Harris et al., 2014; Zhu et al., 2016).
2.6. Types of AAV vectors
The serotype of AAV vectors and the promoter used are significant determinants of the neuronal transduction and expression of the desired transgenes. Compared with other viral vectors, AAVs are non-toxic and permit long-term (months to year) expression (Morsy et al., 1998). Several commercially available promoters including human synapsin (hSyn), calmodulin-dependent protein kinase II (CamkII), cytomegalovirus (CMV or CAG), and EF-1α, as well as human glial fibrillary acidic protein (GFAP) are used to induce DREADD expression (more in detail: Tervo et al., 2016; Campbell and Marchant, 2018). Most of these promoters are primarily neuron-specific, although some possess preferences for cell types. For example, the CamkII predominately targets excitatory neurons in the cortex, but not always (Jennings et al., 2013; Yau and Mcnally, 2015), and GFAP is expressed in astrocytes (Yizhar et al., 2011).
At least 13 serotypes of AAVs have been discovered and these vectors are the top tier of viral vectors for transporting transgenes into the target brain regions (Srivastava, 2016). These serotypes display different tropism and transport efficiencies dependent upon the targeted cell types and tissue regions (Aschauer et al., 2013; Nair et al., 2015). Of these, AAV1, 2, 5, 6, 8, and 9 are common in use of neuroscience studies (Grimm et al., 2008). The expression of reporter genes varies significantly, depending upon the experimental conditions, including the serotypes used and the cell types targeted (Aschauer et al., 2013). These differences in serotype characteristics may stem from variation in capsids on the surface of viruses that can alter their affinity to binding partners on the targeted cell surface (Keenan et al., 2017). For example, AAV2 shows minimal ability to spread from the injection site compared to other serotypes (Mastakov et al., 2002), whereas AAV5, 8, and 9 likely display widespread transgene expression (Burger et al., 2004; Broekman et al., 2006; Cearley and Wolfe, 2006; Murlidharan et al., 2014). The cell entry of AAVs is mediated by interactions between glycans or proteins expressed on the cell membrane and AAV capsid proteins (Lykken et al., 2018). Therefore, the tendency of AAV vectors to label in an anterograde or retrograde direction results in the cellular distribution of cognate receptors to their surface proteins. Most of the AAV serotypes show anterograde labeling to infect the soma when injected into the rodent central nervous system, but AAV1, 5, 8, and 9 also exhibit retrograde transport, with the degree of tropism varying by the regions/tissues (Rothermel et al., 2013; Castle et al., 2014; 2016). A recently developed synthetic AAV capsid based on AAV2 showed highly effective and selective retrograde labeling, is also available as retro-AAV (Tervo et al., 2016). In addition, recent studies demonstrated that AAV1 (and likewise AAV9) is also transported anterogradely down the axon, with trans-synaptic labeling (Castle et al., 2014; Zingg et al., 2020). This indicates that AAV1-mediated transgene expression in pre-synaptic neurons can drive its expression in post-synaptic neurons, although the partial retrograde tropism would be confounded. In all the cases, pilot studies for checking AAV efficiency that will be used are highly recommended at certain time points after viral injection into the targeted brain regions. For example, in our preliminary work, one serotype-promoter combination (i.e., AAV 9-CAG-tdTomato) was effective for both anterograde and retrograde infection of cortical neurons, but only anterograde labeling for thalamic neurons when the virus was injected into thalamus. It is interesting to note that there are also two AAV 9 capsid variants, called PHP.eB and PHP.S, which enable noninvasive gene delivery via tropism specific transduction. PHP.eB efficiently transduces the majority of CNS neurons throughout the mouse brain and spinal cord, whereas the PHP.S variant is ideal for the introduction of transgene expression in peripheral neurons, such as the DRG, trigeminal ganglia, cardiac ganglia, and enteric nervous system.
3. Practice in chemogenetic applications
3.1. The advancement of DREADD application
Several virus types have been employed to deliver transgenes into the desired brain regions. Numerous successful studies have reported AAV vectors encoding modulator genes, such as DREADDs, and channelrhodopsins (receptors for optogenetics) to investigate specific neural circuits and cellular activities regulating targeted behavioral expression. Interestingly, these methodologies have also been employed for clinical treatments, such as gene therapy, of immune and neurological diseases (Weinberg et al., 2013; O’Connor and Boulis, 2015; Santiago-Ortiz and Schaffer, 2016; Grimm and Buning, 2017; Keenan et al., 2017). These results have stimulated investment in AAV production to improve the ability of AAV capsids for selective transduction and cell-type specificity (Tervo et al., 2016; Chan et al., 2017; Jüttner et al., 2019).
The technical basics required for the delivery of DREADD transgenes via AAV stereotaxic infusion are available in a number of biomedical methodological articles (cf. Lowery and Majewska, 2010; Stoica et al., 2013; Correia et al., 2017). For virus delivery, AAV infusion volume is a considerable factor influencing the extent to which the AAVs will transduce neurons and spread through tissues. Then, the incubation time following an AAV injection is a matter for permitting viral particles to internalize cells and to transfect DREADD transgenes into tissues. After attaching to the cells, viral components start to internalize into the cells within 8 min, and reach the perinuclear region within 40 min (Bartlett et al., 2000). However, the transfection of DREADD transgenes appears to require a longer incubation time; 12 h after AAV infusion is not sufficient to detect the fluorescence tags attached to the virus vectors around the injection site (Aschauer et al., 2013). The fluorescence tags attached downstream of DREADDs genes are visible through a microscope after 3 days of AAV infusion (Burnett and Knashes, 2016). Considering these colonized processes along with recovery period of animals from surgical infusion, the common incubation time for obtaining sufficient and stable DREADD expression is set as 2 to 3 weeks after virus injection (Smith et al., 2016).
A key advantage of chemogenetics is the ability to remotely control defined neuronal populations by a site-specific or systemic activator drug injection. This technique is especially ideal to modify neuronal activity over a prolonged time periods (the range of minutes to hours). The DREADD receptors are expressed in targeted neuronal membranes, which lack the ability to be activated by endogenous ligands, are sensitive to the inert exogenous ligand. The variants of DREADD include hM3Dq, an engineered M3 muscarinic receptor, which leads to the activation of the phospholipase C cascade altering intracellular calcium level, resulting in apparent neuronal firing (Armbruster et al., 2007). hM4Di is an inhibitory variant of DREADD, an engineered M4 muscarinic acetylcholine receptor, which induces the hyperpolarization of cells by decreasing cAMP signaling and increasing rectifying potassium channels (Armbruster et al., 2007; Rogan and Roth, 2011). Thus, the effectiveness of DREADD ligand administration for the inhibitory effect of hM4Di is weaker than those for the activation effect of hM3Dq (Farrell and Roth, 2013; Mahler et al., 2014). The differences in the sequential mechanisms of DREADD synthesis are also a considerable factor when applying the DREADD methods to studies on neural circuitry function. With an understanding of these characteristics of DREADD and its ligand interaction, chemogenetic manipulations can achieve gene delivery to discrete brain regions for highly specific, temporal anatomical control over neural activity in these sites. These chemogenetic features bring researchers who perform traditional studies on the interactions between drugs and behavior an innovative approach to dissect region- or circuit-specific mechanisms of brain function in the context of drug treatment. It is noteworthy that optogenetic manipulation via virally introduced photosensitive receptors in the targeted cells is alternative to gain further control of neural activity with a high spatio-temporal resolution. There are several drawbacks and advantages in the optogenetics compared to chemogenetics. One of the best advantages in optogenetics is a higher temporal resolution to rapidly (range of milliseconds) control neuronal activity which is likely mimicking the nature of neuronal activity (Kim et al., 2016). It requires permanently implanted optic probes attached with a fixed head-skull attachment that might cause limitations on certain behavioral measurements unless wireless devices are used. However, it is important to note that both chemogenetics and optogenetics should be considered as equivalent methods for manipulating neural activities; i.e. the techniques can be exchangeable within the same experimental designs. The choice of the methods should be depended on the resource availability and researcher’s experimental designs.
3.2. Region-specific application of DREADD manipulations
Chemogenetic methods also enable researchers to address intractable problems in interpreting conventional behavioral pharmacology studies. Administration of drugs both systemically and site-specifically induces functional heterogenous behavioral responses through the target receptors, because they are generally distributed throughout neural circuits and pre-synaptic and post-synaptic locations that orchestrate to produce integrative neuronal activities associated with drug-induced behavioral responses (Figure 2). Therefore, there are continual inconsistencies and disagreements between expected experimental outputs and drug effects on specific behaviors. Furthermore, behavioral responses expressed consequent to drug treatment are thought to be formed with association between environmental contexts and internal states consisting of several sequential micro-behavior components (Whissell et al., 2016). For example, drug-induced behavior consists of several sub-behavioral elements, including perception, learning, exploration, and emotional motives. Within certain brain regions, synaptic and neuronal processes are sequentially regulating these different behavioral components.
Figure 2.
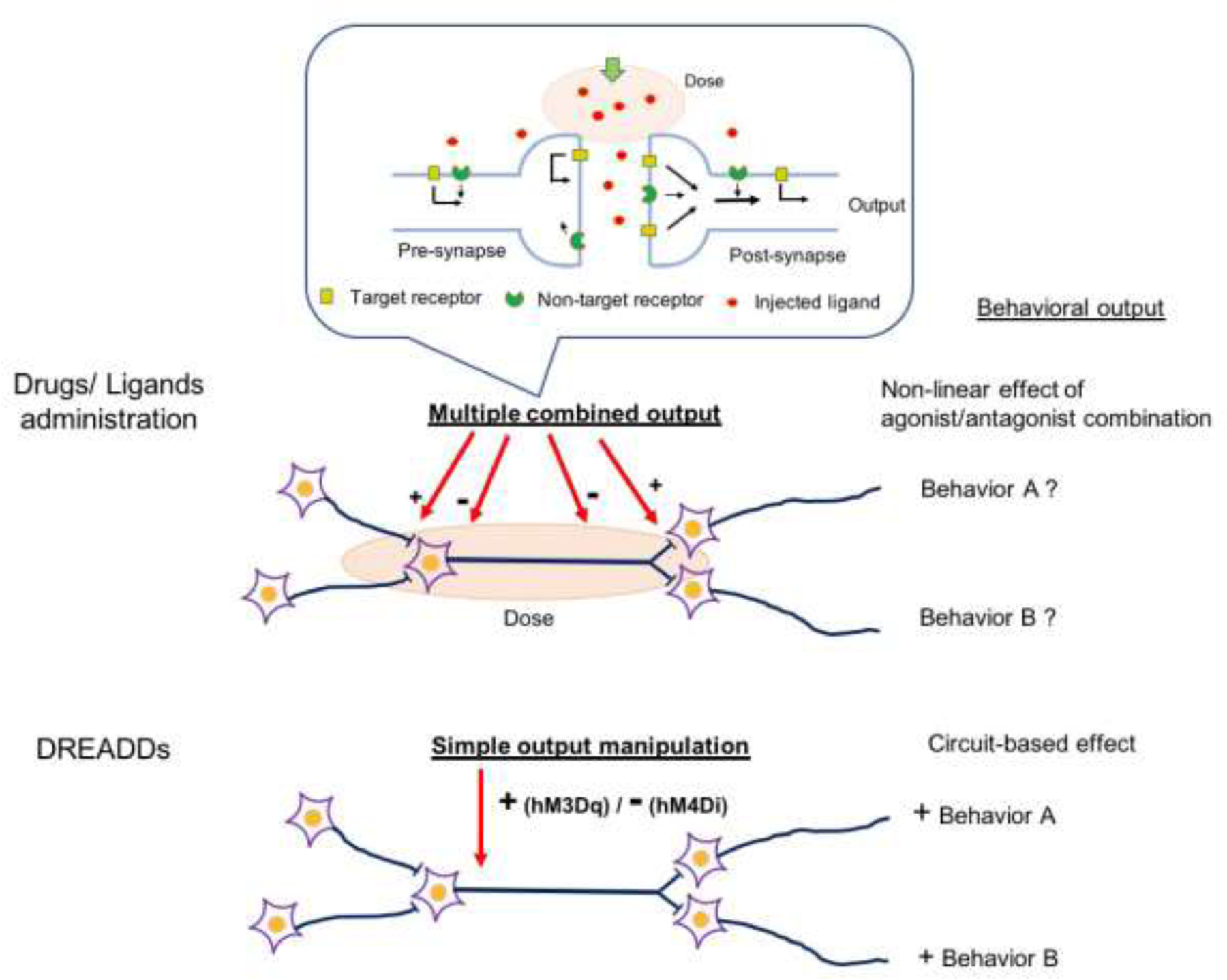
A schematic diagram of putative processing models in the differences of neuronal manipulation by drugs/ligands administration and the DREADD application. Drug administration induces humoral processes widely distributing drugs throughout the body via osmotic and circulation drive. Compounds of the drugs penetrate into the neurons and synaptic clefts and ultimately bind to several receptors in its affinity dependent manner. The locations of receptors include pre- and post-synapses and axonal tracts. Therefore, the total outputs of neural firing stimulated by drug administration will reflect combined effects of agonism and antagonism of drug stimulations, which would be read out as non-linear quantitative effects on the output of neuronal firing. On the other hand, the DREADDs application can archive cell- or circuit-specific manipulation of the simple output from the target neuronal node, which allows to investigate the neuron specific function on regulating particular behavior.
As chemogenetic manipulation provides better spatial-temporal resolutions, researchers are now able to focus on understanding a particular sub-behavioral component among the complex behavioral sequence derived by drug treatments. Thus, the excitatory and inhibitory DREADD approaches serve as a better replacement for traditional electrical stimulation and brain-lesion techniques respectively, in order to focus on the specific behavioral expressions subsequent or consequent to the behaviors that are also associated with drug treatment. The traditional electrical or surgical manipulation roughly clips around the target region/tissue, which significantly lacks a specificity of target cell or molecule manipulation. Moreover, each synapse, both in pre-synaptic and post-synaptic locations is known to contain multiple types of receptors; therefore, drug administration generally triggers signal transduction that aggregates activates of multiple receptors in synapses and neurons. Signal transduction processes are then initiated by the drug binding to multiple receptors, resulting in simultaneously modulation of different states of neuronal activity. Neural signal initiated by the drug administration would thus be depolarized to make a decision on to express desired behavior.
Multiple repeated administrations of a target receptor ligand via the intracranial cannulas are also problematic. A major drawback of microinjection methods is that the repeated infusion of the drug accrues damage at the target tissue and induces saturation of target receptor receptivity. In addition, the injection of a fluid into a target site influences cerebrospinal fluid flow and stimulates synaptic ligand-receptor binding at the injection site, and consequently, the cannula often becomes dysfunctional over time by clogging inside, which are a common problem when using cannula-guided microinjection. The DREADD techniques also require a delivery of viral vector into a target site, but thereafter, can achieve site-specific, repeated accurate manipulation of the target site by systemic ligand injections. Several studies have also demonstrated local infusions of the DREADD ligand into the brain region where DREADDs are expressed to trigger selective neural manipulation (Mahler et al., 2014; Stachniak et al., 2014; Lichtenberg et al., 2017; McGlinchey and Aston-Jones, 2017).
An additional advantage of using AAV-DREADD system for neuronal manipulation is the ability to use a Cre recombinase system combined with a DREADD-inserted loxP cassette, which permits the restriction of DREADDs expression in defined neuronal populations. Given the usefulness of the Cre-loxP system, numerous region- and molecular-specific Cre and inducible Cre transgenic mouse lines have been made available and are still under development, as described above. Cre-dependent viral injection into Cre-expressing mouse lines allows the restricted expression of DREADD in cells that express Cre. A possible caveat for the use of Cre-driver mice is the risk of off-target effects by Cre-induced tumors and tissue toxicity (Pfeifer et al., 2001; Janbandhu et al., 2014).
3.3. Circuit-specific application of DREADD manipulation
One of the most innovative advantages of chemogenetics is the use of DREADD transgenes to manipulate the activity of targeted neuronal circuits in behavior. This strategy permits the selective interrogation of certain neuronal circuits (e.g., a node of neuronal projection) and thus unified the manipulation of tangled synaptic processes that are associated with behavioral outputs. A dual viral vector axon targeting approach combined with wild-type animals would be commonly adopted to target specific projection neurons, in which the Cre recombinase is carried by an AAV vector retrograde (cf. anterograde is also applicable) transport through the projection (Fig. 1B). The axonal terminals around the injection site of the AAV/Cre vector should be anatomically connected with the brain region in which another AAV vector encoding Cre-dependent DREADDs (e.g., DIO; double-floxed inverse orientation) is injected. The Cre-dependent DREADDs will be expressed only when the AAV vectors recognize the neurons infected with AAV/Cre (Fenno et al., 2014). However, this projection targeting approach still tends to cause inappropriate expression of transgenes in the non-targeted projection neurons. The use of AAV/Cre vectors, in which Cre recombinase expression is tightly regulated under a neuronal subtype specific promoter offer us to solve this problem (Gompf et al., 2015). Under the genetic constructs, the recombination of Cre-dependent DREADDs is restricted in specific neuronal subtype, which allow researchers to target a specific neuronal projection in wild-type animals (Gompf et al., 2015; Wakabayashi et al., 2021).
Alternatively, a two-recombinase approach which employs Cre-driver mice and AAV/Flp, is for synergizing the restricted modulator gene expression in specific projection neurons to study neural tracing connectivity across the brain network (Wickersham et al., 2007; Fenno et al., 2014). In this strategy, Cre-dependent flippase (Flp) encoded in a retrograde virus is injected at the axonal terminal of a Cre driver mouse, while an AAV encoding Flp-dependent DREADDs is injected into the cell body side of the targeted projection neurons (Fig. 1C). Flp-mediated recombination then occurs at the recombination sites and allows inverse-flanked DREADD. This axon-genetic projection targeting approach enables highly specific and selective projection targeting compared with the axon projection targeting methods.
To achieve more accurate temporal dynamics of neural functional studies, an inducible Cre system is developed for more sophisticated controls of the Cre activation at the precise time and in a specific cell type (Fig. 1D). An exogenous inducer such as tetracycline or tamoxifen can temporally induce Cre recombinase expression or activation in the defined cell population via systemic or site-specific injection (Kim et al., 2018), and AAV vectors carrying Cre-dependent DREADDs transduce the DREADD receptors into the target regions in which Cre is temporally expressed. This strategy allows researchers to determine the magnitude of the behavioral effects selectively manipulated by the target brain region/circuit in which Cre recombinase is expressed or activated in a temporal and cell-type specific manner.
One of the main purposes of research into behavioral pharmacology is to elucidate the neurochemical processes underlying the effects of a drug on behavior, and this research has yielded invaluable assays in isolating neurochemical mechanisms of drug action and in relating the effects to observed changes in behavior (Zimmermann and Poling, 2016). However, systemically administered drugs are delivered to the entire body, including the central nervous system via blood circulation, and diffusively bind to various receptors in an affinity dependent manner. Drugs are widespread to reach receptors located throughout the pre- and post-synapses and even dendrites in various types of neurons, resulting in the production of multiplex outputs via signal transduction processes as a set of multiple neuronal firings. Furthermore, drug action is not limited to the central nervous system but also is an interaction with external environmental variables, and also has an effect on the peripheral tissues and body itself all that can play a role in the regulation of behavior either directly or indirectly. This nature of drug action is accompanied by practical difficulties in the investigation of neurochemical mechanisms underlying drug action when aiming to identify cell- or circuit-specific targets of drug activity. Thus, many behavioral pharmacology studies have explored the relationship between the dose of drugs and the occurrence of an operated/targeted behavioral responses, which has contributed to the progression of studies on rate-dependent drug effects to control behavioral outcomes (Quisenberry et al., 2016). These classical strategies must be revised with the advent of chemogenetics particularly for application to the investigation of neurochemical mechanisms underlying drug action on behavior.
Behavior is produced via the intermittent signaling outputs of patterning interaction among synaptic activities through inter-connected neural circuits. A selective manipulation of particular neurons that are connected from/to the axonal terminals of other inter-connected neurons, either by chemogenetics or drug stimulation, would induce multiple orchestrated interactions in the neural system, which is “read out” as a discrete sequence of behavior. The network-wide activity of neural circuits will be responsible for certain behavioral expression that also interacts with environment and circumstances. Likewise, the drug action must be considered as an output resulting from functional relationships between several variables of drug action across the central nervous system and external variables, including drug treatment and environmental factors. Consequently, chemogenetic manipulation achieves the precise control of neuronal activities generated by a node of specific neurons or circuits, as the functional investigation of the particular neurons or circuits will be executed by probing each piece or node of components comprising the circuitry system that regulates a set of expressions of integrated behavior. This is nothing special, as drug manipulations are implemented based on humoral regulation, whereas chemogenetic manipulations are achieved by handling wire-connected circuitry (Figure 2).
3.4. Serotonergic circuits interrogation using DREADD manipulation
The DREADDs technique has been used widely to identify neuronal subtypes and neural circuits responsible for certain behavioral expression in a particular environment. Inhibitory DREADDs have provided an invaluable tool to determine the temporal necessity of certain anatomical nodes in neuronal functions, which could serve as a pseudo brain-lesion technique. Chemical or electrical lesions are valuable approaches to elucidate the loss of function in the lesioned region/node of interest; however, these methods significantly lack the specificity of cell- or molecular-target manipulation, and thus, it is difficult to parse out lesion effects from compensatory changes and vulnerable fluctuations following permanent tissue damage. Here we will show how the DREADDs technique can archive better replications and replacements with traditional methodologies, to dissect cell specific processes in 5-HT neurons (Figure 3).
Figure 3.
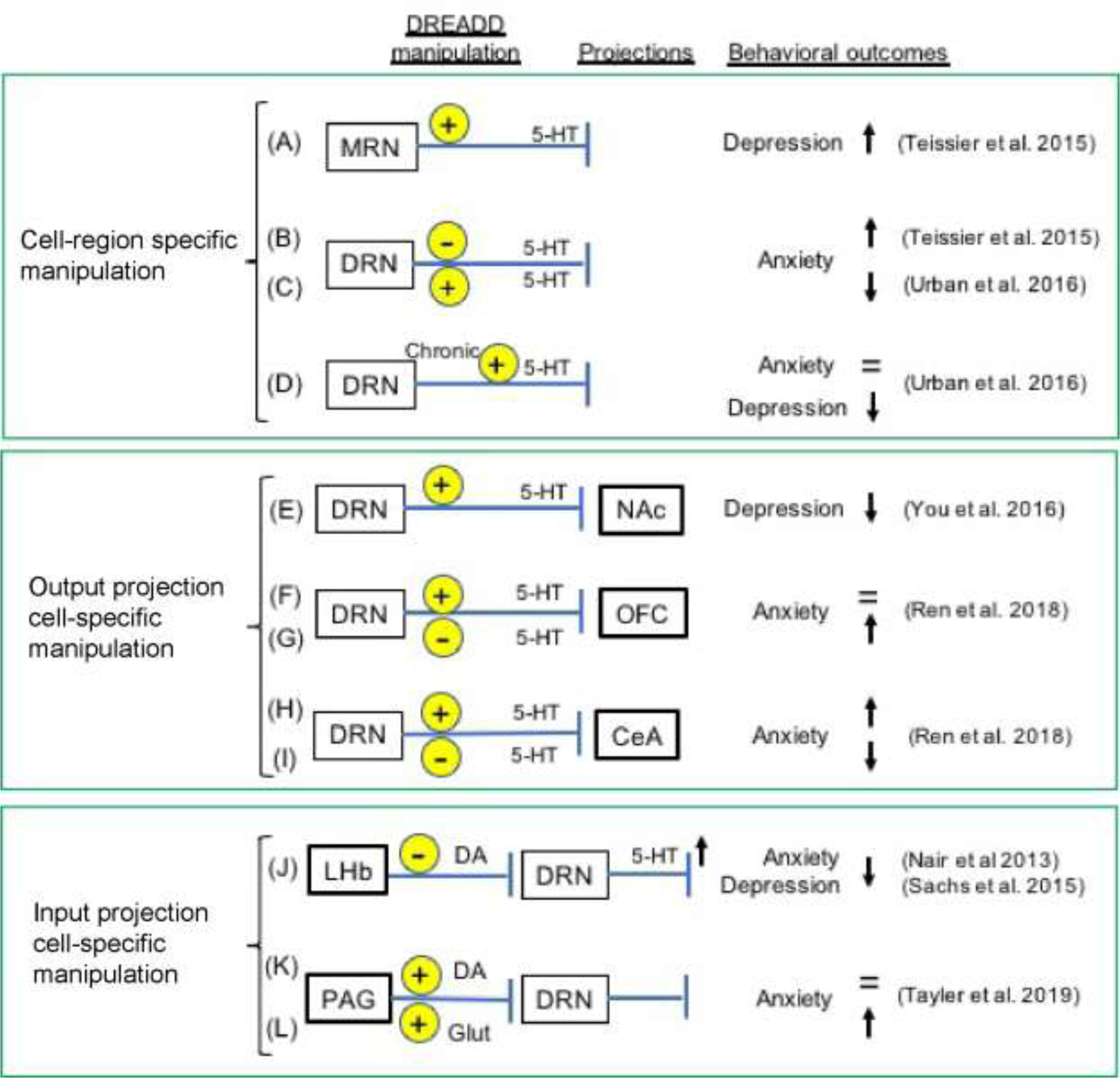
A schematic of summarized research investigating serotonergic circuits in the raphe nuclei, using chemogenetic manipulation. Cell-region specific manipulation; designed to manipulate region-dependent (MRN: median raphe, DRN: dorsal raphe) cell-type (5-HT: serotonergic neuron) specific activities (A-D). Output projection-cell specific manipulation; designed to modulate serotonergic projecting neurons to targeted regions (NAc; nucleus accumbens, OFC; orbitofrontal cortex, and CeA; central amygdala)(E-I). Input projection-cell specific manipulation; designed to manipulate specific projecting neurons (DA; dopaminergic, and glutamate; glutamatergic) from afferent regions (LHb; the lateral habenula, PAG; the periaqueductal gray) to the DRN (serotonergic) neurons (J-L).
The lateral habenula (LHb), a brain region connected to the brainstem nuclei, is involved in dopaminergic nuclei and serotonergic raphe nuclei (Geisler and Trimble, 2008). 5-HT neurons in the dorsal raphe nucleus are inhibited by electrical stimulation of the LHb (Wang and Aghajanian, 1977), whereas electrical lesions of the LHb enhance 5-HT activation in the dorsal raphe nucleus accompanied by decreased depression-like behaviors (Yang et al., 2008).
Inhibitory DREADDs can replicate the loss of function in the LHb in a temporal, reversal order. Interestingly, chemogenetic inhibition of the LHb temporally increases 5-HT levels in the dorsal raphe and reduces expression of anxiety and depression-like behaviors in rodent models (Fig. 3J) (Nair et al., 2013; Sachs et al., 2015).
The majority of the 5-HT signaling system originates in the raphe nuclei, which are involved in the processing of mood and motivation, along with several fundamental physiological and cognitive responses. Serotonergic neurons possess two discrete modes of firing: tonic firing is a “clock-like” neuronal activity (Jacobs and Azmitia, 1992) that would be associated with extra-synaptic 5-HT levels; and the burst firing is the rapid, high-amplitude firing associated with intra-synaptic 5-HT release (Quentin et al., 2018). These complicated mode differences in 5-HT activity are likely to be translated into certain behaviors, but this remains unclear. A pharmacological lesion in the dorsal raphe 5-HT nucleus facilitates anxiety-like behaviors in rats (Sena et al., 2003), whereas electrolytic lesion in the median raphe nucleus suppresses these anxietic behaviors (Jacobs and Cohen, 1976). Chemogenetic selective manipulations on the median and dorsal raphe nuclei provide evidence for the differentiated function of raphe nuclei in controlling emotional behavior, in which the activation of 5-HT neurons in the median raphe (Fig. 3A) and the inhibition of 5-HT neurons in the dorsal raphe induced increased anxiety and depression-like behaviors (Fig. 3B) (Teissier et al., 2015). Furthermore, selective manipulation of the dorsal raphe 5-HT neurons is potently relevant to the expression of anxiety-like behaviors (Fig. 3B,C), whereas those of the median raphe 5-HT neurons primarily influence changes in depression-like behaviors (Fig. 3A) (Teissier et al., 2015). These studies demonstrate the reasonable feasibility of chemogenetic approaches showing a highly cell- and region-specific manipulation. Although DREADDs can be used to modulate neural activity transiently in single test sessions, they are particularly well-suited for studies in which a brain area must be manipulated repeatedly over days. The acute inhibition of 5-HT neurons of the dorsal raphe nucleus by DREADD enhances the expression of anxiety-like behaviors but decreases depression-like behaviors (Fig. 3B), while an activation of these 5-HT neurons reduces the anxiety-like behavior (Fig. 3C) (Urban et al., 2016; You et al., 2016). In contrast, chronic stimulation of DREADD receptors in the dorsal raphe does not affect anxiety-like behavior, but maintains the suppression of depression-like behaviors (Fig. 3D) (Urban et al., 2016). This difference in the time-course behavior implies a temporal resolution in the dorsal raphe serotonergic states regulating emotional behaviors, and this would be associated with phase differences in serotonergic firing modes (Quentin et al., 2018).
Chemogenetic manipulations of neural circuitry can also provide more profound and accurate information regarding the cell-specific neuronal circuity associated with cognate behaviors, which classical drug manipulation is unable to accomplish. The 5-HT neurons densely spread its projections to several brain regions; consequently, each tract of projections has a different regulatory function for several behaviors. A specific circuit manipulation using DREADDs demonstrated that the serotonergic dorsal raphe nucleus afferents to the nucleus accumbens facilitates anti-depression-like behavior, whereas inhibition yielded cocaine-seeking behavior (Fig. 3E) (You et al., 2016). The dorsal raphe 5-HT neurons are also projected to several different brain regions, including the orbitofrontal cortex and the central nucleus of the amygdala (Spannuth et al., 2011). The cell-specific circuit manipulation revealed that activation of the dorsal raphe 5-HT neurons projected to the amygdala promoted anxiety-like behavior (Fig. 3H), whereas inhibition of the 5-HT projections suppressed those behaviors (Fig. 3I) (Ren et al., 2018). In contrast, the DREADD-mediated inhibition of the dorsal raphe 5-HT neurons projected to the orbitofrontal cortex facilitated anxiety-like and depression-related behaviors (Fig. 3G), while activation of those 5-HT neurons had no effect on these behaviors (Fig. 3F) (Ren et al., 2018). These gain- and loss of function experiments nicely providing cell-specificity, illustrating compelling evidence that the dorsal raphe 5-HT system contained parallel sub-projections that result in different (even opposite) outputs in behavioral functions. The result of circuit-specific behavioral function in the dorsal raphe 5-HT neurons was confirmed by optogenetic manipulation, in which optical activation of the dorsal raphe 5-HT neurons projected to the amygdala enhanced anxiety-like behavior (Bernabe et al., 2020). Furthermore, a selective input to the dorsal raphe nucleus has been also investigated using DREADDs manipulation, in which the dopaminergic neurons in the ventrolateral periaqueductal gray (PAG) projected to the dorsal raphe were not involved in the regulation of anxiety-like behavior (Fig. 3K), but in analgesia, whereas the glutamatergic neurons in the PAG projected to the dorsal raphe stimulated anxiety-like behavior (Fig. 3L) (Taylor et al., 2019). Collectively, these technical challenges using chemogenetics provide crucial information on the specific function of rich and complex circuitry and the cell populations regulating emotional and other related behaviors that have been intimated by conventional drug studies, thereby providing new strategies for pharmacological interrogation and therapeutic treatment.
4. Future remarks
The field of experimental studies of drug effects on behavior (that is, behavioral pharmacology) must be combined with the most current functional analytical techniques in order to: 1) obtain a better understanding of the cognate neurological mechanism regulating behavior; and then 2) ultimately provide better therapeutic strategies to the patients who experience clinical problems in behavioral adaptation to psychiatric diseases and in coping with drug-related health issues. The neurochemical mechanisms of drug action in the central nervous system are a complex interactive process, which hampers the efforts to identify a simple one-on-one relationship between drug and behavior. The current chemogenetic techniques can provide a highly intense spatial and temporal resolution for more accurate manipulation of the brain function associated with specific behavior. Furthermore, chemogenetics facilitates a decision of paradigm change in the framework and methodology of conventional behavioral pharmacology.
It is important to be aware that there are indeed technical limitations in the chemogenetic manipulations highlighted in this review. The clinical application of chemogenetics in conjunction with specific cell or circuit function therapies, also has some critiques that need to be overcome. In this context, the use of chemogenetics offers a powerful approach to change the concept of drugs for controlling behavior and therapeutic strategies for managing issues relating to drug use and behavioral adaptation. We anticipate that greater progress in behavioral intervention therapy as well as psychopharmacology will be provided by coupling with chemogenetic brain stimulation that allows a more integrated manipulation of particular behavioral phases with advanced chemogenetic techniques in the future.
Highlights:
A next-step advent of psychopharmacology will be precipitated by chemogenetics
Chemogenetics achieve with a better temporal and spatial resolution in manipulation of neural circuits than conventional psychopharmacological methods
Several practical characteristics of chemogenetics employed in mouse model system are introduced.
One-on-one serotonergic circuit-based control over behavioral processes can be revealed by chemogenetic approach
Acknowledgments
Funding
This study was supported by FAU Brain Institute Neuroscience Pilot Award Program and National Institute on Drug Abuse grant DA040882 (AO), and MEXT KAKENHI Grant Number 19K24681 (HA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Alcacer C, Andreoli L, Sebastianutto I, Jakobsson J, Fieblinger T, Cenci MA (2017) Chemogenetic stimulation of striatal projection neurons modulates responses to Parkinson’s disease therapy. J Clin Invest 127(2):720–734. doi: 10.1172/JCI90132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL (2009) Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63: 27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JA, Yost JM, Setola V, Chen X, Sassano MF, Chen M, et al. (2011) Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc. Natl. Acad. Sci. U.S.A 108, 18488–18493. doi: 10.1073/pnas.1104807108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen WE, DeNardo LA, Chen MZ, Liu CD, Loh KM, Fenno LE, Ramakrishnan C, Deisseroth K, Luo L (2017) Thirst-associated preoptic neurons encode an aversive motivational drive. Science 357(6356):1149–1155. doi: 10.1126/science.aan6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmedov D, Mendoza-Rodriguez MG, Rajendran K, Rossi M, Wess J, Berdeaux R (2017) Gs-DREADD knock-in mice for tissue-specific, temporal stimulation of cyclic AMP signaling. Mol. Cell Biol 37(9):e00584–00516. doi: 10.1128/MCB.00584-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, Chen B, Hen R (2018) Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature 559(7712):98–102. doi: 10.1038/s41586-018-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster MN, Li X, Pausch MH, Herlitze S, Roth BL (2007) Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA 104(12): 5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigas F. (2015) Developments in the field of antidepressants, where do we go now? Eur Neuropyschopharmacol 25(5):657–670. doi: 10.1016/j.euroneuro.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Aschauer DF, Kreuz S, Rumpel S (2013) Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One 8(9): e76310. doi: 10.1371/journal.pone.0076310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM (2012) Deconstruction of a neural circuit for hunger. Nature 488(7410):172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Sternson SM (2018) Chemogenetic tools for causal cellular and neuronal biology. Physiol Rev 98(1): 391–418. doi: 10.1152/physrev.00009.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JS, Wilcher R, Samulski RJ (2000) Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J Virol 74(6):2777–2785. doi: 10.1128/jvi.74.6.2777-2785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, Gray JA, Roth BL (2009) The expanded biology of serotonin. Annu Rev Med 60: 355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabe C, Caliman I, Truitt WA, Molosh AI, Lowry CA et al. (2020) Using loss- and gain-of-function approaches to target amygdala-projecting serotonergic neurons in the dorsal raphe nucleus that enhance anxiety-related and conditioned fear behaviors. J. Psychopharmacol 34(4): 400–411. doi: 10.1177/0269881119900981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekman ML, Comer LA, Hyman BT, Sena-Esteves M (2006) Adeno-associated virus vectors serotyped with AAV8 capsid are more efficient than AAV-1 or −2 serotypes for widespread gene delivery to the neonatal mouse brain. Neuroscience 138, 501–510. doi: 10.1016/j.neuroscience.2005.11.057 [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Chavkin C (2010) Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berl.) 210:137–147. doi: 10.1007/s00213-010-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S et al. (2004) Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol. Ther 10, 302–317. doi: 10.1016/j.ymthe.2004.05.024 [DOI] [PubMed] [Google Scholar]
- Burnett CJ, Krashes MJ (2016) Resolving behavioral output via chemogenetic designer receptors exclusively activated by designer drugs. J Neurosci 36(36): 9268–9282. doi: 10.1523/JNEUROSCI.1333-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbel EJ, Marchant NJ (2018) The use of chemogenetics in behavioural neuroscience: receptor variants, targeting approaches and caveats. Br J Pharmacol 175(7): 994–1003. doi: 10.1111/bph.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Soden ME, Zweifel LS, Palmiter RD (2013) Genetic identification of a neural circuit that suppresses appetite. Nature 503(7474):111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle MJ, Gershenson ZT, Giles AR, Holzbaur EL, Wolfe JH (2014) Adeno-associated virus serotypes 1, 8, and 9 share conserved mechanisms for anterograde and retrograde axonal transport. Hum Gene Ther 25(8):705–720. doi: 10.1089/hum.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle MJ, Turunen HT, Vandenberghe LH, Wolfe JH (2016) Controlling AAV tropism in the nervous system with natural and engineered capsids. Methods Mol Biol 1382:133–149. doi: 10.1007/978-1-4939-3271-9_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cearley CN, Wolfe JH (2006) Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol. Ther 13, 528–537. doi: 10.1016/j.ymthe.2005.11.015 [DOI] [PubMed] [Google Scholar]
- Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu WL, Sánchez-Guardado L, Lois C, Mazmanian SK et al. (2017) Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci 20(8):1172–1179. doi: 10.1038/nn.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WH, Lin SK, Lane HY, Wei FC, Hu WH, Lam YWF, Jann MW (1998) Reversible metabolism of clozapine and clozapine N-oxide in schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry 22(5):723–739. doi: 10.1016/s0278-5846(98)00035-9. [DOI] [PubMed] [Google Scholar]
- Charnay Y, Léger L (2010) Brain serotonergic circuitries. Dialogues Clin Neurosci 12(4):471–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Choo H, Huang XP, Yang X, Stone O, Roth BL, Jin J (2015) The first structure-activity relationship studies for designer receptors exclusively activated by designer drugs. ACS Chem Neurosci 6(3):476–484. doi: 10.1021/cn500325v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Ahanonu B, Grewe BF, Wang D, Schnitzer MJ, Scherrer G (2019) An amygdalar neural ensemble that encodes the unpleasantness of pain. Science 363(6424):276–281. doi: 10.1126/science.aap8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia PA, Matias S, Mainen ZF (2017) Stereotaxic adeno-associated virus injection and cannula implantation in the dorsal raphe nucleus of mice. Bio protoc 7(18):e2549. doi: 10.21769/BioProtoc.2549.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B, Nakai H, Xiao W (2010) Characterization of genome integrity for oversized recombinant AAV vector. Mol Ther 18(1):87–92. doi: 10.1038/mt.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiselé JL, Bertrand S, Galzi JL, Devillers-Thiéry A, Changeux JP, Bertrand D (1993) Chimaeric nicotinic-serotonergic receptor combines distinct ligand binding and channel specificities. Nature 366(6454):479–483. doi: 10.1038/366479a0. [DOI] [PubMed] [Google Scholar]
- Farrell MS, Roth BL (2013) Pharmacosynthetics: reimagining the pharmacogenetic approach. Brain Res 1511:6–20. doi: 10.1016/j.brainres.2012.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno LE, Mattis J, Ramakrishnan C, Hyun M, Lee SY, He M, Tucciarone J et al. (2014) Targeting cells with single vectors using multiple-feature Boolean logic. Nat Methods 11(7):763–772. doi: 10.1038/nmeth.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MS, Pei Y, Wan Y, Yadav PN, Daigle TL, Urban DJ. et al. (2013). A Gαs DREADD mouse for selective modulation of cAMP production in striatopallidal neurons. Neuropsychopharmacology, 38(5):854–862. doi: 10.1038/npp.2012.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois A, Low SA, Sypek EI, Christensen AJ, Sotoudeh C, Beier KT, Ramakrishnan C, Ritola KD, Sharif-Naeini R, Deisseroth K et al. (2017) Brainstem-spinal cord inhibitory circuit for mechanical pain modulation by GABA and enkephalins. Neuron 93(4):822–839. doi: 10.1016/j.neuron.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson R, Lagerström MC, Lundin LG, Schiöth HB (2003) The G-protein-coupled receptors in the human genome form five main families. Mol Pharmacol 63(6):1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- Garr E, Delamater AR (2020) Chemogenetic inhibition in the dorsal striatum reveals regional specificity of direct and indirect pathway control of action sequencing. Neurobiol Learn Mem 169:107169. doi: 10.1016/j.nlm.2020.107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Trimble M (2008) The lateral habenula: no longer neglected. CNS Spectr 13(6):484–489. doi: 10.1017/s1092852900016710. [DOI] [PubMed] [Google Scholar]
- Gerak LR, Maguire DR, France CP (2019) Behavioral pharmacology of drugs acting at Mu opioid receptors. Handb Exp Pharmacol 258:127–145. doi: 10.1007/164_2019_265. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Paletzki R, Heintz N (2013) GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron 80(6):1368–1383. doi: 10.1016/j.neuron.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y, Giguere PM, Nichols DE, Roth B (2018) A chemogenetic platform for spatio-temporal control of b-arrestin translocation and signaling at G protein-coupled receptors. bioRxiv [Google Scholar]
- Goutaudier R, Coizet V, Carcenac C, Carnicella S (2020) Compound 21, a two-edged sword with both DREADD-selective and off-target outcomes in rats. PLoS One 15(9):e0238156. doi: 10.1371/journal.pone.0238156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Lee JS, Wang L, Desai T, Akache B, Storm TA, Kay MA (2008) In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J. Virol 82(12):5887–5911. doi: 10.1128/JVI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Büning H (2017) Small but increasingly mighty: Latest advances in AAV vector research, design, and evolution. Hum Gene Ther 28(11):1075–1086. doi: 10.1089/hum.2017.172. [DOI] [PubMed] [Google Scholar]
- Grutter T, Prado de Carvalho L, Dufresne V, Taly A, Edelstein SJ, Changeux J-P (2005) Molecular tuning of fast gating in pentameric ligand-gated ion channels. Proc. Natl. Acad. Sci. USA, 102(50):18207–18212. doi: 10.1073/pnas.0509024102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Podriguez LA, Ellis RJ, Richie CT, Harvey BK et al. (2017) Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357(6350):503–507. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompf HS, Budygin EA, Fuller PM, Bass CE (2015) Targeted genetic manipulations of neuronal subtypes using promoter-specific combinatorial AAVs in wild-type animals. Front Behav Neurosci 2015.00152 doi: 10.3389/fnbeh.2015.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenthner CJ, Miyamichi K, Yang HH, Heller HC, Luo L (2013) Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron 78(5):773–784. doi: 10.1016/j.neuron.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Hirokawa KE, Sorensen SA, Gu H, Mills M, Ng LL, Bohn P et al. (2014) Anatomical characterization of Cre driver mice for neural circuit mapping and manipulation. Front Neural Circuits 8:76. doi: 10.3389/fncir.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausen AC, Ruud J, Jiang H, Hess S, Varbanov H, Kloppenburg P, Bruning JC (2016) Insulin-dependent activation of MCH neurons impairs locomotor activity and insulin sensitivity in obesity. Cell Rep 17(10):2512–2521. doi: 10.1016/j.celrep.2016.11.030. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH (1998) Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc. Natl. Acad. Sci. USA, 95(25):15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP (1994) International union of pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev 46(2):157–203. [PubMed] [Google Scholar]
- Jacobs BL, Azmitla EC (1992) Structure and function of the brain serotonin system. Physiol Rev 72(1):165–229. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Cohen A (1976) Differential behavioral effects of lesions of the medial or dorsal raphe nuclei in rats: open field and pain-elicited aggression. J Comp Physiol Psychol 90(1):102–108. [DOI] [PubMed] [Google Scholar]
- Janbandhu VC, Moik D, Fassler R (2014) Cre recombinase induces DNA damage and tetraploidy in the absence of LoxP sites. Cell Cycle 13(3):462–470. doi: 10.4161/cc.27271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendryka M, Palchaudhuri M, Ursu D, van der Veen B, Liss B, Kätzel D, Nissen W, Pekcec A (2019) Pharmacokinetic and pharmacodynamic actions of clozapine-N-oxide, clozapine, and compound 21 in DREADD-based chemogenetics in mice. Sci Rep 9(1):4522. doi: 10.1038/s41598-019-41088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings KA, Lesch KP, Sharp T, Cragg SJ (2010) Non-linear relationship between 5-HT transporter gene expression and frequency sensitivity of 5-HT signals. J Neurochem 115(4):965–973. doi: 10.1111/j.1471-4159.2010.07001.x. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD (2013) Distinct extended amygdala circuits for divergent motivational states. Nature 496(7444):224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, 1990. Behavioral pharmacology, drug abuse, and the future. Behav Pharmacol 1(4):385–393. [PubMed] [Google Scholar]
- Jüttner J, Szabo A, Gross-Scherf B, Morikawa RK, Rompani SB, Hantz P, Szikra T, Esposti F, Cowan CS, Bharioke A et al. (2019) Targeting neuronal and glial cell types with synthetic promoter AAVs in mice, non-human primates and humans. Nat Neurosci 22(8):1345–1356. doi: 10.1038/s41593-019-0431-2. [DOI] [PubMed] [Google Scholar]
- Keenan WT, Fernandez DC, Shumway LJ, Zhao H, Hattar S (2017) Eye-drops for activation of DREADDs. Front Neural Circuits 11:93. doi: 10.3389/fncir.2017.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Ahrlund-Richter S, Wang X, Deisseroth K, Carlen M (2016) Prefrontal parvalbumin neurons in control of attention. Cell 164:208–218. doi: 10.1016/j.cell.2015.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kim M, Im S-K, Fang S (2018) Mouse Cre-LoxP system: general principles to determine tissue-specific roles of target genes. Lab Anim Res 34(4):147–159. doi: 10.5625/lar.2018.34.4.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Moratos-Flier E, Roth BL, Lowell BB (2011) Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 121(4):1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK (2005) Transduction of receptor signals by β-arrestins. Science 308: 512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Lichtenberg NT, Pennington ZT, Holley SM, Greenfield VY, Cepeda C, Levine MS et al. (2017) Basolateral amygdala to orbitofrontal cortex projections enable cue-triggered reward expectations. J Neurosci 37(35):8374–8384. doi: 10.1523/JNEUROSCI.0486-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery RL, Majewska AK (2010) Intracranial injection of adeno-associated viral vectors. J Vis Exp (45):2140. doi: 10.3791/2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykken EA, Shyng C, Edwards RJ, Rozenberg A, Gray SJ (2018) Recent progress and considerations for AAV gene therapies targeting the central nervous system. J Neurodev Disord 10(1):16. doi: 10.1186/s11689-018-9234-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren DA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, Espana RA, Clark SD. (2016) Clozapine N-Oxide administration produces behavioral effects in Long-Evans rats: implications for designing DREADD experiments. eNeuro 3(5):0219–0216. doi: 10.1523/ENEURO.0219-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus CJ, Lee PH, Atasoy D, Su HH, Looger LL, Sternson SM (2011) Chemical and genetic engineering of selective ion channel-ligand interactions. Science 333(6047):1292–1296. doi: 10.1126/science.1206606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus CJ, Lee PH, Bonaventura J, Zemla R, Gomez JL, Ramirez MH, Hu X, Galvan A, Basu J, Michaelides M, Sternson SM (2019) Ultrapotent chemogenetics for research and potential clinical applications. Science 364(6436), eaav5282. doi: 10.1126/science.aav5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones G (2014) Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci 17(4):577–585. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Brodnik ZD, Cox BM, Buchta WC, Bentzley BS, Quintanilla J. et al. (2019) Chemogenetic manipulations of ventral tegmental area dopamine neurons reveal multifaceted roles in cocaine abuse. J Neurosci 39(3):503–518. doi: 10.1523/JNEUROSCI.0537-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manvich DF, Webster KA, Foster SL, Farrell MS, Ritchie JC, Porter JH, Weinshenker D (2018) The DREADD agonist clozapine N-oxide (CNO) is reverse-metabolized to clozapine and produces clozapine-like interoceptive stimulus effects in rats and mice. Sci Rep 8(1): 3840. doi: 10.1038/s41598-018-22116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Whitaker LR, Bossert JM, Harvey BK, Hope BT, Kaganovsky K et al. (2016) Behavioral and physiological effects of a novel k-opioid receptor-based DREADD in rats. Neuropsychopharmacology 41(2):402–409. doi: 10.1038/npp.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastakov MY, Baer K, Kotin RM, During MJ (2002) Recombinant adeno-associated virus serotypes 2- and 5-mediated gene transfer in the mammalian brain: quantitative analysis of heparin co-infusion. Mol. Ther 5, 371–380. doi: 10.1006/mthe.2002.0564 [DOI] [PubMed] [Google Scholar]
- Matias S, Lottem E, Dugue GP, Mainen ZF (2017) Activity patterns of serotonin neurons underlying cognitive flexibility. eLife, 6:e20552. doi: 10.7554/eLife.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCudden CR, Hains MD, Kimple RJ, Siderovski DP, Willard FS (2005) G-protein signaling: back to the future. CMLS Cell. Mol. Life Sci 62, 551–577. doi: 10.1007/s00018-004-4462-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinshey EM, Aston-Jones G (2017) Dorsal hippocampus drives context-induced cocaine seeking via inputs to lateral septum. Neuropsychopharmaology 43(5):987–1000. doi: 10.1038/npp.2017.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger D, Clifford J, Chiba H, Chambon P (1995) Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc Natl Acad Sci U S A 92(15):6991–5. doi: 10.1073/pnas.92.15.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger D, Chambon P (2001) Site- and time-specific gene targeting in the mouse. Methods 24(1):71–80. doi: 10.1006/meth.2001.1159. [DOI] [PubMed] [Google Scholar]
- Morsy MA, Gu M, Motzel S, Zhao J, Lin J, Su Q, Allen H, Franlin L, Parks RJ, Graham FL, Kochanek S, Bett AJ, Caskey CT (1998) An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc. Natl. Acad. Sci. USA, 95(14):7866–7871. doi: 10.1073/pnas.95.14.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murlidharan G, Samulski RJ, Asokan A (2014) Biology of adeno-associated viral vectors in the central nervous system. Front Mol Neurosci 7:76. doi: 10.3389/fnmol.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Miyakawa N, Takuwa H, Hiro Y, Oyama K, Ji B, Takahashi M et al. (2020) Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat Neurosci doi: 10.1038/s41593-020-0661-3 [DOI] [PubMed] [Google Scholar]
- Nair SG, Smirnov D, Neumajer JF (2015) DREADD’ed addiction: using designer receptors to delineate neural circuits underlying drug-seeking behaviors. In: Thiel G (Ed). Designer Receptors Exclusively Activated by Designer Drugs Humana Press: New York, pp. 129–146. [Google Scholar]
- Nair SG, Strand NS, Neumaier JF (2013) DREADDing lateral habenula: a review of methodological approaches for studying lateral habenula function. Brain Res 1511:93–101. doi: 10.1016/j.brainres.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Wess J (2012) Design and functional characterization of a novel, arrestin-biased designer G protein-coupled receptor. Mol. Pharmacol 82(4):575–582. doi: 10.1124/mol.112.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Cui Z, Li C, Meister J, Cui Y, Fu O, Smith AS, et al. (2016) Gs-coupled GPCR signalling in AgRP neurons triggers sustained increase in food intake. Nat Commun 7:10268. doi: 10.1038/ncomms10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navabpour S, Kwapis JI, Jarome TJ (2020) A neuroscientist’s guide to transgenic mice and other genetic tools. Neurosci Biobehav Rev 108:732–748. doi: 10.1016/j.neubiorev.2019.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DM, Boulis NM (2015) Gene tyerapy for neurodegenerative diseases. Trends Mol Med 21(8):504–512. doi: 10.1016/j.molmed.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Pfeifer A, Brandon EP, Kootstra N, Gage FH, Verma IM (2001) Delivery of the Cre recombinase by a self-deleting lentiviral vector: Efficient gene targeting in vivo. PNAS 98(20):11450–11455. doi: 10.1073/pnas.201415498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quentin E, Belmer A, Maroteaux L (2018) Somato-dendritic regulation of raphe serotonin neurons; a key to antidepressant action. Front Neurosci 12:982. doi: 10.3389/fnins.2018.00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quisenberry AJ, Snider SE, Bickel WK (2016) The return of rate dependence. Behav Anal (Wash D C) 16(4):215–220. doi: 10.1037/bar0000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Walker JK, Bohn LM (2005) Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther 314:1195–1201. doi: 10.1124/jpet.105.087254 [DOI] [PubMed] [Google Scholar]
- Paper J, Morrison RD, Daniels JS, Howell L, Bachevalier J, Wichmann T, Galvan A (2017) Metabolism and distribution of clozapine-N-oxide: Implications for nonhuman primate chemogenetics. ACS Chem Neurosci 8(7):1570–1576. doi: 10.1021/acschemneuro.7b00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CS, Soriano P (2007) High-efficiency FLP and PhiC31 site-specific recombination in mammalian cells. PLoS ONE 2, e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Friedmann D, Xiong J, Liu CD, Ferguson BR, Weerakkody T, DeLoach KE, Ran C, Pun A, Sun Y et al. (2018) Anatomically defined and functionally distinct dorsal raphe serotonin subsystems. Cell 175(2):472–487.e20. doi: 10.1016/j.cell.2018.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L (2000) Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol 417(2):181–194. [PubMed] [Google Scholar]
- Rogan SC, Roth BL (2011) Remote control of neuronal signaling. Pharmacol Rev 63(2):291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JA, Hoggan MD, Shatkin AJ (1966) Nucleic acid from an adeno-associated virus: chemical and physical studies. Proc Natl Acad Sci USA, 56(1):86–92. doi: 10.1073/pnas.56.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL (2016) DREADDs for neuroscientists. Neuron, 89(4):638–649. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel M, Brunert D, Zabawa C, Díaz-Quesada M, Wachowiak M (2013) Transgene expression in target-defined neuron populations mediated by retrograde infection with adeno-associated viral vectors. J Neurosci 33(38):15195–15206. doi: 10.1523/JNEUROSCI.1618-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runegaard AH, Fitzpatrick CM, Drucker Woldbye DP, Andreasen JT, Sorensen AT, Gether U (2019) Modulating dopamine signaling and behavior with chemogenetics: concepts, progress, and challenges. Pharmacol Rev 71(2):123–156. doi: 10.1124/pr.117.013995. [DOI] [PubMed] [Google Scholar]
- Sachs BD, Ni JR, Caron MG (2015). Brain 5-HT deficiency increases stress vulnerability and impairs antidepressant responses following psychosocial stress. Proc Natl Acad Sci USA, 112(8):2557–2562. doi: 10.1073/pnas.1416866112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai K, Zhao S, Takatoh J, Rodriguez E, Lu J, Leavitt AD, Fu M, Han B-X, Wang F (2016) Capturing and manipulating activated neuronal ensembles with CANE delineates a hypothalamic social-fear circuit. Neuron 92(4):739–753. doi: 10.1016/j.neuron.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Ortiz JL, Schaffer DV (2016) Adeno-associated virus (AAV) vectors in cancer gene therapy. J Control Release 240:287–301. doi: 10.1016/j.jconrel.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Streicher JM, Meltzer HY, Bohn LM (2014). Clozapine acts as an agonist at serotonin 2A receptors to counter MK-801-induced behaviors through a βarrestin2-independent activation of Akt. Neuropsychopharmacology 39(8):1902–1913. doi: 10.1038/npp.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena LM, Bueno C, Pobbe RL, Andrade TG, Zangrossi H Jr., Viana MB (2003) The dorsal raphe nucleus exerts opposed control on generalized anxiety and panic-related defensive responses in rats. Behav Brain Res 142(1–2):125–133. doi: 10.1016/s0166-4328(02)00399-6. [DOI] [PubMed] [Google Scholar]
- Shields BC, Kahuno E, Kim C, Apostolides PF, Brown J, Lindo S, et al. (2017) Deconstructing behavioral neuropharmacology with cellular specificity. Science, 356, 6333, eaaj2161. doi: 10.1126/science.aaj2161 [DOI] [PubMed] [Google Scholar]
- Smith KS, Bucci DJ, Luikart BW, Mahler SV (2016) DREADDs: use and application in behavioral neuroscience. Behav Neurosci 130(2):137–155. doi: 10.1037/bne0000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spannuth BM, Hale MW, Evans AK, Lukkes JL, Campeau S, Lowry CA (2011) Investigation of a central nucleus of the amygdala/dorsal raphe nucleus serotonergic circuit implicated in fear-potentiated startle. Neuroscience 179:104–119. doi: 10.1016/j.neuroscience.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A (2016) In vivo tissue-tropism of adeno-associated viral vectors. Curr. Opin. Virol 21: 75–80. doi: 10.1016/j.coviro.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachniak TJ, Ghosh A, Sternson SM (2014) Chemogenetic synaptic silencing of neural circuits localizes a hypothalamusàmidbrain pathway for feeding behavior. Neuron 8(4):797–808. doi: 10.1016/j.neuron.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Jennings JH, Ung RL, Blair GA, Weinberg RJ, Neve RL, Boyce F, Mattis J, Ramakrishnan C, et al. (2013) A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron 80(4):1039–1053. doi: 10.1016/j.neuron.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steculorum SM, Ruud J, Karakasilioti I, Backes H, Engström Ruud L, Timper K, Hess ME, Tsaousidou E, Mauer J, Vogt MC, Paeger L, Bremser S, Klein AC et al. (2016). AgRP neurons control systemic insulin sensitivity via myostatin expression in brown adipose tissue. Cell 165(1):125–138. doi: 10.1016/j.cell.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM, Roth BL. (2014). Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci 37:387–407. doi: 10.1146/annurev-neuro-071013-014048. [DOI] [PubMed] [Google Scholar]
- Stiedl O, Pappa E, Konradsson-Geuken A, Ove Ogren S (2015) The role of the serotonin receptor subtypes 5-HT1A and 5-HT7 and its interaction in emotional learning and memory. Front Pharmacol 6:162. doi: 10.3389/fphar.2015.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica L, Ahmed SS, Gao G, Sena Esteves M (2013) Gene transfer to the CNS using recombinant adeno-associated virus. Curr Protoc Microbio Chapter 14:Unit14D.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NE, Pei JZ, Zhang J, Vlasov KY, Davis T, Taylor E, Weng F-J, Van Dort CJ, Solt K, Brown EN (2019) The role of glutamatergic and dopaminergic neurons in the periaqueductal gray/dorsal raphe: separating analgesia and anxiety. eNeuro 6(1):0018–18,2019. doi: 10.1523/ENEURO.0018-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teissier A, Chemiakine A, Inbar B, Bagchi S, Ray RS, Palmiter RD, Dymecki SM, Moore H, Ansorge MS (2015) Activity of raphe serotonergic neurons controls emotional behaviors. Cell Rep 13(9):1965–1976. doi: 10.1016/j.celrep.2015.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo DG, Hwang BY, Viswanathan S, Gaj T, Lavzin M, Pitola KD, Lindo S, Michael S, Kuleshova E et al. (2016) Designer AAV variant permits efficient retrograde access to projection neurons. Neuron 92(2):372–382. doi: 10.1016/j.neuron.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KJ, Khajehali E, Bradley SJ, Navarrete JS, Huang XP, Slocum S et al. (2018) DREADD agonist 21 is an effective agonist for muscarinic-based DREADDs in vitro and in vivo. ACS Pharmacol Transl Sci 1(1):61–72. doi: 10.1021/acsptsci.8b00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M (2003) 5-HT1A receptor knockout mouse as a genetic model of anxiety. Eur J Pharmacol 463(1–3):177–184. doi: 10.1016/s0014-2999(03)01280-9. [DOI] [PubMed] [Google Scholar]
- Urban DJ, Roth BL (2015) DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu. Rev. Pharmacol. Toxicol 55, 399–417. doi: 10.1146/annurev-pharmtox-010814-124803. [DOI] [PubMed] [Google Scholar]
- Urban DJ, Zhu H, Marcinkiewcz CA, Michaelides M, Oshibuchi H, Rhea D et al. (2016) Elucidation of the behavioral program and neuronal network encoded by dorsal raphe serotonergic neurons. Neuropsychopharmacology 41(5):1404–1415. doi: 10.1038/npp.2015.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upright NA, Baxter MG. (2020) Effect of chemogenetic actuator drugs on prefrontal cortex-dependent working memory in nonhuman primates. Neuropsychopharmacology, 10.1038/s41386-020-0660-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haaren F (2016) The behavioral pharmacology of prescription drugs: their role in problem behavior and skill acquisition. Behavior Analysis: Research and Practice, 16, 190–199. 10.1037/bar0000049 [DOI] [Google Scholar]
- Vardy E, Robinson JE, Le C, Olsen RH, Diberto JF, Giguere PM et al. (2015) A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron 86(4):936–946. doi: 10.1016/j.neuron.2015.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhou FM (2019) cAMP-producing chemogenetic and adenosine A2a receptor activation inhibits the inwardly rectifying potassium current in striatal projection neurons. Neuropharmacology 148:299–243. doi: 10.1016/j.neuropharm.2019.01.014. [DOI] [PubMed] [Google Scholar]
- Wang RY, Aghajanian GK (1977) Physiological evidence for habenula as major link between forebrain and midbrain raphe. Science 197(4298):89–91. doi: 10.1126/science.194312. [DOI] [PubMed] [Google Scholar]
- Wakabayashi KT, Feja M, Leigh MPK, Baindur AN, Suarez M, Meyer PJ, Bass CE (2021) Chemogenetic activation of mesoaccumbal gamma-aminobutyric acid projections selectively tunes responses to predictive cues when reward value is abruptly decreased. Biol Psychiatry 89(4):366–375. doi: 10.1016/j.biopsych.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg MS, Samulski RJ, McCown TJ (2013) Adeno-associated virus (AAV) gene therapy for neurological disease. Neuropharmacology 69:82–88. doi: 10.1016/j.neuropharm.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham IR, Lyon DC, Barnard RJO, Mori T, Finke S, Conzelmann K-K et al. (2007) Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53(5):639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AA, Ingram WM, Levine S, Resnik J, Kamel CM, Lish JR, Elizalde DI, Janowski SA et al. (2012) Reduced levels of serotonin 2A receptors underlie resistance of Egr3-deficient mice to locomotor suppression by clozapine. Neuropsychopharmacology 37(10):2285–2298. doi: 10.1038/npp.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Bergman J, Vanderschuren L, Ellenbroek B (2019) Announcement of special issue: translational research in behavioural pharmacology. Behav Pharmacol 29(8):667. doi: 10.1097/FBP.0000000000000458. [DOI] [PubMed] [Google Scholar]
- Whissell PD, Tohyama S, Martin LJ (2016) The use of DREADDs to deconstruct behavior. Front Genet 7:70. doi: 10.3389/fgene.2016.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JO, Mcnally GP (2015) Pharmacogenetic excitation of dorsomedial prefrontal cortex restores fear prediction error. J Neurosci 35(1):74–83. doi: 10.1523/JNEUROSCI.3777-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K (2011) Optogenetics in neural systems. Neuron 71(1):9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Yang LM, Hu B, Xia YH, Zhang BL, Zhao H (2008) Lateral habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal raphe nucleus. Behav Brain Res 188(1):84–90. doi: 10.1016/j.bbr.2007.10.022. [DOI] [PubMed] [Google Scholar]
- You IJ, Wright SR, Garcia-Garcia AL, Tapper AR, Gardner PD, Koob GF, Leonardo ED et al. (2016) 5-HT1A Autoreceptors in the Dorsal Raphe Nucleus Convey Vulnerability to Compulsive Cocaine Seeking. Neuropsychopharmacology 41(5):1210–1222. doi: 10.1038/npp.2015.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Aryal DK, Olsen RH, Urban DJ, Swearingen A, Forbes S, Roth BL, Hochgeschwender U (2016) Cre-dependent DREADD (Designer Receptors Exclusively Activated by Designer Drugs) mice. Genesis 54(8):439–446. doi: 10.1002/dvg.22949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Belluscio L, Hen R (2000) G(olf)alpha mediates dopamine D1 receptor signaling. J Neurosci 20(16):RC91. doi: 10.1523/JNEUROSCI.20-16-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann ZJ, Poling A (2016) The discipline of behavioral pharmacology. Behavior Analysis: Research and Practice 16(4):156–158. 10.1037/bar0000057 [DOI] [Google Scholar]
- Zingg B, Chou XL, Zhang ZG, Mesik L, Liang F, Tao HW, Zhang LI (2017) AAV-mediated anterograde transsynaptic tagging: Mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron 93(1):33–47. doi: 10.1016/j.neuron.2016.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg B, Peng B, Huang J, Tao HW, Zhang LI (2020) Synaptic specificity and application of anterograde transsynaptic AAV for probing neural circuitry. J Neurosci 40(16):3250–3267. doi: 10.1523/JNEUROSCI.2158-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


