Abstract
As the world embarks on mass vaccination for COVID-19, we are beginning to encounter unintended dilemmas in imaging oncology patients; particularly with regards to FDG PET/CT. In some cases, vaccine-related lymphadenopathy and FDG uptake on PET/CT can mimic cancer and lead to confounding imaging results. These cases where findings overlap with cancer pose a significant dilemma for diagnostic purposes, follow-up, and management leading to possible treatment delays, unnecessary repeat imaging and sampling, and patient anxiety. These cases can largely be avoided by optimal coordination between vaccination and planned imaging as well as preemptive selection of vaccine administration site. This coordination hinges on patient, oncologist, and radiologists’ awareness of this issue and collaboration. Through close communication and patient education, we believe this will eliminate significant challenges for our oncology patients as we strive to end this pandemic.
Keywords: Oncologic imaging, COVID-19 vaccine, FDG PET/CT
Introduction
As the world embarks on mass vaccination for COVID-19, we are beginning to encounter unintended dilemmas in imaging oncology patients; particularly with regards to 18-fluorodeoxyglucose PET/CT (FDG PET/CT). In some cases, vaccine-related lymphadenopathy and FDG uptake on FDG PET/CT can mimic cancer and lead to confounding imaging results. Many of these cases of confusing results can be avoided by coordination of vaccination with planned imaging and avoiding the sequence of imaging being performed shortly after vaccination. Given the current structure of mass vaccination in the US, this type of coordination is expected to be particularly challenging, and radiology and oncology teams and patients will need to work together to share information and optimize imaging.
Background
Multiple traditional vaccines administered into the deltoid muscle have been shown to result in ipsilateral lymphadenopathy that can manifest on imaging studies[1], [2], [3], [4], [5]. In addition, transient FDG uptake can also be seen on FDG PET/CT in the spleen following vaccination[6]. Prior radiology studies show that these findings tend to resolve after 12–14 days[1], [3], [5], but can occasionally persist for up to 4–6 weeks after vaccination. In recent clinical trials, both FDA-approved vaccines (Moderna and Pfizer-BioNTech) list axillary lymphadenopathy as the second most commonly reported side effect, after pain at the injection site[7], [8], [9]. In the Moderna trial, this side effect was more commonly reported in younger patients (under age 85)[7]. As expected, transient COVID-19 vaccine-related lymphadenopathy and tracer uptake is being observed in axillary, supraclavicular, and cervical lymph nodes on the ipsilateral side of the deltoid vaccination[10], [11], [12]. Many cases can be attributed to vaccination based on pattern and natural history of disease. However, even in cases where vaccine information is available, some instances of vaccination will result in overlapping findings and the vaccine-pattern uptake cannot be distinguished from cancer, rendering the study nondiagnostic despite adequate information about vaccination (Fig. 1, Fig. 2, Fig. 3, Fig. 4 .). These findings will pose a significant challenge for follow-up, especially in cases of high grade or aggressive malignancies, and those requiring urgent initiation of treatment. Some authors of this paper recently published a manuscript on the imaging findings and a proposed algorithm[[11] for follow up, recognizing that there are significant barriers with access and insurance approval. Optimal coordination between imaging and vaccination can avoid inconclusive and confusing results leading to treatment delays, unnecessary repeat imaging and sampling, and patient anxiety.
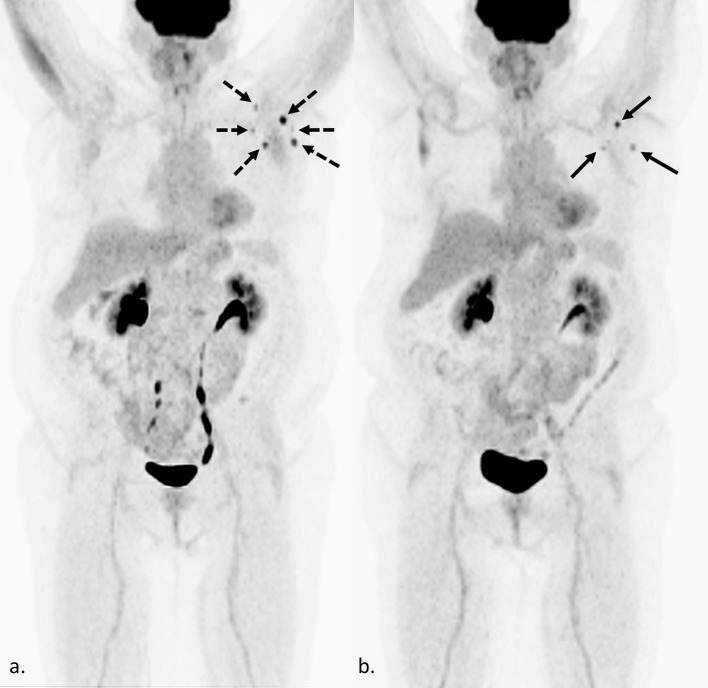
Fig. 1.
Confounding findings on FDG PET/CT in a 57-year-old woman with metastatic breast cancer 4 weeks after Moderna COVID-19 vaccination. Maximum intensity projection (MIP) (a.) shows an increase in size, number, and degree of FDG uptake in left axillary lymph nodes (dashed black arrows), compared to the previous FDG PET/CT performed 6 months prior (b. black arrows). As this is her only site of metastatic disease, and she received the Moderna vaccine 4 weeks prior, it is unclear if the increase is due to recent vaccination or an increase in her tumor burden. In this case, an increase in tumor burden was favored based on trends of prior imaging and tumor markers.
Fig. 2.
Confounding findings on FDG PET/CT in a 51 year-old man with new Hodgkin’s lymphoma after recent vaccination. MIP (a), axial fusion (b and c), and axial attenuation correction (d) images demonstrate FDG avid lymphadenopathy above and below the diaphragm (white arrows). It is unclear if the left axillary and supraclavicular uptake (white dashed arrows) are from a recent COVID-19 vaccination to the left deltoid three weeks prior, but would not result in a change in stage. However, increased uptake in the spleen (black dashed arrows), more avid than liver, could be due to either vaccination or disease involvement and would result in a change in stage.
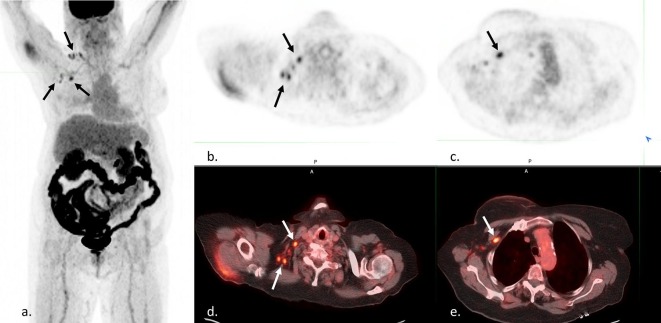
Fig. 3.
Confounding findings on FDG PET/CT in a 73 year-old woman with history of breast cancer to evaluate for recurrence. MIP (a), axial PET (b and c), and axial fusion (d and e) images demonstrate FDG-avid right axillary and supraclavicular lymph nodes (black and white arrows). This patient reported a recent Moderna COVID-19 vaccination in the left deltoid 5 days prior at a mass vaccination site. No records about vaccine administration were available to the reading radiologist or primary care physician. Initially it was unclear if the findings represented a recurrence of a right breast cancer or incorrect reporting/recording of vaccination side. Ultimately, the patient was able to be reached, and a right-sided vaccination was confirmed.
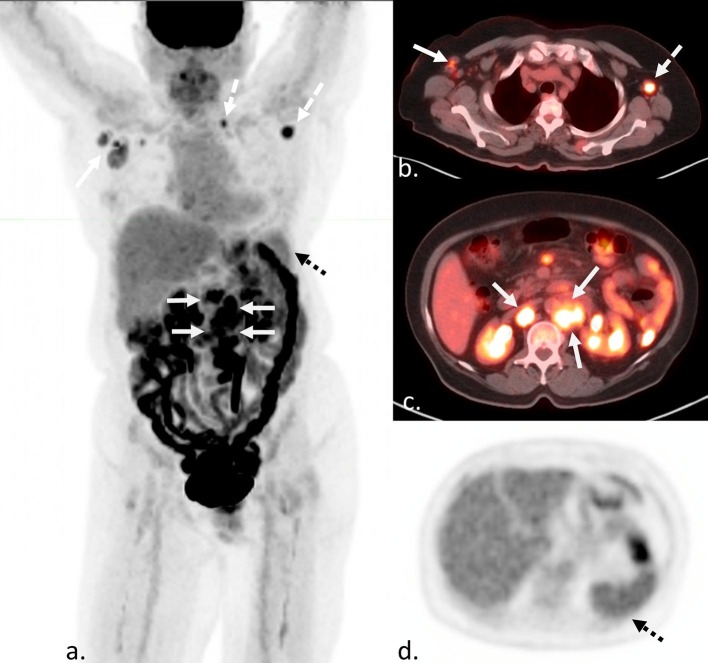
Fig. 4.
Confounding findings on FDG PET/CT in a 43 year-old man with lymphadenopathy and suspected diagnosis of lymphoma. MIP (a), and axial fusion (b, c, and d) FDG PET/CT shows an FDG avid retroperitoneal mass (white arrows), internal mammary lymphadenopathy (white dashed arrows), and left greater than right axillary lymphadenopathy (black arrows). This patient reported receiving a Moderna COVID-19 vaccine 1 week prior to the left deltoid. It is unclear if the findings in the left axilla are related to recent vaccination or lymphoma. While these findings would not change disease stage, this site should be avoided for biopsy due to potential confounding findings, and another more technically challenging and invasive site must be considered for highest yield sampling of representative disease.
Challenges
-
1.
Patient and physician awareness: Many patients and physicians caring for oncology patients are not aware that vaccination can produce false positive findings on FDG PET/CT. Therefore, they are not aware that simple measures such as vaccine site selection and optimal scheduling can easily avoid confusing results. Many instances of vaccine-related findings on imaging will be easily attributable to vaccination and not pose a significant diagnostic dilemma. However, certain cancers will require special consideration. Cancers that might be prone to potential overlapping or confounding findings include those that primarily manifest in the lymph nodes/spleen (lymphoma [and other conditions like Castleman’s]), cancers with laterality that could potentially involve vaccine pattern lymph nodes (breast, trunk or upper extremity melanoma/sarcoma, lung cancer [especially upper lobe], and head and neck cancers); and other advanced cancers that have previously shown involvement to vaccine pattern lymph nodes.[11] Other cancers, such as bone marrow related hematologic malignancies, localized abdominal or pelvic, or contralateral cancers might not pose a significant diagnostic dilemma and abnormal findings in these setting may be more readily attributable to a recent vaccination.
-
2.
Workflow: For stable patients on treatment or surveillance, imaging is often ordered far in advance. Many of the imaging studies being performed at present, in the midst of the pandemic and a mass vaccination effort, may have been ordered and arranged months to a year prior. Therefore, a potential conflict between vaccination and imaging might not be on the radar for the patient or ordering physician. Furthermore, some patients may have chosen to defer routine surveillance scans due to the ongoing pandemic and are now overdue for scans around the same time as vaccination has become available to them.
-
3.
Vaccination information sharing: While some of the COVID-19 vaccinations in oncology patients are being administered in the clinics, infusion centers, or in-system primary care offices, the vast majority of patients are seeking vaccination arrangements on their own. Many venues are being utilized, including mass vaccination sites, pharmacies, malls, grocery stores, senior centers, churches, public health locations – very few of which are integrated with health systems and electronic medical records (EMR). Therefore, the oncology team may not be aware of patients’ vaccination status or plans to be able to provide guidance about administration sites and timing with planned imaging.
-
4.
Information available to the radiologist: In cases where vaccine-related findings and a particular cancer overlap, radiologists need access to information about vaccine timing and administration site to be able to interpret or advise follow up to clarify confounding findings. Again, with limited EMR integration regarding vaccination, this information is not always readily available or volunteered by patients unless specifically asked. Other clinical factors and information might be helpful to the radiologist when interpreting these challenging cases, such as tumor markers and clinical status. Having adequate access to prior imaging can also be helpful in troubleshooting.
Solutions
-
1.
Patient communication: All patients undergoing treatment for malignancy should be notified of the potential impact that COVID-19 vaccine may have on subsequent imaging studies and/or physical exam. Patients should be reassured that COVID-19 vaccination should not be postponed or eschewed, but simply that the date and location of the injection(s) needs to be conveyed to their physicians, documented in their oncology record, and be considered for optimal coordination with planned imaging.
-
2.
Vaccine site selection and timing of planned imaging: When possible, it is best to avoid the sequence of FDG PET/CT soon after vaccination to reduce false positive or confounding results. If a patient has a cancer with laterality, patients should be instructed to receive the vaccine in the contralateral arm to avoid confusing results. The CDC currently recommends vaccination in the deltoid[13], and further information is needed before considering alternate sites in specific cases.
FDG PET/CT should not be delayed when clinically indicated or urgently needed. If feasible, imaging prior to vaccination is ideal to avoid overlapping findings. If not feasible, imaging is advised to be performed no sooner than 2 weeks after vaccination. It is our opinion that 4–6 weeks is a more optimal timeframe, but this is not always practical in oncology patients, especially when it delays treatment initiation. The mRNA vaccine technology may be more immunogenic than traditional vaccines[14], resulting in potentially more potent and longer lasting effects; and a longer time interval might be required. A recent research letter published in Radiology found that 29% of patients vaccinated with mRNA biotechnology undergoing FDG PET/CT showed persistent axillary tracer uptake at 7–10 weeks.[15] We might also observe differences in imaging between the first and second shots of a two-shot series as well as varied immune responses in different age groups, mirroring trends in clinically reported side effects seen in clinical trials.
-
3.
Data sharing: The more information that the oncology team has about patient vaccination status and planning, the better the timing with planned imaging can be coordinated and optimized. EMR’s can be optimized, perhaps through patient portal entry, to pull vaccination information into the record and have it easily available to the oncology care team to consider for imaging planning and the radiologist for interpretation. At our institution, technologists performing FDG PET/CT interview patients and convey this information to the reading radiologist; but it is not widely available for review by oncologists or radiologists reading in other modalities outside of FDG PET/CT that might also be affected. While adding pre-vaccination questions about malignancy could be considered, this issue only affects a small subset of the public and would be near impossible to implement on a wide scale due to the vast number and types of sites offering vaccination.
Conclusion
While most cases of vaccine-pattern uptake on FDG PET/CT can be attributed to recent vaccination, some cases will result in overlapping findings with cancer and pose a significant dilemma for diagnostic purposes, follow-up, and management leading to possible treatment delays, unnecessary repeat imaging and sampling, and patient anxiety. These cases can largely be avoided by optimal coordination between vaccination and planned imaging as well as preemptive selection of vaccine administration side/site. This coordination hinges on patient, oncology physician, and radiologists’ awareness of this issue and collaboration. Through close communication and patient education, we believe this will eliminate significant challenges for our oncology patients as we strive to end this pandemic.
Funding
None.
Acknowledgments
Acknowledgements
Thank you to Jonathan Goodman MPhil, a writer for Cancer Therapy Advisor, for the thoughtful interview questions that helped turn the focus on this issue from problems to solutions.
Disclosures
Lacey J. McIntosh, DO, MPH: Consultant, Bioclinica, but without conflicts of interest for this paper.
Max P. Rosen, MD, MPH: None.
Kriti Mittal, MD, MS: Advisory board: Dendreon, Bayer, Aptitude Health, Aveo Oncology; paid honorarium: MedPage Today; but without specific conflicts of interest for this paper.
Giles F. Whalen, MD: None.
Venu G. Bathini, MD: None.
Tasneem Ali, MD: None.
Kathryn L. Edmiston, MD: None.
William V. Walsh, MD: None.
Jonathan M. Gerber, MD: None.
References
- 1.Shirone N., Shinkai T., Yamane T., et al. Axillary lymph node accumulation on FDG-PET/CT after influenza vaccination. Ann Nucl Med. 2012;26:248–252. doi: 10.1007/s12149-011-0568-x. [DOI] [PubMed] [Google Scholar]
- 2.Thomassen A., Lerberg Nielsen A., Gerke O., et al. Duration of 18F-FDG avidity in lymph nodes after pandemic H1N1v and seasonal influenza vaccination. Eur J Nucl Med Mol Imaging. 2011;38:894–898. doi: 10.1007/s00259-011-1729-9. [DOI] [PubMed] [Google Scholar]
- 3.Panagiotidis E., Exarhos D., Housianakou, et al. FDG uptake in axillary lymph nodes after vaccination against pandemic (H1N1) Eur Radiol. 2010;20:1251–1253. doi: 10.1007/s00330-010-1719-5. [DOI] [PubMed] [Google Scholar]
- 4.Coates E.E., Costner P.J., Nason M.C., et al. Lymph node activation by PET/CT following vaccination with licensed vaccines for human papilloma viruses. Clin Nucl Med. 2017;42(5):329–334. doi: 10.1097/RLU.0000000000001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burger I.A., Husmann L., Hany T.F., Schmid D.T., Schaefer N.G. Incidence and intensity of F-18 FDG uptake after vaccination with H1N1 vaccine. Clin Nucl Med. 2011;36(10):848–853. doi: 10.1097/RLU.0b013e3182177322. [DOI] [PubMed] [Google Scholar]
- 6.Mingos M, Howard S, Giacalone N, Kozono D, Jacene H. Systemic Immune Response to Vaccination on FDG-PET/CT. Nucl Med Mol Imaging. 2016 Dec;50(4):358–61. doi: 10.1007/s13139-015-0385-6. Epub 2015 Dec 9. PMID: 27994693; PMCID: PMC5135690. [DOI] [PMC free article] [PubMed]
- 7.Local reactions, systemic reactions, adverse events, and serious adverse events: Moderna COVID-19 vaccine. Centers for Disease Control and Prevention; [February 11, 2021]; Available from: https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html.
- 8.Local reactions, systemic reactions, adverse events, and serious adverse events: Pfizer-BioNTech COVID-19 vaccine. Centers for Disease Control and Prevention; [February 13, 2021]; Available from: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html.
- 9.Baden LR, El Sahly HM, Essink B, Kotloff J, Frey S, et al for the COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403–16. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed]
- 10.Edmonds CE, Zuckerman SP, Conant EF. Management of Unilateral Axillary Lymphadenopathy Detected on Breast MRI in the Era of Coronavirus Disease (COVID-19) Vaccination. AJR 2021 Feb 5 [published online]. Accepted manuscript. doi: 10.2214/AJR.21.25604.
- 11.McIntosh LJ, Bankier AA, Vijayaraghavan GR, Licho R, Rosen MP. COVID-19 vaccination-related uptake on FDG PET/CT: An emerging dilemma and suggestions for management. AJR 2021 Accepted Feb 26 [in press]. 10.2214/AJR.21.25728. [DOI] [PubMed]
- 12.Eifer M., Eshet Y. Imaging of COVID-19 Vaccination at FDG PET/CT. Radiology. 2021 doi: 10.1148/radiol.2020210030. Jan 28 [published online] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.COVID-19 vaccine FAQs for healthcare professionals. Centers for Disease Control and Prevention; [February 24, 2021]; Available from: https://www.cdc.gov/vaccines/covid-19/hcp/faq.html.
- 14.Pardi N., Hogan M., Porter F., et al. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eshet Y, Tau N, Alhoubani Y, Kanana N, Domachevsky L, Eifer M. Prevalence of Increased FDG PET/CT Axillary Lymph Node Uptake Beyond 6 Weeks after mRNA COVID-19 Vaccination. Radiology 2021: Apr 27: 210886. doi: 10.1148/radiol.2021210886. Online ahead of print. [DOI] [PMC free article] [PubMed]