Abstract
Successful arthrodesis at the craniocervical junction and atlantoaxial joint can be more challenging than in other segments of the cervical spine. Different techniques for spinal fixation in this region have been well described, along with auxiliary methods to improve fusion rates. The occipital vascularized bone graft is a novel technique that can be used to augment bony arthrodesis in the supra-axial cervical spine. It provides the benefits of a vascularized autologous graft, such as accelerated healing, earlier fusion, and increased strength. This technique can be learned with relative ease and may be particularly helpful in cases with high risk of nonunion or pseudoarthrosis in the upper cervical spine.
Keywords: vascularized bone graft, spinoplastic reconstruction, occipital graft, autograft, spinal fusion
The occipitocervical junction is an area of special consideration in upper cervical instability due to its unique anatomy and protective qualities. 1 While successful arthrodesis is critical for achieving favorable outcomes in patients undergoing craniocervical and atlantoaxial stabilization, certain characteristics of this anatomic area can make this difficult to achieve. 1 2 The vertebral levels between the occiput and atlas as well as the atlas and axis lack intervertebral disks; there are cartilaginous synovial articulations only. The C1–C2 junction has the widest range of motion of any spinal segment. 3 Thus, the high degree of movement combined with the sharp angle at this junction requires the most rigid fixation device to promote arthrodesis. 1
Primary techniques for occipitocervical fusion include fixation methods (screw/rod, wiring/rod, screw/plate), nonvascularized onlay bone grafts (iliac crest, interlaminar rib), and/or vascularized bone grafts (VBGs) (free bone grafts, pedicled bone grafts). 2 4 5 6 Revision surgery in the occipitocervical region is technically challenging due to altered anatomy and the proximity of vital structures such as the medulla oblongata, spinal cord, and vertebral arteries. With failure rates as high as 50%, revision surgery in this area may benefit from augmentation with modalities such as VBGs. 2 3
VBGs are preferred over non-VBGs for several reasons, the majority of which stems from the readily-available blood supply of these grafts. The vascularity of these bone grafts results in an increased number of osteogenic cells, faster arthrodesis times, biomechanical support during early bone healing, and primary union at the graft–host interface. 2 5 Cell viability is maintained in the bone graft, and primary bone healing occurs at the fusion site as opposed to the creeping substitution into the scaffold of necrotic non-VBG. 7 This ultimately results in progressive resorption, loss of structural integrity, and pseudoarthrosis and hardware failure. 5 The occipital VBG is a novel pedicled bone graft based on the semispinalis capitis muscle that exemplifies the benefits of a VBG in an accessible and effective manner without prolonging operative time. The occipital VBG has been described in cadaveric studies and successfully implemented in patients to supplement revision arthrodesis in this anatomically challenging area. 2
Indications
The main indication for primary occipitocervical fusion is instability of the atlantoaxial joint with evidence of cord compression such as abnormal neurological signs in the extremities, unrelieved pain, and progressive severe subluxation. 1 4 Such instability may be acute or chronic, stemming from congenital, traumatic, degenerative, inflammatory, infectious, and neoplastic etiologies. 6
The indication for revision occipitocervical surgery is nonunion with persistent or progressive symptoms due to pseudoarthrosis and instrumentation failure. 3 Risk factors contributing to nonunion include immunosuppressive drug use, nonsteroidal anti-inflammatory drug use, malnutrition, osteoporosis, and smoking. 2 3 Other indications for vascularized bone grafting for augmentation of hardware include an irradiated field, previously operated field, or chronic osteomyelitis. 7 The occipital VBG can be used to augment both primary occipitocervical arthrodesis and revision arthrodesis; this pedicled VBG may be better suited for those in which free VBG transfer may be too morbid of a procedure.
Techniques
Bohl et al first described a pedicled VBG for the posterior occipitocervicothoracic spine through a cadaveric study; the technique was then performed in human subjects by Reece et al for revision arthrodesis of the atlantoaxial joint. 2 The procedure begins in the prone position with the neck neutrally positioned. The prior surgical incision is used; this midline incision extends from the greater occipital protuberance to the desired distal point of fixation. The prior hardware is dissected out and removed. New transarticular screws are placed to achieve joint fixation for the remainder of the procedure. The joint(s) are decorticated in preparation for later arthrodesis. The fascial plane between the trapezius muscle and the semispinalis capitis muscle is identified and dissected, with protection of the overlying trapezius muscle for eventual primary closure. A piece of occipital bone bordered inferiorly by the foramen magnum and medially by the median nuchal line is then identified; the superior and lateral borders depend on the size of bone graft required. Subperiosteal dissection then occurs around the occipital bone to be rotated, taking care not to disrupt the periosteum or semispinalis capitis muscle attachments to this piece of bone, which would comprise the bone graft's vascular pedicle ( Figs. 1 and 2 ). 2 5
Fig. 1.
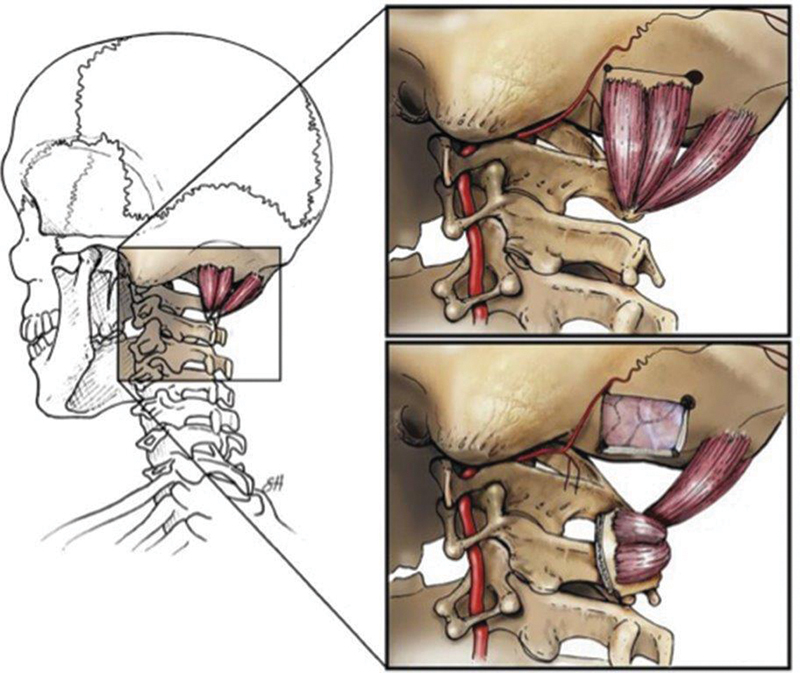
Illustration of the pedicled, vascularized occipital bone graft. The vascularized bone graft is pedicled on the splenius capitis muscle and rotated in between the C1 and C2 lamina to augment arthrodesis. (Reprinted with permission from Scott Holmes, CMI, Baylor College of Medicine.)
Fig. 2.
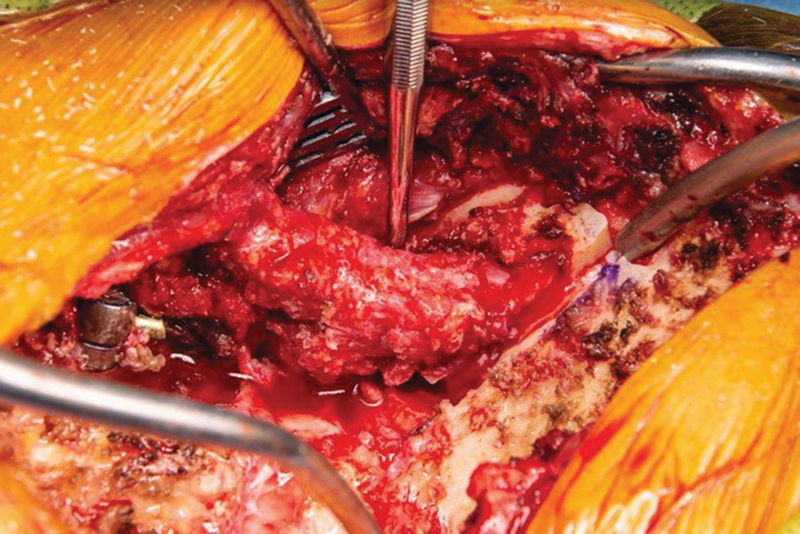
Intraoperative photograph showing the harvesting of the occipital bone graft. The cuts of the craniectomy are visible. The lateral border of the intact muscle pedicle is exposed with the dissector.
The semispinalis capitis muscle is a Mathes–Nahai type 2 muscle, with a dominant deep cervical artery coming off the costocervical trunk, meaning that this muscle and any bony attachments can be rotated from the occiput to as caudal as the upper thoracic spine without compromising its dominant vascular supply. The blood supply to the semispinalis capitis pedicle is based on cervical and superior subcostal branches; therefore, the semispinalis pedicle should not be mobilized below the inferior cervical levels. 5 The occipital bone is then removed using high-speed drills and/or osteotomes as needed. Finally, the graft is secured to the fusion site using fixation methods such as plates, screws, rods, or wires.
It is essential to ensure that the bone graft is contacting the bony surfaces above and below the joint(s) to be arthrodesed, decorticated at the points of bony contact, and compressed into place. These considerations help facilitate primary bone healing and remodeling according to Wolff's Law. 2 8 Bohl et al also demonstrated that full-thickness bone grafts are better for occipitocervical fusions, as these grafts retain bicortical structure and can provide better biomechanical support. 5 Comparatively, split-thickness bone grafts can fracture easily during harvest and may be better suited for subaxial fusions when graft osteogenicity is more important than graft rigidity. 5 Mean occipital VBG dimensions in the cadaveric study were 5.9 cm (length) × 3.26 cm (width) × 0.9 cm (full-thickness). 5
Clinical Outcomes
To date, two patients have received occipitocervical fusions augmented with an occipital VBG. The first was a 72-year-old woman with numerous risk factors for pseudoarthrosis including nicotine use, long segment fusion, and history of cervical pseudoarthrosis. The patient presented 6 months following a motor vehicle collision complaining of persistent neck pain. Imaging showed C1–C2 instability with subaxial stenosis, as well as a prior C1–C6 posterior instrumented fusion with broken hardware. While her subaxial fusion was solid, C1–C2 had not successfully fused, leading to hardware fracture. The surgical team and patient agreed upon revision surgery with augmentation of her C1–C2 arthrodesis using an occipital bone graft ( Fig. 3 ). She tolerated the procedure well, with significant improvement in her neck pain reported on postoperative day 1. At 3, 6, and 18 months postoperatively, imaging showed intact hardware with no subluxation of C1 on C2 and partial fusion of the levels ( Fig. 4 ). 2
Fig. 3.
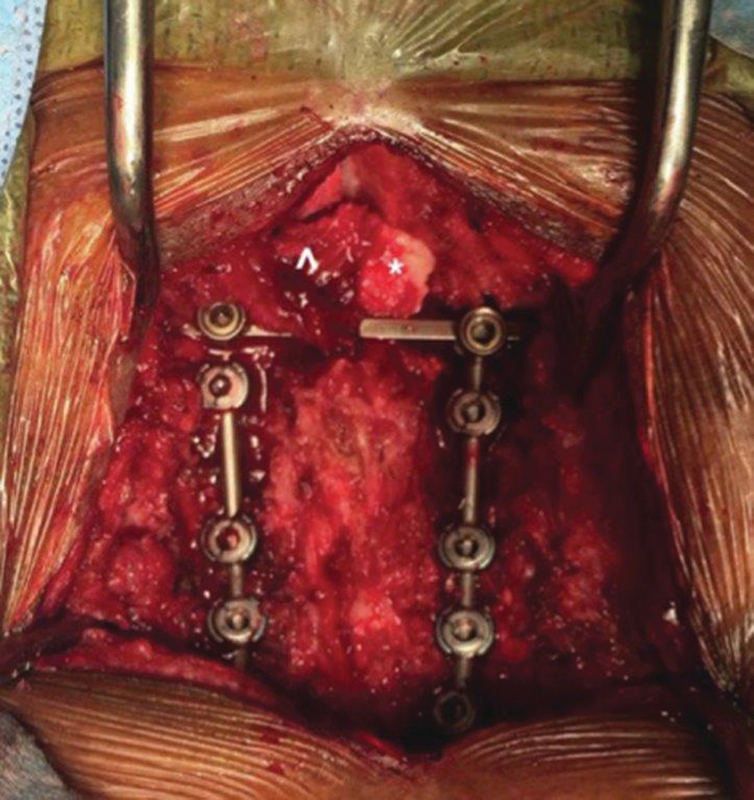
Intraoperative photograph of a cervical spine instrumented fusion. The muscle pedicle is intact (^) and the occipital bone graft (*) is held in place across the C1 and C2 lamina with a cross-connector to apply compression.
Fig. 4.
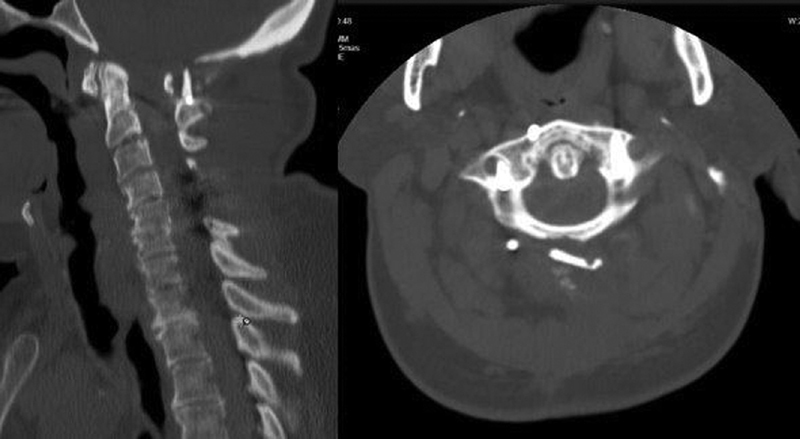
Postoperative computed tomography reconstruction of the patient 3 (left) and 6 months (right) following occipital bone graft fusion augmentation showing partial fusion from C1 to C2.
The second patient was a 75-year-old woman with a history of previous C2-pelvis fixation, pseudoarthrosis, and proximal junctional failure. The patient experienced a mechanical fall and sustained a C2 fracture with transverse atlantal ligament tear, a displaced C1 ring, and an injured vertebral artery. The recommended surgical plan was an extension of her fusion to the occiput and augmentation of her arthrodesis with an occipital bone graft. She tolerated the procedure well and was discharged to a skilled nursing facility. Three weeks following her procedure, she was readmitted for an occipital pressure ulcer and concern for seeding of the surgical site with infection. She underwent a surgical exploration and washout of surgical site infection. The occipital graft was inspected and found to be viable and vascularized, despite the infection. The VBG was left in place, and the patient's surgical site infection was treated successfully with intravenous antibiotics. At a 10-month postoperative visit, the patient was clinically stable with radiographic evidence of bone graft fusion at C1 and partial fusion at the occiput and C2. There was no radiographic or clinical evidence of screw haloing or other signs of pseudoarthrosis ( Fig. 5 ).
Fig. 5.
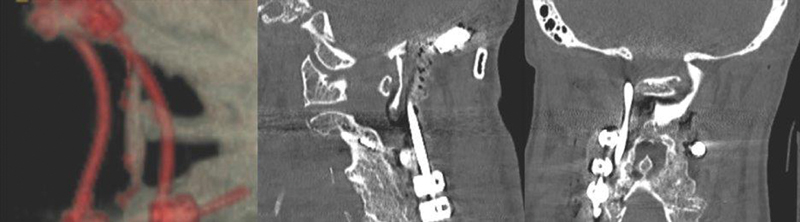
Postoperative computed tomography reconstruction of a patient 10 months following occipital bone graft fusion augmentation. There is solid fusion from the cranium to the C2 lamina.
Alternatives
There are several alternatives to the occipital VBG including augmentation with bone morphogenic protein (BMP), periosteal flap, other VBGs, and free microsurgical bone graft transfer. The following alternatives are presented in order of least to most invasive method.
Bone Morphogenic Protein
There have been reports of using human BMP with absorbable collagen sponge in posterior cervical spine fusion in both primary arthrodesis and salvage arthrodesis. 9 10 11 Lu et al describe the use of BMP in a 4-month-old patient with Down syndrome who had failed two prior attempts of stabilization. He had nonunion of his craniovertebral junction, which was ultimately remained fused at 4 years postoperatively after the third attempt, which used BMP. 10 Mladenov et al similarly describe a 6-year-old patient with mucopolysaccharidosis type 1 (Hurler's disease) who presented with progressive muscle weakness in the lower extremities and sleep apnea. Magnetic resonance imaging revealed spinal cord compression caused by C1–C2 instability and soft tissue apposition. Initial surgery with C1–C2 laminectomy and iliac crest bone grafting from occiput to C4 resulted in unstable nonunion; revision surgery with posterior decortication, repeat iliac crest bone grafting, and BMP application resulted in solid fusion at 18-month follow-up with improvement of presenting symptoms. 9
Evidence has shown BMP (more specifically, rhBMP-2, and rhBMP-7) to be effective osteoinductive agents when used as an adjunct to instrumentation or allograft in spinal surgery. However, the safety profile of BMP is still under investigation. Reported adverse events associated with BMP use include ectopic bone formation, bone resorption or remodeling at the graft site, hematoma, neck swelling, and painful seroma. Other concerns include the theoretical carcinogenicity and teratogenic effects, the high cost of the product, and the lack of evidence guiding proper dosage. 12
Periosteal Turndown Flap Technique
In 1984, Koop et al described a periosteal flap technique as a relatively safe and simple method to augment graft fusion by providing more osteogenic cells; this procedure was reviewed and confirmed by Yasmeh et al. 13 14 Koop et al reported a retrospective review of 13 patients who underwent posterior cervical fusion with the periosteal flap technique. Two patients received wire instrumentation and all patients were kept immobilized with halo external fixation. Only one patient, who had received bank-rib allograft, developed nonunion. 14 Yasmeh et al report the successful use of the periosteal flap technique in a 6-year-old male who presented with an occiput to C2 distraction injury following a pedestrian versus automobile trauma. The patient underwent posterior spinal fusion from occiput to C3 using sublaminar wires and autologous iliac crest graft with the periosteal turndown flap technique. There was evidence of solid fusion mass and no neurological deficits at 5 months follow-up. 13
In this procedure, the occiput is exposed by staying above the periosteum, which is reflected at the occiput using a combination of forceps, a Penfield dissector, and a 15 blade to elevate the layer. The attachment is preserved at the caudal base of the flap, which can be divided into two adjacent flaps or reflected whole. The exposed intended area of fusion is then decorticated. Cancellous bone strips are harvested from iliac crest and placed surrounding the decorticated occipitocervical posterior elements to bridge the occipitocervical region. Additional autograft or allograft may be used as necessary. The periosteal flaps are reflected caudally and sutured in place to the spinous process of the most inferior cervical level to be fused. The distal portion of the flap is folded underneath itself, placing the osteophyte rich occipital surface of the flap against the defect. The periosteal turndown technique can be used in the presence or absence of instrumentation. Additional cables and hardware can be added to provide additional security to the bone graft and spinal fixation, respectively. 13
Other Vascularized Bone Grafts
In the same cadaveric study describing the occipital VBG, Bohl et al describe a scapular VBG that could be mobilized on a subscapular pedicle and reach from occiput to T7, spanning up to 8 levels. Mean cadaveric scapular VBG dimensions were 15.1 cm (length) × 1.9 cm (width) × 0.8 cm (thickness). The dissection is based on identifying and protecting the descending branch of the transverse cervical artery, the blood supply to the subscapularis pedicle. However, bilateral harvesting of the scapular VBG is necessary if bilaterally posterior spinal graft support is desired. The medial scapula harvest also results in some potential for functional arm or shoulder movement deficits. 5
Of note, Bohl et al performed a cadaveric study for far-lateral vascularized rib grafts for posterior spinal defects. Upper rib vascularized rib grafts only reached to C2 (occiput to C4) ipsilaterally and C3 (occiput to C5) contralaterally. Furthermore, 41.7% of the intercostal vessel pedicles were torn during harvest and rotation, making the far-lateral vascularized rib graft a riskier option with less reach compared with the occipital VBG and scapular VBG. 15
Free Fibula Vascularized Bone Graft
The vascularized free fibula bone graft has been described in cervical spine arthrodesis, usually through an anterior approach. 16 17 The vascularized fibula graft has multiple advantages including lengthiness (∼25 cm on average), lack of angulation, thick cortex, and versatility for recipient site. 7 Fusion rates from 90 to 96% have been reported in uncomplicated two- and three-level fusions using allograft fibula. 16 In particular, a vascularized free fibula bone graft is especially appealing in the context of osteomyelitis or radiation; these grafts have shown to be good adjuncts to plating and immobilization instrumentation. 18
The data on successful vascularized free fibula bone graft transfer through a posterior approach is limited. Jandali et al describe a case report for a 13-year-old boy with a malignant peripheral nerve sheath tumor of the skull base and upper cervical spine who had undergone radiation and chemotherapy. He underwent further tumor resection, C1 laminectomy, removal of the C1 lateral mass, and occipital condyle resection. This defect was reconstructed with a 24 cm fibula flap, a saphenous vein graft anastomosed as an arteriovenous loop, and placement of posterior hardware from the occiput to C4. There was radiographic evidence on computed tomography of bony fusion at 23 weeks postoperatively, but the cervical hardware began to erode through irradiated skin at 15 months postoperatively. The patient was taken back to the operating room for hardware removal, where the fibula was still viable and fused as a sheet of bone to the cervical spinal column. 7
The potential complications of a free fibula flap include nonunion, the recurrence or development of osteomyelitis, failure of fixation, fibular fracture, cutaneous flap necrosis, sensory disturbances, contracture of flexor hallucis longus, and valgus deformity of the donor ankle. 17 Furthermore, the procedure is technically challenging and may require long operative times that are not well suited for all surgical candidates. 2
Conclusion
The literature is robust in supporting vascularized bone grafting to augment spinal fusion, with an increasing number of studies being published that demonstrate the anatomic and clinical feasibility of rotating VBGs into spinal fusion beds. 2 5 7 19 20 Bone structural viability and strength are maintained throughout the healing process. Patients undergoing VBG augmentation are inherently at higher risk for complications and have numerous risk factors for pseudoarthrosis, poor wound healing, and surgical site infections. Furthermore, the occipitocervical junction is a critical area with high mobility, predisposing it to nonunion and hardware failure. The occipital VBG is a novel technique for augmenting fusion in this anatomic area. Alternatives exist for vascularized and nonvascularized augmentation in this area, including BMP, the periosteal turndown flap, scapular vascularized flap, and the free fibula bone transfer. The occipital VBG may be the most anatomically accessible, learnable, effective, and least morbid technique for augmenting fusion in this region.
Funding Statement
Funding None.
Footnotes
Conflicts of Interest Dr. Ropper receives consulting fees from Globus Medical and Stryker. All authors, including Dr. Ropper, have no financial interests in relation to the content of this article.
References
- 1.Lee D J, Ahmadpour A, Ament J D, Goodarzi A, Panchal R R. When is occipitocervical fusion necessary for upper cervical injuries? Semin Spine Surg. 2017;29:20–26. [Google Scholar]
- 2.Reece E M, Vedantam A, Lee S. Pedicled, vascularized occipital bone graft to supplement atlantoaxial arthrodesis for the treatment of pseudoarthrosis. J Clin Neurosci. 2020;74:205–209. doi: 10.1016/j.jocn.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Shroeder G D, Baaj A A, Vaccaro A R. Boca Raton, FL: Taylor & Francis Group, LLC; 2020. Revision Spine Surgery: Pearls and Pitfalls. [Google Scholar]
- 4.Newman P, Sweetnam R. Occipito-cervical fusion. An operative technique and its indications. J Bone Joint Surg Br. 1969;51(03):423–431. [PubMed] [Google Scholar]
- 5.Bohl M A, Mooney M A, Catapano J S. Pedicled vascularized bone grafts for posterior occipitocervical and cervicothoracic fusion: a cadaveric feasibility study. Oper Neurosurg (Hagerstown) 2018;15(03):318–324. doi: 10.1093/ons/opx258. [DOI] [PubMed] [Google Scholar]
- 6.Winegar C D, Lawrence J P, Friel B C. A systematic review of occipital cervical fusion: techniques and outcomes. J Neurosurg Spine. 2010;13(01):5–16. doi: 10.3171/2010.3.SPINE08143. [DOI] [PubMed] [Google Scholar]
- 7.Jandali S, Diluna M L, Storm P B, Low D W. Use of the vascularized free fibula graft with an arteriovenous loop for fusion of cervical and thoracic spinal defects in previously irradiated pediatric patients. Plast Reconstr Surg. 2011;127(05):1932–1938. doi: 10.1097/PRS.0b013e31820cf4a6. [DOI] [PubMed] [Google Scholar]
- 8.Shaffer J W, Davy D T, Field G A, Bensusan J S, Kellis G J. The superiority of vascularized compared to nonvascularized rib grafts in spine surgery shown by biological and physical methods. Spine. 1988;13(10):1150–1154. doi: 10.1097/00007632-198810000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Mladenov K V, Kunkel P, Stuecker R. The use of recombinant human BMP-2 as a salvage procedure in the pediatric spine: a report on 3 cases. Eur Spine J. 2010;19 02:S135–S139. doi: 10.1007/s00586-009-1179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu D C, Sun P P.Bone morphogenetic protein for salvage fusion in an infant with Down syndrome and craniovertebral instability. Case report J Neurosurg 2007106(6, Suppl):480–483. [DOI] [PubMed] [Google Scholar]
- 11.Oluigbo C O, Solanki G A. Use of recombinant human bone morphogenetic protein-2 to enhance posterior cervical spine fusion at 2 years of age: technical note. Pediatr Neurosurg. 2008;44(05):393–396. doi: 10.1159/000149907. [DOI] [PubMed] [Google Scholar]
- 12.Benglis D, Wang M Y, Levi A D.A comprehensive review of the safety profile of bone morphogenetic protein in spine surgery Neurosurgery 2008620502ONS423–ONS431., discussion ONS431 [DOI] [PubMed] [Google Scholar]
- 13.Yasmeh S, Quinn A, Harris L. Periosteal turndown flap for posterior occipitocervical fusion: a technique review. Eur Spine J. 2017;26(09):2303–2307. doi: 10.1007/s00586-017-5085-8. [DOI] [PubMed] [Google Scholar]
- 14.Koop S E, Winter R B, Lonstein J E. The surgical treatment of instability of the upper part of the cervical spine in children and adolescents. J Bone Joint Surg Am. 1984;66(03):403–411. [PubMed] [Google Scholar]
- 15.Bohl M A, Hlubek R J, Turner J D, Kakarla U K, Preul M C, Reece E M. Far-lateral vascularized rib graft for cervical and lumbar spinal arthrodesis: cadaveric technique description. Plast Reconstr Surg Glob Open. 2019;7(04):e2131. doi: 10.1097/GOX.0000000000002131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rolle M L, Henn R E, Low D W, Storm P B. Occipital cervical fusion with a vascularized free fibular graft. Int J Spine Res. 2019;1(01):23–25. [Google Scholar]
- 17.Minami A, Kaneda K, Satoh S, Abumi K, Kutsumi K. Free vascularised fibular strut graft for anterior spinal fusion. J Bone Joint Surg Br. 1997;79(01):43–47. doi: 10.1302/0301-620x.79b1.7112. [DOI] [PubMed] [Google Scholar]
- 18.Lee M J, Ondra S L, Mindea S A, Fine N A, Dumanian G A. Indications and rationale for use of vascularized fibula bone flaps in cervical spine arthrodeses. Plast Reconstr Surg. 2005;116(01):1–7. doi: 10.1097/01.prs.0000169710.53269.bc. [DOI] [PubMed] [Google Scholar]
- 19.Yelizarov V G, Minachenko V K, Gerasimov O R, Pshenisnov K P. Vascularized bone flaps for thoracolumbar spinal fusion. Ann Plast Surg. 1993;31(06):532–538. doi: 10.1097/00000637-199312000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Davis J B, Taylor A N. Muscle pedicle bone grafts; experimental study. AMA Arch Surg. 1952;65(02):330–336. doi: 10.1001/archsurg.1952.01260020343016. [DOI] [PubMed] [Google Scholar]


