Abstract
Wound complications occur in up to 19% of patients undergoing complex spine surgery. The role of the plastic surgeon in complex and redo spine surgery is important and evolving. Classically, plastic surgeons have been involved in the management of patients who develop wound complications following surgery. This involves reconstruction of posterior trunk defects with locoregional fasciocutaneous, muscle, and free tissue transfers. There has also been an increasing role for plastic surgeons to become involved in prophylactic closures of complex and/or redo spine surgeries for high-risk populations. Identification of patients with comorbidities and likelihood for multiple reoperations who are prophylactically treated with complex closure with or without local muscle flaps could significantly decrease the postoperative wound complications.
Keywords: spine surgery, complications, plastic surgery, complex closure
Historically, the plastic surgeon has played a limited role in spine reconstruction. With expanding indications, increasing complexity, and improvement in implants and techniques, the number of spine surgery cases is increasing, as are the associated wound complications. 1 2 Wound complications following spinal surgery can be devastating and occur in up to 19% of patients undergoing complex spine surgery. 3 These patients oftentimes possess one or more comorbidities that place them at an increased risk of complications. The main risk factors for wound complications are history of radiation, active cerebrospinal fluid (CSF) leak, infection, malnutrition, obesity, diabetes, multiple previous surgeries, use of hardware, long operative time, > 6 operative spinal levels, and surgical approach. 4 Patients who have one or more of these risks factors exhibit a 40% wound complication rate. 5 Postoperative wound complications most commonly include wound dehiscence, superficial or deep surgical-site infections, exposure of hardware leading to removal and potential deformity, longer hospital stays, prolonged antibiotics, and multiple returns to the operating room. 3
Following spine surgery, patients spend the majority of their postoperative recovery lying directly on their incision, compromising an already oftentimes-tenuous closure. Adding any amount of moisture creates an unfavorable environment for wound healing further leading to wound compromise. Given the aforementioned reasons, the role of the plastic surgeon in setting of wound management associated with complex spine surgery has become commonplace. Recent trends in spine surgery involve plastic surgeons prophylactically to decrease the risk of postoperative wound formation. Identifying at-risk patients prior to spine surgery is essential, as there is ample evidence that prophylactic muscle flap surgery after complex spine procedures can reduce the risk of wound complications. 4
Patient Presentation and Management Principles
Classically, the postoperative spine surgery patient has presented to the plastic surgeon with an open or threatened posterior trunk wound with or without exposed hardware ( Figs. 1 and 2 ). The overarching goals of reconstruction of posterior trunk defects are to protect vital underlying structures and provide stable, layered, soft-tissue closure. It is important to identify and address dural tears or CSF leaks that will prevent healing or lead to further complications. CSF leaks may increase the rate of wound infection, surgical site dehiscence, pseudomenigomyelocele, meningitis, and cerebellar herniation. 6 Preoperative workup includes imaging to determine the extent of the wound and involvement of underlying structures, as well as to assess the underlying hardware and to plan further spinal instrumentation. An X-ray is helpful to evaluate presence and location of hardware, while a computed tomography scan helps to determine if there are any fluid collections either above or below musculature, where the hardware is in relation to the wound, and the extent of bony involvement. Other factors to consider when assessing the wound include timing of the last procedure, patient obesity, history of radiation, underlying osteomyelitis, history of multiple previous procedures, and nutritional deficiency. Preoperative patient nutrition should be closely evaluated, with nutritional laboratories aiming to have albumin > 2.0 g/dL and prealbumin > 20 mg/dL. Vitamin C, A, Folate, and Zinc should also be repleted prior to surgery for optimal benefit.
Fig. 1.
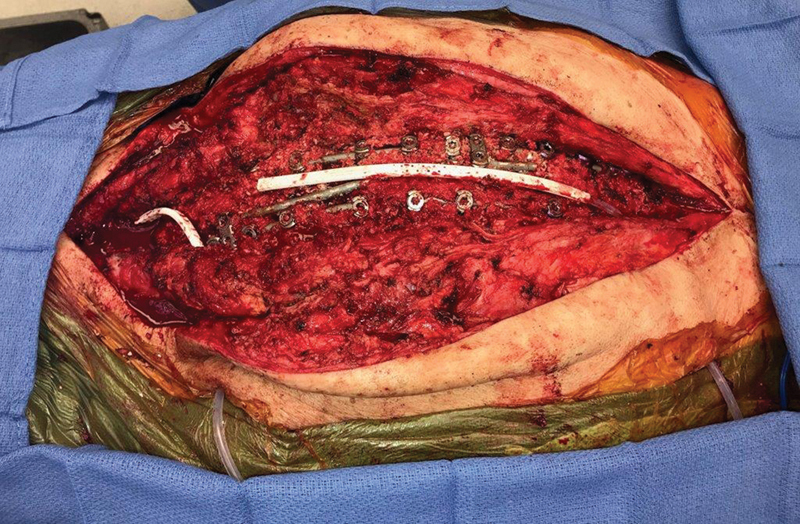
Open spinal wound with exposed hardware.
Fig. 2.
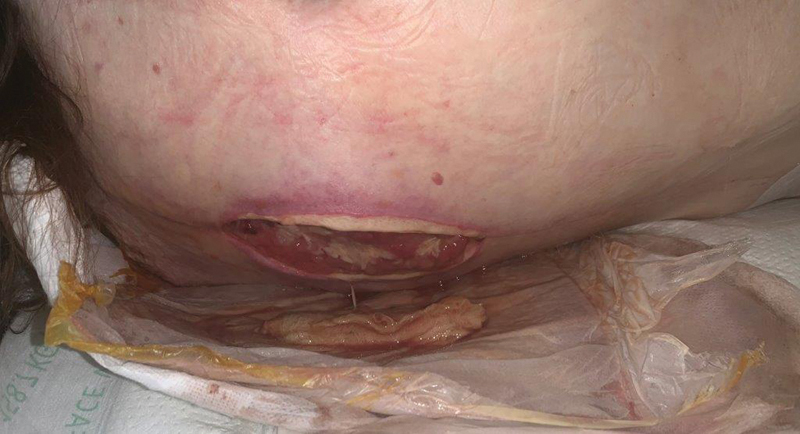
Open, draining spinal wound.
Timing of management is critical. If the patient presents within 4 to 6 weeks of wound development or drainage, the best next steps are exploration and debridement of nonviable tissue. 7 Cultures are taken at this time to guide antibiotic treatment. During the acute presentation, soft tissue reconstruction with hardware preservation is the goal and is often achievable. For chronic wounds, defined as wounds present for more than 6 weeks, bacterial colonization of hardware becomes a concern and the wound cannot be managed with only soft tissue reconstruction. Chronic wounds usually necessitate hardware removal, closure versus application of negative pressure, and delayed reconstruction. 7
Treatment principles of posterior trunk wounds include timely debridement of all devitalized, infected, or fibrotic tissue, skin, and bone. In addition, appropriate systemic management with antibiotics to control infection is paramount based on initial culture data. Once these principles are addressed, definitive reconstruction can be planned. It is important to realize that patients may require serial debridements prior to reconstruction. The number of necessary debridements depends on the status of the wound. Proceeding to reconstruction prior to fully debriding a wound will result in failure. It is essential that the plastic surgeon perform or assist in the debridement to ensure that only healthy tissue remains. In cases that involve malignancy, reconstruction should ensue only after the permanent margins are free of tumor. Most commonly, radical debridement and reconstruction can take place in a single stage.
As with most reconstructive challenges, adhering to the reconstructive ladder is helpful ( Fig. 3 ). Some of these postoperative spine wounds heal by secondary intention, although the majority will require debridement and at least a complex layered closure and subsequent healing by primary intention. Healing by secondary intention with local wound care is only possible for wounds that are superficial with an intact fascia and no exposed hardware. Negative pressure wound therapy (NPWT) is commonly used in open wounds prior to definitive closure. Multiple studies have confirmed the safety and efficacy of its use. 8 NPWT promotes a healthy wound bed with reduction of periwound edema and represents an effect adjunct in the management of complicated spine wounds. 9 Negative pressure should not be used over grossly necrotic tissue. Skin grafts generally do not provide the durable coverage that is required given the location of the wound and underlying hardware. Tissue expansion is an option to consider should there be a paucity of skin at the midline and if there is durable fascial coverage of the spine. Expanded skin lateral to the defect can then be advanced to provide coverage of the midline. In general, however, both skin grafts and tissue expansion have limited usefulness in this patient population. Lastly and most importantly on the reconstructive ladder, locoregional muscle groups and occasionally free tissue transfer remain the mainstay for closure of these spine wounds.
Fig. 3.
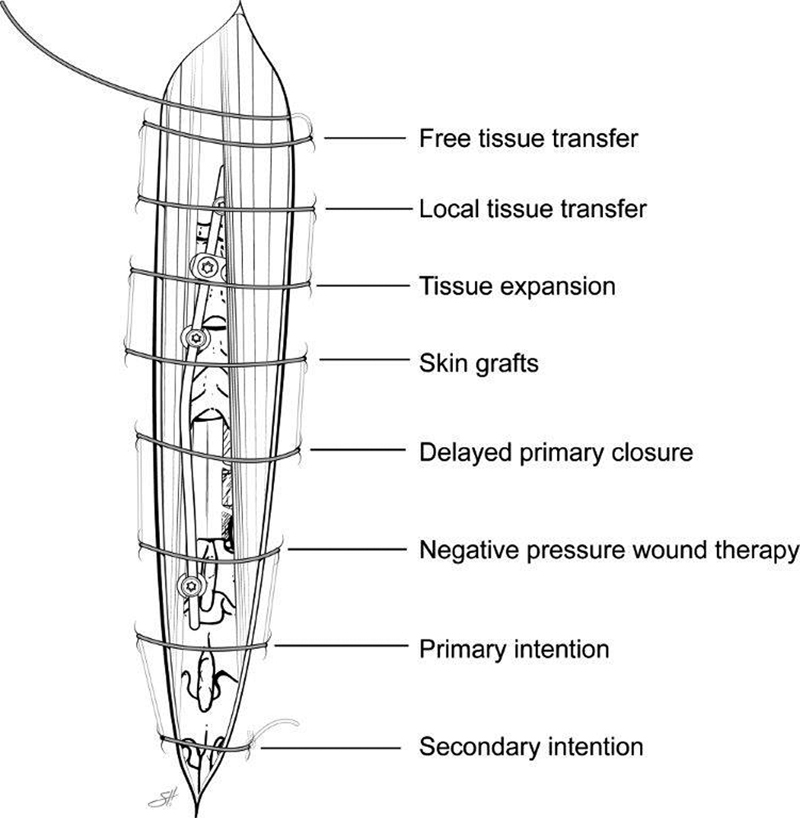
The reconstructive ladder. Reprinted with permission of Baylor College of Medicine.
Muscle Flap Coverage
Muscle flaps are the gold standard for reconstruction of larger posterior trunk defects. Not only does the muscle flap fill in dead space but it also inhibits bacterial growth that can cause infection by providing increased blood flow and improving oxygen and nutrient exchange. 10 Obliteration of dead space is essential, as dead space leads to seroma, infection, hardware failure, and wound breakdown. A study by Calderon et al examined infection and necrosis rates between musculocutaneous flaps and fasciocutaneous flaps at three different levels of the flap (proximal to distal). They found necrosis rates are higher in fasciocutaneous flaps than musculocutaneous flaps, and more in the distal portion of the fasciocutaneous when compared with the proximal level. There was no significant difference in necrosis when comparing distal to proximal areas of the musculocutaneous flap supporting the benefit of muscle flaps over fasciocutaneous flaps. 11 Mathes and Nahai classified muscle flaps based on their blood supply, forever changing the specialty of plastic surgery. 12 Understanding the blood supply of each muscle is essential in utilizing muscle flaps for spinal reconstruction.
The paraspinous muscles are Mathes–Nahai type IV segmental muscles with blood supply from the dorsal segmental branches off the aorta, giving rise to medial and lateral perforators. This segmental muscle group extends from the thoracic region to the lumbosacral region. Given this location, this muscle flap is best for midline defects from high thoracic to the low lumbar region. In the cervical region, the muscle is thin and tapers under the trapezius, making it a poor option for cervical defects. The paraspinous muscle can either be advanced to the midline bilaterally ( Fig. 4A–C ) or used as a turnover flap. Skin flaps are raised superficial to the thoracolumbar fascia by releasing the thoracolumbar fascia laterally so that the muscle can be mobilized medially. Complete mobilization of the muscle requires dissection deep to the paraspinous muscles ( Fig. 5A–C ). 13
Fig. 4.
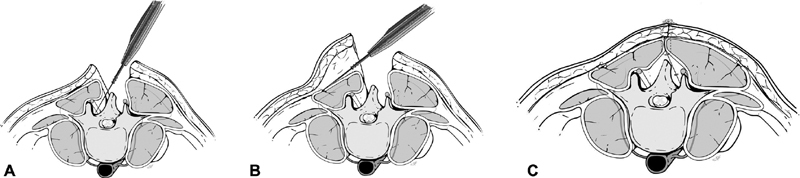
Cross-section of paraspinous advancement flap. ( A ) Medial dissection. ( B ) Lateral dissection. ( C ) Advancement and closure of bilateral paraspinous muscle flaps over spinal hardware. Reprinted with permission of Baylor College of Medicine.
Fig. 5.
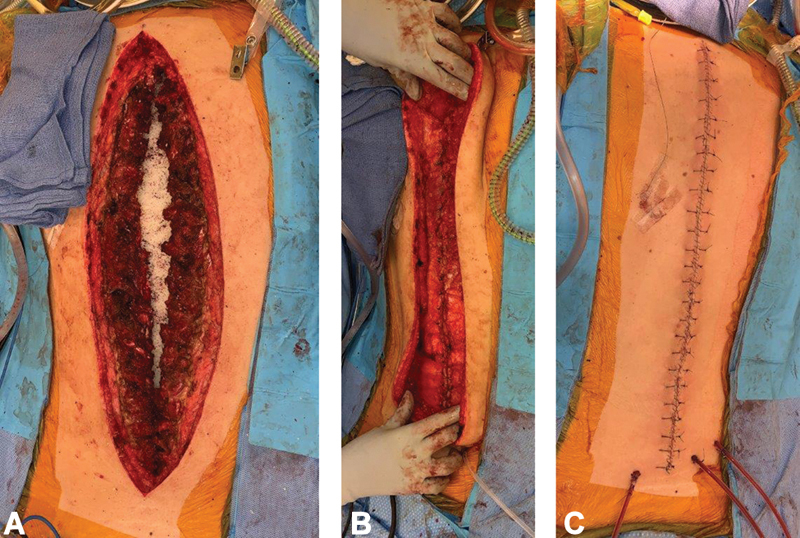
Case example of paraspinous advancement flap. ( A ) Vancomycin powder placed over exposed spinal hardware. ( B ) Advancement and closure of bilateral paraspinous muscle flaps. ( C ) Skin closure.
The latissimus flap is a second line option to the paraspinous muscle flap. The latissimus muscle is a Mathes–Nahai type V flap with its primary blood supply from the thoracodorsal artery and multiple segmental perforating branches from lumbar and intercostal arteries. It can be raised as either a musculocutaneous or a muscle only flap. This muscle is flat, broad, and arises from the posterior third of the outer ridge of the iliac crest, from the lumbar and sacral spinous processes (T7 to T12, L1 to L5) and from the thoracolumbar fascia. This flap is used cautiously in patients with a history of thoracotomy given the potential compromise to the muscle and/or vascular pedicle. The latissimus muscle is ideal for reconstructing lower cervical, thoracic, and lumbar defects, as it can provide 10 to 12 cm of length. The latissimus muscle flap can be advanced, rotated, or used as a turnover flap. When used as an advancement flap, the blood supply is based on the thoracodorsal artery. When used as a turnover flap, the blood supply is based on the lumbar and intercostal perforators. Dissection for a turnover flap should begin at the insertion to divide the thoracodorsal pedicle, as starting at the midline risks dividing the perforators, which are consistently located 5 cm from the midline. 14 The latissimus muscle is optimal for nonfused spinal wounds, or when there has been radiation to the paraspinous muscles. When used with a skin paddle, it is important to make the skin paddle perpendicular to the defect to minimize the closing tension. The donor site morbidity from the harvest of the latissimus flap is relatively low, with the most common complication being seroma formation.
The trapezius muscle is a Mathes–Nahai type II flap with the transverse cervical artery providing the dominant blood supply and intercostal perforators and branches of the occipital artery providing minor blood supply. This flap can be harvested as a musculocutaneous or muscle flap only. This triangular muscle covers the midline of the back from the occiput to T12. The trapezius is indicated for high thoracic and cervical wounds, where the paraspinous muscles have limited mobility and size. It can be rotated or advanced and must be carefully elevated off the paraspinous and rhomboid muscles. 15 16 Caution should be taken when designing the skin paddle on the trapezius and avoid extending the skin island more than 1 cm over the underlying muscle, as this move has been an increased incidence of skin flap loss ( Figs. 6 and 7 ).
Fig. 6.
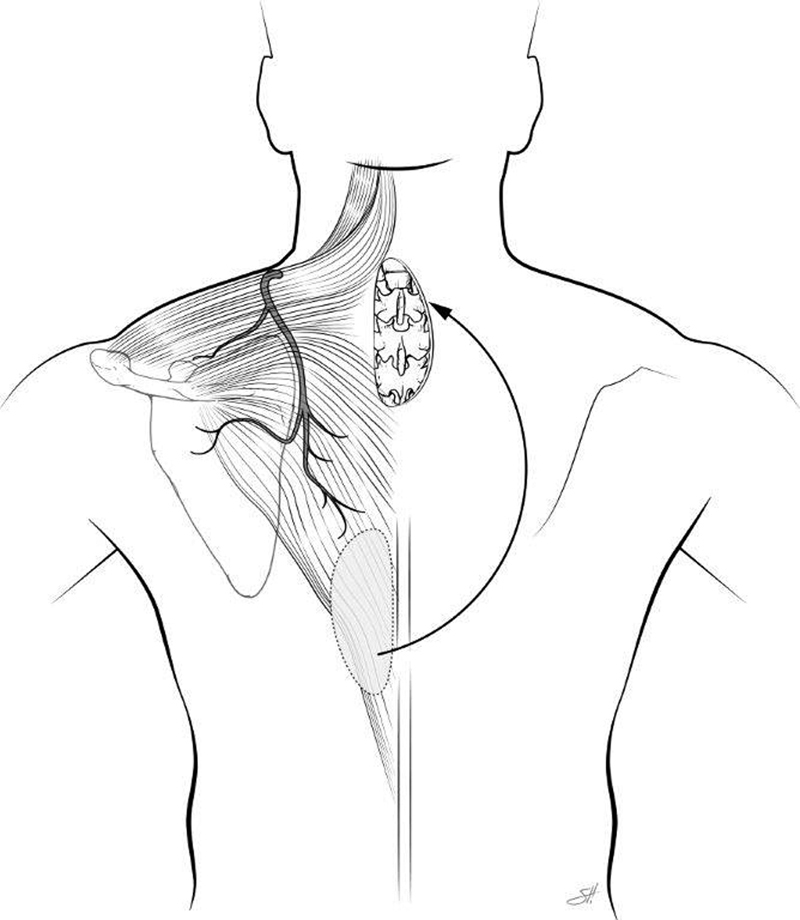
Diagram of trapezius flap. Reprinted with permission of Baylor College of Medicine.
Fig. 7.
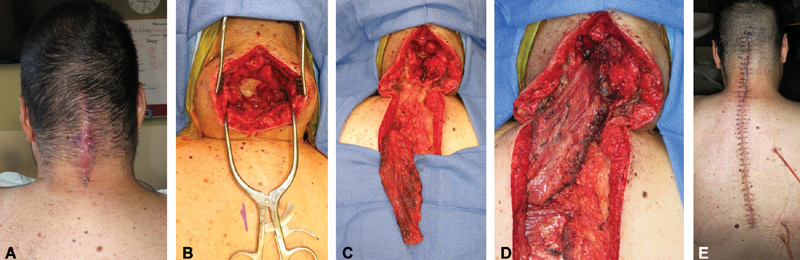
Case example of trapezius muscle flap to cover exposed cervical spinal hardware. ( A ) Exposed cervical spinal hardware. ( B ) Intraoperative exposure. ( C ) Trapezius muscle flap prior to inset. ( D ) Inset of trapezius muscle flap to cover cervical operative levels. ( E ) Postoperative closure.
The lumbosacral region is a difficult area to reconstruct given the limited availability of tissues. Options for this region include the gluteus maximus muscle, the vertical rectus abdominis muscle (VRAM) flap pull through, an omentum flap, and free tissue transfer. The gluteus muscle is a type III muscle with two dominant pedicles—the superior and inferior gluteal arteries—that can be harvested as muscle alone or as a perforator flap based on the gluteal perforating vessels. The superior gluteal artery-based perforator flap includes skin and fat from the mid/upper buttock over the gluteus muscle. The blood supply is from the aforementioned superior gluteal artery and venae from perforates through the gluteus maximus. The point of rotation is based on the artery and can be used to reach to the ipsilateral ischium and sacrum. A V to Y fasciocutaneous gluteal flap can be used as local advancement flaps for smaller midline defects in the lumbosacral region.
Total en bloc sacrectomy defects pose a reconstructive challenge. The goal in reconstruction is to provide stable soft-tissue coverage and obliterate the large dead space. Local options tend not to be adequate. The V to Y gluteal flap may provide stable soft tissue closure but may not fill the dead space adequately. The pedicled VRAM flap simultaneously obliterates the dead space and prevents the herniation of bowel from the peritoneal cavity into the sacral defect where spinal hardware is used. 17 In addition to soft tissue reconstruction, the pelvic floor is reconstructed with permanent mesh or an acellular dermal matrix. 18 The use of the pedicled omental flap has been reported in the treatment of complex spinal wounds after en bloc resection of spine tumors. The blood supply to the omentum is via the right and left gastroepiploic vessels that arise from the celiac and superior mesenteric arteries. The omentum is highly vascular and has the unique ability to fight infection as well as the ability to reconstruct CSF leaks. 19
Free tissue transfer is considered in patients who have had previous advancement flaps, scarred erector spinae muscles from redo surgeries, neuromuscular conditions, and extensive trauma with soft tissue loss with wide debridement. In these circumstances, local muscles are not options for coverage. Similarly, patients who have had wide-field radiation to their back do not have muscle and skin optimal for reconstruction of posterior trunk defects. In addition, the latissimus and trapezius muscle flaps generally do not provide adequate coverage for defects larger than 12 cm. Coverage for these patients is best using free tissue transfer. The primary difficulty in using free flaps for posterior trunk defects is finding appropriate recipient vessels. Options for recipient vessels include the superior gluteal artery, which is a large caliber vessel and often relatively unaffected with radiation, as well as the intercostal arteries.
Vein grafts can be used in an arteriovenous (AV) loop fashion to anastomose to the common femoral artery or external carotid. However, vein grafts can be prone to twist and spasm and require some time to mature prior to final anastomosis. While some elect immediate free tissue transfer, others stage the final reconstruction and allow the AV loop to mature ( Fig. 8 ). 20
Fig. 8.
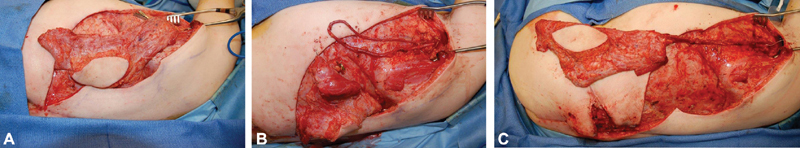
Case example of arteriovenous (AV) loop. ( A ) Staged island flap. ( B ) Formation of AV loop. ( C ) Anastomosis of island flap to AV loop.
Complications
Even with well-planned, meticulous spinal reconstruction, complications remain significant. Complications include hematoma, seroma, partial or total flap loss, CSF leaks, and continued wound dehiscence or necrosis. To decrease the risk of complications, it is important to obtain hemostasis prior to closure, close any dead space, and ensure any CSF leaks are controlled. More recently, plastic surgeons are getting involved in prophylactic closure in patients with significant comorbidities. Prophylactic complex closure has been shown to be a good option in patients with a history of previous hardware infection, previous scarring, radiation, CSF leaks, or a large reconstruction of greater than six to seven vertebral bodies. 21 Data has shown that there is a 6.8% lower rate of postoperative wound complications after closure with local muscle flaps in this high-risk population. 4
Postoperative Considerations
Postoperative management includes the use of incisional NPWT for 5 to 7 days. NPWT is thought to decrease infection rates by protecting the incision from external infectious sources, removing fluid and infectious material from the surgical site, increasing wound microcirculation and tissue oxygen saturation levels, decreasing lateral wound tension, and increasing incisional apposition. 22 Dyck et al have shown that the use of NPWT in the prevention of postoperative infection in high-risk patients who underwent spine surgery resulted in 50% reduction in surgical site infections. 23 In addition, others have shown not only a significant reduction in the incidence of postoperative wound infection but also wound dehiscence. 24 Following reconstruction, the patient receives q2H turns and lateral positioning during recovery so that pressure is avoided on the closure. Antibiotics usage should be determined based on cultures. There are generally no activity restrictions per the plastic surgery service. We ensure that the surgical site is adequately drained with one to two Jackson–Pratt drains, placed subcutaneously, which are removed sequentially when the output is less than 30cc over the course of 24 hours for 3 consecutive days. There are generally also two deep drains placed and managed by the spine service.
Conclusion
The management of posterior trunk defects depends on the anatomic location of the defect ( Fig. 9 ). For cervical defects, the trapezius and latissimus muscle flaps provide the best reconstructive option. Thoracic defects are best managed with paraspinous muscle flaps as a first-line option and the latissimus muscle as a second option. The best coverage options for lumbosacral defects are paraspinous muscle flaps, latissimus muscle flap, or superior gluteal artery perforator flaps. In the rare circumstance that the locoregional flaps are not an option, free tissue transfer is the final avenue. A plastic surgeon should be involved early and often in posterior trunk defects. As previously mentioned, plastic surgeons have historically become involved in these cases only after a wound breakdown. However, it is important to keep in mind that prophylactic closure is often the best option for the patient to heal from surgery, and therefore a plastic surgeon should be consulted prior to surgery.
Fig. 9.
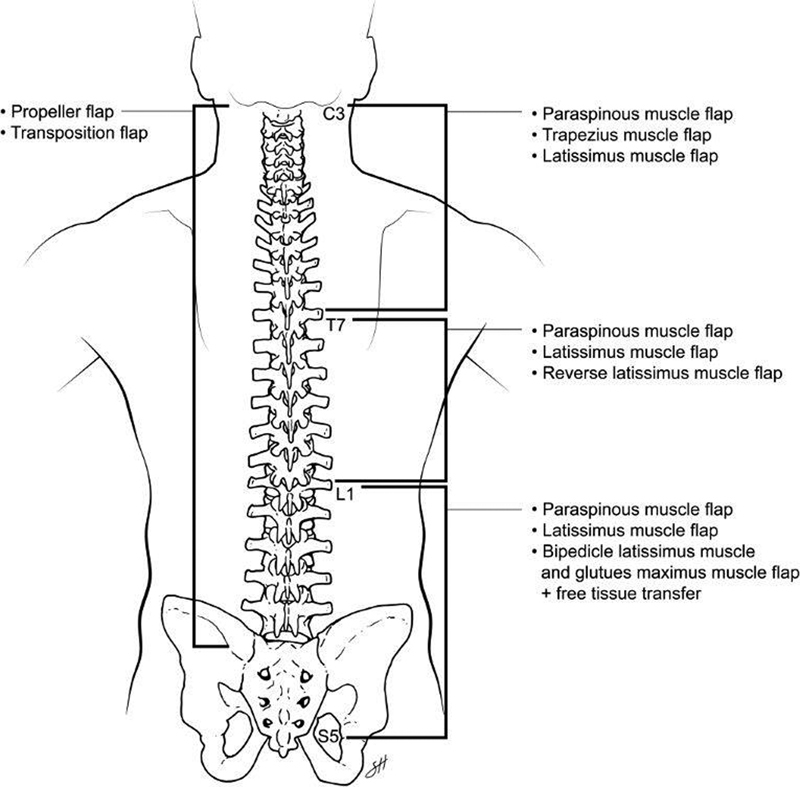
Summary diagram demonstrating how the management of spinal defects depends on the anatomic location of the defect. Reprinted with permission of Baylor College of Medicine.
Funding Statement
Funding None.
Footnotes
Conflicts of Interest Dr. Hansen is part of the Speakers Bureau for Smith and Nephew, but this commitment has no conflict with this report.
References
- 1.Verma R, Siddiqi F, Lipetz J S, Samujh C, Silber J S. Advances in technology and surgical technique in spine surgery. Am J Orthop. 2007;36(08):413–417. [PubMed] [Google Scholar]
- 2.Reeg S E. A review of comorbidities and spinal surgery. Clin Orthop Relat Res. 2001;384(384):101–109. doi: 10.1097/00003086-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Maruo K, Berven S H. Outcome and treatment of postoperative spine surgical site infections: predictors of treatment success and failure. J Orthop Sci. 2014;19(03):398–404. doi: 10.1007/s00776-014-0545-z. [DOI] [PubMed] [Google Scholar]
- 4.Cohen L E, Fullerton N, Mundy L R. Optimizing successful outcomes in complex spine reconstruction using local muscle flaps. Plast Reconstr Surg. 2016;137(01):295–301. doi: 10.1097/PRS.0000000000001875. [DOI] [PubMed] [Google Scholar]
- 5.Nasser R, Yadla S, Maltenfort M G. Complications in spine surgery. J Neurosurg Spine. 2010;13(02):144–157. doi: 10.3171/2010.3.SPINE09369. [DOI] [PubMed] [Google Scholar]
- 6.Saint-Cyr M, Nikolis A, Moumdjian R. Paraspinous muscle flaps for the treatment and prevention of cerebrospinal fluid fistulas in neurosurgery. Spine. 2003;28(05):E86–E92. doi: 10.1097/01.BRS.0000048656.90401.4C. [DOI] [PubMed] [Google Scholar]
- 7.Mathes D W, Thornton J F, Rohrich R J. Management of posterior trunk defects. Plast Reconstr Surg. 2006;118(03):73e–83e. doi: 10.1097/01.prs.0000233130.93861.15. [DOI] [PubMed] [Google Scholar]
- 8.Lee R, Beder D, Street J. The use of vacuum-assisted closure in spinal wound infections with or without exposed dura. Eur Spine J. 2018;27(10):2536–2542. doi: 10.1007/s00586-018-5612-2. [DOI] [PubMed] [Google Scholar]
- 9.Chen K, Lin J T, Sun S B, Lin J, Kong J Z, Tian N F. Vacuum-assisted closure combined with a closed suction irrigation system for treating postoperative wound infections following posterior spinal internal fixation. J Orthop Surg Res. 2018;13(01):321. doi: 10.1186/s13018-018-1024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eshima I, Mathes S J, Paty P. Comparison of the intracellular bacterial killing activity of leukocytes in musculocutaneous and random-pattern flaps. Plast Reconstr Surg. 1990;86(03):541–547. doi: 10.1097/00006534-199009000-00026. [DOI] [PubMed] [Google Scholar]
- 11.Calderon W, Chang N, Mathes S J. Comparison of the effect of bacterial inoculation in musculocutaneous and fasciocutaneous flaps. Plast Reconstr Surg. 1986;77(05):785–794. doi: 10.1097/00006534-198605000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Mathes S J, Nahai F. Classification of the vascular anatomy of muscles: experimental and clinical correlation. Plast Reconstr Surg. 1981;67(02):177–187. [PubMed] [Google Scholar]
- 13.Mericli A F, Tarola N A, Moore J H, Jr, Copit S E, Fox J W, IV, Tuma G A. Paraspinous muscle flap reconstruction of complex midline back wounds: risk factors and postreconstruction complications. Ann Plast Surg. 2010;65(02):219–224. doi: 10.1097/SAP.0b013e3181c47ef4. [DOI] [PubMed] [Google Scholar]
- 14.Bostwick J, III, Scheflan M, Nahai F, Jurkiewicz M J. The “reverse” latissimus dorsi muscle and musculocutaneous flap: anatomical and clinical considerations. Plast Reconstr Surg. 1980;65(04):395–399. doi: 10.1097/00006534-198004000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Mathes S J, Stevenson T R. Reconstruction of posterior neck and skull with vertical trapezius musculocutaneous flap. Am J Surg. 1988;156(04):248–251. doi: 10.1016/s0002-9610(88)80284-8. [DOI] [PubMed] [Google Scholar]
- 16.Disa J J, Smith A W, Bilsky M H. Management of radiated reoperative wounds of the cervicothoracic spine: the role of the trapezius turnover flap. Ann Plast Surg. 2001;47(04):394–397. doi: 10.1097/00000637-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Kim J E, Pang J, Christensen J M. Soft-tissue reconstruction after total en bloc sacrectomy. J Neurosurg Spine. 2015;22(06):571–581. doi: 10.3171/2014.10.SPINE14114. [DOI] [PubMed] [Google Scholar]
- 18.Junge K, Krones C J, Rosch R, Fackeldey V, Schumpelick V. Mesh reconstruction preventing sacral herniation. Hernia. 2003;7(04):224–226. doi: 10.1007/s10029-003-0137-x. [DOI] [PubMed] [Google Scholar]
- 19.Sambri A, Gasbarrini A, Cialdella S, De Iaco P, Boriani S. Pedicled omental flaps in the treatment of complex spinal wounds after en bloc resection of spine tumors. J Plast Reconstr Aesthet Surg. 2017;70(09):1267–1271. doi: 10.1016/j.bjps.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Matschke J, Armbruster R, Reeps C, Weitz J, Dragu A. AV loop free flap: an interdisciplinary approach for perineal and sacral defect reconstruction after radical oncological exenteration and radiation in a colorectal cancer patient. World J Surg Oncol. 2019;17(01):154. doi: 10.1186/s12957-019-1698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chieng L O, Hubbard Z, Salgado C J, Levi A D, Chim H. Reconstruction of open wounds as a complication of spinal surgery with flaps: a systematic review. Neurosurg Focus. 2015;39(04):E17. doi: 10.3171/2015.7.FOCUS15245. [DOI] [PubMed] [Google Scholar]
- 22.Horch R E.Incisional negative pressure wound therapy for high-risk wounds J Wound Care 201524(4, Suppl):21–28. [DOI] [PubMed] [Google Scholar]
- 23.Dyck B A, Bailey C S, Steyn C. Use of incisional vacuum-assisted closure in the prevention of postoperative infection in high-risk patients who underwent spine surgery: a proof-of-concept study. J Neurosurg Spine. 2019;31(03):430–439. doi: 10.3171/2019.2.SPINE18947. [DOI] [PubMed] [Google Scholar]
- 24.Adogwa O, Fatemi P, Perez E. Negative pressure wound therapy reduces incidence of postoperative wound infection and dehiscence after long-segment thoracolumbar spinal fusion: a single institutional experience. Spine J. 2014;14(12):2911–2917. doi: 10.1016/j.spinee.2014.04.011. [DOI] [PubMed] [Google Scholar]


