Abstract
Purpose
The purpose of the current meta-analysis was to evaluate whether multidisciplinary team improved overall survival of colorectal cancer.
Methods
PubMed, EMBASE, and Cochrane Library database were searched from inception to October 25, 2020. The hazard ratio (HR) and 95% confidence (CI) of overall survival (OS) were calculated.
Results
A total of 11 studies with 30814 patients were included in this meta-analysis. After pooling the HRs, the MDT group was associated with better OS compared with the non-MDT group (HR = 0.81, 95% CI 0.69-0.94, p = 0.005). In subgroup analysis of stage IV colorectal cancer, the MDT group was associated with better OS as well (HR = 0.73, 95% CI 0.59-0.90, p = 0.004). However, in terms of postoperative mortality, no significant difference was found between MDT and non-MDT groups (OR = 0.84, 95% CI 0.44-1.61, p = 0.60).
Conclusion
MDT could improve OS of colorectal cancer patients.
1. Introduction
Colorectal cancer ranks the third largest cancer in the world and the second leading cause of cancer-related death, resulting in approximately 600,000 deaths each year [1, 2]. Metastases occur in 20% to 30% of patients at the time of diagnosis [3, 4]. Surgery is the main treatment for patients with colorectal cancer; in addition, other treatment including radiotherapy and chemotherapy can improve the prognosis as well [5–7].
The core of the multidisciplinary team (MDT) is a patient-centered focus model by regularly organizing experts in related disciplines to discuss and make the most suitable treatment plan for patients [8]. MDT has proven to be effective in the treatment of breast cancer, oral cancer, and prostate cancer [9–11]. In recent years, MDT has become an increasingly popular form of diagnosis and treatment in colorectal cancer [12, 13]. The MDT of colorectal cancer is composed of experts in multiple fields including surgery, oncology, radiology, pathology, and other related disciplines [14, 15].
Previous studies reported survive benefit of MDT on colorectal cancer [16, 17]; however, other studies held negative point [18, 19]. Therefore, the purpose of the current meta-analysis was to evaluate whether MDT improved OS of colorectal cancer.
2. Methods
The current meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [20].
2.1. Literature Search Strategy
PubMed, EMBASE, and Cochrane Library database were searched by two authors, respectively. The literature search was performed on October 25, 2020. The search strategy focused on two items including multidisciplinary team and colorectal cancer. For the multidisciplinary team, the search strategy was as follows: (multidisciplinary) OR (multidisciplinary) OR (MDT). For colorectal cancer, the search strategy was as follows: (colorectal cancer) OR (colon cancer) OR (rectal cancer) OR (colorectal neoplasm) OR (colon neoplasm) OR (rectal neoplasm) OR (colorectal tumor) OR (colon tumor) OR (rectal tumor). After that, we combined the two search items using “AND,” and the scope of search strategy was limited in title and abstract.
2.2. Inclusion and Exclusion Criteria
The screening of the studies was carried out by two authors independently. Initially, titles and abstracts were screened to exclude irrelevant studies, and after that, full texts were required for further screening. Case reports, nonhuman studies, conference abstracts, comments, and letters to editor were excluded. All included studies were checked for duplicate medical records from the same or overlapping cohort of patients. Finally, all the included studies were crosschecked by the two authors.
2.3. Data Extraction and Quality Assessment
The data were extracted by two authors. The extracted data included the first author, publishing year, country, study design, study date, sample size, sex, tumor location, and HRs with associated 95% CIs or p value for OS. HRs were extracted from multivariate analyses and/or univariate analyses or estimated from Kaplane-Meier survival curves [21, 22]. Any discrepancies between the two authors were resolved by a third author for consensus. The quality of the included studies was assessed based on the Newcastle-Ottawa Scale (NOS) [23], and a NOS score ≥ 7 was considered high-quality studies [24].
2.4. Statistical Analysis
Pooled HRs and 95%CIs were calculated for OS of colorectal cancer. For dichotomous variables, odds ratios (ORs) were calculated, respectively. 95% confidence intervals (CI) were calculated. The value of I2 and the result of the Chi-squared test were used to assess the statistical heterogeneity [25, 26]. It was considered high heterogeneity when I2 > 50%, the random effect model was used, and p < 0.1 was considered statistically significant. While the fixed effect model was used for the studies with I2 ≤ 50%, p < 0.05 was considered statistically significant. This meta-analysis was performed with RevMan 5.3 (The Cochrane Collaboration, London, United Kingdom).
3. Result
3.1. Search Results
A total of 4972 studies were screened in database, and eleven studies [16–19, 27–33] with 30814 patients were included for meta-analysis according to the inclusion and exclusion criteria, and the flowchart of study selection was shown in Figure 1.
Figure 1.
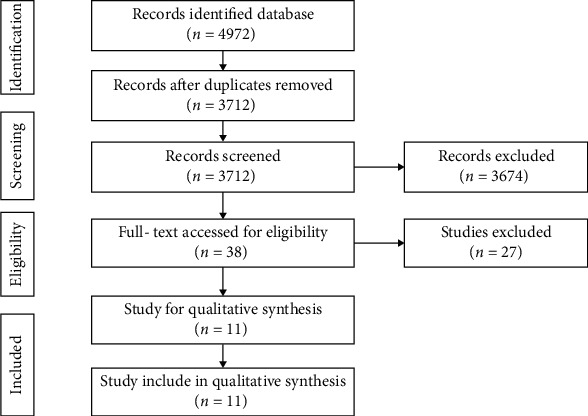
Flowchart of study selection.
3.2. Characteristics of the Included Studies
The 11 studies were published from 2011 to 2020, seven of which were from China, and the others were from The Netherlands, Australia, Denmark and The United Kingdom, respectively. There were 6 retrospective studies and 5 cohort studies. The sample size, sex, tumor stage and NOS score were shown in Table 1.
Table 1.
Characteristics of the studies included in the meta-analysis.
| Author | Year published | Country | Study design | Study date | Sample size | Gender (male/female) | Tumor staging | NOS | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MDT | Non-MDT | MDT | Non-MDT | MDT | Non-MDT | ||||||
| van der Vlies | 2020 | The Netherlands | Cohort | 2015-2018 | 127 | 306 | 65/62 | 191/115 | I/II/III/IV | I/II/III/IV | 8 |
| Li | 2020 | China | Retrospective | 2014-2018 | 46 | 83 | 32/14 | 54/29 | IV | IV | 7 |
| Lin | 2018 | China | Retrospective | 2006-2009 | 326 | 325 | 155/171 | 181/144 | I/II/III/IV | I/II/III/IV | 8 |
| Hsu | 2016 | China | Cohort | 2005-2008 | 3515 | 22251 | 1978/1537 | 12609/9642 | I/II/III/IV | I/II/III/IV | 8 |
| Ye | 2012 | China | Cohort | 1999-2006 | 298 | 297 | 166/132 | 180/117 | I/II/III/IV | I/II/III/IV | 8 |
| Chien | 2018 | China | Retrospective | 2007-2017 | 75 | 86 | 42/33 | 43/43 | IV | IV | 7 |
| Lan | 2016 | China | Retrospective | 2001-2010 | 439 | 636 | 261/178 | 429/207 | IV | IV | 8 |
| Rogers | 2017 | Australia | Retrospective | 2009-2012 | 363 | 257 | 227/136 | 140/117 | I/II/III/IV | I/II/III/IV | 7 |
| Wille | 2013 | Denmark | Cohort | 2001-2006 | 344 | 467 | 193/151 | 245/222 | I/II/III/IV | I/II/III/IV | 8 |
| Du | 2011 | China | Retrospective | 2001-2009 | 101 | 162 | 57/44 | 88/74 | I/II/III/IV | I/II/III/IV | 7 |
| Macdermid | 2009 | The United Kingdom | Cohort | 1997-2005 | 134 | 176 | 62/72 | 96/80 | II/III/IV | II/III/IV | 7 |
Abbreviations: MDT: multidisciplinary team; NOS: Newcastle-Ottawa Scale.
3.3. Impact of MDT on Overall Survival
All the 11 studies reported MDT on OS. After pooling the HRs, a significant difference was found between the MDT group and non-MDT group (HR = 0.81, 95% CI 0.69-0.94, p = 0.005). These results suggested that the MDT group was associated with better OS (Figure 2).
Figure 2.
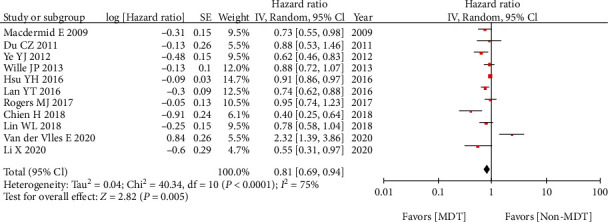
Impact of MDT on overall survival.
3.4. Subgroup Analysis of MDT on Overall Survival of Stage IV Colorectal Cancer
Five studies reported the HRs of stage IV colorectal cancer, and in order to explore MDT on OS of the specific tumor stage, we performed subgroup analysis of stage IV colorectal cancer. The MDT group was associated with better OS compared with the non-MDT group in terms of stage IV colorectal cancer (HR = 0.73, 95% CI 0.59-0.90, p = 0.004) (Figure 3).
Figure 3.
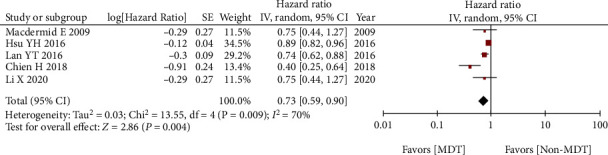
Subgroup analysis of MDT on overall survival of stage IV colorectal cancer.
3.5. Impact of Other Factors on Overall Survival
There were other factors reported influencing OS, and we conducted subgroup analysis of the factors including sex, age, differentiation, tumor stage, and neoadjuvant chemotherapy. After pooling up the HRs, old age (HR = 1.38, 95% CI 1.09-1.74, p = 0.006), poor differentiation (HR = 1.35, 95% CI 1.06-1.72, p = 0.01), and higher tumor stage (HR = 1.77, 95% CI 1.19-2.65, p = 0.005) were related to poor OS. The results of subgroup analysis were shown in Table 2.
Table 2.
Factors analysis of hazard ratios for overall survival.
| Factors | No. of studies | Effects model | HR (95% CI) | p | Heterogeneity | |
|---|---|---|---|---|---|---|
| I 2 (%) | p | |||||
| Sex | 5 | Random | 1.24 (0.93-1.66) | 0.14 | 64 | 0.02 |
| Age | 5 | Random | 1.38 (1.09-1.74) | 0.006 | 53 | 0.07 |
| Neoadjuvant chemotherapy | 2 | Fixed | 0.70 (0.45-1.10) | 0.12 | 0 | 0.59 |
| Differentiation | 3 | Fixed | 1.35 (1.06-1.72) | 0.01 | 47 | 0.15 |
| Tumor stage | 2 | Random | 1.77 (1.19-2.65) | 0.005 | 87 | 0.006 |
Notes: HR: hazard ratio; 95% CI: 95% confidence interval.
3.6. Postoperative Mortality between MDT and Non-MDT Groups
Four studies reported the postoperative mortality between MDT and non-MDT groups. After pooling up all the data, no significant difference was found between MDT and non-MDT groups (OR = 0.84, 95% CI 0.44-1.61, p = 0.60) (Figure 4).
Figure 4.
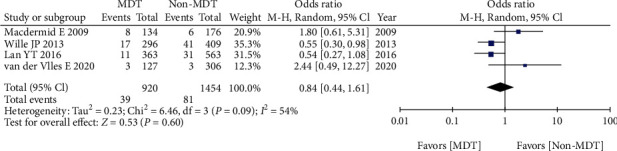
Postoperative mortality between MDT and non-MDT groups.
4. Discussion
A total of 11 studies with 30814 patients were included in this meta-analysis. After pooling the HRs, the MDT group was associated with better OS compared with the non-MDT group. In subgroup analysis of stage IV colorectal cancer, the MDT group was associated with better OS as well. However, in terms of postoperative mortality, no significant difference was found between MDT and non-MDT groups.
Despite the reduction in mortality with changes in screening tests and lifestyle, the prognosis of patients with metastatic colorectal cancer was still poor [34, 35]. There were many prognostic factors including tumor stage, tumor location, and obesity which could affect the OS [36, 37]. In this meta-analysis, we found that age, degree of differentiation, tumor stage, and MDT were prognostic factors of colorectal cancer. These results were similar with previous studies [29, 31].
A population-based study reported the postoperative mortality after resection for colorectal cancer that was related to advanced age and tumor stage [38], and another population-based study reported similar results [39]. In this meta-analysis, we explored whether MDT had effect on the postoperative mortality, and no difference was found between MDT and non-MDT groups. Therefore, MDT might not affect postoperative mortality; although, accurate clinical data and imaging were accessed [40].
Although surgery played an important role in colorectal cancer, the requirement for cooperation of experts from various disciplines was increasing [41]. At present, most doctors in the world recognized the positive effect of MDT and accepted MDT as a main treatment for cancer [42]. MDT has demonstrated advantages not only in the field of digestive tract tumors but also in various fields of oncology. Many experts were involved in MDT and could easily deal with conflicting viewpoints, and this meant all experts could participate in a personalized tumor treatment based on clinical data and imaging. In this meta-analysis, after pooling the HRs, the MDT group was associated with better OS compared with the non-MDT group. In subgroup analysis of stage IV colorectal cancer, the MDT group was associated with better OS as well. There were many factors accounting for the current results. MDT could reduce the number of imperfect decisions made by individual physicians [12]. Moreover, patients could benefit from MDT including highly accurate staging, good treatment cohesion, high quality of life, and long-tem survival [31–33].
MDT could bring survival benefits, and the model of MDT had been in progress and explored [28]. The MDT meeting was challenging for involved experts, and self-education was important for surgeons, radiologists, pathologists, and oncologists participating in the meeting [28, 29]. However, van der Vlies et al. raised up a concern about negative outcome of MDT on OS, which was related to implementation of a preoperative MDT on patient management [18]. But if no adequate investment in team training, MDT might not be able to bring benefits to patients and health-care professionals [19].
Some limitations existed in this meta-analysis. Firstly, RCTs were not included in this meta-analysis, and only retrospective studies and cohort studies that subjected to inherent bias were included. Secondly, most of the studies included were from Asia, and the results might be applied to Asian patients. Thirdly, significant heterogeneity existed among the studies, and it seemed unlikely that any hospital system did multidisciplinary team therapy and then abandoned it, which meant that patients treated before the adoption of such teamwork and then incorporated into the study that might have received care in an earlier era when treatment options were less advanced. Alternatively, patients with truly poor prognosis or multiple comorbidities might not have been referred to the multidisciplinary team, which would have resulted in a selection bias. Although the effects of heterogeneity were reduced using the random-effect model method, this effect could not be completely eliminated. Furthermore, the included studies did not explicate the composition of MDT in detail, and the participation criteria of MDT remained unclear. Therefore, multicenter, multiregional, prospective, and high quality RCTs should be carried out in the future.
In conclusion, MDT could improve overall survival of colorectal cancer patients and had no influence on postoperative mortality.
Acknowledgments
Thanks are due to all the authors of this article.
Data Availability
All data included in this study are available upon request by contact with the corresponding author.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer Journal for Clinicians. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Rawla P., Sunkara T., Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Gastroenterology Review. 2019;14(2):89–103. doi: 10.5114/pg.2018.81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R. L., Miller K. D., Fedewa S. A., et al. Colorectal cancer statistics, 2017. CA: a Cancer Journal for Clinicians. 2017;67(3):177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 4.Leufkens A. M., van den Bosch M. A., van Leeuwen M. S., Siersema P. D. Diagnostic accuracy of computed tomography for colon cancer staging: a systematic review. Scandinavian Journal of Gastroenterology. 2011;46(7-8):887–894. doi: 10.3109/00365521.2011.574732. [DOI] [PubMed] [Google Scholar]
- 5.Brenner H., Kloor M., Pox C. P. Colorectal cancer. Lancet. 2014;383(9927):1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 6.McCaw Z. R., Kim D. H., Wei L. J. Risk-benefit comparisons between shorter and longer durations of adjuvant chemotherapy in high-risk stage II colorectal cancer. JAMA Oncology. 2020;6(8):1301–1302. doi: 10.1001/jamaoncol.2020.2256. [DOI] [PubMed] [Google Scholar]
- 7.Wasan H. S., Gibbs P., Sharma N. K., et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-global): a combined analysis of three multicentre, randomised, phase 3 trials. The Lancet Oncology. 2017;18(9):1159–1171. doi: 10.1016/S1470-2045(17)30457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ioannidis A., Konstantinidis M., Apostolakis S., Koutserimpas C., Machairas N., Konstantinidis K. M. Impact of multidisciplinary tumor boards on patients with rectal cancer. Molecular and Clinical Oncology. 2018;9(2):135–137. doi: 10.3892/mco.2018.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kesson E. M., Allardice G. M., George W. D., Burns H. J. G., Morrison D. S. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ. 2012;344(apr26 1, article e2718) doi: 10.1136/bmj.e2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colasante A., Augurio A., Basilico R., et al. A multidisciplinary group for prostate cancer management: a single institution experience. Oncology Letters. 2018;15(2):1823–1828. doi: 10.3892/ol.2017.7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X., Zhao J., Shen Z., et al. Multidisciplinary approach in improving survival outcome of early-stage gastric cancer. The Journal of Surgical Research. 2020;255:285–296. doi: 10.1016/j.jss.2020.05.058. [DOI] [PubMed] [Google Scholar]
- 12.Jung S. M., Hong Y. S., Kim T. W., et al. Impact of a multidisciplinary team approach for managing advanced and recurrent colorectal cancer. World Journal of Surgery. 2018;42(7):2227–2233. doi: 10.1007/s00268-017-4409-5. [DOI] [PubMed] [Google Scholar]
- 13.Fernando C., Frizelle F., Wakeman C., Frampton C., Robinson B. Colorectal multidisciplinary meeting audit to determine patient benefit. ANZ Journal of Surgery. 2017;87(11):E173–E177. doi: 10.1111/ans.13366. [DOI] [PubMed] [Google Scholar]
- 14.Tomlinson J. S., Jarnagin W. R., DeMatteo R. P., et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. Journal of Clinical Oncology. 2007;25(29):4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 15.Adam R., Wicherts D. A., de Haas R. J., et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? Journal of Clinical Oncology. 2009;27(11):1829–1835. doi: 10.1200/JCO.2008.19.9273. [DOI] [PubMed] [Google Scholar]
- 16.Lin W. L., Sun J. L., Chang S. C., et al. Effectiveness of the multidisciplinary team model in treating colorectal cancer. Gastroenterology Nursing. 2018;41(6):491–496. doi: 10.1097/SGA.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 17.Li X., Chen Q., Bi X., et al. Effects of multidisciplinary team on the outcomes of colorectal cancer patients with liver metastases. Annals of Palliative Medicine. 2020;9(5):2741–2748. doi: 10.21037/apm-20-193. [DOI] [PubMed] [Google Scholar]
- 18.van der Vlies E., Smits A. B., Los M., et al. Implementation of a preoperative multidisciplinary team approach for frail colorectal cancer patients: influence on patient selection, prehabilitation and outcome. Journal of Geriatric Oncology. 2020;11(8):1237–1243. doi: 10.1016/j.jgo.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Wille-Jørgensen P., Sparre P., Glenthøj A., et al. Result of the implementation of multidisciplinary teams in rectal cancer. Colorectal Disease. 2013;15(4):410–413. doi: 10.1111/codi.12013. [DOI] [PubMed] [Google Scholar]
- 20.Moher D., Liberati A., Tetzlaff J., Altman D. G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine. 2009;6(7, article e1000097) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parmar M. K., Torri V., Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine. 1998;17(24):2815–2834. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Tierney J. F., Stewart L. A., Ghersi D., Burdett S., Sydes M. R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(1):p. 16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 24.Wells G. A., Shea B., O'Connell D., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 2017.
- 25.Ioannidis J. P. A. Interpretation of tests of heterogeneity and bias in meta-analysis. Journal of Evaluation in Clinical Practice. 2008;14(5):951–957. doi: 10.1111/j.1365-2753.2008.00986.x. [DOI] [PubMed] [Google Scholar]
- 26.Higgins J. P., Thompson S. G., Deeks J. J., Altman D. G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lan Y. T., Jiang J. K., Chang S. C., et al. Improved outcomes of colorectal cancer patients with liver metastases in the era of the multidisciplinary teams. International Journal of Colorectal Disease. 2016;31(2):403–411. doi: 10.1007/s00384-015-2459-4. [DOI] [PubMed] [Google Scholar]
- 28.Rogers M. J., Matheson L., Garrard B., et al. Comparison of outcomes for cancer patients discussed and not discussed at a multidisciplinary meeting. Public Health. 2017;149:74–80. doi: 10.1016/j.puhe.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 29.du C. Z., Li J., Cai Y., Sun Y. S., Xue W. C., Gu J. Effect of multidisciplinary team treatment on outcomes of patients with gastrointestinal malignancy. World Journal of Gastroenterology. 2011;17(15):2013–2018. doi: 10.3748/wjg.v17.i15.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacDermid E., Hooton G., MacDonald M., et al. Improving patient survival with the colorectal cancer multi-disciplinary team. Colorectal Disease. 2009;11(3):291–295. doi: 10.1111/j.1463-1318.2008.01580.x. [DOI] [PubMed] [Google Scholar]
- 31.Hsu Y. H., Kung P. T., Wang S. T., Fang C. Y., Tsai W. C. Improved patient survivals with colorectal cancer under multidisciplinary team care: a nationwide cohort study of 25,766 patients in Taiwan. Health Policy. 2016;120(6):674–681. doi: 10.1016/j.healthpol.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Ye Y. J., Shen Z. L., Sun X. T., et al. Impact of multidisciplinary team working on the management of colorectal cancer. Chinese Medical Journal. 2012;125(2):172–177. [PubMed] [Google Scholar]
- 33.Chen C. H., Hsieh M. C., Lao W. T., Lin E. K., Lu Y. J., Wu S. Y. Multidisciplinary team intervention associated with improved survival for patients with colorectal adenocarcinoma with liver or lung metastasis. American Journal of Cancer Research. 2018;8(9):1887–1898. [PMC free article] [PubMed] [Google Scholar]
- 34.Unseld M., Drimmel M., Siebenhüner A., et al. Optimizing treatment sequence for late-line metastatic colorectal cancer patients using trifluridine/tipiracil and regorafenib. Clinical Colorectal Cancer. 2018;17(4):274–279. doi: 10.1016/j.clcc.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Wang S., Feng Y., Swinnen J., Oyen R., Li Y., Ni Y. Incidence and prognosis of liver metastasis at diagnosis: a pan-cancer population-based study. American Journal of Cancer Research. 2020;10(5):1477–1517. [PMC free article] [PubMed] [Google Scholar]
- 36.Mo S., Cai X., Zhou Z., et al. Nomograms for predicting specific distant metastatic sites and overall survival of colorectal cancer patients: a large population-based real-world study. Clinical and Translational Medicine. 2020;10(1):169–181. doi: 10.1002/ctm2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stillwell A. P., Ho Y., Veitch C. Systematic review of prognostic factors related to overall survival in patients with stage IV colorectal cancer and unresectable metastases. World Journal of Surgery. 2011;35(3):684–692. doi: 10.1007/s00268-010-0891-8. [DOI] [PubMed] [Google Scholar]
- 38.Manfredi S., Jooste V., Gay C., Faivre J., Drouillard A., Bouvier A.-M. Time trends in colorectal cancer early postoperative mortality. A French 25-year population-based study. International Journal of Colorectal Disease. 2017;32(12):1725–1731. doi: 10.1007/s00384-017-2918-1. [DOI] [PubMed] [Google Scholar]
- 39.Morris E. J. A., Taylor E. F., Thomas J. D., et al. Thirty-day postoperative mortality after colorectal cancer surgery in England. Gut. 2011;60(6):806–813. doi: 10.1136/gut.2010.232181. [DOI] [PubMed] [Google Scholar]
- 40.Yu L., Wang L., Tan Y., et al. Accuracy of magnetic resonance imaging in staging rectal cancer with multidisciplinary team: a single-center experience. Journal of Cancer. 2019;10(26):6594–6598. doi: 10.7150/jca.32685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rougier P., Neoptolemos J. P. The need for a multidisciplinary approach in the treatment of advanced colorectal cancer: a critical review from a medical oncologist and surgeon. European Journal of Surgical Oncology. 1997;23(5):385–396. doi: 10.1016/S0748-7983(97)93715-X. [DOI] [PubMed] [Google Scholar]
- 42.Lamb B. W., Wong H. W. L., Vincent C., Green J. S. A., Sevdalis N. Teamwork and team performance in multidisciplinary cancer teams: development and evaluation of an observational assessment tool. BMJ Quality and Safety. 2011;20(10):849–856. doi: 10.1136/bmjqs.2010.048660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included in this study are available upon request by contact with the corresponding author.


