Abstract
目的
探讨右美托咪定(DEX)预防老年脊柱手术患者术后认知功能障碍(POCD)发生率的免疫调节机制及合理剂量。
方法
选择行择期脊柱手术患者120例,随机分为4组:S组(n=30,0.2 μg·kg-1·h-1 DEX),M组(n=30,0.5 μg·kg-1·h-1 DEX),L组(n= 30,0.8 μg·kg-1·h-1 DEX),C组(n=30,等容量生理盐水);S组、M组、L组麻醉诱导前使用0.3 μg/kg负荷量DEX泵注10 min,C组则使用等容量生理盐水泵注;全身麻醉诱导气管插管后对S组、M组、L组分别静脉泵注DEX 0.2、0.5、0.8μg·kg-1·h-1至手术结束前40 min,记录苏醒时间和低血压、窦性心动过缓的发生,术前(D0)及术后1 d(D1)、3 d(D2)、7 d(D3)评估患者MMSE评分及检测Aβ、TNF-α、IL-1β和IL-6的血清浓度。
结果
与DO比较,4组患者术后D1时间点的Aβ、TNF-α、IL-1β和IL-6血清水平明显升高(P < 0.05);M组、L组Aβ血清水平D3时间点下降至术前水平(P > 0.05),而C组、S组仍较高水平;M组、L组的TNF-α、IL-1β和IL-6的血清水平在D2时间点恢复术前水平(P > 0.05),而C组、S组D3时间点才恢复。与C组比较,M组、L组的POCD发生率显著减少(P < 0.05),但M组、L组两组之间没有显著差异。L组患者低血压和心动过缓的发生率最高(P < 0.01),苏醒时间(Tw)也比其他3组长(P < 0.05)。
结论
围术期使用DEX0.3 μg/kg负荷剂量泵注10 min后,持续泵注0.5 μg·kg-1·h-1 DEX可控制Aβ和促炎性细胞因子血清水平,有效预防老年脊柱手术患者POCD发生。
Keywords: DEX, 术后认知功能障碍, β-淀粉样蛋白, 细胞因子, 老年
Abstract
Objective
To explore the immunomodulatory mechanism and optimal dose of dexmedetomidine (DEX) for preventing postoperative cognitive dysfunction (POCD) in elderly patients undergoing spinal surgery.
Methods
A total of 120 elderly patients undergoing elective spinal surgery with general anesthesia were randomized into 4 groups to receive a loading dose of 0.3 μg/kg DEX for 10 min before anesthesia induction followed by maintenance doses of 0.2, 0.5, and 0.8 μg · kg-1·h-1 (low-, medium-, and high-dose DEX groups, respectively) or an equal volume of normal saline (control group). DEX and saline was discontinued 40 min before the end of the surgery. Before induction (D0) and on day 1 (D1), day 3 (D2) and day 7 (D3) after the operation, the cognitive function of the patients was assessed using the MMSE scale and their serum levels of β-amyloid (Aβ), TNF-α, IL-1β and IL-6 were measured. The occurrence of adverse effects including bradycardia and hypotension and the recovery time of the patients were recorded.
Results
Compared with those on D0, serum levels of Aβ, IL-1β, IL-6, and TNF-α on D1 were markedly increased in all the groups (P < 0.05); the levels of Aβ decreased to the baseline level on D3 in medium- and high-dose DEX groups (P > 0.05) but remained high in the other two groups. On D2, TNF-α, L-1β and IL-6 recovered their baseline levels in medium- and high-dose DEX groups (P > 0.05) but remained elevated in the other two groups. The incidences of POCD in medium- and high-dose DEX groups were comparable but significantly lower than that in the control group (P < 0.05). The incidences of hypotension and bradycardia were the highest in high-dose DEX group (P < 0.01), which also had longer recovery time than the other 3 groups (P < 0.05).
Conclusion
With a loading dose of 0.3 μg/kg followed by a maintenance doses of 0.5 μg · kg-1·h-1, DEX can effectively reduce the incidence of POCD in elderly patients undergoing spinal surgery by inhibiting the production of Aβ and pro-inflammatory cytokines.
Keywords: dexmedetomidine, postoperative cognitive dysfunction, β-amyloid, cytokine, elderly
术后认知功能障碍(POCD)是老年患者常见的术后神经系统并发症,可延长患者住院时间,降低生活质量,增加术后死亡率[1]。年龄、手术是诱发POCD的危险因素[2-3],随着我国人口老龄化趋势,越来越多老年患者需要接受手术[4],因此,防治老年患者POCD也越来越受到关注,但POCD发病机制尚未有确切结论。多项对POCD发病机制的研究集中关注神经细胞炎症反应的分子机制[5],沉积于小胶质细胞内的Aβ激活炎症小体NLRP3,增加TNF-α、IL-1β和IL-6等细胞因子的释放[6]。Aβ、TNF-α、IL-1β和IL-6不仅能准确地反应脑部炎症反应,还与POCD的发生和发展密切相关。如何避免或减轻围术期神经炎症反应,可能是防治POCD的关键。右美托咪定(DEX)是一种高选择性α2-肾上腺素受体激动剂,在不引起呼吸抑制的情况下,为手术患者提供良好的镇静和镇痛作用,但容易出现低血压和心动过缓。DEX可抑制脑缺血模型的IL-6、TNF-α等促炎性细胞因子释放,产生神经保护作用[7];DEX为临床防治POCD提供了新的选择,应用前景较好,部分临床研究报道了DEX在预防POCD中的作用[8-9],然而也有部分临床研究发现术中使用DEX并不能预防POCD的发生[10-11]。回顾以往的研究,临床研究因用药时机、输注时间和输注剂量不同而导致结果不同。未来,还需加大临床研究,加深基础机制研究,明确DEX在POCD防治中的作用及机制[12]。
近年来,已有研究明确DEX预防POCD与输注时机及输注时间密切相关,老年患者非心脏手术后尽早期输注DEX可降低POCD发生率,提高术后6月、1年及2年生存率,提高患者生活质量[13];此外,围术期长时间接受DEX输注可明显改善认知功能障碍的发生[14];Lee等[8]研究证明DEX的输注剂量在预防POCD的重要性,然而当前研究未提出DEX干预POCD的临床合理输注剂量,本研究围绕术中输注DEX预防老年患者POCD的合理剂量这一目标,设计临床研究探索DEX输注剂量对预防POCD影响的量效关系,我们假设高剂量DEX更能降低老年患者POCD发生率。
为了验证这一假设,本研究通过对全身麻醉下接受脊柱手术老年患者静脉泵注不同剂量DEX,通过检测简易智力状态量表(MMSE),评估不同剂量DEX对老年脊柱手术患者POCD的影响,这一设计为术中输注DEX预防老年患者POCD的临床合理剂量提供参考。此外,本研究测定老年脊柱手术患者Aβ、IL-1β、TNF-α和IL-6的血清水平,为DEX对POCD的免疫调节作用提供理论依据,就DEX抗神经炎症损伤的相关机制进行探讨。
1. 资料和方法
1.1. 一般资料
本研究根据《药物临床试验质量管理规范》进行。每位受试者术前均告知研究并签署知情同意书。本研究获得了南方医科大学附属宝安医院伦理委员会的批准。
纳入2015年10月~2017年4月在南方医科大学附属宝安医院老年脊柱手术患者(≥65岁)120名。纳入标准:(1)美国麻醉医师协会(ASA)分级Ⅰ~Ⅲ级;(2)65岁≤年龄≤90岁;(3)术前简易智力状态量表(MMSE)评分≥24;(4)术前无窦性心动过缓各种传导阻滞或低血压的患者。排除标准:(1)患者有严重的视力障碍、听力障碍或不能言语;(2)有麻醉药物过敏史;(3)患有可能影响意识水平的精神和神经疾病的患者;(4)严重肝肾功能异常:Child-Pugh分级C级和尿毒症期;(5)中途拒绝继续参与研究的患者。实验分组:本实验采用区组随机双盲对照研究。采用计算机产生分组序列,分组序列装入不透光信封存于麻醉护士处。将符合纳入标准的患者按手术安排时间顺序和分组序列顺序匹配,分配四组:C组(n=30)、S组(n=30)、M组(n=30)、L组(n=30)。4组试验药物均由麻醉护士使用透明注射器配置不同浓度药物或生理盐水,以相同速度静脉输注,麻醉护士不知晓研究内容。
1.2. 方法
术前禁食6 h,禁饮2 h,避免术前用药。入室后于右前臂开放静脉通道,左侧桡动脉行动脉置管监测外周动脉血压,所有受试者均由同一位麻醉医师进行气管插管全身麻醉。3组DEX组(S组、M组、L组)的患者在麻醉诱导前使用负荷剂量0.3 μg/kg的盐酸DEX注射液(200 μg/2 mL江苏恒瑞医药股份有限公司批准文号:国药准字H20090248)泵注10 min,4组患者均使用1~2 mg/kg丙泊酚中/长链脂肪乳注射液(50 mL∶ 0.5 g预充注射器北京费森尤斯卡比医药有限公司批准文号:国药准字J20160098)和2~3 µg/kg枸橼酸芬太尼注射液(0.1 mg/mL宜昌人福药业有限责任公司批准文号:国药准字H42022076),0.15 mg/kg注射用液顺笨磺酸阿曲库铵[5 mg/瓶上海东英(江苏)药业有限公司批准文号:国药准字H20060927)]诱导后置入气管导管。麻醉维持期间S组、M组、L组患者分别以0.2 μg/(kg·h)、0.5 μg/(kg·h)、0.8 μg/(kg·h)的DEX静脉泵注维持,对照组C组患者则使用相同容量的生理盐水作为安慰剂泵注;术中丙泊酚和注射用盐酸瑞芬太尼(1 mg/瓶宜昌人福药业有限责任公司批准文号:国药准字H20030198)持续泵注维持麻醉,按需间断静脉推注顺式阿曲库铵。机械通气使PetCO2维持35~45 mmHg,监测麻醉深度BIS值(Aspect Medical System, Norwood, MA, USA)维持40~60 [15],保持血液动力学稳定,维持MAP波动在基线10%以下[16]。预计手术结束前5 min停用全部患者的丙泊酚和瑞芬太尼。预计手术结束前40 min停止S组、M组和L组DEX泵注和C组患者生理盐水的泵注。给予氟比洛芬酯联合芬太尼静脉自控镇痛缓解术后镇痛,VAS评分控制在3分以内。
1.3. 观察指标和采血测量
记录患者术中低血压(收缩压 < 90 mmHg,或比基础值减少20%以上)和心动过缓(心率 < 55次/min,或比基础值减少20%以上)的发生;记录手术时间(Te)和手术结束到睁开眼睛的苏醒时间(Tw)。
麻醉诱导前(D0)和术后1 d(D1)、术后3 d(D2)和术后7 d(D3)采用MMSE评估受试者认知功能,评估由未参与本实验的工作人员完成。MMSE对术后认知功能领域的检测结果与术前基线相比,当患者术后功能领域降低至少一个标准差(SD)则被认定为认知下降,当患者有两个或以上神经功能领域出现认知下降时,则被认定出现POCD。
分别在D0、D1、D2、D34个时间点抽取受试者静脉血10 mL测定血清Aβ、TNF-α、IL-1β和IL-6的含量。通过插入右侧前臂静脉的留置导管抽取血液样本,放入肝素抗凝管,30 min内送往医院实验室,在4 ℃离心力2000×g下离心10 min,分离血浆样本-80 ℃保存检验。用市售酶ELISA试剂盒(Thermo Fisher Scientific Co. Ltd Boston, USA),采用ELISA技术测定Aβ和TNF-α、IL-1β、IL-6的血清水平。
1.4. 统计学方法
本项研究使用20.0版SPSS(SPSS Inc.Chicago, IL, USA)软件统计分析。计量资料以均数±标准差表示,分类变量以频数(%)表示。Aβ、TNF-α、IL-1β、IL-6血清水平比较采用单因素方差分析和LSD-t检验,计数资料采用χ2检验。P < 0.05被认为差异有统计学意义。
2. 结果
2.1. 4组受试者一般资料比较
4组共纳入120名老年脊柱手术患者,4组患者一般资料统计(年龄、性别比例、身高、体质量、ASA分级、手术时间)比较差异均无统计学意义(P > 0.05,表 1)。
1.
4组受试者一般资料比较
Demographic and clinical data of the patients in the 4 groups
| Chracteristics | Group S | Group M | Group L | Control group | P |
| Age (years) | 74.7±2.6 | 71.2±3.5 | 69.8±4.3 | 73.4±5.1 | 0.627 |
| Gender (M/F) | 17/13 | 16/14 | 18/12 | 17/13 | 0.819 |
| Weight (kg) | 60.3±4.2 | 61.5±3.7 | 63.2±5.3 | 62.4±4.5 | 0.782 |
| Height (m) | 1.67±0.08 | 1.63±0.11 | 1.65±0.07 | 1.69±0.12 | 0.526 |
| ASA*grade (Ⅰ/Ⅱ/Ⅲ) | 2/21/7 | 4/16/10 | 5/18/7 | 4/17/9 | |
| Operative time (h) | 2.62±0.57 | 2.42±0.41 | 2.78±0.29 | 2.37±0.36 | 0.263 |
2.2. 围手术期认知功能的评估
与D0比较,C组和S组受试者的MMSE评分在D1、D2、D3时间点显著降低(P < 0.05),而M组和L组受试者的MMSE评分则无显著统计学差异。与C组相比,M组和L组D1、D2、D3时间点MMSE评分显著升高(P < 0.05),S组无显著统计学差异(表 2)。
2.
4组患者MMSE评分
Mini Mental-State Examination (MMSE) scores in the 4 groups(Mean±SD)
| Group | Time | |||
| D0 | D1 | D2 | D3 | |
| MMSE: Mini Mental-State Examination. D0: Prior to induction of anesthesia (baseline). D1: 1 day after surgery. D2: 3 days after surgery. D3: 7 days after surgery. *P < 0.05 vs before surgery; #P < 0.05 vs control group. | ||||
| Control | 27.7±0.7 | 22.6±1.2* | 24.1±0.8* | 24.3±0.5* |
| S | 27.6±0.4 | 23.7±0.6* | 24.8±1.4* | 25.7±1.2* |
| M | 28.0±1 | 27.3±1.3# | 27.7±1.1# | 28.2±1.3# |
| L | 28.4±0.6 | 27.8±1.1# | 28.2±0.6# | 28.1±0.7# |
2.3. 术后POCD的发生率
术后第1天C组13名受试者和S组11名受试者出现POCD,两组之间没有显著统计学差异(P=0.598)。与C组相比,M组(5名患者)和L组(3名患者)出现POCD的患者数量显著减少(分别P < 0.05和P < 0.01),但两组之间没有显著统计学差异(表 3)。
3.
4组受试者POCD的发生率
Incidence of POCD in the 4 groups (n, %)
| Group | Time | ||
| D1 | D2 | D3 | |
| D1: 1 day after surgery. D2: 3 days after surgery. D3: 7 days after surgery. *P < 0.05 vs control group. #P < 0.01 vs control group. | |||
| Control | 13 (43.3) | 12 (40.0) | 12 (40.0) |
| S | 11 (36.7) | 10 (33.3) | 11 (36.7) |
| M | 5 (16.7)* | 5 (16.7)* | 4 (13.3)* |
| L | 3 (10.0)# | 2 (6.7)# | 2 (6.7)# |
2.4. 不良反应的发生率和苏醒时间
围术期L组受试者低血压和心动过缓的发生率最高(P < 0.01),其苏醒时间(Tw)也比其他3组长(P < 0.05),其余3组差异无统计学意义(表 4)。
4.
4组患者不良反应的发生率及苏醒时间
Incidence of adverse effects and recovery time of the patients in the 4 groups
| Group | Adverse effect (n, %) | Recoverytime (min, Mean±SD) | |
| Bradycardia | Hypotension | ||
| *P < 0.05 vs control group; #P < 0.05 vs group M. | |||
| Control | 7 (23.3) | 8 (26.7) | 18.2±2.7 |
| S | 8 (26.7) | 11 (36.6) | 15.3±2.2 |
| M | 12 (40.0) | 10 (33.3) | 16.7±3.4 |
| L | 20 (66.7)*# | 21 (70.0)*# | 27.3±3.8*# |
2.5. DEX对Aβ的影响
4组受试者术前Aβ水平无统计学差异(P > 0.05),与术前(D0)血清水平相比较, 各组Aβ血清水平在D1时间点均明显增加,随后呈逐渐下降趋势;术后7 d C组和S组的Aβ仍有显著较高水平(P < 0.01),而M组和L组的Aβ则下降至术前水平(P > 0.05,图 1)。与C组比较,D1、D2、D3时间点Aβ血清水平在M组和L组显著降低(P < 0.01),S组无统计学意义(P > 0.05)。
1.
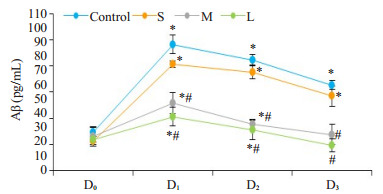
4组患者Aβ的变化趋势
Changes in serum levels of Aβ in the 4 groups. *P < 0.05 vs before surgery; #P < 0.05 vs control group.
2.6. DEX对血清IL1β、TNF-α、IL6的影响
与术前相比(D0),各组TNF-α、IL1β和IL6的血清水平在D1时间点增加至高峰后呈逐渐下降趋势。在C组和S组的TNF-α、IL1β和IL6血清水平在D2时间点仍处于显著较高水平(P < 0.01),D3时间点可下降至正常(P > 0.05);M组和L组的TNF-α、IL1β和IL6血清水平在D2时间点就下降到正常(P > 0.05)。与C组比较,D1、D2时间点TNF-α、IL1β和IL6血清水平在M组和L组显著降低(P < 0.01),S组无统计学意义(P > 0.05,图 2A~C)。
2.
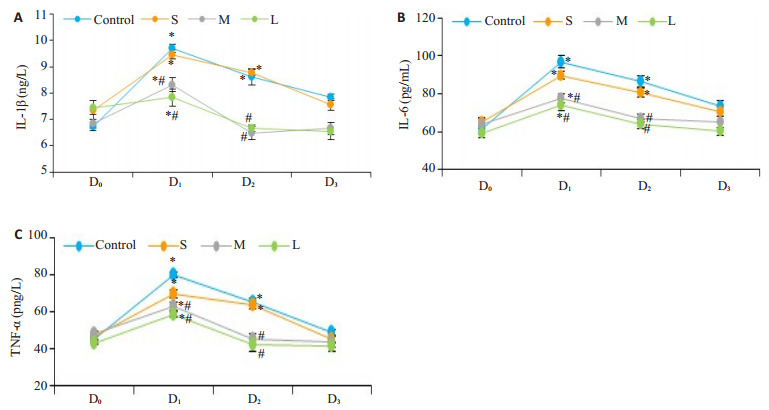
4组患者不同时间点IL-1β、IL-6、TNF-α变化趋势
Changes in serum levels of IL-1β (A)、IL-6 (B) and TNF-α (C) in the 4 groups. *P < 0.05 vs before surgery; #P < 0.05 vs control group.
3. 讨论
POCD通常发生于老年患者,其临床表现为意识紊乱、觉醒水平降低、性格改变和记忆力减弱或错构等,可能发展成不可逆的阿尔默海茨病[17]。Tan等[18]研究显示,老年患者的POCD发生率平均高达47%。Shoair等[19]研究显示,即使在大型非心脏手术术后3个月,仍有15.9%的老年患者表现POCD。本研究结果显示C组患者D1时间点POCD的发生率为43%,结果与相关文献相近,说明老年脊柱手术POCD发生率较高,脊柱手术的创伤应激可能是促使老年脊柱手术患者POCD发生的重要因素之一。控制手术创伤应激是临床麻醉重要要求,探索安全有效的麻醉药物,对提高麻醉质量和减少POCD发生具有重要意义。
DEX的神经保护作用已有相关研究报道,Sato等[20]使用DEX处理脑缺血大鼠神经系统,明确DEX的体外神经保护作用,而临床上DEX干预对POCD的影响临床试验结果尚不统一,关于DEX干预和POCD发生的量效关系研究也甚少。本研究对老年脊柱手术患者泵注三种不同剂量的DEX,通过MMSE评估POCD发生率,结果表明:与术前相比,术后1 d到7 d C组和S组MMSE评分显著降低,S组POCD发生率虽有所下降,但与C组比较无统计学差异(P > 0.05),说明围术期使用小剂量DEX不能显著减少POCD的发生;M组和L组术后第1~7天的MMSE评分与术前比较无明显变化(P > 0.05),与C组比较,POCD发生率显著降低。本试验使用中、大剂量DEX可显著减少POCD的发生率,考虑到DEX的镇静作用呈剂量依赖性的,我们推断DEX在足够的血药浓度下才能充分发挥神经保护作用,与部分临床试验显示[10-11]DEX对POCD无显著影响的结果比较,可能与低剂量输入DEX不能有效控制手术创伤应激反应,而未能明显抑制神经炎性反应有关,以上研究显示出术中使用中、大剂量DEX可能影响POCD发生,并且术中DEX输注剂量可能为干预POCD提供新的选择。本研究应用DEX的不良反应统计结果显示,老年患者大剂量使用DEX后低血压和心动过缓发生率最高(P < 0.01),苏醒时间(Tw)明显长于其他3组(P < 0.05),且与C组相比差异有统计学意义(P < 0.01),说明围术期大剂量DEX辅助麻醉并不适合老年患者,美国食品药品监督管理局和欧洲药品管理局在DEX的注册文件显示,65岁以上患者的心动过缓和低血压发生率较高[21],鉴于DEX的临床广泛使用和安全考虑,围术期中等剂量DEX更适合老年患者的临床使用。
研究显示[22],老年小鼠经腹部手术发展POCD后,β前体蛋白转录活性增强,Aβ含量显著增加。Aβ是体内β-淀粉样前体蛋白正常代谢产物之一,低浓度Aβ可刺激未成熟神经元的分化,随着浓度的增加,Aβ出现神经毒性效应,包括树突和轴突收缩,以及成熟分化神经元细胞的减少或缺失[23-24]。Aβ的神经毒性引起老年患者发生POCD的机制可能与增强氧化应激、诱导神经元凋亡、突触功能障碍、中枢胆碱能损伤和Tau蛋白磷酸化加速有关[25-27]。因此,抑制或减少Aβ的产生和沉积可能是成功防治POCD的关键。本研究结果表明:四组患者术后血清Aβ含量均升高,术后1 d达到峰值,说明老年患者经历脊柱手术创伤应激可能是诱发POCD的因素;由于停止手术创伤应激,术后血清Aβ含量开始下降,M组和L组术后7 d Aβ含量可恢复到术前水平,但C组和S组含量仍高于术前水平,M组和L组术后Aβ含量明显低于C组,说明围术期使用中、大剂量DEX干预可显著减少Aβ分泌,调节手术创伤应激产生的相关神经炎症。
小胶质细胞为位于中枢神经系统的一类巨噬细胞,小胶质细胞通过细胞表面受体与可溶性Aβ结合,活化的小胶质细胞和星形胶质细胞释放炎性细胞因子,如TNF-α、IL-6和IL-1β,激活补体系统,引起中枢神经系统的炎症反应,影响神经元突触连接功能,引起海马损伤发展为POCD [28-30]。Taniguchi等[31]发现DEX可剂量依赖性地降低内毒素注射后极高的死亡率和细胞因子血清浓度,DEX的神经保护作用是否能通过抑制炎性细胞因子,控制神经炎性反应,减少POCD发生率尚不明确[32]。本研究还发现,围手术期使用中、大剂量DEX可降低IL-1β、IL-6和TNF-α的血清水平,与C组比较,M组和L组在术后1 d、3 d的IL-1β、IL-6和TNF-α血清浓度显著降低,这些变化与Aβ血清含量的变化相一致,说明围术期使用中、大剂量DEX可显著控制炎症反应。本研究C组Aβ和IL-6、TNF-α、IL-1β在术后D1、D2时间点上升,相应时间点的MMSE评分降低,与D0比较差异均有统计学意义,POCD发生率分别为13例(43.3%)、12例(40.0%),通过对比MMSE评分和POCD发生率,发现血清Aβ和IL-6、TNF-α、IL-1β的波动变化还与POCD发生密切相关。在You [33]的调查结果也被提到,MMSE与Aβ等炎性相关生物标志物呈负相关。Aβ等炎性相关生物标志物升高联合MMSE评分下降可为POCD诊断提供关键指标。然而,DEX干预后Aβ与细胞因子变化趋势不同,DEX组术后3 d TNF-α、IL-1β和IL-6血清水平下降至基线值正常水平,但Aβ血清水平在术后7 d才下降至正常水平。据报道,IL-1β活性形式的形成是一个复杂的过程,通常需要两个独立的步骤,分别是诱导pro-IL-1β和激活caspase-1[34]。本研究发现,IL-1β血清水平迅速下降至正常水平,并先于Aβ血清水平的下降,说明DEX可能通过抑制Aβ分泌阻断proIL-1β的诱导和caspase-1的激活,IL-1β分泌在促炎反应中产生炎症级联反应起关键作用,最终导致神经元功能障碍和神经元死亡,一定剂量下DEX干预可抑制IL-1β的分泌发挥抗神经炎反应的作用。当前研究没有注册,可能存在小偏移,但是我们严格按照CONSORT条目清单步骤进行。此发现为我们进一步探索DEX预防POCD的发病机制及寻找干预靶点提供新的思路及依据。目前没有文献报道阻断IL-1β分泌在干预POCD发生发展中的作用,因此对其具体调控机制及下游通路仍需进一步研究。
综上所述,本研究发现DEX改善POCD的作用呈剂量相关,围术期使用负荷剂量0.3 μg/kg DEX泵注10 min,维持剂量0.5和0.8 μg/(kg·h)[优先为0.5 μg/(kg·h)]可能通过抑制Aβ和促炎性细胞因子的分泌,降低炎症反应,减少老年脊柱手术患者POCD发病率。
致谢
作者感谢徐楚红博士(中国武汉华中科技大学协和医院临床药理学系)对数据分析的帮助!
Biography
李智,硕士,主治医师,E-mail: lizhi0441@yeah.net
Funding Statement
深圳市科技创新委员会(JCYJ20170306144452663)
Contributor Information
李 智 (Zhi LI), Email: lizhi0441@yeah.net.
陈 建颜 (Jianyan CHEN), Email: chenjianyan@yahoo.com.
References
- 1.Czyż-Szypenbejl K, Mędrzycka-Dąbrowska W, Kwiecień-Jaguś K, et al. The occurrence of postoperative cognitive dysfunction (POCD)-systematic review. Psychiatr Pol. 2019;53(1):145–60. doi: 10.12740/PP/90648. [Czyż-Szypenbejl K, Mędrzycka-Dąbrowska W, Kwiecień-Jaguś K, et al. The occurrence of postoperative cognitive dysfunction (POCD)-systematic review[J]. Psychiatr Pol, 2019, 53(1): 145-60.] [DOI] [PubMed] [Google Scholar]
- 2.Evered LA, Silbert BS. Postoperative cognitive dysfunction and noncardiac surgery. AnesthAnalg. 2018;127(2):496–505. doi: 10.1213/ANE.0000000000003514. [Evered LA, Silbert BS. Postoperative cognitive dysfunction and noncardiac surgery[J]. AnesthAnalg, 2018, 127(2): 496-505.] [DOI] [PubMed] [Google Scholar]
- 3.Lin X, Chen Y, Zhang P, et al. The potential mechanism of postoperative cognitive dysfunction in older people. Exp Gerontol. 2020;130:110791. doi: 10.1016/j.exger.2019.110791. [Lin X, Chen Y, Zhang P, et al. The potential mechanism of postoperative cognitive dysfunction in older people[J]. Exp Gerontol, 2020, 130: 110791.] [DOI] [PubMed] [Google Scholar]
- 4.Nathan N. Beyond Emergence: Understanding postoperative cognitive dysfunction (POCD) AnesthAnalg. 2018;127(2):323. doi: 10.1213/ANE.0000000000003598. [Nathan N. Beyond Emergence: Understanding postoperative cognitive dysfunction (POCD)[J]. AnesthAnalg, 2018, 127(2): 323.] [DOI] [PubMed] [Google Scholar]
- 5.Lv G, Li C, Wang W, et al. Silencing SP1 alleviated sevofluraneinduced POCD development via cholinergic anti-inflammatory pathway. Neurochem Res. 2020;45(9):2082–90. doi: 10.1007/s11064-020-03070-7. [Lv G, Li C, Wang W, et al. Silencing SP1 alleviated sevofluraneinduced POCD development via cholinergic anti-inflammatory pathway[J]. Neurochem Res, 2020, 45(9): 2082-90.] [DOI] [PubMed] [Google Scholar]
- 6.Kotekar N, Shenkar A, Nagaraj R. Postoperative cognitive dysfunction-current preventive strategies. Clin Interv Aging. 2018;13:2267–73. doi: 10.2147/CIA.S133896. [Kotekar N, Shenkar A, Nagaraj R. Postoperative cognitive dysfunction-current preventive strategies[J]. Clin Interv Aging, 2018, 13: 2267-73.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaye AD, Chernobylsky DJ, Thakur P, et al. DEXmedetomidine in enhanced recovery after surgery (ERAS) protocols for postoperative pain. Curr Pain Headache Rep. 2020;24(5):21. doi: 10.1007/s11916-020-00853-z. [Kaye AD, Chernobylsky DJ, Thakur P, et al. DEXmedetomidine in enhanced recovery after surgery (ERAS) protocols for postoperative pain[J]. Curr Pain Headache Rep, 2020, 24(5): 21.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee C, Lee CH, Lee G, et al. The effect of the timing and dose of DEXmedetomidine on postoperative delirium in elderly patients after laparoscopic major non-cardiac surgery: a double blind randomized controlled study. http://europepmc.org/abstract/MED/29549829. J ClinAnesth. 2018;47:27–32. doi: 10.1016/j.jclinane.2018.03.007. [Lee C, Lee CH, Lee G, et al. The effect of the timing and dose of DEXmedetomidine on postoperative delirium in elderly patients after laparoscopic major non-cardiac surgery: a double blind randomized controlled study[J]. J ClinAnesth, 2018, 47: 27-32.] [DOI] [PubMed] [Google Scholar]
- 9.Wang WX, Wu Q, Liang SS, et al. DEXmedetomidine promotes the recovery of neurogenesis in aged mouse with postoperative cognitive dysfunction. Neurosci Lett. 2018;677:110–16. doi: 10.1016/j.neulet.2018.03.043. [Wang WX, Wu Q, Liang SS, et al. DEXmedetomidine promotes the recovery of neurogenesis in aged mouse with postoperative cognitive dysfunction[J]. Neurosci Lett, 2018, 677: 110-16.] [DOI] [PubMed] [Google Scholar]
- 10.Kim JA, Ahn HJ, Yang M, et al. Intraoperative use of DEXmedetomidine for the prevention of emergence agitation and postoperative delirium in thoracic surgery: a randomized-controlled trial. Can JAnaesth. 2019;66(4):371–9. doi: 10.1007/s12630-019-01299-7. [Kim JA, Ahn HJ, Yang M, et al. Intraoperative use of DEXmedetomidine for the prevention of emergence agitation and postoperative delirium in thoracic surgery: a randomized-controlled trial[J]. Can JAnaesth, 2019, 66(4): 371-9.] [DOI] [PubMed] [Google Scholar]
- 11.Deiner S, Luo X, Lin HM, et al. Intraoperative infusion of DEXmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. JAMA Surg. 2017;152(8):e171505. doi: 10.1001/jamasurg.2017.1505. [Deiner S, Luo X, Lin HM, et al. Intraoperative infusion of DEXmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial[J]. JAMA Surg, 2017, 152(8): e171505.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.贾 谜谜, 闫 琪, 马 尚文, et al. 右美托咪定在围术期神经认知障碍防治中的研究进展. https://www.cnki.com.cn/Article/CJFDTOTAL-MZAQ202004022.htm. 麻醉安全与质控. 2020;4(4):240–4. [贾谜谜, 闫琪, 马尚文, 等. 右美托咪定在围术期神经认知障碍防治中的研究进展[J]. 麻醉安全与质控, 2020, 4(4): 240-4.] [Google Scholar]
- 13.Zhang DF, Su X, Meng ZT, et al. Impact of DEXmedetomidine on long-term outcomes after noncardiac surgery in elderly: 3-year follow-up of a randomized controlled trial. Ann Surg. 2019;270(2):356–63. doi: 10.1097/SLA.0000000000002801. [Zhang DF, Su X, Meng ZT, et al. Impact of DEXmedetomidine on long-term outcomes after noncardiac surgery in elderly: 3-year follow-up of a randomized controlled trial[J]. Ann Surg, 2019, 270 (2): 356-63.] [DOI] [PubMed] [Google Scholar]
- 14.Su X, Meng ZT, Wu XH, et al. DEXmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. http://www.sciencedirect.com/science/article/pii/S0140673616305803. Lancet. 2016;15, 388(10054):1893–902. doi: 10.1016/S0140-6736(16)30580-3. [Su X, Meng ZT, Wu XH, et al. DEXmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial[J]. Lancet, 2016, 15, 388 (10054): 1893-902.] [DOI] [PubMed] [Google Scholar]
- 15.Poon YY, Chang HC, Chiang MH, et al. "A real-world evidence" in reduction of volatile anesthetics by BIS-guided anesthesia. Sci Rep. 2020;10(1):11245. doi: 10.1038/s41598-020-68193-x. [Poon YY, Chang HC, Chiang MH, et al. "A real-world evidence" in reduction of volatile anesthetics by BIS-guided anesthesia[J]. Sci Rep, 2020, 10(1): 11245.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown CH, Neufeld KJ, Tian J, et al. Effect of targeting mean arterial pressure during cardiopulmonary bypass by monitoring cerebral autoregulation on postsurgical delirium among older patients: a nested randomized clinical trial. JAMA Surg. 2019;154(9):819–26. doi: 10.1001/jamasurg.2019.1163. [Brown CH, Neufeld KJ, Tian J, et al. Effect of targeting mean arterial pressure during cardiopulmonary bypass by monitoring cerebral autoregulation on postsurgical delirium among older patients: a nested randomized clinical trial[J]. JAMA Surg, 2019, 154(9): 819-26.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belrose JC, Noppens RR. Anesthesiology and cognitive impairment: a narrative review of current clinical literature. BMC Anesthesiol. 2019;19(1):241. doi: 10.1186/s12871-019-0903-7. [Belrose JC, Noppens RR. Anesthesiology and cognitive impairment: a narrative review of current clinical literature[J]. BMC Anesthesiol, 2019, 19(1): 241.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan CB, Ng J, Jeganathan R, et al. Cognitive changes after surgery in the elderly: Does minimally invasive surgery influence the incidence of postoperative cognitive changes compared to open colon surgery? http://www.karger.com/Article/Abstract/357804. Dement Geriatr Cogn Disord. 2015;39(3/4):125–31. doi: 10.1159/000357804. [Tan CB, Ng J, Jeganathan R, et al. Cognitive changes after surgery in the elderly: Does minimally invasive surgery influence the incidence of postoperative cognitive changes compared to open colon surgery?[J]. Dement Geriatr Cogn Disord, 2015, 39(3/4): 125-31.] [DOI] [PubMed] [Google Scholar]
- 19.Shoair OA, Grasso Ii MP, Lahaye LA, et al. Incidence and risk factors for postoperative cognitive dysfunction in older adults undergoing major noncardiac surgery: a prospective study. J Anaesthesiol Clin Pharmacol. 2015;31(1):30–6. doi: 10.4103/0970-9185.150530. [Shoair OA, Grasso Ii MP, Lahaye LA, et al. Incidence and risk factors for postoperative cognitive dysfunction in older adults undergoing major noncardiac surgery: a prospective study[J]. J Anaesthesiol Clin Pharmacol, 2015, 31(1): 30-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato K, Kimura T, Nishikawa T, et al. Neuroprotective effects of a combination of DEXmedetomidine and hypothermia after incomplete cerebral ischemia in rats. http://europepmc.org/abstract/MED/19860751. Acta Anaesthesiol Scand. 2018;54(3):377–82. doi: 10.1111/j.1399-6576.2009.02139.x. [Sato K, Kimura T, Nishikawa T, et al. Neuroprotective effects of a combination of DEXmedetomidine and hypothermia after incomplete cerebral ischemia in rats[J]. Acta Anaesthesiol Scand, 2018, 54(3): 377-82.] [DOI] [PubMed] [Google Scholar]
- 21.Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of DEXmedetomidine. Clin Pharmacokinet. 2017;56(8):893–913. doi: 10.1007/s40262-017-0507-7. [Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of DEXmedetomidine[J]. Clin Pharmacokinet, 2017, 56(8): 893-913.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karthick C, Nithiyanandan S, Essa MM, et al. Time-dependent effect of oligomeric amyloid-beta (1-42)-induced hippocampal neurodegeneration in rat model of Alzheimer's disease. Neurol Res. 2019;41(2):139–50. doi: 10.1080/01616412.2018.1544745. [Karthick C, Nithiyanandan S, Essa MM, et al. Time-dependent effect of oligomeric amyloid-beta (1-42)-induced hippocampal neurodegeneration in rat model of Alzheimer's disease[J]. Neurol Res, 2019, 41(2): 139-50.] [DOI] [PubMed] [Google Scholar]
- 23.Shutes BL, Gee SW, Sargel CL, et al. DEXmedetomidine as single continuous sedative during noninvasive ventilation: typical usage, hemodynamic effects, and withdrawal. Pediatr Crit Care Med. 2018;19(4):287–97. doi: 10.1097/PCC.0000000000001451. [Shutes BL, Gee SW, Sargel CL, et al. DEXmedetomidine as single continuous sedative during noninvasive ventilation: typical usage, hemodynamic effects, and withdrawal[J]. Pediatr Crit Care Med, 2018, 19(4): 287-97.] [DOI] [PubMed] [Google Scholar]
- 24.Elsherbini A, Kirov AS, Dinkins MB, et al. Association of Aβ with ceramide-enriched astrosomes mediates Aβ neurotoxicity. Acta Neuropathol Commun. 2020;8(1):60. doi: 10.1186/s40478-020-00931-8. [Elsherbini A, Kirov AS, Dinkins MB, et al. Association of Aβ with ceramide-enriched astrosomes mediates Aβ neurotoxicity[J]. Acta Neuropathol Commun, 2020, 8(1): 60.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rojanathammanee L, Puig KL, Combs CK. Pomegranate polyphenols and extract inhibit nuclear factor of activated T-cell activity and microglial activation in vitro and in a transgenic mouse model of Alzheimer disease. J Nutr. 2013;143(5):597–605. doi: 10.3945/jn.112.169516. [Rojanathammanee L, Puig KL, Combs CK. Pomegranate polyphenols and extract inhibit nuclear factor of activated T-cell activity and microglial activation in vitro and in a transgenic mouse model of Alzheimer disease[J]. J Nutr, 2013, 143(5): 597-605.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doens D, Fernández PL. Microglia receptors and their implications in the response to amyloid β for Alzheimer's disease pathogenesis. J Neuroinflammation. 2014;11:48. doi: 10.1186/1742-2094-11-48. [Doens D, Fernández PL. Microglia receptors and their implications in the response to amyloid β for Alzheimer's disease pathogenesis[J]. J Neuroinflammation, 2014, 11: 48.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen WZ, Lu KL, Wang JW, et al. Activation of mTOR signaling leads to orthopedic surgery-induced cognitive decline in mice throμgh β-amyloid accumulation and tau phosphorylation. Mol Med Rep. 2016;14(4):3925–34. doi: 10.3892/mmr.2016.5700. [Shen WZ, Lu KL, Wang JW, et al. Activation of mTOR signaling leads to orthopedic surgery-induced cognitive decline in mice throμgh β-amyloid accumulation and tau phosphorylation[J]. Mol Med Rep, 2016, 14(4): 3925-34.] [DOI] [PubMed] [Google Scholar]
- 28.Trivedi, Daksha, Cochrane Review Summary: Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Prim Health Care Res Dev. 2017:1–2. doi: 10.1017/S1463423617000202. [Trivedi, Daksha, Cochrane. Review Summary: Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations[J]. Prim Health Care Res Dev, 2017: 1-2.] [DOI] [PubMed] [Google Scholar]
- 29.Schaefer T, Koenigsperger S, Olotu C, et al. Biomarkers and postoperative cognitive function: could it be that easy? http://www.researchgate.net/publication/329329983_Biomarkers_and_postoperative_cognitive_function_could_it_be_that_easy. Curr Opin Anaesthesiol. 2019;32(8):1119–20. doi: 10.1097/ACO.0000000000000676. [Schaefer T, Koenigsperger S, Olotu C, et al. Biomarkers and postoperative cognitive function: could it be that easy[J]? Curr Opin Anaesthesiol, 2019, 32(8): 1119-20.] [DOI] [PubMed] [Google Scholar]
- 30.Le Y, Liu S, Peng M, et al. Aging differentially affects the loss of neuronal dendritic spine, neuroinflammation and memory impairment at rats after surgery. PLoS One. 2014;9(9):e106837. doi: 10.1371/journal.pone.0106837. [Le Y, Liu S, Peng M, et al. Aging differentially affects the loss of neuronal dendritic spine, neuroinflammation and memory impairment at rats after surgery[J]. PLoS One, 2014, 9(9): e106837.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taniguchi T, Kurita A, Kobayashi K, et al. Dose- and time-related effects of DEXmedetomidine on mortality and inflammatory responses to endotoxin-induced shock in rats. J Anesth. 2008;22(3):221–8. doi: 10.1007/s00540-008-0611-9. [Taniguchi T, Kurita A, Kobayashi K, et al. Dose- and time-related effects of DEXmedetomidine on mortality and inflammatory responses to endotoxin-induced shock in rats[J]. J Anesth, 2008, 22 (3): 221-8.] [DOI] [PubMed] [Google Scholar]
- 32.Subramaniam B, Shankar P, Shaefi S, et al. Effect of intravenous acetaminophen vs placebo combined with propofol or DEXmedetomidine on postoperative delirium among older patients following cardiac surgery: the DEXACET randomized clinical trial. JAMA. 2019;321(7):686–96. doi: 10.1001/jama.2019.0234. [Subramaniam B, Shankar P, Shaefi S, et al. Effect of intravenous acetaminophen vs placebo combined with propofol or DEXmedetomidine on postoperative delirium among older patients following cardiac surgery: the DEXACET randomized clinical trial[J]. JAMA, 2019, 321(7): 686-96.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You JC, Jones E, Cross DE, et al. Association of β-amyloid burden with sleep dysfunction and cognitive impairment in elderly individuals with cognitive disorders. http://www.researchgate.net/publication/336593302_Association_of_b-Amyloid_Burden_With_Sleep_Dysfunction_and_Cognitive_Impairment_in_Elderly_Individuals_With_Cognitive_Disorders/download. JAMA Network Open. 2019;2(10):115–9. doi: 10.1001/jamanetworkopen.2019.13383. [You JC, Jones E, Cross DE, et al. Association of β-amyloid burden with sleep dysfunction and cognitive impairment in elderly individuals with cognitive disorders[J]. JAMA Network Open, 2019, 2(10): 115-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malik A, Kanneganti TD. Inflammasome activation and assembly at a glance. J Cell Sci. 2017;130(23):3955–63. doi: 10.1242/jcs.207365. [Malik A, Kanneganti TD. Inflammasome activation and assembly at a glance[J]. J Cell Sci, 2017, 130(23): 3955-63.] [DOI] [PMC free article] [PubMed] [Google Scholar]


