Abstract
目的
探讨拟康氏木霉胞外多糖(EPS)联合奥沙利铂(Oxa)用药对结肠癌细胞HCT116的协同抑制作用。
方法
实验分为Control组(0 μg/mL)、Oxa组(8 μg/mL Oxa)、EPS组(100 μg/mL EPS)、EPS+Oxa组(8 μg/mL Oxa+100 μg/mL EPS)。CCK-8检测细胞活力,CompuSyn软件拟合Fa-CI曲线评价联用效果。流式检测凋亡和周期、划痕实验和transwell实验检测细胞迁移能力,Oxa和EPS相关基因与结直肠癌相关基因取交集进行PPI分析以及GO和KEGG富集分析。
结果
Oxa单用或与EPS联用处理HCT116细胞,均可使HCT116细胞活力受到抑制,并呈现剂量与时间依耐性,两者联用有明显的协同作用(CI < 1)。两药联用组的细胞总凋亡率与Oxa组以及对照组比较均显著升高(P < 0.05);联合用药组处于S期的比例高于其他各组。EPS和Oxa均具有抑制HCT116细胞迁移的作用,两者联用后抑制作用更为明显。KEGG分析显示主要涉及耐药、凋亡、血管新生等通路。
结论
EPS联合奥沙利铂可协同抑制HCT116细胞增殖,促进该细胞凋亡和细胞S周期阻滞、抑制细胞迁移,铂耐药、PI3K-Akt、MAPK等信号通路发挥了关键作用。
Keywords: 多糖, 奥沙利铂, 结直肠癌, 协同, 拟康氏木霉
Abstract
Objective
To explore the synergistic inhibitory effect of polysaccharide from Trichoderma pseudokoningii (EPS) and oxaliplatin (Oxa) on colorectal cancer (CRC) HCT116 cells.
Methods
HCT116 cells were treated with 8 μg/mL Oxa and 100 μg/mL EPS alone or in combination, and the changes in cell viability was assessed with CCK-8 assay. CompuSyn software was used for fitting the Fa-CI curve to evaluate the combined effect of the two agents. Flow cytometry was performed to analyze cell apoptosis and cell cycle changes, and wound healing assay and Transwell assay were used to examine the migration ability of the treated cells. Oxa- and EPS-related genes and CRC-related genes were intersected for protein-protein interaction (PPI) analysis and GO and KEGG enrichment analyses.
Results
Treatment with Oxa alone or in combination with EPS significantly inhibited the viability of HCT116 cells in a dose- and time-dependent manner, and the two agents exhibited a significant synergistic effect (CI < 1). The combined treatment with Oxa and EPS resulted in a significantly higher total cell apoptosis rate and a higher percentage of cells in S phase than Oxa alone and the control treatment (P < 0.05). EPS and Oxa alone both inhibited the migration of HCT116 cells, and their combination produced a stronger inhibitory effect. GO enrichment analysis of the key genes related with Oxa, EPS and CRC suggested that these genes were involved mainly in such biological processes as exogenous apoptosis signaling, cell response to chemical stress, and reactive oxygen metabolism; KEGG analysis showed that these genes were involved in the pathways of drug resistance, apoptosis and angiogenesis.
Conclusion
EPS and Oxa can synergistically inhibit the proliferation of HCT116 cells possibly through the PI3K-Akt, MAPK, VEGF, and p53 signaling pathways.
Keywords: polysaccharide, oxaliplatin, colorectal cancer, synergy, Trichoderma pseudokoningii
在世界范围内,结直肠癌(CRC)是导致癌症和癌症死亡的第2大常见原因[1]。CRC被诊断的可能性在40岁以后逐渐增加,50岁以后急剧上升,80岁后诊断率达到高峰[2]。近年来,我国结直肠癌的发病率和死亡率呈上升趋势,已成为世界上每年新增病例和死亡人数最多的国家,严重威胁着我国居民的健康[3]。有针对性的筛查,更复杂的诊断程序和早期的治疗干预可以及时地降低与结直肠癌相关的发病率和死亡率[4-5]。但是作为筛查金标准的结肠镜检查和作为主要治疗手段的手术切除均易产生术后感染性并发症,应激引起的炎症对结直肠癌患者的长期生存结局有负面影响[6]。
靶向治疗和化疗是CRC常用的疗法,可以提高患者长期生存率和减少复发[7]。而常规化疗由于缺乏特异性,效果有限,对患者产生不良副作用[8-9]。奥沙利铂(Oxa)是首个被证实具有抗CRC活性的铂类药物,其机制可能与形成铂-DNA复合物阻碍DNA合成从而引起细胞死亡有关,Oxa对DNA复制的抑制作用强于顺铂等其他铂类药物,因此以Oxa为基础的化疗被推荐为转移性结直肠癌的一线治疗方案[10-11]。然而,据美国食品和药物管理局报道,Oxa可导致胃肠道、血液学和神经系统等严重不良反应,通常会导致停止治疗[12]。Oxa引起的这些严重不良事件主要与患者使用剂量相关,长期反复使用奥沙利铂还会导致耐药性的产生从而影响其临床应用[13-14]。因此,为了克服奥沙利铂的耐药和毒性,迫切需要开发新的化学增敏剂。
近年来,多糖发挥奥沙利铂增敏作用时有报道。Zhang等[15]发现香菇多糖与Oxa具有显著的协同抗肝癌作用并可减轻用药的副作用。黑根霉胞外多糖对CT26结肠癌移植瘤小鼠具有抑瘤作用[16]并且和Oxa发挥协同抗结肠癌作用[17]。拟康氏木霉胞外多糖(EPS)是从玉米秸秆中分类得到的拟康氏木霉中提取分离得到的均一多糖,已发现其具有免疫活性,可促进小鼠树突状细胞成熟[18]并诱导巨噬细胞活化[19]。前期研究发现EPS可抑制包括人结肠癌细胞HCT116在内的多种肿瘤细胞增殖并诱导其凋亡[20-22]。为了进一步了解拟康氏木霉胞外多糖是否可增加奥沙利铂在CRC化疗中的敏感性,本实验以人结肠癌细胞HCT116为研究对象,进行了体外实验研究,并通过网络药理学的方法探讨了作用机制。
1. 材料和方法
1.1. 试剂与仪器
1640培养基、胎牛血清(FBS)(Gibco);CCK-8试剂盒、Annexin V-FITC/PI双染细胞凋亡检测试剂盒、细胞周期检测试剂盒(碧云天)。细胞培养箱(Gold-SIM);倒置荧光显微镜(OLYMPUS);流式细胞仪(BD)。
1.2. 材料
拟康氏木霉胞外多糖(EPS)为本实验室自制,制备方法参考本课题组发表的文献[22],过DEAE-Sepharose快速流动柱和葡聚糖凝胶G-75柱上后收集主要组分并冻干得到白色纯化的EPS。凝胶渗透色谱(GPC)测定平均相对分子质量为31 900 D。气相色谱法测定EPS的组分糖分为鼠李糖,木糖、岩藻糖、甘露糖、葡萄糖和半乳糖比率为16.2∶14.4∶1:25.8∶23.6∶48.1。数据与我们的相同以前的结果相同。
1.3. 细胞培养
人结肠癌细胞株(HCT116)购于上海生命科学研究院细胞库,在含10% FBS的1640培养基中培养,培养箱温度设置为37 ℃,CO2含量设置为5%。
1.4. 方法
1.4.1. CCK-8法检测细胞活力
实验分为Oxa组、EPS组、Oxa+EPS组。Oxa组给予不同浓度(0、2、4、8、16、32、64 μg/mL)Oxa处理,EPS组给予不同浓度(0、25、50、100、200、400、800 μg mL)EPS处理。Oxa+EPS组中每组的Oxa给药浓度与单用组相同,Oxa与EPS给药浓度比为1∶12.5。取对数生长期HCT116细胞接种于96孔板中,2×103/孔,培养24 h后,各组加药,每组设3个复孔。加药培养24、48、72 h后,弃去原培养基,每孔加入100 μL培养基和10 μL CCK-8试剂,1 h后测A450 nm处吸光值A,数据以均数±标准差表示。细胞增殖抑制率=1-(A实验组-A调零孔)(/A空白对照组-A调零孔)×100%。CompuSyn软件拟合Fa-CI曲线。
1.4.2. 流式检测细胞凋亡和细胞周期
实验分为Control组、Oxa组、EPS组、Oxa+EPS组。取对数生长期细胞接种于6孔培养板种6孔,24 h后吸弃培养基,其中3个孔各加2 mL完全培养基,前2孔细胞一分为三用作空白管和PI单阳管以及FITC单阳管,另一孔细胞用作实验组对照。EPS组加2 mL浓度为100 μg/mLEPS、Oxa组加2 mL浓度为8 μg/mL Oxa、Oxa+EPS组组加入1 mL 200 μg/mLEPS和1 mL 16 μg/mL Oxa。24 h后使用不含EDTA胰酶消化后,用PBS洗涤细胞后加入195 μL Annexin V-FITC结合液轻轻重悬细胞,空白管不加染料,单阳管只加10 μL PI染色液或5 μL Annexin V-FITC,各实验管2种染料均加入,避光室温孵育5 min。过细胞筛后立即使用流式细胞仪检测,使用Flowjo软件分析,以早期凋亡率与晚期凋亡率的和作为观察指标。细胞周期检测实验组种板和加药与检测凋亡相同,细胞用胰酶消化收集细胞后,用冰冷的PBS洗涤2次,加入70%乙醇吹打均匀,4 ℃过夜,1000 r/min离心3 min,沉淀细胞。吸除上清,加入约1 mL预冷的PBS,重悬细胞。再次离心沉淀细胞,加入当日现配的含RNase A的碘化丙啶染色液,37 ℃避光温浴30 min,过细胞筛后上机检测细胞周期。
1.4.3. 划痕实验和Transwell小室迁移实验
实验分为Control组(0 μg/mL)、Oxa组(8 μg/mL Oxa)、EPS组(100 μg/mL EPS)、Oxa+EPS组(100 μg/mL EPS+8 μg/mL Oxa)。记号笔于6孔板背面划横线,将对数生长期细胞种于六孔板中,细胞数以隔夜长满为宜。第2天用1 mL枪头垂直于背后的横线划痕,PBS洗涤后分别加药,药物使用1%血清培养基配制,以1%血清培养基为对照,培养24 h。0 h,24 h拍照,每组记录3处划痕。1% 血清培养液悬浮细胞,5×105/孔细胞铺于Transwell上层小室,下室加10%血清培养基配制的各组600 μL,培养16 h后,取出Transwell小室,弃去孔中培养液,用PBS洗2遍,甲醇固定30 min,0.1%结晶紫染色20 min,用棉签轻轻擦掉上层未迁移细胞,用PBS洗3遍。将小室适当风干,显微镜拍照。
1.4.4. 统计学处理统计
数据皆采用均数±标准差表示,统计学分析采用SPSS 18.0软件,采用单因素方差分析法进行组间比较。P < 0.05为差异有统计学意义,实验独立重复3次。
1.4.5. 核心靶点的获取
应用pubchem(<a href="https://pubchem.ncbi.nlm.nih.gov/" target="_blank">https://pubchem.ncbi.nlm.nih.gov/</a>)查找奥沙利铂对应的2D结构,并将结果导入pharmMapper数据库获得对应的靶点,查阅所以拟康氏木霉胞外多糖相关文献找出对应靶基因,与奥沙利铂对应靶基因合并作为药物靶点。GeneCards(<a href="https://www.genecards.org/" target="_blank">https://www.genecards.org/</a>)检索“Colorectal cancer”获得疾病对应靶点,将药物靶点和疾病靶点取交集,获得疾病与药物相关靶点,再使用Cytoscape中的MCODE插件通过计算获得核心靶点。
1.4.6. GO富集分析和KEGG富集分析
Bioconductor数据库获取核心基因的GO注释信息,使用R包clusterProfiler,进行GO功能富集分析和KEGG通路注释分析,根据P值选择富集高的进行可视化生成柱状图。
2. 结果
2.1. EPS对奥沙利铂抑制HCT116细胞增殖的作用
CCK-8结果显示,两者均能抑制HCT116细胞增殖并并呈现剂量与时间依耐性(图 1)。Oxa单用或与EPS联用处理HCT116细胞,均可使HCT116细胞活力受到抑制,并呈现剂量与时间依耐性(图 2A)。由CompuSyn软件拟合Fa-CI曲线,结果显示两者联用有较好的协同作用(图 2B)。
1.
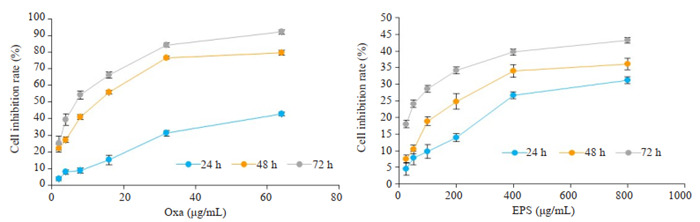
奥沙利铂与EPS对HCT116细胞增殖活力的影响
Effects of oxaliplatin and polysaccharide from Trichoderma pseudokoningii (EPS) on proliferation of HCT116 cells.
2.
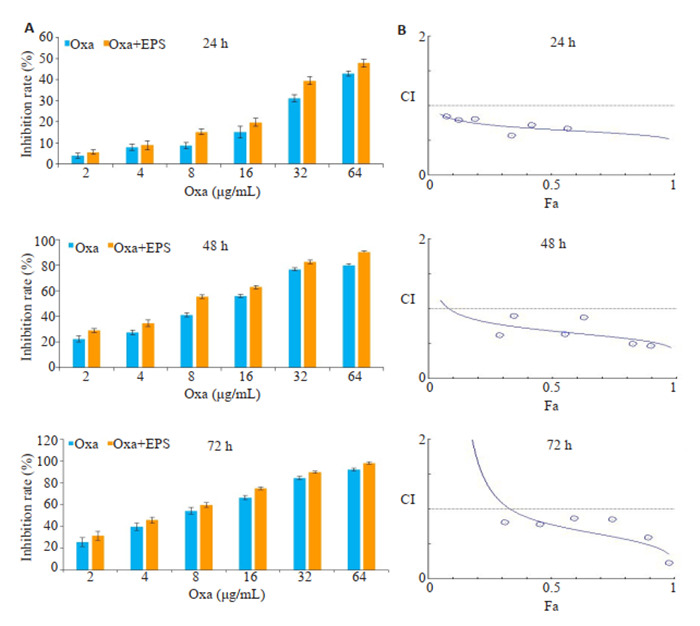
EPS与奥沙利铂联用对结肠癌细胞增殖的协同抑制作用
EPS and oxaliplatin (Oxa) synergistically inhibit HCT116 cell proliferation. A: Inhibition rates of HCT116 cells incubated with Oxa and with Oxa + EPS (1∶12.5) for 24, 48, and 72 h determined by CCK-8 assay. Data are presented as Mean ± SD of 3 independent experiments. B: Combination index (CI) for EPS and oxaliplatin in HCT116 cells.
2.2. EPS联用奥沙利铂对HCT116细胞凋亡的影响
流式细胞仪检测后使用Flowjo软件分析,左上象限为坏死细胞,左下为正常细胞,右上为晚期凋亡细胞,右下为晚期凋亡细胞,以右上+右下获得的总比例作为凋亡的指标。HCT116细胞经100 μg/mL EPS或8 μg/mL Oxa处理24 h后,细胞总凋亡率均明显高于对照组(P < 0.05,图 3);两药联合组的细胞总凋亡率较Oxa组以及对照组均显著升高(P < 0.05)。
3.
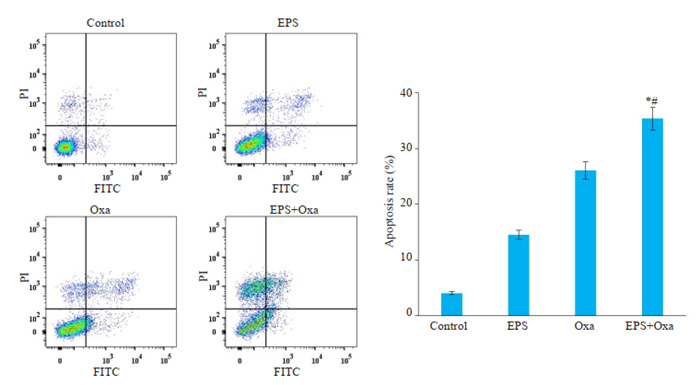
流式细胞术检测各给药组对人结肠癌细胞HCT116细胞凋亡的影响
Flow cytometric analysis of apoptosis rates of HCT116 cells treated with 100 μg/mL EPS, 8 μg/mL oxaliplatin, or both (Mean±SD, n=3). *P < 0.05 vs control group; #P < 0. 05 vs Oxa group.
2.3. EPS联用奥沙利铂对HCT116细胞周期的影响
与空白对照组比较,各组S期的细胞比例均出现升高,两药联用后趋势更加明显,G2/M比例无显著差别。两药联用后S期细胞比例高于单用一种药物。由此可见,两种药物均可将HCT116细胞阻滞于S期,使得细胞周期不能顺利进入G2/M期,且两药联用有协同作用(图 4)。
4.
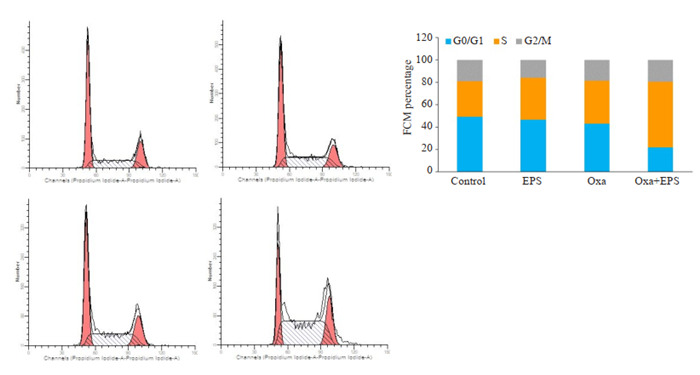
流式细胞术检测各给药组作用于人结肠癌细胞HCT116后诱导细胞周期的作用
Flow cytometry for analyzing cell cycle changes in HCT116 cells treated with 100 μg/mL EPS, 8 μg/mL oxaliplatin, or both.
2.4. EPS联用奥沙利铂对HCT116细胞迁移能力的影响
划痕实验和Transwell小室实验是常用的评估细胞迁移能力的实验。100倍显微镜下观察EPS联用Oxa对结肠癌细胞迁移能力的影响,发现两药均有一定的抑制迁移作用,两者联用后抑制迁移作用更为明显(图 5A)。两药联用后作用抗迁移增强。Transwell小室实验结果与划痕实验结果一致(图 5B)。
5.
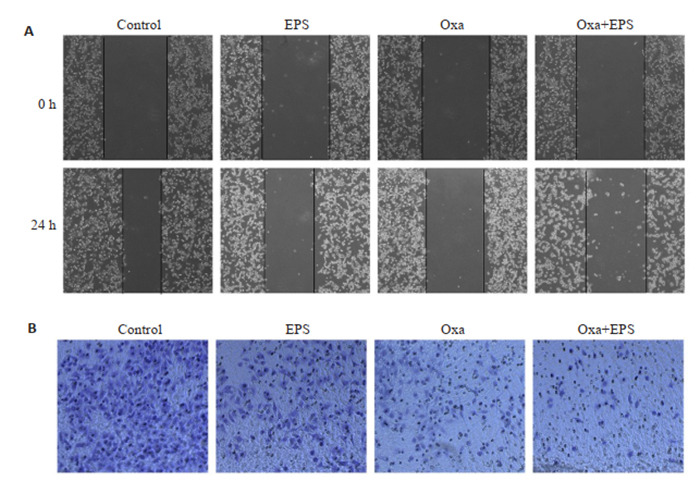
拟康氏木霉胞外多糖联用奥沙利铂对HCT116细胞的影响
Effect of EPS (100 μg/mL) combined with oxaliplatin (8 μg/mL) on migration ability of HCT116 cells. A: Wound healing assay (Original magnification: × 100). B: Transwell migration assay (×100).
2.5. 核心靶基因
通过查找数据库和查阅文献,共获得与奥沙利铂和拟康氏木霉胞外多糖相关基因191个,与CRC相关基因7542个,共有交集基因125个。将125个交集基因导入string构建PPI网络后导出文件,使用R包获得连接数前30的基因,利用Cytoscape软件中的插件cytoHubba和MCODE找出关键子网络,共获得5个关键蛋白模块,其中4个得分超过5(图 6),将4个模块中包含的55个基因作为核心基因用于后续分析。
6.
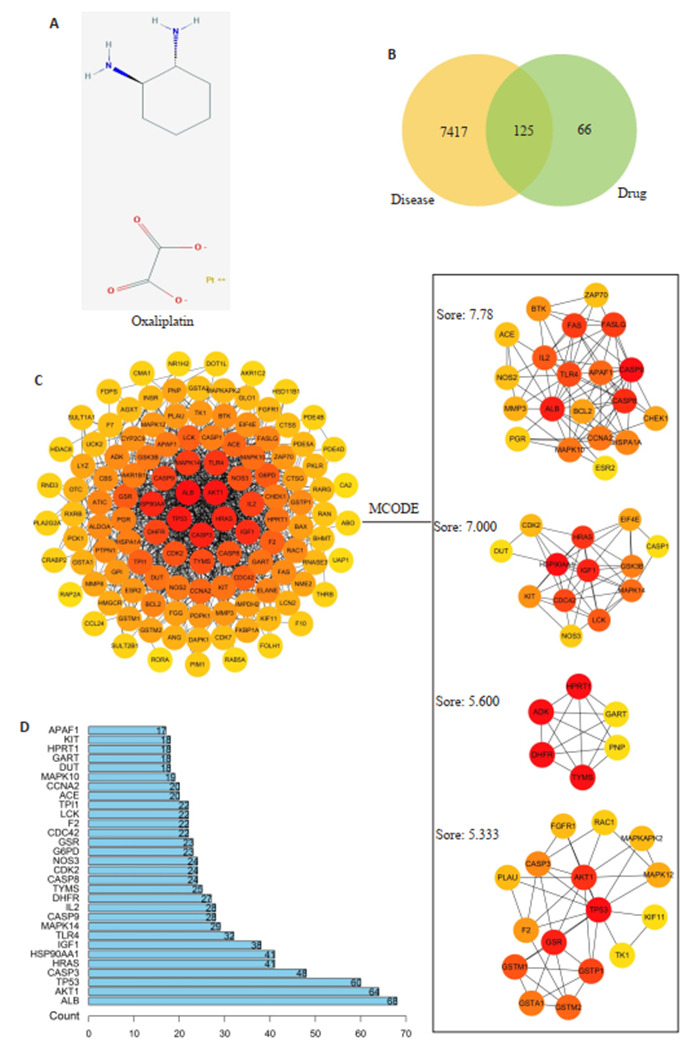
核心靶点的筛选
Screening of the core targets. A: Oxaliplatin 2D structure. B: Drug-disease intersection genes. C: PPI network and key protein modules. D: Target connection top 30 genes.
2.6. GO富集分析
核心基因GO富集后共得到1651条,其中生物过程(BP)1526条,细胞成分(CC)12条,分子功能(MF)113条。根据P值选择BP、CC、MF富集前10个进行可视化并生成气泡图(图 7),与生物过程相关的主要有外源性凋亡信号通路、细胞对化学应激的反应、活性氧代谢过程等;与细胞成分相关的主要包括突膜筏、膜微区、胞质小泡等;与分子功能相关的主要半胱氨酸型内肽酶活性参与细胞凋亡过程、谷胱甘肽转移酶活性、抗氧化活性等。
7.
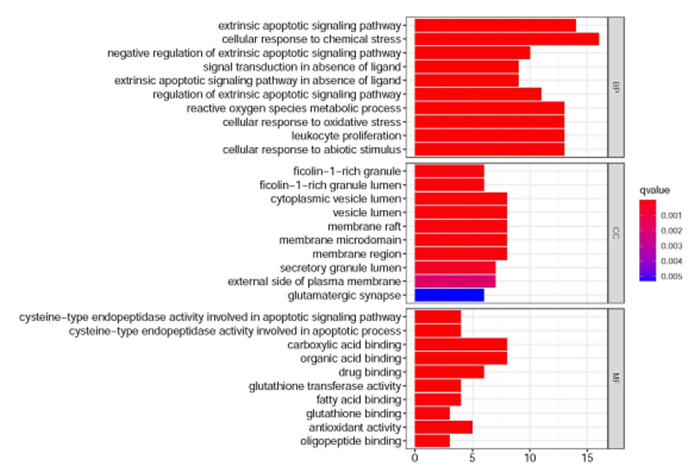
GO富集分析(排名前10)
GO Enrichment analysis of the top 10 genes.
2.7. KEGG富集分析
核心靶基因进行KEGG分析显示,关键靶基因富集在143条通路上,涉及耐药、凋亡、血管新生等通路。根据P值排名前30的结果形成KEGG功能富集条形图(图 8),提示奥沙利铂和拟康氏木霉胞外多糖联用可以通过多条通路作用于CRC,其中排在首位的是铂耐药通路,一系列通路存在节点基因重合并呈现交叉调控。以富集基因最多的PI3K-Akt信号通路(hsa04151)为例,该通路与凋亡信号通路、细胞周期信号通路、MAPK信号通路、VEGF信号通路、p53信号通路等通路均有交叉(图 9)。
8.
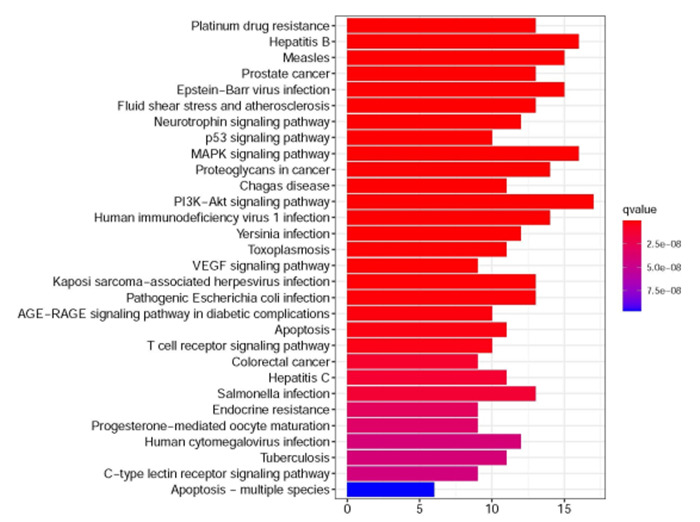
KEGG富集分析(排名前30)
KEGG enrichment analysis of the top 30 genes.
9.
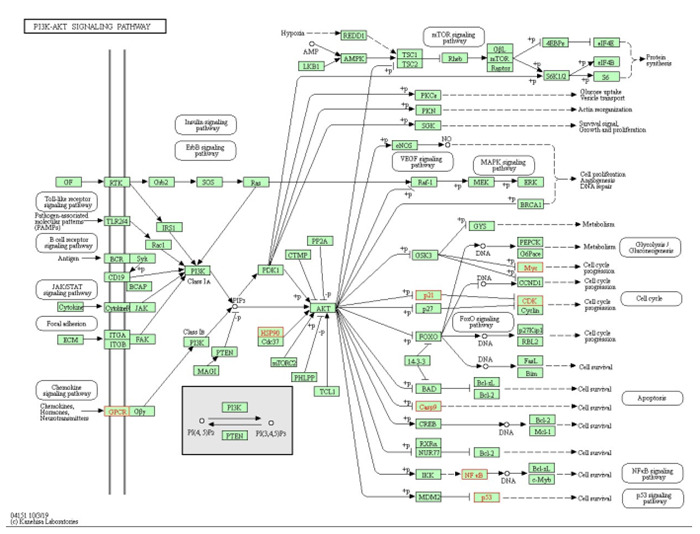
PI3K-Akt信号通路图(hsa04151)
PI3K-Akt signaling pathway (hsa04151).
3. 讨论
常规化疗药物联合增敏剂的使用,可以提高癌细胞对常规药物敏感性,减少毒副作用和化疗耐药性。因此,人们越来越关注“化疗增敏剂”的研究与开发。奥沙利铂是第三代铂类药物,对转移性CRC具有很好的疗效,是晚期结肠癌和转移性结肠癌治疗的一线化疗药物[23],但在治疗中易引起严重过敏反应及其他副作用[24],长期使用还会产生耐药[25],因此急需开发有效的增敏药物。已报导奥沙利铂的潜在增敏剂有姜黄素、人参皂苷、胡椒酮等[26-28],它们大多从植物提取分离,往往受限于原材料和提取分离工艺,不利于后期产业化生产。真菌多糖作为一种天然产物,具有使用安全,毒性低,生产不受季节气候和场地影响,是理想的候选药物[29-30]。
在EPS过去的研究中,主要涉及抗肿瘤及提供免疫作用,本研究首次探讨了EPS与其他抗肿瘤药物的联合用药作用。两药合用并非简单的效果叠加,经常会产生协同或拮抗效果[31]。本研究发现,EPS和奥沙利铂联用对HCT116的抑制增殖活性、引起细胞周期阻滞、诱导凋亡作用均表现出了协同活性。在对HCT116细胞的迁移活力的研究中,我们发现两者在划痕实验和小室迁移实验中显示两者均可抑制HCT116细胞迁移活力,合用后均显示出1+1大于2的效果。这提示我们EPS还可能通过抑制迁移发挥抗肿瘤活性,这一点在以往研究中被忽略了,下一步我们打算对EPS抑制肿瘤的EMT作用做进一步研究,但EPS的IC50超过了1 mg/mL,单独用药虽然有一定的效果,但是用药剂量过大,不利于后期作为抗肿瘤药物开发应用。之前本课题组的研究人员希望通过硒化或硫酸酯化的方法进行多糖改性[32],以获得以活性更高的多糖,本研究为EPS的研究提示了新的方向。
网络药理学主要用于中药机制的研究,适合复杂成分机制的研究,EPS联用Oxa的协同作用涉及增殖、凋亡、细胞周期、迁移等多种表型,因此我们使用网络药理学的方法进行机制的分析。网络药理学分析发现,联合用药使活性氧代谢和半胱氨酸型内肽酶活性参与细胞凋亡等一列生物过程和分子功能发生了改变,铂耐药以及细胞增殖与凋亡相关的一系列信号通路受到了调控。其中富集最多基因的通路是PI3K-Akt信号通路,该信号通路在病理和生理过程中起着至关重要的作用。细胞处于应激状态时,PI3K/Akt通路是一个关键的调节因子[33-34]。激活或干扰PI3K/Akt通路与许多人类恶性肿瘤密切相关,因此具有重要意义[35]。该通路是潜在的抗肿瘤药物的治疗靶点。趋化因子受体9、炎症相关细胞因子toll样受体和白细胞介素-6是PI3K-Akt通路的一些重要的上游调节因子[36-38]。该通路与其他几种通路存在相互作用,如缺氧诱导因子,丝裂原活化蛋白激酶(MAPK)、Notch、Wnt、JNK和Ras[39-42]。PI3K-Akt通路活化可促进细胞增殖、生长和参与血管生成,因此影响细胞增殖、侵袭、迁移、凋亡和新陈代谢[43-45]。Akt通过磷酸化palladin和vimentin,参与细胞迁移和侵袭。PI3K/Akt信号通路在EPS联用Oxa抑制HCT116细胞的迁移中的作用,将在后续的研究中进行实验验证。
综上所述,拟康氏木霉胞外多糖和奥沙利铂联用,可以增加人结肠癌HCT116细胞对奥沙利铂的敏感性,减少奥沙利铂的用量,起到增效减毒的作用,在此过程中铂耐药、PI3K/Akt、P53等多条信号通路被调控。协同作用的发挥,是多靶点、多通路共同作用的结果。该研究为对真菌多糖开发为肿瘤治疗或辅助治疗药物具有重要意义,值得进一步进行体内实验验证并探讨其临床应用的可能。
Biography
李萍,硕士,实验师,E-mail: liping919@wnmc.edu.cn
Funding Statement
国家自然科学基金(81402818);重庆市教育委员会人文社会科学研究项目(17SKG294);安徽省高校自然科学研究项目(KJ2017A257); 安徽省大学生创新创业训练项目(S201910368082)
Supported by National Natural Science Foundation of China (81402818)
Contributor Information
李 萍 (Ping LI), Email: liping919@wnmc.edu.cn.
刘 筱琴 (Xiaoqin LIU), Email: 243791775@qq.com.
王 国栋 (Guodong WANG), Email: guodong201@csu.edu.cn.
References
- 1.Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145–64. doi: 10.3322/caac.21601. [Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020[J]. CA Cancer J Clin, 2020, 70(3): 145-64.] [DOI] [PubMed] [Google Scholar]
- 2.Virostko J, Capasso A, Yankeelov TE, et al. Recent trends in the age at diagnosis of colorectal cancer in the US national cancer data base, 2004-2015. Cancer. 2019;125(21):3828–35. doi: 10.1002/cncr.32347. [Virostko J, Capasso A, Yankeelov TE, et al. Recent trends in the age at diagnosis of colorectal cancer in the US national cancer data base, 2004-2015[J]. Cancer, 2019, 125(21): 3828-35.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.中国抗癌协会大肠癌专业委员会中国结直肠肿瘤早诊筛查策略制订专家组 中国结直肠肿瘤早诊筛查策略专家共识. 中华胃肠外科杂志. 2018;21(10):1081–6. doi: 10.3760/cma.j.issn.1671-0274.2018.10.001. [中国抗癌协会大肠癌专业委员会中国结直肠肿瘤早诊筛查策略制订专家组. 中国结直肠肿瘤早诊筛查策略专家共识[J]. 中华胃肠外科杂志, 2018, 21(10): 1081-6.] [DOI] [Google Scholar]
- 4.Meester RGS, Peterse EFP, Knudsen AB, et al. Optimizing colorectal cancer screening by race and sex: Microsimulation analysis Ⅱ to inform the american cancer society colorectal cancer screening guideline. Cancer. 2018;124(14):2974–85. doi: 10.1002/cncr.31542. [Meester RGS, Peterse EFP, Knudsen AB, et al. Optimizing colorectal cancer screening by race and sex: Microsimulation analysis Ⅱ to inform the american cancer society colorectal cancer screening guideline[J]. Cancer, 2018, 124(14): 2974-85.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotwal AA, Schonberg MA. Cancer screening in the elderly: a review of breast, colorectal, lung, and prostate cancer screening. http://europepmc.org/abstract/MED/28731949. Cancer J. 2017;23(4):246–53. doi: 10.1097/PPO.0000000000000274. [Kotwal AA, Schonberg MA. Cancer screening in the elderly: a review of breast, colorectal, lung, and prostate cancer screening[J]. Cancer J, 2017, 23(4): 246-53.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibutani M, Nakao S, Maeda K, et al. Inflammation caused by surgical stress has a negative impact on the long-term survival outcomes in patients with colorectal cancer. Anticancer Res. 2020;40(6):3535–42. doi: 10.21873/anticanres.14342. [Shibutani M, Nakao S, Maeda K, et al. Inflammation caused by surgical stress has a negative impact on the long-term survival outcomes in patients with colorectal cancer[J]. Anticancer Res, 2020, 40(6): 3535-42.] [DOI] [PubMed] [Google Scholar]
- 7.Grapsa D, Syrigos K, Saif MW. Bevacizumab in combination with fluoropyrimidine-irinotecan-or fluoropyrimidine-oxaliplatin-based chemotherapy for first-line and maintenance treatment of metastatic colorectal cancer. Expert Rev Anticancer Ther. 2015;15(11):1267–81. doi: 10.1586/14737140.2015.1102063. [Grapsa D, Syrigos K, Saif MW. Bevacizumab in combination with fluoropyrimidine-irinotecan-or fluoropyrimidine-oxaliplatin-based chemotherapy for first-line and maintenance treatment of metastatic colorectal cancer[J]. Expert Rev Anticancer Ther, 2015, 15(11): 1267-81.] [DOI] [PubMed] [Google Scholar]
- 8.Negarandeh R, Salehifar E, Saghafi F, et al. Evaluation of adverse effects of chemotherapy regimens of 5-fluoropyrimidines derivatives and their association with DPYD polymorphisms in colorectal cancer patients. BMC Cancer. 2020;20(1):560. doi: 10.1186/s12885-020-06904-3. [Negarandeh R, Salehifar E, Saghafi F, et al. Evaluation of adverse effects of chemotherapy regimens of 5-fluoropyrimidines derivatives and their association with DPYD polymorphisms in colorectal cancer patients[J]. BMC Cancer, 2020, 20(1): 560.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smedman TM, Guren TK, Line PD, et al. Transplant oncology: assessment of response and tolerance to systemic chemotherapy for metastatic colorectal cancer after liver transplantation-a retrospective study. Transpl Int. 2019;32(11):1144–50. doi: 10.1111/tri.13471. [Smedman TM, Guren TK, Line PD, et al. Transplant oncology: assessment of response and tolerance to systemic chemotherapy for metastatic colorectal cancer after liver transplantation-a retrospective study[J]. Transpl Int, 2019, 32(11): 1144-50.] [DOI] [PubMed] [Google Scholar]
- 10.McQuade RM, Stojanovska V, Bornstein JC, et al. Colorectal cancer chemotherapy: the evolution of treatment and new approaches. http://europepmc.org/abstract/MED/28079003. Curr Med Chem. 2017;24(15):1537–57. doi: 10.2174/0929867324666170111152436. [McQuade RM, Stojanovska V, Bornstein JC, et al. Colorectal cancer chemotherapy: the evolution of treatment and new approaches[J]. Curr Med Chem, 2017, 24(15): 1537-57.] [DOI] [PubMed] [Google Scholar]
- 11.Okazaki S, Schirripa M, Loupakis F, et al. Tandem repeat variation near the HIC1 (hypermethylated in cancer 1) promoter predicts outcome of oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Cancer. 2017;123(22):4506–14. doi: 10.1002/cncr.30880. [Okazaki S, Schirripa M, Loupakis F, et al. Tandem repeat variation near the HIC1 (hypermethylated in cancer 1) promoter predicts outcome of oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer[J]. Cancer, 2017, 123(22): 4506-14.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibrahim A, Hirschfeld S, Cohen MH, et al. FDA drug approval summaries: oxaliplatin. Oncologist. 2004;9(1):8–12. doi: 10.1634/theoncologist.9-1-8. [Ibrahim A, Hirschfeld S, Cohen MH, et al. FDA drug approval summaries: oxaliplatin[J]. Oncologist, 2004, 9(1): 8-12.] [DOI] [PubMed] [Google Scholar]
- 13.Chang H, Tao YL, Jiang W, et al. Optimize the dose of oxaliplatin for locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy followed by radical surgery and adjuvant chemotherapy. BMC Cancer. 2020;20(1):498. doi: 10.1186/s12885-020-06988-x. [Chang H, Tao YL, Jiang W, et al. Optimize the dose of oxaliplatin for locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy followed by radical surgery and adjuvant chemotherapy[J]. BMC Cancer, 2020, 20(1): 498.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu HH, Chen MC, Baskaran R, et al. Oxaliplatin resistance in colorectal cancer cells is mediated via activation of ABCG2 to alleviate ER stress induced apoptosis. J Cell Physiol. 2018;233(7):5458–67. doi: 10.1002/jcp.26406. [Hsu HH, Chen MC, Baskaran R, et al. Oxaliplatin resistance in colorectal cancer cells is mediated via activation of ABCG2 to alleviate ER stress induced apoptosis[J]. J Cell Physiol, 2018, 233 (7): 5458-67.] [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Li Q, Wang J, et al. Polysaccharide from Lentinus edodes combined with oxaliplatin possesses the synergy and attenuation effect in hepatocellular carcinoma. Cancer Lett. 2016;377(2):117–25. doi: 10.1016/j.canlet.2016.04.037. [Zhang Y, Li Q, Wang J, et al. Polysaccharide from Lentinus edodes combined with oxaliplatin possesses the synergy and attenuation effect in hepatocellular carcinoma[J]. Cancer Lett, 2016, 377(2): 117-25.] [DOI] [PubMed] [Google Scholar]
- 16.Zhu L, Cao J, Chen G, et al. Anti-tumor and immunomodulatory activities of an exopolysaccharide from Rhizopus nigricans on CT26 tumor-bearing mice. Int Immunopharmacol. 2016;36:218–24. doi: 10.1016/j.intimp.2016.04.033. [Zhu L, Cao J, Chen G, et al. Anti-tumor and immunomodulatory activities of an exopolysaccharide from Rhizopus nigricans on CT26 tumor-bearing mice[J]. Int Immunopharmacol, 2016, 36: 218-24.] [DOI] [PubMed] [Google Scholar]
- 17.王颜 天池, 鹿 艳, 韩 军, et al. 黑根霉胞外多糖联合奥沙利铂对二甲肼诱导的大鼠结肠癌的抑制作用及对Survivin/caspase-3/caspase-7的影响. 中国药理学通报. 2019;35(5):690–4. doi: 10.3969/j.issn.1001-1978.2019.05.020. [王颜天池, 鹿艳, 韩军, 等. 黑根霉胞外多糖联合奥沙利铂对二甲肼诱导的大鼠结肠癌的抑制作用及对Survivin/caspase-3/caspase-7的影响[J]. 中国药理学通报, 2019, 35(5): 690-4.] [DOI] [Google Scholar]
- 18.Xu Y, Li J, Ju J, et al. Exopolysaccharide from Trichoderma pseudokoningii promotes maturation of murine dendritic cells. Int J Biol Macromol. 2016;92:1155–61. doi: 10.1016/j.ijbiomac.2016.06.064. [Xu Y, Li J, Ju J, et al. Exopolysaccharide from Trichoderma pseudokoningii promotes maturation of murine dendritic cells[J]. Int J Biol Macromol, 2016, 92: 1155-61.] [DOI] [PubMed] [Google Scholar]
- 19.Wang G, Zhu L, Yu B, et al. Exopolysaccharide from Trichoderma pseudokoningii induces macrophage activation. Carbohydr Polym. 2016;149:112–20. doi: 10.1016/j.carbpol.2016.04.093. [Wang G, Zhu L, Yu B, et al. Exopolysaccharide from Trichoderma pseudokoningii induces macrophage activation[J]. Carbohydr Polym, 2016, 149: 112-20.] [DOI] [PubMed] [Google Scholar]
- 20.李 萍, 沈 学彬, 周 玉燕, et al. 拟康氏木霉胞外多糖对人结肠癌细胞HCT116增殖及凋亡的影响. 中国药理学通报. 2019;35(11):1621–6. doi: 10.3969/j.issn.1001-1978.2019.11.025. [李萍, 沈学彬, 周玉燕, 等. 拟康氏木霉胞外多糖对人结肠癌细胞HCT116增殖及凋亡的影响[J]. 中国药理学通报, 2019, 35(11): 1621-6.] [DOI] [Google Scholar]
- 21.Wang G, Liu C, Liu J, et al. Exopolysaccharide from Trichoderma pseudokoningii induces the apoptosis of MCF-7 cells through an intrinsic mitochondrial pathway. Carbohydr Polym. 2016;136:1065–73. doi: 10.1016/j.carbpol.2015.09.108. [Wang G, Liu C, Liu J, et al. Exopolysaccharide from Trichoderma pseudokoningii induces the apoptosis of MCF-7 cells through an intrinsic mitochondrial pathway[J]. Carbohydr Polym, 2016, 136: 1065-73.] [DOI] [PubMed] [Google Scholar]
- 22.Huang T, Lin J, Cao J, et al. An exopolysaccharide from Trichoderma pseudokoningii and its apoptotic activity on human leukemia K562 cells. Carbohydr Polym. 2012;89(2):701–8. doi: 10.1016/j.carbpol.2012.03.079. [Huang T, Lin J, Cao J, et al. An exopolysaccharide from Trichoderma pseudokoningii and its apoptotic activity on human leukemia K562 cells[J]. Carbohydr Polym, 2012, 89(2): 701-8.] [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Aladelokun O, Ideta T, et al. Inhibition of PGE2/EP4 receptor signaling enhances oxaliplatin efficacy in resistant colon cancer cells through modulation of oxidative stress. Sci Rep. 2019;9(1):4954. doi: 10.1038/s41598-019-40848-4. [Huang H, Aladelokun O, Ideta T, et al. Inhibition of PGE2/EP4 receptor signaling enhances oxaliplatin efficacy in resistant colon cancer cells through modulation of oxidative stress[J]. Sci Rep, 2019, 9(1): 4954.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang JH, King TM, Chang MC, et al. Oxaliplatin-induced severe anaphylactic reactions in metastatic colorectal cancer: case series analysis. World J Gastroenterol. 2012;18(38):5427–33. doi: 10.3748/wjg.v18.i38.5427. [Wang JH, King TM, Chang MC, et al. Oxaliplatin-induced severe anaphylactic reactions in metastatic colorectal cancer: case series analysis[J]. World J Gastroenterol, 2012, 18(38): 5427-33.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Huang L, Shi H, et al. Ursolic acid enhances the therapeutic effects of oxaliplatin in colorectal cancer by inhibition of drug resistance. http://europepmc.org/abstract/MED/29034540. Cancer Sci. 2018;109(1) doi: 10.1111/cas.13425. [Zhang Y, Huang L, Shi H, et al. Ursolic acid enhances the therapeutic effects of oxaliplatin in colorectal cancer by inhibition of drug resistance[J]. Cancer Sci, 2018, 109(1): .] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zangui M, Atkin SL, Majeed M, et al. Current evidence and future perspectives for curcumin and its analogues as promising adjuncts to oxaliplatin: state-of-the-art. Pharmacol Res. 2019;141:343–56. doi: 10.1016/j.phrs.2019.01.020. [Zangui M, Atkin SL, Majeed M, et al. Current evidence and future perspectives for curcumin and its analogues as promising adjuncts to oxaliplatin: state-of-the-art[J]. Pharmacol Res, 2019, 141: 343-56.] [DOI] [PubMed] [Google Scholar]
- 27.Shan K, Wang Y, Hua H, et al. Ginsenoside Rg3 combined with oxaliplatin inhibits the proliferation and promotes apoptosis of hepatocellular carcinoma cells via downregulating PCNA and cyclin D1. Biol Pharm Bull. 2019;42(6):900–5. doi: 10.1248/bpb.b18-00852. [Shan K, Wang Y, Hua H, et al. Ginsenoside Rg3 combined with oxaliplatin inhibits the proliferation and promotes apoptosis of hepatocellular carcinoma cells via downregulating PCNA and cyclin D1[J]. Biol Pharm Bull, 2019, 42(6): 900-5.] [DOI] [PubMed] [Google Scholar]
- 28.Zhang P, Shi L, Zhang T, et al. Piperlongumine potentiates the antitumor efficacy of oxaliplatin through ROS induction in gastric cancer cells. Cell Oncol: Dordr. 2019;42(6):847–60. doi: 10.1007/s13402-019-00471-x. [Zhang P, Shi L, Zhang T, et al. Piperlongumine potentiates the antitumor efficacy of oxaliplatin through ROS induction in gastric cancer cells[J]. Cell Oncol: Dordr, 2019, 42(6): 847-60.] [DOI] [PubMed] [Google Scholar]
- 29.李 萍, 秦 国正, 王 国栋, et al. 拟康氏木霉菌丝多糖提取工艺优化. 食品科学. 2013;34(10):35–8. doi: 10.7506/spkx1002-6630-201310008. [李萍, 秦国正, 王国栋, 等. 拟康氏木霉菌丝多糖提取工艺优化[J]. 食品科学, 2013, 34(10): 35-8.] [DOI] [Google Scholar]
- 30.林 俊, 李 萍, 陈 靠山. 近5年多糖抗肿瘤活性研究进展. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGZY201308003.htm. 中国中药杂志. 2013;38(8):1116–25. [林俊, 李萍, 陈靠山. 近5年多糖抗肿瘤活性研究进展[J]. 中国中药杂志, 2013, 38(8): 1116-25.] [PubMed] [Google Scholar]
- 31.袁 守军. 多药合用药效学协同、相加和拮抗定量计算新方法的建立. 中国药理学与毒理学杂志. 2016;30(12):1316–32. doi: 10.3867/j.issn.1000-3002.2016.12.011. [袁守军. 多药合用药效学协同、相加和拮抗定量计算新方法的建立[J]. 中国药理学与毒理学杂志, 2016, 30(12): 1316-32.] [DOI] [Google Scholar]
- 32.秦 国正, 邵 太丽, 李 萍, et al. 黑根霉胞外多糖硫酸酯的制备及其抗肿瘤活性. http://www.j-smu.com/oa/DArticle.aspx?type=view&id=2019101227. 南方医科大学学报. 2019;39(10):1227–31. doi: 10.12122/j.issn.1673-4254.2019.10.15. [秦国正, 邵太丽, 李萍, 等. 黑根霉胞外多糖硫酸酯的制备及其抗肿瘤活性[J]. 南方医科大学学报, 2019, 39(10): 1227-31.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhattacharya D, Singh MK, Chaudhuri S, et al. T11TS impedes glioma angiogenesis by inhibiting VEGF signaling and pro-survival PI3K/Akt/ENOS pathway with concomitant upregulation of PTEN in brain endothelial cells. J Neuro Oncol. 2013;113(1):13–25. doi: 10.1007/s11060-013-1095-5. [Bhattacharya D, Singh MK, Chaudhuri S, et al. T11TS impedes glioma angiogenesis by inhibiting VEGF signaling and pro-survival PI3K/Akt/ENOS pathway with concomitant upregulation of PTEN in brain endothelial cells[J]. J Neuro Oncol, 2013, 113(1): 13-25.] [DOI] [PubMed] [Google Scholar]
- 34.Yu Y, Zhang Y, Wang S, et al. Inhibition effects of patchouli alcohol against influenza a virus through targeting cellular PI3K/Akt and ERK/MAPK signaling pathways. Virol J. 2019;16(1):163. doi: 10.1186/s12985-019-1266-x. [Yu Y, Zhang Y, Wang S, et al. Inhibition effects of patchouli alcohol against influenza a virus through targeting cellular PI3K/Akt and ERK/MAPK signaling pathways[J]. Virol J, 2019, 16(1): 163.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porta C, Figlin RA. Phosphatidylinositol-3-kinase/Akt signaling pathway and kidney cancer, and the therapeutic potential of phosphatidylinositol-3-kinase/Akt inhibitors. J Urol. 2009;182(6):2569–77. doi: 10.1016/j.juro.2009.08.085. doi: 10.1016/j.juro.2009.08.085. [Porta C, Figlin RA. Phosphatidylinositol-3-kinase/Akt signaling pathway and kidney cancer, and the therapeutic potential of phosphatidylinositol-3-kinase/Akt inhibitors[J]. J Urol, 2009, 182 (6): 2569-77.] [DOI] [PubMed] [Google Scholar]
- 36.Zegeye MM, Lindkvist M, Fälker K, et al. Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signalingmediated pro-inflammatory response in human vascular endothelial cells. Cell Commun Signal. 2018;16(1):55. doi: 10.1186/s12964-018-0268-4. [Zegeye MM, Lindkvist M, Fälker K, et al. Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signalingmediated pro-inflammatory response in human vascular endothelial cells[J]. Cell Commun Signal, 2018, 16(1): 55.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li B, Wang Z, Zhong Y, et al. CCR9-CCL25 interaction suppresses apoptosis of lung cancer cells by activating the PI3K/Akt pathway. Med Oncol. 2015;32(3):66. doi: 10.1007/s12032-015-0531-0. [Li B, Wang Z, Zhong Y, et al. CCR9-CCL25 interaction suppresses apoptosis of lung cancer cells by activating the PI3K/Akt pathway[J]. Med Oncol, 2015, 32(3): 66.] [DOI] [PubMed] [Google Scholar]
- 38.Li B, Xi P, Wang Z, et al. PI3K/Akt/mTOR signaling pathway participates in Streptococcus uberis-induced inflammation in mammary epithelial cells in concert with the classical TLRs/NF-ĸB pathway. Vet Microbiol. 2018;227:103–11. doi: 10.1016/j.vetmic.2018.10.031. [Li B, Xi P, Wang Z, et al. PI3K/Akt/mTOR signaling pathway participates in Streptococcus uberis-induced inflammation in mammary epithelial cells in concert with the classical TLRs/NF-ĸB pathway[J]. Vet Microbiol, 2018, 227: 103-11.] [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z, Yao L, Yang JH, et al. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia (Review) http://www.ncbi.nlm.nih.gov/pubmed/30106145. Mol Med Rep. 2018;18(4):3547–54. doi: 10.3892/mmr.2018.9375. [Zhang Z, Yao L, Yang JH, et al. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia (Review)[J]. Mol Med Rep, 2018, 18 (4): 3547-54.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu SF, Gao F, Wen L, et al. Osteocalcin induces proliferation via positive activation of the PI3K/Akt, P38 MAPK pathways and promotes differentiation through activation of the GPRC6A-ERK1/2 pathway in C2C12 myoblast cells. Cell Physiol Biochem. 2017;43(3):1100–12. doi: 10.1159/000481752. [Liu SF, Gao F, Wen L, et al. Osteocalcin induces proliferation via positive activation of the PI3K/Akt, P38 MAPK pathways and promotes differentiation through activation of the GPRC6A-ERK1/2 pathway in C2C12 myoblast cells[J]. Cell Physiol Biochem, 2017, 43(3): 1100-12.] [DOI] [PubMed] [Google Scholar]
- 41.Rosenberg L, Yoon CH, Sharma G, et al. Sorafenib inhibits proli-feration and invasion in desmoid-derived cells by targeting Ras/MEK/ERK and PI3K/Akt/mTOR pathways. Carcinogenesis. 2018;39(5):681–8. doi: 10.1093/carcin/bgy038. [Rosenberg L, Yoon CH, Sharma G, et al. Sorafenib inhibits proli-feration and invasion in desmoid-derived cells by targeting Ras/MEK/ERK and PI3K/Akt/mTOR pathways[J]. Carcinogenesis, 2018, 39(5): 681-8.] [DOI] [PubMed] [Google Scholar]
- 42.Shorning BY, Dass MS, Smalley MJ, et al. The PI3K-AKT-mTOR pathway and prostate cancer: at the crossroads of AR, MAPK, and WNT signaling. Int J Mol Sci. 2020;21(12):4507–53. doi: 10.3390/ijms21124507. [Shorning BY, Dass MS, Smalley MJ, et al. The PI3K-AKT-mTOR pathway and prostate cancer: at the crossroads of AR, MAPK, and WNT signaling[J]. Int J Mol Sci, 2020, 21(12): 4507-53.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.李 勤, 许 娟秀. miR-34a-5p通过调控CDK6表达和PI3K/AKT信号通路调节滋养层细胞的增殖、浸润和凋亡. http://www.j-smu.com/oa/DArticle.aspx?type=view&id=20200179. 南方医科大学学报. 2020;40(1):79–86. doi: 10.12122/j.issn.1673-4254.2020.01.13. [李勤, 许娟秀. miR-34a-5p通过调控CDK6表达和PI3K/AKT信号通路调节滋养层细胞的增殖、浸润和凋亡[J]. 南方医科大学学报, 2020, 40(1): 79-86.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mao SY, Wu Y, Wang RL, et al. Overexpression of GAS6 promotes cell proliferation and invasion in bladder cancer by activation of the PI3K/AKT pathway. Onco Targets Ther. 2020;13:4813–24. doi: 10.2147/OTT.S237174. [Mao SY, Wu Y, Wang RL, et al. Overexpression of GAS6 promotes cell proliferation and invasion in bladder cancer by activation of the PI3K/AKT pathway[J]. Onco Targets Ther, 2020, 13: 4813-24.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Y, Guo W, Gu X, et al. Repression of deoxynivalenol-triggered cytotoxicity and apoptosis by mannan/β-glucans from yeast cell wall: Involvement of autophagy and PI3K-AKT-mTOR signaling pathway. Int J Biol Macromol. 2020;164:1413–21. doi: 10.1016/j.ijbiomac.2020.07.217. [Zhao Y, Guo W, Gu X, et al. Repression of deoxynivalenol-triggered cytotoxicity and apoptosis by mannan/β-glucans from yeast cell wall: Involvement of autophagy and PI3K-AKT-mTOR signaling pathway[J]. Int J Biol Macromol, 2020, 164: 1413-21.] [DOI] [PubMed] [Google Scholar]


