Abstract
The present study was conducted to clarify the therapeutic effect of cornuside on experimental autoimmune encephalomyelitis (EAE) and its influence on T helper 17 (Th17) cell and regulatory T (Treg) cell infiltration into the central nervous system. Rats were randomly placed into four treatment groups: control, EAE, EAE+cornuside, and EAE+prednisolone. The neurological function scores of rats were assessed daily. On the second day after EAE rats began to show neurological deficit symptoms, the four groups were treated with normal saline, normal saline, cornuside (150 mg/kg), and prednisolone (5 mg/kg), respectively. The treatment was discontinued after two weeks, and the spinal cord was obtained for hematoxylin and eosin (H&E) and luxol fast blue staining, as well as retinoic acid receptor-related orphan receptor γ (RORγ) and forkhead box protein P3 (Foxp3) immunohistochemical staining. Blood was collected for Th17 and Treg cell flow cytometry testing, and the serum levels of interleukin (IL)-17A, IL-10, transforming growth factor-β (TGF-β), IL-6, IL-23, and IL-2 were measured via enzyme-linked immunosorbent assay (ELISA). Compared with rats in the EAE group, rats in the EAE+cornuside and EAE+prednisolone groups began to recover from neurological deficits earlier, and had a greater degree of improvement of symptoms. Focal inflammation, demyelination, and RORγ-positive cell infiltration were reduced by cornuside or prednisolone treatment, whereas the Foxp3-positive cell numbers were not significantly different. Meanwhile, the number of Th17 cells and the IL-17A, IL-6, and IL-23 levels were lower in the blood after cornuside or prednisolone treatment, whereas the number of Treg cells or the levels of IL-10, TGF-β, and IL-2 were not markedly different. Cornuside can alleviate symptoms of EAE neurological deficits through its anti-inflammatory and immunosuppressive effects, and Th17 cells may be one of its therapeutic targets.
Keywords: Cornuside, Experimental autoimmune encephalomyelitis, Multiple sclerosis, Inflammation
1 Introduction
Multiple sclerosis (MS) is the predominant neuroimmune disease and the chief contributor to nontraumatic neurological disability in young people, with the degree of disability increasing with the disease duration (Stenager, 2019; Hauser and Cree, 2020). The condition causes varying degrees of motor, sensory, cognitive, and visual dysfunctions (Oh et al., 2018; Dobson and Giovannoni, 2019). Currently, MS is treated with corticosteroids in the acute phase and immunomodulators in the remission phase as disease-modifying therapies, while both feature insufficient efficacy or severe side effects (McCall, 2019; McCool et al., 2019; Li et al., 2020). Therefore, novel treatment options need to be actively explored.
The pathology of MS is marked by chronic inflammation, demyelination, and axonal loss (Pegoretti et al., 2020). Experimental autoimmune encephalomyelitis (EAE) is a widely used animal model of MS (Burrows et al., 2019). Different types of EAE can mimic the pathological manifestations and pathogenesis of various MS stages (Lassmann and Bradl, 2017). Both MS and EAE pathogenesis are dominated by CD4+ T lymphocyte-mediated cellular immunity (Segal, 2019). It is well-known that T helper 17 (Th17)/regulatory T (Treg) cell homeostasis contributes greatly to the pathogenesis of EAE (Fasching et al., 2017; Li et al., 2019). Th17 cells cross the blood–brain barrier to trigger an inflammatory cascade and recruit more immune cells to infiltrate the central nervous system (CNS) (Balasa et al., 2020). In MS, Treg cells exert immune tolerance by inhibitory cytokine release, cell–cell contact-dependent suppression, and other mechanisms (Kleinewietfeld and Hafler, 2014).
Cornuside is one of the essential constituents of cornel iridoid glycosides extracted from the Chinese herb Corni Fructus (Cornus officinalis Sieb. et Zucc., known in China as "Shanzhuyu"). According to the traditional Chinese medicine (TCM) theory, Yin deficiency of the liver and kidney is an important syndrome in MS (Fan and Wu, 2014; Zhao et al., 2018). Corni Fructus has been widely used to tonify the liver and the kidney in TCM (Dong et al., 2018; Huang et al., 2018). It has high safety in clinical application, and only exhibits limited toxicity at high concentrations (Huang et al., 2018). Many TCM prescriptions used to treat MS, for example, Liuwei Dihuang Pill and Zuogui Pill, include Corni Fructus as their principal component (Fan and Wang, 2018; Huang et al., 2018). In addition, previous studies have shown that cornel iridoid glycosides could alleviate symptoms of EAE, reduce EAE CNS inflammation, and more importantly, inhibit CD3+ T lymphocyte infiltration into the CNS (Yin et al., 2014; Qu et al., 2016, 2019).
In view of the above, we hypothesized that cornuside could improve EAE, and that T lymphocytes, especially Th17 and Treg cells, are involved in the mechanism of action. Therefore, the aim of the present study was to determine whether cornuside could alleviate the symptoms and improve the pathology of EAE, as well as inhibit the infiltration of Th17 cells into the CNS or enhance the infiltration of Treg cells, in order to ultimately provide a basis for the potential applicability of cornuside for MS treatment.
2 Materials and methods
2.1. Materials
The cornuside (CAS No. 131189-57-6) used in this study was provided by Chengdu Herbpurify Co., Ltd., China, with a purity of 99.647%. It has the chemical formula C24H30O14, and a molecular weight of 542.49.
2.2. Animals
The experimental subjects were 6‒8-week-old female Lewis rats with a body weight of 170‒200 g, which were acquired from Shanghai SLAC Laboratory Animal Co., Ltd., Shanghai, China. The rats were placed in a specific pathogen-free animal barrier environment in the laboratory animal research center, and housed under 12-h light/dark cycle with access to food and water ad libitum. A one-week period was allowed for acclimation prior to the experiment. The study was approved by the Laboratory Animal Management and Ethics Committee of Zhejiang Chinese Medical University (Hangzhou, China).
2.3. EAE induction and neurological function assessment
The induction of EAE was performed using guinea pig spinal cord homogenate (GPSCH) immune emulsion (Pitarokoili et al., 2017). The immune emulsion was a 1:2 (volume ratio) mixture of 50% GPSCH (1 g spinal cord:1 mL normal saline) and complete Freund's adjuvant (CFA) (Sigma, USA). The CFA emulsion consisted of incomplete Freund's adjuvant and heat-inactivated dried Mycobacterium tuberculosis (1 mg/mL). On the day of EAE induction, rats were subcutaneously injected with 0.1 mL of immune emulsion (injected into two sides of the base of the tail, 0.05 mL per injection). The second day of induction was considered Day 1 post-immunization.
Rats were scored daily for neurological deficit symptoms starting on Day 1 post-immunization. The 5-point EAE score criteria were: 0, no symptoms of neurological deficit; 1, tail weakness; 2, mild paralysis of the hind limbs; 3, one hind limb severely paralyzed; 4, both hind limbs severely paralyzed; 5, all limbs paralyzed, near-death state or death (Schneider et al., 2009).
2.4. Grouping and treatment
Rats were randomly separated into four groups: control, EAE, EAE+cornuside, and EAE+prednisolone; and were treated with normal saline, normal saline, cornuside (150 mg/kg), and prednisolone (5 mg/kg), respectively, by gavage. Drug treatment was performed from the next day after the onset of neurological deficit symptoms in EAE rats (11th day after immunization) until the end of a two-week treatment period (24th day post-immunization).
2.5. Histopathology
On Day 25 post-immunization, the spinal cords of all rats were obtained, fixed, paraffin-embedded, sectioned (4 μm), and stained with hematoxylin and eosin (H&E). Demyelination was detected by Luxol fast blue (LFB) staining.
2.6. Immunohistochemical staining
After dewaxing the slices and antigen repair at high temperature and pressure, 3% H2O2 solution was added for 10 min to eliminate the effect of endogenous enzyme. Primary antibodies were then added and samples were incubated at 37 ℃ for 60 min. Next, secondary antibodies were added and samples were incubated at 37 ℃ for 60 min. Subsequently, diaminobenzidine treatment for 1 min was performed for color development. The nuclei were stained with Harris hematoxylin solution for 1 min; sections were dehydrated with ethanol, transparentized with xylene, and sealed with natural glue.
In order to assess Th17 cell and Treg cell infiltration, the primary antibodies were selected as rabbit polyclonal anti-retinoic acid receptor-related orphan receptor γ (anti-RORγ) (dilution 1:350, Abcam, Cambridge, UK) and mouse anti-forkhead box protein P3 (anti-Foxp3) (dilution 1:100, Abcam), respectively. The secondary antibodies chosen were goat anti-rabbit immunoglobulin G (IgG) (Santa Cruz, USA) or goat anti-mouse IgG (Abcam), respectively.
For the quantitative analysis of Th17 cell and Treg cell infiltration into the spinal cord, four sections per rat were selected and photographed with an Olympus microscope, and 3–5 fields of view were chosen for each section. Positive cells were counted and converted into positive cell ratio. The percentage of positive cells was calculated as (the number of positive cells in the fields of view)/(the total number of cells in the fields of view)×100%.
2.7. Flow cytometry
The numbers of Th17 cells and Treg cells in the blood were determined by blood collection from rats on Day 25 post-immunization and measuring the number of CD4+ interleukin (IL)-17A+ and CD25+ Foxp3+ T cells in the blood using flow cytometry. Peripheral blood mononuclear cells were separated using lymphocyte isolation solution, extracellularly stained with mouse anti-CD4-FITC (Abcam), and then fixed to break the cell membrane. The intracellular staining was performed in mouse anti-IL-17A-PE (Abcam) cells to label Th17 cells. Accordingly, mouse anti-CD25-PE (Abcam) was stained extracellularly, and mouse anti-Foxp3-AF488 (Abcam) was stained intracellularly to label Treg cells. Data were analyzed as the percentage of Th17 cells or Treg cells.
2.8. Enzyme-linked immunosorbent assay
Blood was collected from rats on Day 25 post-immunization to measure the IL-17A, IL-10, IL-6, IL-23, transforming growth factor-β (TGF-β), and IL-2 cytokine levels by enzyme-linked immunosorbent assay (ELISA) using a cytokine-specific assay kit (Deco, Shanghai, China) following the manufacturer's instructions.
2.9. Statistical methods
Statistical analyses were performed by SPSS 19.0 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was employed to analyze the differences between groups. The Fisher's least significant difference (LSD) test was chosen when the variances were congruent, and the Tamhane's T2 test was preferred when the variances were uneven.
3 Results
3.1. Effect of cornuside on the symptoms of neurological deficit in EAE rats
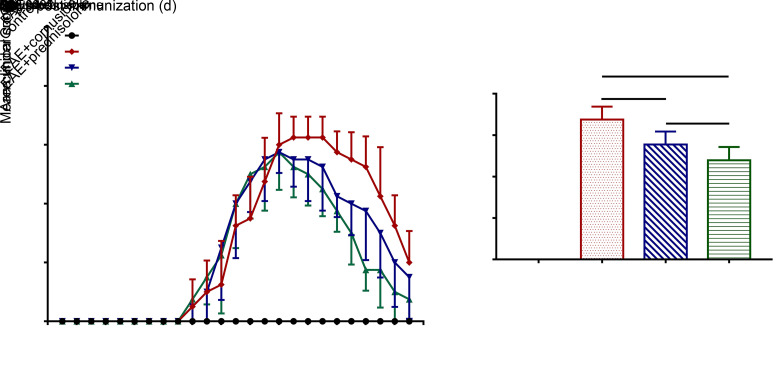
The symptoms of neurological deficit in EAE rats began to appear on Day 10 after immunization, and treatment started on Day 11 post-immunization. The symptoms of EAE+cornuside and EAE+prednisolone group rats reached a peak on Day 16 after immunization before they started to recover, while the symptoms of EAE group rats reached a peak on Day 19 after immunization before they started to recover (Fig. 1a). The area under the curve was used to analyze the disease severity, and results showed that cornuside and prednisolone treatments could reduce the clinical score of EAE (Fig. 1b).
Fig. 1. Cornuside alleviated the symptoms of neurological deficit in experimental autoimmune encephalomyelitis (EAE) rats. (a) The 5-point EAE score in rats from Day 1 to 25 post-immunization. (b) The area under curve used to analyze the disease severity. All data are expressed as mean±standard deviation (n=8 in each group).
3.2. Effect of cornuside on the histopathology of spinal cord of EAE rats
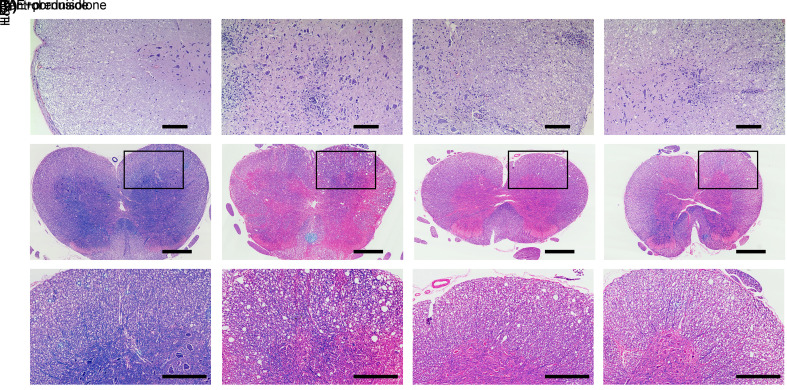
For the purpose of clarifying whether cornuside could ameliorate the histopathology of EAE, the spinal cords of all rats were obtained to perform H&E and LFB staining. As shown in the representative H&E sections, there were no obvious inflammatory foci in the control group, whereas the infiltration of lots of inflammatory cells was observed in the EAE group. Moreover, inflammation was alleviated in the cornuside and prednisolone treatment groups (Fig. 2a). In comparison to the control group, rats in the EAE group exhibited increased demyelination foci, while those in both the cornuside and the prednisolone treatment groups had decreased demyelination foci compared to the EAE group (Figs. 2b and 2c).
Fig. 2. Cornuside reduced inflammation and demyelination in the spinal cord of experimental autoimmune encephalomyelitis (EAE) rats. On the 25th day post-immunization, rat spinal cords were separated, sectioned, and stained with hematoxylin eosin (H&E) or Luxol fast blue (LFB). (a) Representative sections of H&E, scale bar=100 µm. (b) Representative sections of LFB, scale bar=500 µm. (c) Local magnification of LFB sections, scale bar=250 μm.
3.3. Effects of cornuside on the infiltration of Th17 and Treg cells in EAE spinal cord
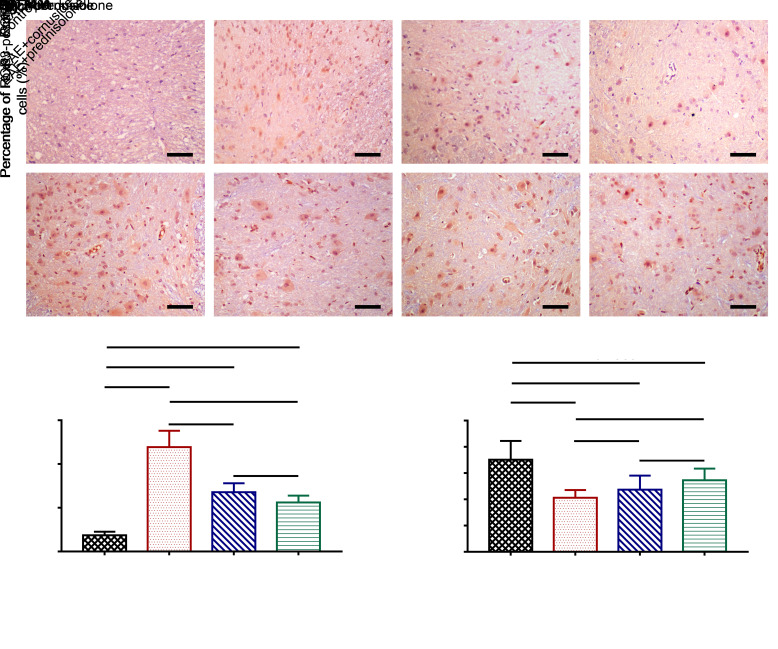
The effects of cornuside on the infiltration of Th17 and Treg cells in the spinal cord were determined by the immunohistochemical staining of rat spinal cord sections. The number of Th17 cells was reflected by RORγ staining, and the number of Treg cells was indicated by Foxp3 staining. Th17 cell infiltration was elevated in the EAE group of rats compared with the control, and cornuside and prednisolone treatments could lower the number of Th17 cells infiltrating into the spinal cord (Figs. 3a and 3c). The number of Treg cells in the spinal cord of rats in the EAE group was reduced compared to the control group, but cornuside treatment failed to increase the number of Treg cells (Figs. 3b and 3d).
Fig. 3. Effects of cornuside on the numbers of T helper 17 (Th17) and regulatory T (Treg) cells in the spinal cord of experimental autoimmune encephalomyelitis (EAE) rats. The rat spinal cord sections were stained by immunohistochemistry. The number of Th17 cells is reflected by retinoic acid receptor-related orphan receptor γ (RORγ) staining (a), while the number of Treg cells is indicated by forkhead box protein P3 (Foxp3) staining (b). Scale bar=25 μm. (c) Th17 cell number. (d) Treg cell number. All data are expressed as mean±standard deviation (n=4 in each group, 4 sections per rat).
3.4. Effects of cornuside on the numbers of Th17 and Treg cells in EAE rat blood, and on the serum levels of IL-17A and IL-10
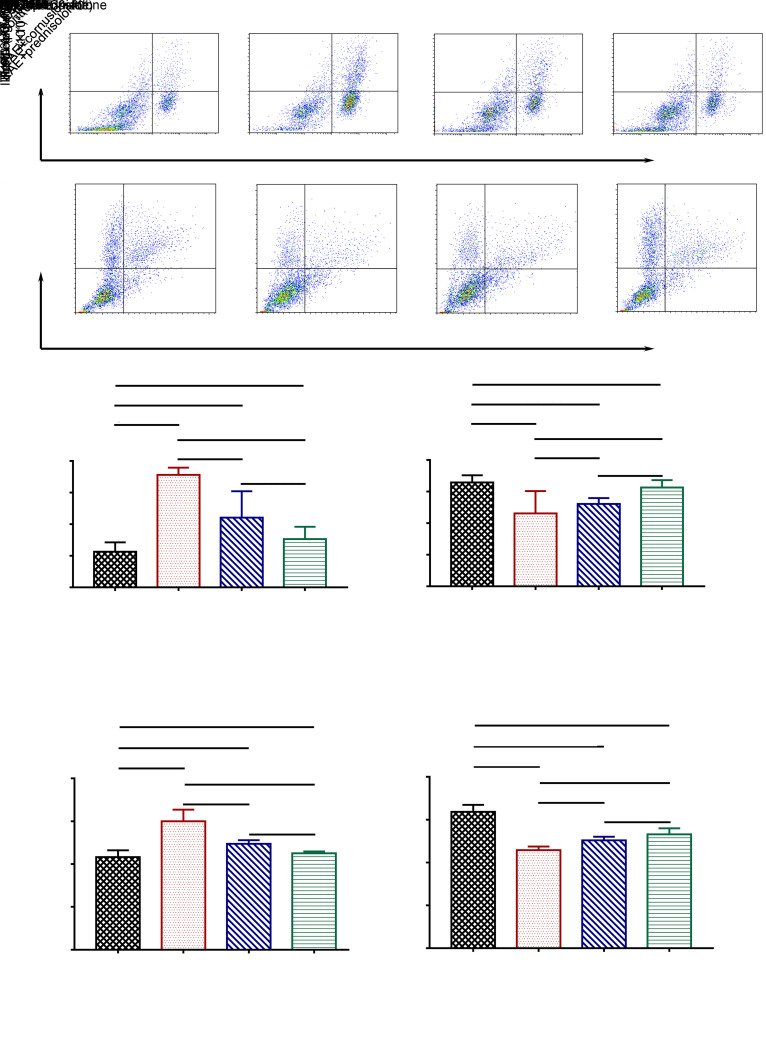
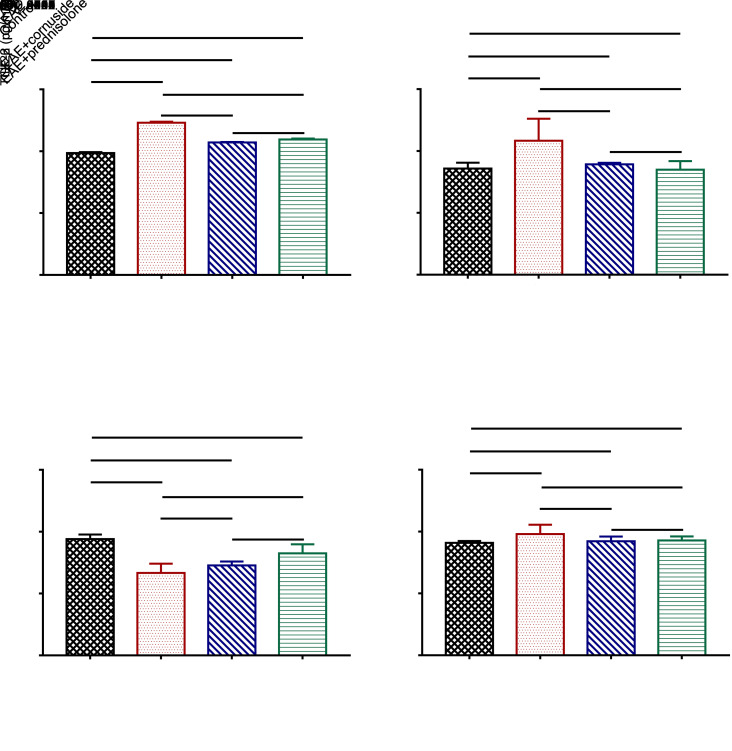
The effects of cornuside on the numbers of Th17 and Treg cells in the rat blood and on the serum levels of IL-17A and IL-10 were determined by flow cytometry or ELISA. Results demonstrated that the number of Th17 cells was increased and the number of Treg cells was reduced in the EAE group compared to the control group, and cornuside treatment could lower the number of Th17 cells in the blood, but had no effect on the number of Treg cells (Figs. 4a‒4d). Meanwhile, the measurement of serum levels of IL-17A and IL-10 by ELISA showed that EAE raised the level of IL-17A and lowered the level of IL-10 in the rat serum. Meanwhile, cornuside treatment reduced IL-17A expression level, but had no impact on IL-10 level (Figs. 4e and 4f).
Fig. 4. Effects of cornuside on the numbers of T helper 17 (Th17) and regulatory T (Treg) cells in experimental autoimmune encephalomyelitis (EAE) rat blood, as well as on levels of interleukin (IL)-17A and IL-10 in EAE rat serum. (a, b) The numbers of Th17 cells (CD4+ IL-17A+) and Treg cells (CD25+ Foxp3+) in rat blood were determined by flow cytometry. (c, d) Statistical analyses of the numbers of Th17 cells and Treg cells. (e, f) The levels of IL-17A and IL-10 in the serum were determined by enzyme-linked immunosorbent assay (ELISA). Data are expressed as mean±standard deviation (n=4 in each group).
3.5. Effects of cornuside on serum TGF-β, IL-6, IL-23, and IL-2 levels in EAE rats
The cytokines TGF-β, IL-6, and IL-23 are critical for the differentiation of naive CD4+ T cells into Th17 cells, while TGF-β and IL-2 are crucial for the differentiation of naive CD4+ T cells into Treg cells (Cerboni et al., 2020; Pawlak et al., 2020). Therefore, we tested the levels of the above cytokines in rat serum to investigate whether cornuside can influence the differentiation direction of naive CD4+ T cells by affecting the levels of the above cytokines. Results indicated that the levels of IL-6 and IL-23 were increased in the EAE group when compared with the control group. Furthermore, the serum levels of IL-6 and IL-23 were decreased in EAE+cornuside and EAE+prednisolone rats compared to the EAE group (Figs. 5a and 5b). Cornuside treatment did not exert noticeable effects on serum TGF-β or IL-2 level in EAE rats (Figs. 5c and 5d).
Fig. 5. Effects of cornuside on the levels of interleukin (IL)-6 (a), IL-23 (b), transforming growth factor-β (TGF-β) (c), and IL-2 (d) in the serum of experimental autoimmune encephalomyelitis (EAE) rats by enzyme-linked immunosorbent assay (ELISA) on Day 25 after immunization. All data are expressed as mean±standard deviation (n=8 in each group).
4 Discussion
In the present study, cornuside improved neurological function and inhibited inflammatory infiltration and demyelination in the spinal cord of EAE rats. In addition, it also lowered the number of Th17 cells infiltrating into the lesion, but had no obvious effect on the number of Treg cells. Similarly, it decreased the number of Th17 cells in the blood and the level of IL-17A in the serum of EAE rats, but had no significant impact on the number of Treg cells or the IL-10 level. Moreover, cornuside reduced the levels of IL-6 and IL-23, but had no noticeable effect on the level of TGF-β or IL-2 in the serum.
Aiming to evaluate the potential of cornuside for treating MS, EAE rats were treated with cornuside, while corticosteroids were used as positive control drug and normal saline as negative control drug. Cornuside was found to alleviate the neurological deficit symptoms of EAE. This study utilized GPSCH immune emulsion to create a model of EAE with spontaneous remission of symptoms (Schneider et al., 2009), and thus it could be seen that normal saline-treated EAE rats also presented spontaneous improvement after the disease peaked. However, the improvement occurred earlier and was more pronounced with cornuside or corticosteroid treatment. Previous studies have used cornel iridoid glycosides to treat EAE. The treatment initiation points in all of these studies were all on the day of induction for EAE (Yin et al., 2014; Qu et al., 2016, 2019), whereas our study started treatment on the day after the onset of EAE symptoms, highlighting the therapeutic potential of cornuside when applied after the occurrence of EAE symptoms.
MS is characterized by inflammatory demyelination of the CNS (Ding et al., 2019; Moser et al., 2020). Intrathecal inflammation is one of the pathological characteristics of MS during disease progression (Monaco et al., 2020). The key driver of neurodegeneration in MS is inflammation; focal inflammatory activity was associated with neurodegeneration in the first five years after the onset of MS (Pulido-Valdeolivas et al., 2020). Autoreactive T cells enter the CNS passing through blood–brain barrier, upregulate inflammatory mediators, activate microglia/macrophages, and recruit more T lymphocytes, thus leading to inflammation and nerve fiber demyelination (Ruiz et al., 2019; Kunkl et al., 2020). The present study showed that cornuside exerts anti-inflammatory effects, and inhibits this inflammatory infiltration and demyelination in the spinal cord of EAE rats.
Mounting research suggests that Th17/Treg cell homeostasis is crucial for MS pathogenesis (Fasching et al., 2017; Lee, 2018). The naive CD4+ T precursor cell is shared by Th17 and Treg cells, and their initial differentiation requires a common cytokine, the TGF-β signal. However, they eventually differentiate into cells with opposite functions, with the former causing autoimmunity, inflammation, and transplant rejection, while the latter suppressing autoimmunity, inhibiting inflammation, and maintaining immune homeostasis (Xie et al., 2010; Lee, 2018; Zhao and Wang, 2018). The secondary transfer of Th17 cells can induce EAE, while the secondary transfer of Treg cells can alleviate the symptoms of EAE (Kleinewietfeld and Hafler, 2014; Segal, 2019). Herein, we further investigated the effect of cornuside on Th17 cells and Treg cells. Our experiments established that cornuside can reduce the amount of Th17 cells infiltrating into the lesion, while it has no obvious impact on the number of Treg cells. The number of Th17 cells and the level of IL-17A in the blood of EAE rats were decreased by cornuside; however, it had no noticeable effect on the number of Treg cells or the level of IL-10. This indicates that Th17 cells, but not Treg cells, are one of the targets of cornuside.
The effect of cornuside of lowering the numbers of Th17 cells in both the lesion and the blood suggests that it may affect the differentiation of naive CD4+ T cells into Th17 cells. This differentiation and subsequent maintenance of steady state are controlled by the key cytokines TGF-β, IL-6, and IL-23 (Durant et al., 2010; Park et al., 2014; Cerboni et al., 2020). Meanwhile, TGF-β and IL-2 act together to decide the differentiation of naive CD4+ T cells into Treg cells (Pawlak et al., 2020). The further examination of the levels of cytokines related to Th17 and Treg cell differentiation in the serum of EAE rats indicated that cornuside could lower the levels of IL-6 and IL-23, suggesting that it may inhibit the differentiation of naive CD4+ T cells into Th17 cells by reducing the levels of IL-6 and IL-23, and thus decrease the number of Th17 cells infiltrating into EAE lesions. Nonetheless, cornuside had no significant influence on the level of TGF-β or IL-2.
5 Conclusions
In summary, cornuside can alleviate EAE symptoms possibly via anti-inflammatory and immunosuppressive effects, and Th17 cells may be one of its principal targets. Therefore, cornuside has the potential to treat MS; however, the detailed mechanism of its effect on Th17 cell infiltration into the CNS with EAE needs to be further investigated and refined.
Acknowledgments
This work was supported by the Traditional Chinese Medical Science and Technology Project of Zhejiang Province (No. 2019ZA063) and the Scientific Research Fund of Zhejiang Chinese Medical University (No. 2019ZY09), China.
Author contributions
Rongbo ZHANG and Qiang YUAN designed the study. Jin LIU and Bin XU established the animal models. Rongbo ZHANG and Jin LIU performed the experimental research, and wrote and edited the manuscript. You WU and Shunli LIANG contributed to the data analysis. All authors have read and approved the final manuscript and, therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines
Rongbo ZHANG, Jin LIU, Bin XU, You WU, Shunli LIANG, and Qiang YUAN declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- Balasa R, Barcutean L, Balasa A, et al. , 2020. The action of Th17 cells on blood brain barrier in multiple sclerosis and experimental autoimmune encephalomyelitis. Hum Immunol, 81(5): 237-243. 10.1016/j.humimm.2020.02.009 [DOI] [PubMed] [Google Scholar]
- Burrows DJ, McGown A, Jain SA, et al. , 2019. Animal models of multiple sclerosis: from rodents to zebrafish. Mult Scler J, 25(3): 306-324. 10.1177/1352458518805246 [DOI] [PubMed] [Google Scholar]
- Cerboni S, Gehrmann U, Preite S, et al. , 2020. Cytokine-regulated Th17 plasticity in human health and diseases. Immunology, 163(1): 13280. 10.1111/imm.13280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding HY, Xie YN, Dong Q, et al. , 2019. Roles of hyaluronan in cardiovascular and nervous system disorders. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 20(5): 428-436. 10.1631/jzus.B1900155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson R, Giovannoni G, 2019. Multiple sclerosis—a review. Eur J Neurol, 26(1): 27-40. 10.1111/ene.13819 [DOI] [PubMed] [Google Scholar]
- Dong Y, Feng ZL, Chen HB, et al. , 2018. Corni Fructus: a review of chemical constituents and pharmacological activities. Chin Med, 13: 34. 10.1186/s13020-018-0191-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant L, Watford WT, Ramos HL, et al. , 2010. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity, 32(5): 605-615. 10.1016/j.immuni.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YP, Wu W, 2014. Syndrome factors of multiple sclerosis in 500 patients. J Beijing Univ Tradit Chin Med, 37(1): 68-72 (in Chinese). 10.3969/j.issn.1006-2157.2014.01.015 [DOI] [Google Scholar]
- Fan YP, Wang SQ, 2018. Standard for clinical diagnosis and treatment of traditional chinese medicine for multiple sclerosis/neuromyelitis optica. J Cap Med Univ, 39(6): 833-835 (in Chinese). 10.3969/j.issn.1006-7795.2018.06.008 [DOI] [Google Scholar]
- Fasching P, Stradner M, Graninger W, et al. , 2017. Therapeutic potential of targeting the Th17/Treg axis in autoimmune disorders. Molecules, 22(1): 134. 10.3390/molecules22010134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SL, Cree BAC, 2020. Treatment of multiple sclerosis: a review. Am J Med, 133(12): 1380-1390.E2. 10.1016/j.amjmed.2020.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhang YW, Dong L, et al. , 2018. Ethnopharmacology, phytochemistry, and pharmacology of Cornus officinalis sieb. et Zucc. J Ethnopharmacol, 213: 280-301. 10.1016/j.jep.2017.11.010 [DOI] [PubMed] [Google Scholar]
- Kleinewietfeld M, Hafler DA, 2014. Regulatory T cells in autoimmune neuroinflammation. Immunol Rev, 259(1): 231-244. 10.1111/imr.12169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkl M, Frascolla S, Amormino C, et al. , 2020. T helper cells: the modulators of inflammation in multiple sclerosis. Cells, 9(2): 482. 10.3390/cells9020482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H, Bradl M, 2017. Multiple sclerosis: experimental models and reality. Acta Neuropathol, 133(2): 223-244. 10.1007/s00401-016-1631-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GR, 2018. The balance of Th17 versus Treg cells in autoimmunity. Int J Mol Sci, 19(3): 730. 10.3390/ijms19030730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HH, Hu FL, Zhang YL, et al. , 2020. Comparative efficacy and acceptability of disease-modifying therapies in patients with relapsing-remitting multiple sclerosis: a systematic review and network meta-analysis. J Neurol, 267(12): 3489-3498. 10.1007/s00415-019-09395-w [DOI] [PubMed] [Google Scholar]
- Li ZF, Nie LL, Chen LP, et al. , 2019. Rapamycin relieves inflammation of experimental autoimmune encephalomyelitis by altering the balance of Treg/Th17 in a mouse model. Neurosci Lett, 705: 39-45. 10.1016/j.neulet.2019.04.035 [DOI] [PubMed] [Google Scholar]
- McCall B, 2019. Alemtuzumab to be restricted pending review, says EMA. Lancet, 393(10182): 1683. 10.1016/S0140-6736(19)30935-3 [DOI] [PubMed] [Google Scholar]
- McCool R, Wilson K, Arber M, et al. , 2019. Systematic review and network meta-analysis comparing ocrelizumab with other treatments for relapsing multiple sclerosis. Mult Scler Relat Disord, 29: 55-61. 10.1016/j.msard.2018.12.040 [DOI] [PubMed] [Google Scholar]
- Monaco S, Nicholas R, Reynolds R, et al. , 2020. Intrathecal inflammation in progressive multiple sclerosis. Int J Mol Sci, 21(21): 8217. 10.3390/ijms21218217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Akgün K, Proschmann U, et al. , 2020. The role of Th17 cells in multiple sclerosis: therapeutic implications. Autoimmun Rev, 19(10): 102647. 10.1016/j.autrev.2020.102647 [DOI] [PubMed] [Google Scholar]
- Oh J, Vidal-Jordana A, Montalban X, 2018. Multiple sclerosis: clinical aspects. Curr Opin Neurol, 31(6): 752-759. 10.1097/WCO.0000000000000622 [DOI] [PubMed] [Google Scholar]
- Park JS, Lee J, Lim MA, et al. , 2014. JAK2-STAT3 blockade by AG490 suppresses autoimmune arthritis in mice via reciprocal regulation of regulatory T cells and Th17 cells. J Immunol, 192(9): 4417-4424. 10.4049/jimmunol.1300514 [DOI] [PubMed] [Google Scholar]
- Pawlak M, Ho AW, Kuchroo VK, 2020. Cytokines and transcription factors in the differentiation of CD4+ T helper cell subsets and induction of tissue inflammation and autoimmunity. Curr Opin Immunol, 67: 57-67. 10.1016/j.coi.2020.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegoretti V, Swanson KA, Bethea JR, et al. , 2020. Inflammation and oxidative stress in multiple sclerosis: consequences for therapy development. Oxid Med Cell Longev, 2020: 7191080. 10.1155/2020/7191080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitarokoili K, Ambrosius B, Gold R, 2017. Lewis rat model of experimental autoimmune encephalomyelitis. Curr Protoc Neurosci, 81: 9.61.1-9.61.20. 10.1002/cpns.36 [DOI] [PubMed] [Google Scholar]
- Pulido-Valdeolivas I, Andorrà M, Gómez-Andrés D, et al. , 2020. Retinal and brain damage during multiple sclerosis course: inflammatory activity is a key factor in the first 5 years. Sci Rep, 10: 13333. 10.1038/s41598-020-70255-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Z, Zheng N, Zhang YF, et al. , 2016. Preventing the BDNF and NGF loss involved in the effects of cornel iridoid glycoside on attenuation of experimental autoimmune encephalomyelitis in mice. Neurol Res, 38(9): 831-837. 10.1080/01616412.2016.1200766 [DOI] [PubMed] [Google Scholar]
- Qu Z, Zheng N, Wei YZ, et al. , 2019. Effect of cornel iridoid glycoside on microglia activation through suppression of the JAK/STAT signalling pathway. J Neuroimmunol, 330: 96-107. 10.1016/j.jneuroim.2019.01.014 [DOI] [PubMed] [Google Scholar]
- Ruiz F, Vigne S, Pot C, 2019. Resolution of inflammation during multiple sclerosis. Semin Immunopathol, 41(6): 711-726. 10.1007/s00281-019-00765-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Schuetz G, Zollner TM, 2009. Acute neuroinflammation in lewis rats—a model for acute multiple sclerosis relapses. J Neuroimmunol, 213(1-2): 84-90. 10.1016/j.jneuroim.2009.05.015 [DOI] [PubMed] [Google Scholar]
- Segal BM, 2019. The diversity of encephalitogenic CD4+ T cells in multiple sclerosis and its animal models. J Clin Med, 8(1): 120. 10.3390/jcm8010120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenager E, 2019. A global perspective on the burden of multiple sclerosis. Lancet Neurol, 18(3): 227-228. 10.1016/S1474-4422(18)30498-8 [DOI] [PubMed] [Google Scholar]
- Xie XJ, Ye YF, Zhou L, et al. , 2010. Th17 promotes acute rejection following liver transplantation in rats. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 11(11): 819-827. 10.1631/jzus.B1000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin LL, Chen YY, Qu Z, et al. , 2014. Involvement of JAK/STAT signaling in the effect of cornel iridoid glycoside on experimental autoimmune encephalomyelitis amelioration in rats. J Neuroimmunol, 274(1-2): 28-37. 10.1016/j.jneuroim.2014.06.022 [DOI] [PubMed] [Google Scholar]
- Zhao PY, Wang YQ, Liu XH, et al. , 2018. Bu Shen Yi Sui capsule promotes remyelination correlating with Sema3A/NRP-1, LIF/LIFR and Nkx6.2 in mice with experimental autoimmune encephalomyelitis. J Ethnopharmacol, 217: 36-48. 10.1016/j.jep.2018.02.014 [DOI] [PubMed] [Google Scholar]
- Zhao ST, Wang CZ, 2018. Regulatory T cells and asthma. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 19(9): 663-673. 10.1631/jzus.B1700346 [DOI] [PMC free article] [PubMed] [Google Scholar]