Abstract
Hypertension is a prevalent systemic disease in the elderly, who can suffer from several pathological skeletal conditions simultaneously, including osteoporosis. Benidipine (BD), which is widely used to treat hypertension, has been proved to have a beneficial effect on bone metabolism. In order to confirm the osteogenic effects of BD, we investigated its osteogenic function using mouse MC3T3-E1 preosteoblast cells in vitro. The proliferative ability of MC3T3-E1 cells was significantly associated with the concentration of BD, as measured by methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay and cell cycle assay. With BD treatment, the osteogenic differentiation and maturation of MC3T3-E1 cells were increased, as established by the alkaline phosphatase (ALP) activity test, matrix mineralized nodules formation, osteogenic genetic test, and protein expression analyses. Moreover, our data showed that the BMP2/Smad pathway could be the partial mechanism for the promotion of osteogenesis by BD, while BD might suppress the possible function of osteoclasts through the OPG/RANKL/RANK (receptor activator of nuclear factor-κB (NF-κB)) pathway. The hypothesis that BD bears a considerable potential in further research on its dual therapeutic effect on hypertensive patients with poor skeletal conditions was proved within the limitations of the present study.
Keywords: Benidipine, Osteoblast, Osteogenesis, MC3T3-E1
1 Introduction
Hypertension continues to be a severe health problem among aged individuals (Wang et al., 2018), who are also prone to certain bone-related pathological conditions. A high number of partially or totally edentulous old adults present alveolar bone deficiency when undergoing dental implant treatment, and hence need alveolar bone augmentation. Hip fractures and osteoporosis are also common illnesses impacting a considerable proportion of the aged population, which feature bone loss and compromised skeletal health conditions (Chen et al., 2016, 2019; Cui et al., 2019). All of these illnesses require enhanced osteogenesis to improve local or systemic bone formation. Hypertension has been demonstrated to be related to bone mass abnormalities, lower regional bone mineral content and density with some relevant etiological factors involved (Metz et al., 1999; Larijani et al., 2004). Therefore, it has been long suggested that antihypertensive drugs may be useful to promote osteogenesis and modulate bone health at the same time. Several types of antihypertensive medication, including loop diuretics, β-blockers, and angiotensin-converting enzyme inhibitors, have been evaluated on their effects on bone metabolism. Nevertheless, their usefulness remains mostly controversial and inconclusive with some adverse complications and high cost (Lynn et al., 2006; Lim et al., 2009; Yang et al., 2012; Ilić et al., 2013; Ghosh and Majumdar, 2014).
Benidipine (BD) is a type of second generation dihydropyridine calcium channel blocker, which can block L-type, N-type, and T-type calcium channels in different kinds of cells. Because of its long-acting relaxant effect on vascular smooth muscle, low cost, and acceptable complications, it is widely used in the treatment of hypertension and vasospastic angina pectoris (Suzuki et al., 2007; Kosaka et al., 2010; Inayoshi et al., 2011; Xue et al., 2017). On account of its dual effect on calcium ion transportation and hypertension, BD is expected to be a favorable medication for hypertensive patients with the above bone-related conditions. Some previous studies have suggested that BD exerts a certain effect on osteoblast differentiation and function (Nishiya and Sugimoto, 2001; Nishiya et al., 2002; Shimizu et al., 2012; Wang et al., 2014; Ma et al., 2015). BD was found to promote the process of alkaline phosphatase (ALP) expression in human osteoblasts in vitro, which is an essential physiological process of osteogenic differentiation and bone formation (Shimizu et al., 2012). The expression of some proteins vital for osteoblast differentiation and function, including osteocalcin (OCN) and Runt-related transcription factor 2 (RUNX2), was upregulated by BD in mice bone marrow stromal cells (BMSCs) (Ma et al., 2015). The possible mechanism and related pathway connecting BD with bone metabolism, however, have neither been addressed nor elucidated.
In the present study, the MC3T3-E1 cell lineage was chosen as the subject to analyze the mechanism of osteocyte proliferation and differentiation in response to BD. After osteogenic induction, osteogenic representative gene and protein expression was measured with or without BD treatment. Our aim was to investigate the effects of BD of various concentrations on preosteoblast proliferation, differentiation, and mineralization in vitro, and to preliminarily discuss the potential contributing mechanisms. This work provides some insights into the potential further application of BD in promoting osteogenesis and thereby improving skeletal disorders in hypertensive patients.
2 Materials and methods
2.1. BD preparation
A solution of BD was prepared by dissolving solid BD hydrochloride (molecular weight: 542.03 g/mol; Kyowa Hakko Kogyo, Japan) in dimethyl sulfoxide (DMSO) solvent (Sigma-Aldrich, USA). The stock solution was stored at -20 °C. Based on a previous study (Wang et al., 2014), the experimental final BD concentrations were set at 0.01, 0.10, and 1.00 μmol/L due to the anticipated promotion of osteogenesis in vitro and limited cytotoxicity of such levels.
2.2. Cell culture
MC3T3-E1 cells (American Type Culture Collection, USA) were meticulously thawed and cultured in minimum essential medium α (MEM α; Gibco, USA) with 10% fetal bovine serum (FBS; Gibco) containing 100 U/mL streptomycin/penicillin (Sigma-Aldrich) in a humidified incubator at 37 °C with 5% CO2. The cell culture medium was replaced every three days and cells were passaged when cell density reached 80% with 0.25% trypsin (Sigma-Aldrich).
2.3. Cell proliferation assays
The cultured MC3T3-E1 cells were seeded in 96-well plates (5×103 cells/well) and incubated for 12 h. Next, BD solution was added at final concentrations of 0.01, 0.10, and 1.00 μmol/L. Cells without BD treatment were set as negative control, while wells without cells were set as blank. Following 48 h of BD treatment, 20 μL 5.0 mg/mL methylthiazolyldiphenyl-tetrazolium bromide (MTT; Sigma-Aldrich) was added to each well, and wells were incubated for further 4 h at 37 °C. The supernatant was removed and 150 μL DMSO was added. Subsequently, absorbance was measured by optical density (OD) at 490 nm. The cell proliferation rate (PR) was calculated by the following formula: PR=(ODsample–ODblank)/(ODcontrol–ODblank)×100%, where ODsample, ODblank, and ODcontrol are the ODs of sample, blank, and negative control, respectively.
A cell density of 1×109 L-1 was set for a second series of cells subjected to BD treatment for 48 h following the same protocol, 70% ethanol (0 °C; Beijing Chemical Works, China) was added, and cells were incubated at 4 °C for 12 h. Following centrifugation and the removal of supernatant, cells were stained with propidium iodide (PI)/RNase buffer (Sigma-Aldrich) for 15 min and fluorescence was measured using a flow cytometer.
2.4. Alkaline phosphatase activity assay
The following type of conventional osteogenic induction medium was used for the ALP assay: MEM α with 10% FBS containing osteogenic induction supplement (50 μg/mL ascorbic acid, 100 nmol/L dexamethasone, and 10 mmol/L disodium β-glycerophosphate; Sigma-Aldrich). MC3T3-E1 cells were seeded in 24-well plates (2×104 cells/well) and incubated in the osteogenic induction medium for 3 d. Subsequently, a series of BD dilutions (with final concentrations of 0.01, 0.10, and 1.00 μmol/L) were added to the osteogenic induction medium for further incubation, while cells without BD treatment were set as control. Cells at 7, 10, and 14 d after the addition of BD were harvested for assay. The ALP activity and the protein content of cells were determined using an ALP detection kit and a bicinchoninic acid (BCA) protein assay kit (Nanjing Jiancheng Bioengineering Institute, China). All results were normalized to protein content.
2.5. Matrix mineralization assay
Cells from the same MC3T3-E1 line were seeded in 24-well plates (2×104 cells/well) and incubated in the osteogenic induction medium as described above for 3 d. Next, a series of BD dilutions (with final concentrations of 0.01, 0.10, and 1.00 μmol/L) were added to the medium for further incubation for 21 d, while cells without BD treatment were set as control. In order to visualize the matrix mineralization, cells were washed three times with phosphate-buffered saline (PBS; Gibco) and fixed in 95% ethanol for 30 min at room temperature. Fixed cells were washed twice with PBS and stained with 1% alizarin red S (ARS, pH=4.2; Sigma-Aldrich) for 30 min at room temperature. Any excess ARS was washed away with distilled water, and the cells were temporarily incubated in PBS. After inspection and imaging, the mineralized matrix was treated with 10% cetylpyridinium chloride (Sigma-Aldrich) for 10 min at room temperature, and quantitative analysis was performed by reading OD values at 570 nm.
2.6. qRT-PCR
For this assay, MC3T3-E1 cells were seeded in 6-well plates (1×105 cells/well). Following osteogenic incubation for 3 d, cells were treated with BD for 7 d with the same formula and the control group setting as described for previous assays. Total RNA was extracted using TRIzol reagent (Invitrogen Life Technologies, USA). Subsequently, complementary DNA (cDNA) was produced from 1 μg total RNA using a PrimeScript RT Reagent kit (TaKaRa, Japan). The qualitative reverse-transcription polymerase chain reaction (qRT-PCR) process was performed using a SYBR Green Master Mix kit (TaKaRa) in a CFX384 Touch real-time PCR detection system (Bio-Rad, USA). The primers used in qRT-PCR protocol are presented in Table 1.
Table 1.
Primer sequences used in qRT-PCR
| Gene | Forward sequence (5'→3') | Reverse sequence (5'→3') |
|---|---|---|
| Bmp2 | TGGCCCATTTAGAGGAGAACC | AGGCATGATAGCCCGGAGG |
| Runx2 | TTCTCCAACCCACGAATGCAC | CAGGTACGTGTGGTAGTGAGT |
| Ocn | GAACAGACTCCGGCGCTA | AGGGAGGATCAAGTCCCG |
| Opg | AAAGCACCCTGTAGAAAACA | CCGTTTTATCCTCTCTACACTC |
| Rankl | TATGATGGAAGGCTCATGGT | TGTCCTGAACTTTGAAAGCC |
| GAPDH | GACTTCAACAGCAACTCCCAC | TCCACCACCCTGTTGCTGTA |
qRT-PCR: qualitative reverse-transcription polymerase chain reaction; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
2.7. Western blot analysis
According to the same method as above, MC3T3-E1 cells were seeded in six-well plates (1×105 cells/well) for incubation in osteogenic induction medium for 3 d and additional BD treatment for 7 d. Cells were then collected and subjected to total protein extraction. The obtained proteins were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) column and transferred onto a polyvinylidene fluoride (PVDF) membrane (Pfizer, USA). Subsequently, the membrane was blocked in 5% bovine serum albumin (BSA) at room temperature for 2 h, and incubated overnight at 4 °C with the primary antibodies (1:1000 dilutions (volume ratio); Bioss Antibodies, China) including rabbit anti-OPG and rabbit anti-RANKL, while glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Sigma-Aldrich) was set as internal control. Following washing in Tris-buffered saline with 0.05% Tween 20 (TBST solution; Sigma-Aldrich), the blots were incubated at room temperature for 1 h with goat anti-rabbit immunoglobulin G (IgG; BOSTER Biological Technology, China) conjugated with horseradish peroxidase as the secondary antibody (1:3000 dilution (volume ratio)). After visualization with an enhanced luminol-based chemiluminescent kit (Thermo Fisher Scientic, USA), the bands were scanned and imagined using the ChemiDoc™ XRS+ system (Bio-Rad).
Following incubation for 3 d to induce osteogenesis, a series of cells were continuously incubated in osteogenic induction medium containing BD (1.00 μmol/L) and a selective inhibitor of bone morphogenetic protein (BMP) type Ⅰ receptor (LDN-193189, 0.10 μmol/L; Celleck Chemicals, USA) for 7 d (BD+LDN group). Meanwhile, groups of cells with BD treatment alone (BD group), LDN-193189 treatment alone (LDN group), and without either (control) were set for comparisons. Finally, all cells were collected and subjected to western blot (WB) analysis with rabbit anti-P-Samd5 (1:1000 dilution (volume ratio); Bioss Antibodies) as the primary antibody.
2.8. Statistical analysis
All statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was carried out to detect any significant difference among the groups overall. The Dunnett test was used for further comparisons between each subgroup of experimental group and the control group, while the Tukey test was accepted for comparisons among subgroups of the experimental group. The P-values in both multiple comparisons were adjusted to control level I error. The control group and each subgroup of the experimental group included three independent parallel samples and all results were presented as mean±standard deviation (SD), while P<0.05 was considered the threshold for declaring statistical significance.
3 Results
3.1. Effect of BD on cell proliferation
3.1.1. MTT assay
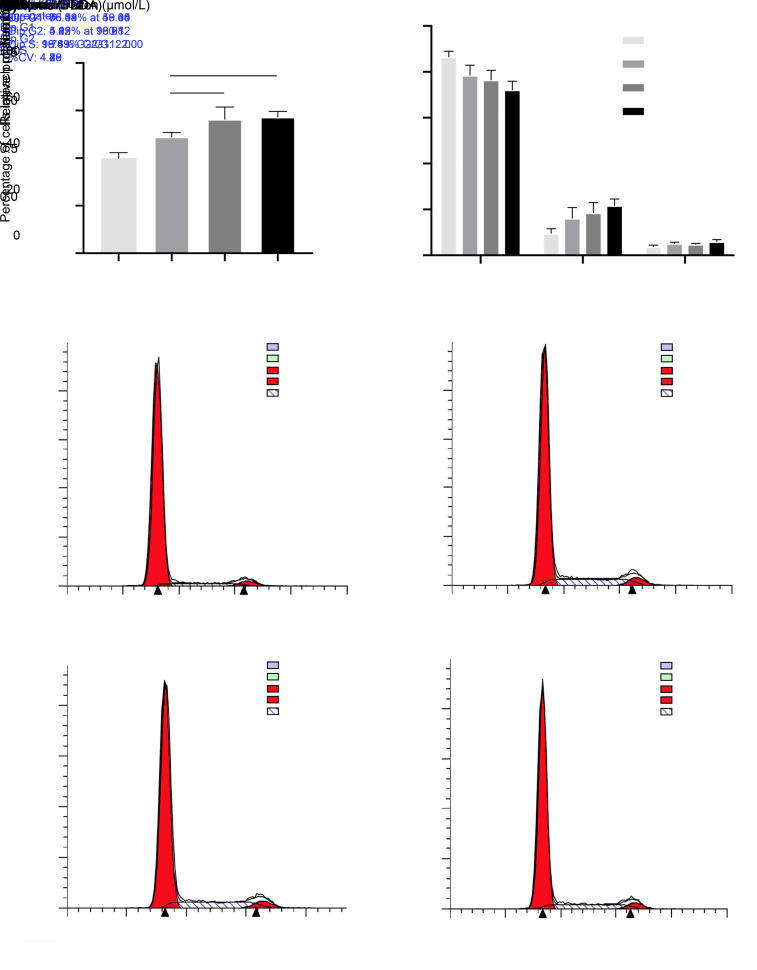
An overall promotion of MC3T3-E1 cell proliferation by BD was observed. Compared with the control group, BD treatment for 48 h at concentrations of 0.01, 0.10, and 1.00 μmol/L promoted the proliferation of cells in a dose-dependent manner, with significant differences (P<0.001 for 0.01 μmol/L group, and P<0.0001 for 0.10 and 1.00 μmol/L groups; Fig. 1a). When compared with the 0.01 μmol/L group, the 0.10 and 1.00 μmol/L groups presented a greater promotion of cell proliferation (P<0.01), with no significant difference observed between the latter two groups.
Fig. 1. Effects of BD on the proliferation rate and cell cycle of MC3T3-E1 cells. The MTT assay (a) and cell cycle assay (b, c) were performed to examine the proliferation of MC3T3-E1 cells after the addition of BD to the basic medium and subsequent incubation for 48 h. (a) BD increased the proliferation rate of cells, especially at 0.10 and 1.00 μmol/L. (b, c) BD decreased the ratio of cells in the G1 and G2/M phases and increased the ratio of cells in the S phase, especially at 1.00 μmol/L. Data are expressed as mean±SD (n=3). * P<0.05, ** P<0.01, *** P<0.001, and **** P<0.0001 represent comparisons with the control group; ## P<0.01 represents comparison between experimental groups. BD: benidipine; MTT: methylthiazolyldiphenyl-tetrazolium bromide.
3.1.2. Cell cycle assay
Figs. 1b and 1c reveal the effect of BD on cell cycle after 48-h treatment. The proportion of cells in the G1 phase in test groups decreased in comparison with the control group, with significant differences observed at concentrations of 0.10 μmol/L (P<0.05) and 1.00 μmol/L (P<0.01). In terms of cells in the S phase, the experimental groups showed higher percentages as the control group, with significant differences at concentrations of 0.10 μmol/L (P<0.05) and 1.00 μmol/L (P<0.01). In addition, the proportion of cells in the G2/M phase in the 1.00 μmol/L treatment group was significantly higher than that in the control group (P<0.01). However, no significant differences among all of above experimental groups were observed. This result indicated that BD promoted the proliferation of MC3T3-E1 cells in vitro.
3.2. Effect of BD on osteogenic differentiation
3.2.1. ALP assay
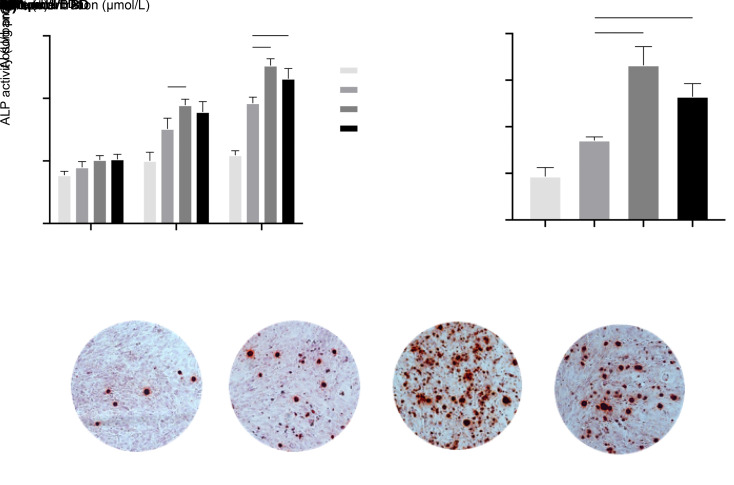
As demonstrated in Fig. 2a, BD generally promoted ALP activity in cells in a time-dependent manner, which was noticeable in the 10- and 14-d clusters. When compared with the control groups, the experimental groups showed a significant improvement of ALP activity in cells upon 10- and 14-d BD treatments, with the most pronounced effect observed in the 0.01 μmol/L group. The concentration of BD made a difference in the results. For the 10-d cluster BD treatments, the ALP activity of cells in the 0.10 μmol/L group was significantly higher than that in the 0.01 μmol/L group (P<0.05). Similarly, this trend was noted in the 14-d cluster.
Fig. 2. Effects of BD on osteogenic differentiation and mineralization of osteogenic-induced MC3T3-E1 cells. The ALP assay (a) and matrix mineralization assay (b, c) were performed to examine ALP activity and mineralization in MC3T3-E1 cells after the addition of BD to the osteogenic medium and subsequent incubation for 14 and 21 d, respectively. (a) BD increased the level of ALP activity in osteogenic-induced MC3T3-E1 cells, especially at 10 and 14 d, with a pronounced effect at 0.10 μmol/L. (b, c) BD promoted the formation of extracellular mineralized nodules, especially at 0.10 μmol/L. Data are expressed as mean±SD (n=3). * P<0.05, ** P<0.01, *** P<0.001, and **** P<0.0001 represent comparisons with the control group; # P<0.05, ## P<0.01, and ### P<0.001 represent comparisons between experimental groups. BD: benidipine; ALP: alkaline phosphatase.
3.2.2. Matrix mineralization assay
Such as the results of the ALP activity assay (Fig. 2b), quantitative analysis indicated that the 21-d BD treatment significantly promoted the extracellular matrix mineralization compared with the control group at concentrations of 0.01 μmol/L (P<0.05), 0.10 μmol/L (P<0.0001), and 1.00 μmol/L (P<0.001). The 0.10 and 1.00 μmol/L groups revealed significantly greater matrix mineralized nodule formation with significant differences compared with the 0.01 μmol/L group (P<0.001 for the 0.10 μmol/L group, and P<0.01 for the 1.00 μmol/L group). Representative images of matrix mineral nodules formed in the osteogenic-induced MC3T3-E1 cells are shown in Fig. 2c, which correspond with the quantitative results. The results of the ALP activity assay and matrix mineralized assay indicated that there was a promoting effect of BD on the osteogenic function of MC3T3-E1 cells.
3.3. Effects of BD on gene and protein expression
3.3.1. Expression of certain crucial bone metabolism-related genes and proteins
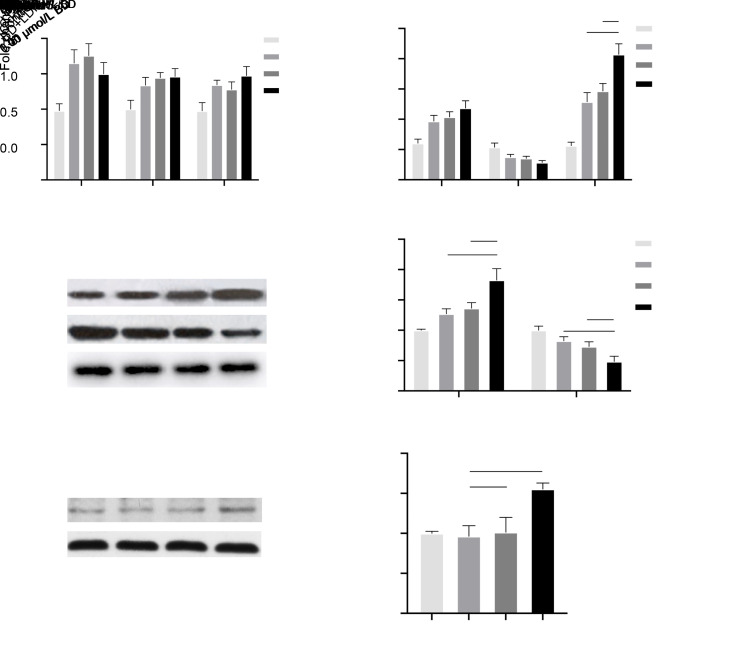
In order to assess the effect of BD on the osteogenic differentiation and maturation of MC3T3-E1 cells, we examined the expression of Bmp2, Ocn, Runx2, Opg, and Rankl genes and their relative proteins. As shown in Fig. 3a, after a 7-d BD treatment, the expression levels of Bmp2, Ocn, and Runx2 were noticeably upregulated. In terms of Bmp2, all test groups had significantly higher expression levels (P<0.01). Meanwhile, those of Ocn in experimental groups were promoted by BD in a dose-dependent manner, with significant differences compared with the control group (P<0.05 for the 0.01 μmol/L group, P<0.01 for the 0.10 μmol/L group, and P<0.01 for the 1.00 μmol/L group). The expression of Runx2 in test groups was also significantly higher at BD concentrations of 0.01 μmol/L (P<0.01), 0.10 μmol/L (P<0.05), and 1.00 μmol/L (P<0.01). Overall, no significant differences were observed among the experimental groups. On the other hand, as demonstrated in Fig. 3b, BD strongly promoted the expression of Opg in all test groups in a dose-dependent manner compared with the control group (P<0.01). Furthermore, the expression of Rankl showed an opposite trend with BD treatment (P<0.01). The ratio of Opg/Rankl was prominently higher in all test groups than in the control group, and the most pronounced effect was observed in the 1.00 μmol/L group when compared with the two other groups (0.01 μmol/L group, P<0.001; 0.10 μmol/L group, P<0.01). Consistently with the results of qRT-PCR, the 7-d BD treatment with the same concentrations raised the expression of OPG proteins, while it suppressed the expression of RANKL protein in a concentration-dependent manner (Figs. 3c and 3d). Moreover, the quantitative WB analysis indicated that BD at 1.00 μmol/L concentration showed the most dominant effect on the expression of OPG and RANKL (Fig. 3d).
Fig. 3. Effects of BD on the expression of some crucial genes and proteins in osteogenic-induced MC3T3-E1 cells. The qRT-PCR (a, b) and WB (c, d) analyses were performed to examine the expression of Bmp2, Ocn, Runx2, Opg, Rankl genes and partial relative proteins in MC3T3-E1 cells after the addition of BD to the osteogenic medium and subsequent 7-d incubation. A further WB assay (e, f) was performed to examine the level of Smad5 phosphorylation (P-Smad5) in MC3T3-E1 cells following BD or LDN-193189 (LDN) addition to the osteogenic medium and subsequent 7-d incubation. (a) BD increased the expression of Bmp2, Ocn, and Runx2. (b) BD increased the expression of Opg and the ratio of Opg/Rankl, while it decreased the expression of Rankl with the most pronounced effect observed at 1.00 μmol/L. (c, d) BD increased the expression of OPG, while it decreased the expression of RANKL, especially at 1.00 μmol/L. (e, f) BD increased the level of Smad5 phosphorylation, while this promoting effect disappeared with the simultaneous use of LDN-193189. Data are expressed as mean±SD (n=3). * P<0.05, ** P<0.01, *** P<0.001, and **** P<0.0001 represent comparisons with the control group; # P<0.05, ## P<0.01, and ### P<0.001 represent comparisons between experimental groups. BD: benidipine; qRT-PCR: qualitative reverse transcription polymerase chain reaction; WB: western blot; LDN-193189: a selective inhibitor of bone morphogenetic protein type Ⅰ receptor; Bmp2: bone morphogenetic protein 2; Ocn: osteocalcin; Runx2: Runt-related transcription factor 2; Opg: osteo-protegerin; Rankl: receptor activator of nuclear factor-κB ligand.
3.3.2. BMP2/Smad pathway
The BMP2/Smad pathway plays an important role in osteogenic differentiation and maturation, where Smad5 acts as an essential intracellular factor. Accordingly, the phosphorylation level of Smad5 in cells was determined by the use of LDN-193189 to address the possible mechanisms accounting for the effect of BD on osteogenic-induced MC3T3-E1 cells. The phosphorylation level of Smad5 was markedly upregulated following 7-d BD treatment compared with the blank control group (Fig. 3e). With the synchronous administration of LDN-193189, however, the phosphorylation level of Smad5 in the BD+LDN group became noticeably lower than that in the BD group, which implied an oppressive effect of LDN-193189 on the promotion of osteogenic processes by BD. The quantitative WB analysis showed the effect even more noticeably (Fig. 3f). This result might indicate that, to some extent, BD affects the osteogenic differentiation and maturation through the BMP2/Smad pathway.
4 Discussion
High blood pressure and osteoporosis are prevalent health problems of the elderly. Some hypertensive patients also present other bone-related illnesses, including encounter alveolar bone deficiency and fractures, which need interventions to improve osteogenesis and bone metabolism. Findings of previous studies are encouraging in that BD has been suggested as an antihypertensive drug that could positively affect the bone modeling and remodeling process (Nishiya and Sugimoto, 2001; Nishiya et al., 2002; Shimizu et al., 2012; Wang et al., 2014; Ma et al., 2015). Therefore, an in vitro study was conducted herein with the aim to investigate the effect of BD on bone metabolism and its potential application in the treatment of hypertensive patients with compromised skeletal health conditions.
The MC3T3-E1 cell lineage is a type of preosteoblast derived from newborn mouse calvaria with high ALP activity and a potential to differentiate into osteoblasts and mineralize in vitro (Sudo et al., 1983). Acting as a stable cell line source in osteogenesis-related research, MC3T3-E1 cells have been widely used in relative in vitro studies (Ma et al., 2018; Liu et al., 2019). In our previous report (Wang et al., 2014), BD at concentrations of 10 and 100 μmol/L was shown to exhibit distinct cytotoxicity to MC3T3-E1 cells in vitro, while BD at low concentrations (<0.001 μmol/L) result in the impaired promotion of cell proliferation and osteogenic differentiation. Thus, aiming to demonstrate the prospective osteogenic-inducing effect, the final concentrations of BD were set at 0.01, 0.10, and 1.00 μmol/L in this study. On the other hand, in clinical use, the concentration of BD in plasma was shown to reach its maximum of around 3 ng/mL (5.5 μmol/L) in 2 h and dropped to near 0.3 ng/mL (0.55 μmol/L) at 7 h following the single oral administration of 8 mg BD to essential hypertensive patients. Interestingly, the anti-hypertensive effect was sustained for almost 24 h, even if the plasma concentration fell back to almost zero (Yao et al., 2006). Considering that the recommended therapeutic doses for mild and moderate hypertension are generally 2 and 4 mg, respectively, it was reasonable to set the in vivo extracellular BD concentrations at 0.01, 0.10, and 1.00 μmol/L compared to plasma concentrations.
The MTT assay and cell cycle assay demonstrated that BD significantly promotes the proliferation of MC3T3-E1 cells in a dose-dependent manner, thus providing a foundation for further osteogenic differentiation (Gao and Liu, 2019). Following osteogenic induction by BD, higher ALP activity and matrix mineralization in osteoblasts were observed in cells treated with BD, especially at high concentrations (0.10 and 1.00 μmol/L). BD has been shown to promote ALP activity and transcription, as well as matrix mineralization in MC3T3-E1 cells (Nishiya and Sugimoto, 2001; Nishiya et al., 2002). Through blocking L-type calcium channels and subsequent Ca2+ influx into cells, BD was suggested to promote ALP activity upon ascorbic acid triggering extracellular collagen accumulation, which comprises the first vital events in osteogenic differentiation (Nishiya and Sugimoto, 2001; Nishiya et al., 2002). The effective threshold concentration of BD in vitro, however, was much lower in these studies than in the present work, which might be due to the different stages of cells influenced to undergo osteogenic differentiation. Shimizu et al. (2012) have also reported relative findings that BD promoted ALP activity in the tibia of ovariectomized spontaneous hypertensive rats, which also supported the promoting effect of BD.
The Bmp2, Runx2, and Ocn genes, as essential regulators of osteoblast-lineage cell differentiation, skeletal development and homeostasis, play vital roles in regulating the osteogenic differentiation and proper function of osteoblasts (Ducy et al., 1999; Mundy et al., 2001; Noël et al., 2004; Huang et al., 2010; Higuchi et al., 2012; Neve et al., 2013; Wu et al., 2016; Ma et al., 2019; Yu et al., 2020). In the present study, qRT-PCR results showed the upregulated expression of Bmp2, Runx2, and Ocn in osteogenic-induced preosteoblasts treated with BD, which was consistent with higher expression of BMP2, RUNX2, and OCN by WB analysis, as reported in our previous study (Wang et al., 2014). After BD treatment in vitro, mice BMSCs presented higher expression of RUNX2 and OCN on the protein level via immunofluorescence analysis and immunostaining analysis (Ma et al., 2015). Moreover, the same effect was demonstrated in their study, which was also observed in an ovariectomized mouse model, indicating the possible application of BD in osteoporosis patients. Therefore, a general promoting effect of BD on the osteogenic differentiation and maturation preosteoblasts was proposed in the present work, with 0.10 and 1.00 μmol/L considered as preferable concentrations.
The WNT/β-catenin signaling pathway has been reported as relevant in the promotion of mouse BMSC osteogenesis by BD, with increased expression of β-catenin and low-density lipoprotein receptor-related protein 5 (LRP5) observed (Ma et al., 2015). Since the higher expression of these two factors had been reported in a number of studies, we focused on a different essential pathway, the BMP2/Smad signaling, which had been proved to regulate osteoblast differentiation and osteogenesis (Cao and Chen, 2005). In addition to 1.00 μmol/L BD, we added LDN-193189, a selective inhibitor of BMP type Ⅰ receptor kinases, into the osteogenic medium. In the subsequent WB analysis, an upregulation of BMP2 expression level was observed with a distinctly higher phosphorylation level of Smad5 after BD treatment, but this effect was suppressed and blocked by LDN-193189. In osteogenic differentiation and bone formation, Smad5 is a crucial regulatory factor in the BMP/Smad pathway (Lee et al., 2002). Once BMPs have bound to the receptors, phospho-Smad5 participates in the formation of intracellular Smad complexes to induce and modulate targeted gene expression, including Runx2 and Osterix (Afzal et al., 2005). Hence, our results might indicate that the BMP2/Smad pathway partially contributes to the promotion of osteogenesis by BD.
In addition to the direct effect of BD in osteogenic-induced preosteoblasts, we also evaluated the changes in Opg and Rankl expression and the proteins thereof after BD treatment to investigate whether BD had any other related effects. The OPG and RANKL proteins, as crucial regulating factors in bone remodeling, play important roles in the reaction between osteoblasts and osteoclasts through the OPG/RANKL/RANK (receptor activator of nuclear factor-κB (NF-κB)) pathway (Sims and Gooi, 2008; Teti, 2011; Chen et al., 2018). The binding of RANKL to RANK, which is highly expressed on the membrane of many cells including osteoclast progenitors and mature osteoclasts, activates the downstream signaling pathways related to the growth and differentiation of osteoclasts (Boyle et al., 2003; Sims and Gooi, 2008). It has been demonstrated that OPG can act as a decay receptor by binding to RANKL and preventing it from binding to RANK (Boyle et al., 2003; Sims and Gooi, 2008). The ratio of RANKL/OPG in human osteoblasts markedly decreased after BD treatment in vitro (Shimizu et al., 2012). In concert with previous findings, the qRT-PCR results of our study showed a remarkable upregulation of Opg expression and a downregulation of Rankl, corresponding with changes in the expression of OPG and RANKL by WB analysis in a dose-dependent manner. While the highest, statistically significant Opg/Rankl ratio was detected in the 1.00 μmol/L group when compared with the control and other groups, a relatively higher BD concentration might be preferable in this regard, which requires further investigation. Moreover, BD was found to show no direct effect on osteoclast differentiation, while a reduced number of differentiated osteoclasts were identified in a co-culture environment of both osteoblast and osteoclast lineage cells from human bone marrow in vitro (Shimizu et al., 2012). Therefore, it was indicated that BD might affect the bone metabolism not only directly through promoting the differentiation of preosteoblasts to boost bone formation, but also indirectly via suppressing the activity of osteoclasts to alleviate bone resorption.
5 Conclusions
In the present study, it was demonstrated that BD promotes the proliferation, osteogenic differentiation and maturation of mice preosteoblasts while suppressing the potential function of osteoclast in vitro, with BMP/Smad and OPG/RANKL/RANK being the possible pathways. Therefore, BD might be a suitable candidate for further research aiming to develop treatments for hypertensive patients that require promoted osteogenesis, such as for alveolar bone augmentation, fractures, and osteoporosis. Considering the instability and limited duration of action of pure BD in local applications, a well-designed drug delivery system is required to achieve the best potential of BD in promoting osteogenesis. Further studies are also needed to determine the dual effect of BD in a certain dose in vivo, and to explore further molecular mechanisms of how BD affects the bone metabolism.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 81600909 and 81800934) and the Zhejiang Provincial Medical Science and Technology Project of China (Nos. 2017RC009, 2018RC012, and 2021KY773).
Author contributions
Baixiang WANG, Jiakang YANG, and Yu WANG performed the experimental research and data analysis, wrote and edited the manuscript. Chenqiu ZHANG created the figures. Lijie FAN and Huiming WANG contributed to the study design, data analysis, and discussion. All authors have read and approved the final manuscript and, therefore, have full access to all data relevant to the study and take responsibility for the integrity and security of such data.
Compliance with ethics guidelines
Baixiang WANG, Jiakang YANG, Lijie FAN, Yu WANG, Chenqiu ZHANG, and Huming WANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Afzal F, Pratap J, Ito K, et al. , 2005. Smad function and intranuclear targeting share a Runx2 motif required for osteogenic lineage induction and BMP2 responsive transcription. J Cell Physiol, 204(1): 63-72. 10.1002/jcp.20258 [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL, 2003. Osteoclast differentiation and activation. Nature, 423(6937): 337-342. 10.1038/nature01658 [DOI] [PubMed] [Google Scholar]
- Cao X, Chen D, 2005. The BMP signaling and in vivo bone formation. Gene, 357(1): 1-8. 10.1016/j.gene.2005.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MM, Zhang YH, Du YP, et al. , 2019. Epidemiological and clinical study of hip fracture in hospitalized elderly patients in Shanghai, China. Arch Osteoporos, 14: 37. 10.1007/s11657-019-0580-7 [DOI] [PubMed] [Google Scholar]
- Chen P, Li ZZ, Hu YH, 2016. Prevalence of osteoporosis in China: a meta-analysis and systematic review. BMC Public Health, 16: 1039. 10.1186/s12889-016-3712-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang ZQ, Duan N, et al. , 2018. Osteoblast‒osteoclast interactions. Connect Tissue Res, 59(2): 99-107. 10.1080/03008207.2017.1290085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui ZY, Meng XY, Feng H, et al. , 2019. Estimation and projection about the standardized prevalence of osteoporosis in mainland China. Arch Osteoporos, 15: 2. 10.1007/s11657-019-0670-6 [DOI] [PubMed] [Google Scholar]
- Ducy P, Starbuck M, Priemel M, et al. , 1999. A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev, 13(8): 1025-1036. 10.1101/gad.13.8.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SW, Liu F, 2019. Novel insights into cell cycle regulation of cell fate determination. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 20(6): 467-475. 10.1631/jzus.B1900197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Majumdar SR, 2014. Antihypertensive medications, bone mineral density, and fractures: a review of old cardiac drugs that provides new insights into osteoporosis. Endocrine, 46(3): 397-405. 10.1007/s12020-014-0167-4 [DOI] [PubMed] [Google Scholar]
- Higuchi A, Ling QD, Hsu ST, et al. , 2012. Biomimetic cell culture proteins as extracellular matrices for stem cell differentiation. Chem Rev, 112(8): 4507-4540. 10.1021/cr3000169 [DOI] [PubMed] [Google Scholar]
- Huang ZN, Ren PG, Ma T, et al. , 2010. Modulating osteogenesis of mesenchymal stem cells by modifying growth factor availability. Cytokine, 51(3): 305-310. 10.1016/j.cyto.2010.06.002 [DOI] [PubMed] [Google Scholar]
- Ilić K, Obradović N, Vujasinović-Stupar N, 2013. The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif Tissue Int, 92(3): 217-227. 10.1007/s00223-012-9671-9 [DOI] [PubMed] [Google Scholar]
- Inayoshi A, Sugimoto Y, Funahashi J, et al. , 2011. Mechanism underlying the block of human Cav3.2 T-type Ca2+ channels by benidipine, a dihydropyridine Ca2+ channel blocker. Life Sci, 88(19-20): 898-907. 10.1016/j.lfs.2011.03.019 [DOI] [PubMed] [Google Scholar]
- Kosaka H, Hirayama K, Yoda N, et al. , 2010. The L-, N-, and T-type triple calcium channel blocker benidipine acts as an antagonist of mineralocorticoid receptor, a member of nuclear receptor family. Eur J Pharmacol, 635(1-3): 49-55. 10.1016/j.ejphar.2010.03.018 [DOI] [PubMed] [Google Scholar]
- Larijani B, Bekheirnia MR, Soltani A, et al. , 2004. Bone mineral density is related to blood pressure in men. Am J Hum Biol, 16(2): 168-171. 10.1002/ajhb.20005 [DOI] [PubMed] [Google Scholar]
- Lee KS, Hong SH, Bae SC, 2002. Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-β and bone morphogenetic protein. Oncogene, 21(47): 7156-7163. 10.1038/sj.onc.1205937 [DOI] [PubMed] [Google Scholar]
- Lim LS, Fink HA, Blackwell T, et al. , 2009. Loop diuretic use and rates of hip bone loss and risk of falls and fractures in older women. J Am Geriatr Soc, 57(5): 855-862. 10.1111/j.1532-5415.2009.02195.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wang D, Qin Y, et al. , 2019. Astragalin promotes osteoblastic differentiation in MC3T3-E1 cells and bone formation in vivo . Front Endocrinol, 10: 228. 10.3389/fendo.2019.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn H, Kwok T, Wong SYS, et al. , 2006. Angiotensin converting enzyme inhibitor use is associated with higher bone mineral density in elderly Chinese. Bone, 38(4): 584-588. 10.1016/j.bone.2005.09.011 [DOI] [PubMed] [Google Scholar]
- Ma J, Wang Z, Zhao JQ, et al. , 2018. Resveratrol attenuates lipopolysaccharides (LPS)-induced inhibition of osteoblast differentiation in MC3T3-E1 cells. Med Sci Monit, 24: 2045-2052. 10.12659/msm.905703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JY, You D, Li WY, et al. , 2019. Bone morphogenetic proteins and inner ear development. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 20(2): 131-145. 10.1631/jzus.B1800084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ZP, Liao JC, Zhao C, et al. , 2015. Effects of the 1, 4-dihydropyridine L-type calcium channel blocker benidipine on bone marrow stromal cells. Cell Tissue Res, 361(2): 467-476. 10.1007/s00441-015-2115-x [DOI] [PubMed] [Google Scholar]
- Metz JA, Morris CD, Roberts LA, et al. , 1999. Blood pressure and calcium intake are related to bone density in adult males. Br J Nutr, 81(5): 383-388. 10.1017/S0007114599000665 [DOI] [PubMed] [Google Scholar]
- Mundy GR, Chen D, Zhao M, et al. , 2001. Growth regulatory factors and bone. Rev Endocr Metab Disord, 2(1): 105-115. 10.1023/a:1010015309973 [DOI] [PubMed] [Google Scholar]
- Neve A, Corrado A, Cantatore FP, 2013. Osteocalcin: skeletal and extra-skeletal effects. J Cell Physiol, 228(6): 1149-1153. 10.1002/jcp.24278 [DOI] [PubMed] [Google Scholar]
- Nishiya Y, Sugimoto S, 2001. Effects of various antihypertensive drugs on the function of osteoblast. Biol Pharm Bull, 24(6): 628-633. 10.1248/bpb.24.628 [DOI] [PubMed] [Google Scholar]
- Nishiya Y, Kosaka N, Uchii M, et al. , 2002. A potent 1, 4-dihydropyridine L-type calcium channel blocker, benidipine, promotes osteoblast differentiation. Calcif Tissue Int, 70(1): 30-39. 10.1007/s00223-001-1010-5 [DOI] [PubMed] [Google Scholar]
- Noël D, Gazit D, Bouquet C, et al. , 2004. Short-term BMP-2 expression is sufficient for in vivo osteochondral differentiation of mesenchymal stem cells. Stem Cells, 22(1): 74-85. 10.1634/stemcells.22-1-74 [DOI] [PubMed] [Google Scholar]
- Shimizu H, Nakagami H, Yasumasa N, et al. , 2012. Links between hypertension and osteoporosis: benidipine ameliorates osteoporosis in ovariectomized hypertensive rats through promotion of osteoblast proliferation and inhibition of osteoclast differentiation. Curr Cardiovasc Risk Rep, 6(4): 274-280. 10.1007/s12170-012-0248-y [DOI] [Google Scholar]
- Sims NA, Gooi JH, 2008. Bone remodeling: multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol, 19(5): 444-451. 10.1016/j.semcdb.2008.07.016 [DOI] [PubMed] [Google Scholar]
- Sudo H, Kodama HA, Amagai Y, et al. , 1983. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol, 96(1): 191-198. 10.1083/jcb.96.1.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Yokoyama K, Akimoto Y, et al. , 2007. Clinical efficacy of benidipine for vasospastic angina pectoris. Arzneimittelforschung, 57(1): 20-25. 10.1055/s-0031-1296581 [DOI] [PubMed] [Google Scholar]
- Teti A, 2011. Bone development: overview of bone cells and signaling. Curr Osteoporos Rep, 9(4): 264-273. 10.1007/s11914-011-0078-8 [DOI] [PubMed] [Google Scholar]
- Wang BX, Bi M, Zhu Z, et al. , 2014. Effects of the antihypertensive drug benidipine on osteoblast function in vitro. Exp Ther Med, 7(3): 649-653. 10.3892/etm.2014.1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZW, Chen Z, Zhang LF, et al. , 2018. Status of hypertension in China: results from the China hypertension survey, 2012‒2015. Circulation, 137(22): 2344-2356. 10.1161/CIRCULATIONAHA.117.032380 [DOI] [PubMed] [Google Scholar]
- Wu MR, Chen GQ, Li YP, 2016. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res, 4: 16009. 10.1038/boneres.2016.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue C, Zhou CC, Yang B, et al. , 2017. Comparison of efficacy and safety between benidipine and hydrochlorothiazide in fosinopril-treated hypertensive patients with chronic kidney disease: protocol for a randomised controlled trial. BMJ Open, 7(2): e013672. 10.1136/bmjopen-2016-013672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SM, Nguyen ND, Eisman JA, et al. , 2012. Association between beta-blockers and fracture risk: a Bayesian meta-analysis. Bone, 51(5): 969-974. 10.1016/j.bone.2012.07.013 [DOI] [PubMed] [Google Scholar]
- Yao K, Nagashima K, Miki H, 2006. Pharmacological, pharmacokinetic, and clinical properties of benidipine hydrochloride, a novel, long-acting calcium channel blocker. J Pharmacol Sci, 100(4): 243-261. 10.1254/jphs.dtj05001x [DOI] [PubMed] [Google Scholar]
- Yu D, Wang J, Qian KJ, et al. , 2020. Effects of nanofibers on mesenchymal stem cells: environmental factors affecting cell adhesion and osteogenic differentiation and their mechanisms. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 21(11): 871-884. 10.1631/jzus.B2000355 [DOI] [PMC free article] [PubMed] [Google Scholar]