Abstract
Background:
The impact of prenatal opioid exposure on brain development remains poorly understood.
Methods:
We conducted a prospective study of term-born infants with and without prenatal opioid exposure. Structural brain MRI was performed between 40–48 weeks postmenstrual age. T2-weighted images were processed using the Developing Human Connectome Project structural pipeline. We compared 63 relative regional brain volumes between groups.
Results:
Twenty-nine infants with prenatal opioid exposure and 42 unexposed controls were included. The groups had similar demographics, except exposed infants had lower birth weights, more maternal smoking and maternal Hepatitis C, fewer mothers with a college degree, and were more likely non-Hispanic White. After controlling for sex, postmenstrual age at scan, birth weight, and maternal education, exposed infants had significantly smaller relative volumes of the deep gray matter, bilateral thalamic ventrolateral nuclei, bilateral insular white matter, bilateral subthalamic nuclei, brainstem, and cerebrospinal fluid. Exposed infants had larger relative volumes of the right cingulate gyrus white matter and left occipital lobe white matter.
Conclusion:
Infants with prenatal opioid exposure had smaller brain volumes in multiple regions compared to controls, with two regions larger in the opioid-exposed group. Further research should focus on the relative contributions of maternal opioids and other exposures.
Introduction
The United States is in the midst of an opioid epidemic, with one infant born exposed to opioids every 15 minutes(1). Opioids cross the placenta and the blood brain barrier(2, 3), but the effects of prenatal opioid exposure on the brain remain poorly characterized. Prenatal opioid exposure has been associated with adverse behavioral and developmental outcomes(4, 5), but it is unclear whether the association is due to direct effects of opioids on the brain and/or to other confounding factors such as polysubstance use, maternal stress, or parenting.
Animal studies have consistently shown negative effects of prenatal opioids on the brain. In experimental models, prenatal exposure to buprenorphine or methadone affects neurotransmitter biosynthesis(6), neurogenesis(7), and myelination(8, 9). In vitro studies in human cells have shown that morphine increases apoptosis in human fetal microglia and neurons(10), suggesting that these animal studies may be applicable to humans. In adult humans, adverse changes in regional brain volumes are seen after even short-term opioid exposure(11, 12). Volumetric studies in older children and adolescents have also shown smaller brain volumes in multiple regions in opioid(13) and polysubstance(14) exposed children. Studies in older children may be confounded by the multiple experiences opioid-exposed children have during infancy and childhood, including adverse home environments and other stressors. Only one previous study has evaluated brain volumes in infants(15). This pilot study found significantly smaller whole brain and basal ganglia volumes and larger lateral ventricular volumes in opioid-exposed infants compared to population means, but no control group was used.
Given the paucity of literature on this topic, additional studies evaluating the effects of prenatal opioid exposure on early brain development are warranted. The objective of our current study was to compare regional brain volumes in term-born infants <8 weeks of age with prenatal opioid exposure and age-matched unexposed controls.
Methods
Subjects
Infants born at >37 weeks gestation with prenatal opioid exposure and no other medical problems were recruited from Cincinnati-area birth hospitals or from the Opioid Exposed Clinic or Neonatal Abstinence Syndrome Clinic at Cincinnati Children’s Hospital. Infants were screened for inclusion/exclusion criteria by trained research coordinators and eligible infants were approached for participation. Healthy control infants born at >37 weeks gestation were recruited from the same birth hospitals, from the Pediatric Primary Care clinic at Cincinnati Children’s using a study flyer in their newborn care packet, or through our Office of Clinical and Translational Research using flyers posted around the hospital and on the website. Infants were recruited between April 2018 and October 2019. Infants with known chromosomal or congenital anomalies, Apgar score at 5 minutes of <7, any requirement for positive pressure ventilation after birth, and any head trauma were excluded from both groups. Opioid exposure was determined by maternal urine toxicology screen at the time of delivery and confirmed with neonatal toxicology screen (meconium or umbilical cord). Lack of opioid or other drug exposure in controls was confirmed by maternal urine toxicology screen at the time of delivery, which is standard clinical practice in our region. Additional information about drug exposure was collected by review of infant medical records and by maternal questionnaire at the time of MRI. Neonatal abstinence syndrome (NAS) was defined as clinically prescribed treatment with opioid replacement medication after birth. Pregnancy and birth history were collected by review of infant medical records. Information about maternal socioeconomic status (education, employment, income) and race was collected by maternal questionnaire at the time of MRI. This study was approved by the Institutional Review Boards at Cincinnati Children’s Hospital, University of Cincinnati Medical Center, Good Samaritan Hospital, and St. Elizabeth Hospital. Written informed consent was obtained from a parent or guardian prior to any study procedures.
Imaging methods
Infants were scanned between 40 and 48 weeks postmenstrual age during sleep with no sedation. Infants were fed, swaddled, fitted with ear protection, placed in the Med-Vac vacuum bag, and moved into the scanner bore. All infants were scanned on the same Philips 3T Ingenia scanner in the Imaging Research Center at Cincinnati Children’s Hospital using a 32 channel receive head coil. Structural MR imaging included a sagittal magnetization prepared inversion recovery 3D T1-weighted gradient echo sequence (shot interval = 2300 milliseconds, repetition time = 7.6 milliseconds, echo time = 3.6 milliseconds, inversion time = 1100 milliseconds, flip angle = 11°, voxel size 1mm × 1mm × 1mm, acceleration (SENSE) = 1 in plane and 2.0 through plane (slice) phase encode, scan time 3 minutes 6 seconds) and an axial 2D T2-weighted fast spin echo sequence (repetition time = 19100–19500 milliseconds, echo time = 166 milliseconds, voxel size 1mm × 1.11mm × 1mm, acceleration (SENSE) = 1.5/in plane phase encode, scan time 3 minutes 50 seconds).
MRI processing
T2-weighted images were processed using the developing Human Connectome Project (dHCP) pipeline(16, 17). The dHCP pipeline automatically performs cortical and sub-cortical volume segmentation, resulting in 87 segmentations and 5 combined regions. All tissue segmentations were visually inspected for accuracy. A representative segmentation is shown in Figure 1. The “deep gray matter” segmentation includes the thalamus, subthalamic nucleus, caudate, lentiform, and intracranial background (Antonios Makropoulos, personal communication, March 2020).
Figure 1.
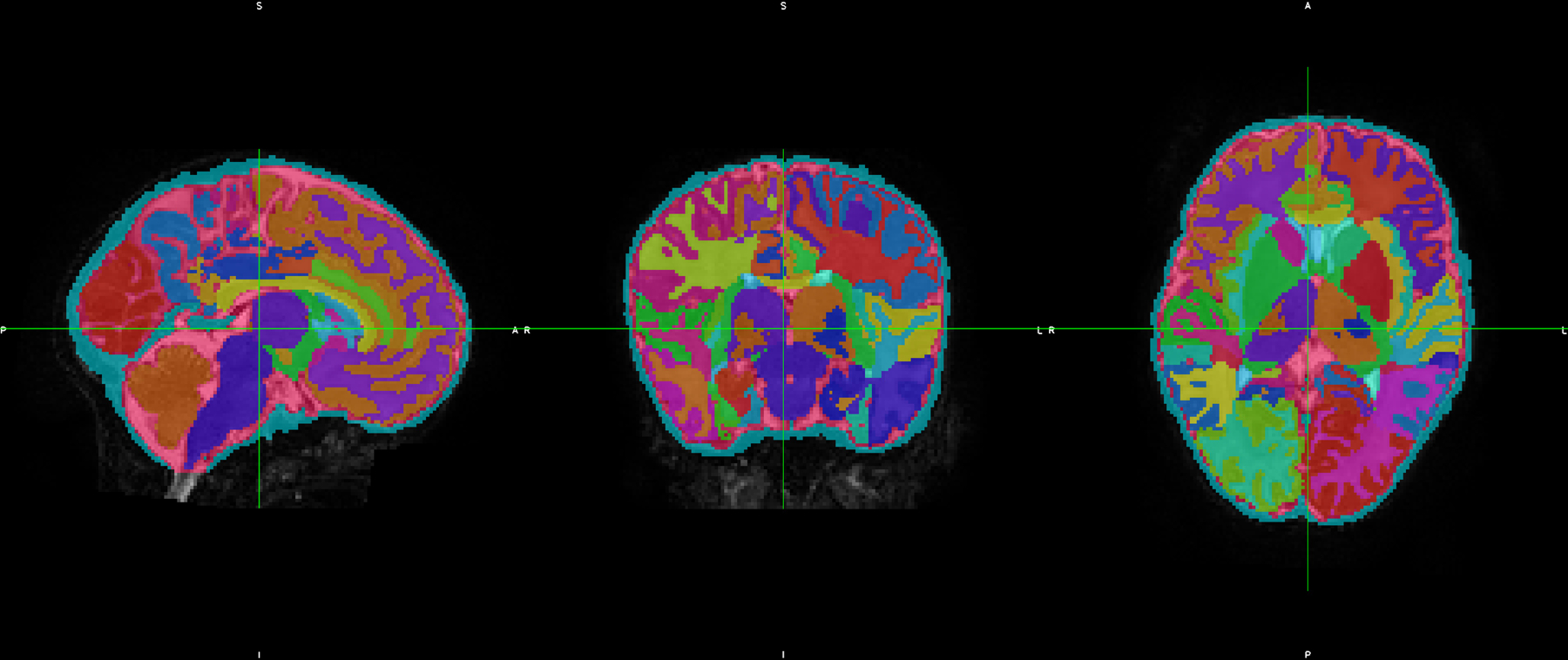
Representative segmentation of a neonatal brain showing different brain regions automatically segmented using the dHCP pipeline
Statistical analysis
All statistical analysis was performed in STATA 16.0 (Stata Corp., College Station, TX). For the purposes of this analyses, we excluded the subsegmentations (e.g., the anterior/posterior parts of structures) and instead included only the “merged regions”. We also excluded the “gyri parahippocampalis et ambiens”, which is a very small brain region that in our prior work has likely shown spurious associations(18). We thus analyzed volume differences in 58 regions of the brain and 5 combined regions (supplementary Table 1). Relative regional volumes were used for all analyses (i.e., the volume of the region divided by the total volume of brain tissue).
Descriptive statistics for the two groups (opioid-exposed and controls) were computed. Groups were compared using two-sided t-tests for continuous variables and Fisher’s exact test for categorical variables. We compared regional brain volumes between the two groups by performing a multivariable linear regression which allowed us to control for the potential confounding effects of infant sex, postmenstrual age (PMA) at scan, birth weight, and maternal education for each of the 63 regions. Since birth weight and maternal education were found to be significantly different between the opioid and unexposed groups, these were also included in the regression model as covariates. We performed a sensitivity analysis removing birth weight from the model, and results were unchanged. Sex and PMA at scan were not significantly different between groups; however, since even small group differences in these variables can affect brain development and volumes, we opted to control for them in the model. We did not include maternal Hepatitis C and maternal smoking in the model due to no Hepatitis C and very little smoking in the control group. We also performed a sensitivity analysis including only infants who were exposed only to opioids and not other substances. Correction for multiple comparisons was not performed given the preliminary nature of this study(19). All significance is reported at p<0.05.
Results
Twenty-nine infants with prenatal opioid exposure and 42 controls with no opioid or other drug exposure were recruited for the study. All scans were successfully processed with the dHCP pipeline and included in the analysis. As shown in Table 1, infants with prenatal opioid exposure and unexposed controls had comparable baseline characteristics, except opioid-exposed infants had smaller birth weights, increased rates of maternal smoking and maternal Hepatitis C, fewer mothers with a college degree, and were more likely non-Hispanic White. None of the infants in either group had known alcohol exposure during pregnancy, although one mother in each group endorsed drinking alcohol in the first trimester before the pregnancy was known. In the opioid-exposed group, 4/29 infants had additional exposures: 2 infants to THC only, one infant to THC and cocaine, and one infant to cocaine and methamphetamines.
Table 1.
Demographics of study population
| Opioid-exposed (n=29) | Controls (n=42) | p value | |
|---|---|---|---|
| Male, n (%) | 10 (34%) | 20 (48%) | 0.27 |
| Gestational age at birth (weeks), mean (SD) | 38.9 (1.1) | 39.0 (0.72) | 0.55 |
| Birth weight (g), mean (SD) | 3048 (299) | 3274 (432) | 0.02 |
| Head circumference at birth (cm), mean (SD) | 34.1 (1.3) | 34.0 (1.4) | 0.89 |
| Postmenstrual age at scan (weeks), mean (SD) | 44.7 (1.2) | 44.0 (2.0) | 0.07 |
| Race/ethnicity | 0.004 | ||
| Non-Hispanic White | 24 | 20 | |
| Non-Hispanic Black | 4 | 20 | |
| Non-Hispanic Asian | 0 | 1 | |
| Hispanic White | 1 | 1 | |
| Maternal smoking, n (%) | 25 (86%) | 3 (7%) | <0.001 |
| Reported maternal alcohol use during pregnancy | 1 (3%) | 1 (2%) | 1.0 |
| Maternal Hepatitis C, n (%) | 19 (66%) | 0 (0%) | <0.001 |
| Maternal college degree, n (%) | 5 (17%) | 24 (57%) | 0.001 |
| Maternal methadone, n (%) | 12 (41%) | n/a | n/a |
| Maternal buprenorphine, n (%) | 15 (52%) | n/a | n/a |
| Maternal heroin and/or fentanyl, n (%) | 12 (41%) | n/a | n/a |
| Neonatal abstinence syndrome requiring opioid treatment, n (%) | 13 (45%) | n/a | n/a |
Two-sided t-test was used to compare continuous variables and Fisher’s exact test was used to compare categorical variables.
Infants with prenatal opioid exposure had decreased relative volumes of the deep gray matter, bilateral ventrolateral nuclei of thalamus, bilateral subthalamic nuclei, bilateral insular white matter, brainstem, and cerebrospinal fluid (CSF), after adjustment for sex, PMA at MRI, birth weight, and maternal education (Table 2, Figure 2). Infants with prenatal opioid exposure had increased relative brain volumes of the white matter of the right cingulate gyrus and white matter of the left occipital lobe. Sensitivity analysis removing the 4 infants with additional exposures besides opioids resulted in 4 regions (overall deep gray, L insular white matter, and bilateral subthalamic nuclei) no longer being significantly different, with p values of 0.09, 0.09, 0.09, and 0.06, respectively. Although all analysis were conducted using relative volumes, for ease of interpretation we presented volumes in mm3. This was accomplished by multiplying the least squares means and corresponding values obtained from the relative volume analysis by the average brain volume of the groups.
Table 2.
Least squares means and standard error of brain volumes and percent differences in exposed and control groups
| Exposed (n=29) | Control (n=42) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LS Mean volume (mm3) | SE | LS Mean volume (mm3) | SE | LS Mean difference | 95% confidence interval (lower bound) | 95% confidence interval (upper bound) | P value | % difference | |
| Deep gray matter | 30657.8 | 307.3 | 31507.5 | 229.3 | −849.6 | −1644.1 | −55.0 | 0.037 | −2.70 |
| R thalamus ventrolateral nucleus | 752.1 | 28.6 | 864.2 | 21.4 | −112.1 | −186.0 | −38.0 | 0.004 | −13.0 |
| L thalamus ventrolateral nucleus | 719.1 | 31.6 | 824.1 | 23.6 | −105.1 | −186.8 | −23.5 | 0.012 | −12.8 |
| R insula white matter | 2659.1 | 44.0 | 2871.6 | 32.8 | −212.5 | −326.1 | −99.0 | 0.000 | −7.4 |
| L insula white matter | 2582.6 | 47.1 | 2745.1 | 35.2 | −162.5 | −284.3 | −40.7 | 0.010 | −5.9 |
| R subthalamic nucleus | 270.6 | 6.3 | 287.6 | 4.7 | −17.0 | −33.2 | −0.8 | 0.040 | −5.9 |
| L subthalamic nucleus | 239.2 | 6.3 | 256.9 | 4.7 | −17.8 | −34.0 | −1.6 | 0.032 | −6.9 |
| Brainstem | 6870.3 | 117.3 | 7258.8 | 87.6 | −388.5 | −691.7 | −85.1 | 0.013 | −5.4 |
| Cerebrospinal fluid | 95493.6 | 3397.3 | 106689.9 | 2535.5 | −11196.3 | −19980.1 | −2412.6 | 0.013 | −10.5 |
| R cingulate gyrus white matter | 3271.1 | 60.7 | 3086.7 | 45.3 | 184.4 | 27.5 | 341.5 | 0.022 | 6.0 |
| L occipital lobe white matter | 7623.2 | 160.9 | 7197.0 | 120.1 | 426.2 | 10.2 | 842.3 | 0.045 | 5.9 |
Percent differences were calculated using the relative volumes adjusted for covariates. For ease of interpretation, we have reported the least square means and standard errors, derived by multiplying the least square means from the original regression analysis by the average brain volume of all subjects.
Figure 2.
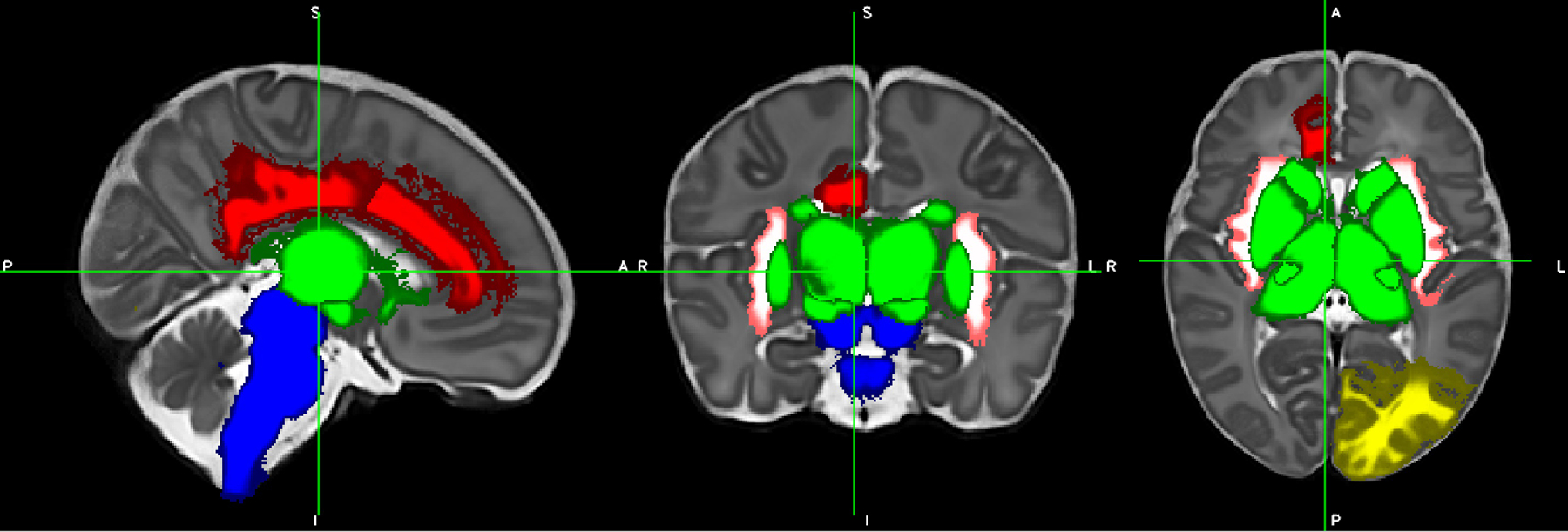
Differences in regional volumes between opioid-exposed and control infants. Blue = brainstem (smaller in exposed infants). White/pink = bilateral insular white matter (smaller in exposed infants). Green = deep gray matter including ventrolateral nuclei and subthalamic nuclei (smaller in exposed infants). Red = right cingulate gyrus white matter (larger in exposed infants). Yellow = left occipital lobe white matter (larger in exposed infants). Cerebrospinal fluid volume (not pictured) was also smaller in opioid-exposed infants.
Discussion
We found that infants with prenatal opioid exposure had smaller overall deep gray matter, thalamus, subthalamic nucleus, insular white matter, brainstem, and CSF volumes compared to unexposed infants, but larger volumes of white matter in the right cingulate gyrus and left occipital lobe. This study builds on previous literature in opioid and polysubstance-exposed infants and children showing smaller regional brain volumes in exposed children compared with controls, with significant overlap in regions found in previous literature.
Opioids are known to cross the human placenta and the blood-brain barrier(2). Opioid exposure could potentially change brain volumes through direct neurotoxicity or indirect vascular or metabolic effects(20, 21). Smaller gray matter volumes could be explained by decreased neurogenesis, increased apoptosis, or decreased density. Opioids induce neuronal apoptosis in animal models(22, 23) and human fetal neurons(10). Opioids decrease neurogenesis(7, 24) and decrease dendritic spine density, both of which could explain smaller brain volumes(25, 26). Smaller white matter volumes could be explained by altered microglia(27) and decreased myelin production(9, 27) or Wallerian degeneration after damage to gray matter(28). However, larger white matter volumes could also be pathologic, reflecting inappropriate pruning or increased connections in pathways related to stress(29, 30).
Few volumetric studies have been performed on children exposed to opioids in utero. A small study of 16 newborns with prenatal opioid exposure showed smaller whole brain and basal ganglia volumes compared to population norms, but no control group was used(15). Another study in 16 opioid-exposed children aged 10–14 years showed smaller volumes of basal ganglia, thalamus, and cerebellar white matter when compared to matched controls(13). A third small study in 14 children with prenatal substance exposure (10 exposed to opioids) and 14 controls found reduced regional volumes of multiple areas, including the cortex, amygdala, accumbens, putamen, pallidum, brainstem, and cerebellum in the substance-exposed group(14). Other studies in infants and children with prenatal opioid exposure have found differences in white matter microstructure(31, 32) and reductions in head circumference(33–35). We did not find differences in head circumference or overall brain volume in infants in our study.
We found that the ventrolateral thalami, subthalamic nuclei, and overall deep gray matter were smaller in infants exposed to opioids. The thalamus is an important structure in drug addiction(36). The ventrolateral thalami are integral structures in the dopaminergic reward circuit(37). Smaller thalamic gray matter volumes have been found for subjects using various substances, including alcohol, cocaine, nicotine, methamphetamine, opioids, and cannabinoids(36). The thalamus had lower glucose metabolism on PET scanning in adult subjects with opioid dependence than healthy controls(38). However, a meta-analysis of imaging gray matter abnormalities in opioid-dependent adults did not find significant decreases in thalamic volumes(39). The subthalamic nucleus is part of the dopamine-rich basal ganglia, which play a role in addiction(40). The basal ganglia are smaller in children with prenatal substance exposure in multiple studies(13–15).
Brainstem volumes were also smaller in opioid-exposed infants. The brainstem is rich in opioid receptors, particularly in the ventral tegmental area of the midbrain(41). One small study of children exposed to opioids and other substances in utero also found the brainstem to be smaller compared with controls(14).
We found smaller insular white matter bilaterally in infants exposed to opioids. The insula is thought to be essential in drug addiction(42) as it acts as a hub for many regions relevant to substance use(39). In both animal studies(43) and studies of adult tobacco users(44), lesions of the insula appear to eliminate addiction to smoking. Decreased gray matter has been found in the insula of adult opioid users(12, 45, 46), including adults with chronic back pain randomized to 1 month of oral morphine as compared to those randomized to placebo(12).
Cerebrospinal fluid volumes were smaller in opioid-exposed infants. Overall brain volumes did not differ between groups, nor did ventricular volumes. This decreased CSF volume in the opioid-exposed group with similar brain and ventricle volumes suggests that the regional volumetric decreases we found in the opioid-exposed group are not due to atrophy but rather maldevelopment of specific structures.
We found larger volumes of the right cingulate gyrus white matter in infants exposed to opioids prenatally. The anterior cingulate gyrus is highly connected to limbic structures in the brain and is a frontocortical area strongly associated with drug addiction(47). In the study discussed above, in which adults with back pain were randomized to 1 month of oral morphine or placebo, morphine administration was associated with increased gray matter in the cingulate cortex.(12) A similar study in adults with back pain administered morphine also showed dosage-correlated volumetric increase in the right ventral posterior cingulate(11). Chronic stress increases white matter connectivity in the cingulum in mice(29) and humans(30).
Opioid-exposed infants also had larger volumes of the left occipital lobe white matter. Multiple white matter tracts in the occipital lobe connect the visual cortex to other areas of the brain(48). Several case series have shown that children exposed to opioids in utero have altered visual development(49–51), but the mechanism by which larger white matter volumes could explain these visual changes is unclear.
Strengths of our study include high-resolution imaging at 3T with regional volumes determined by an automated pipeline, decreasing human error. We had rigorous information on drug exposure, with maternal urine toxicology at the time of delivery for opioid-exposed infants and controls, and biological samples (cord or meconium) for all opioid-exposed infants. We imaged infants before 8 weeks of age, which allowed for the minimization of the effects of caregiving and the home environment compared with imaging in older children. Limitations include the small sample size, although our study is larger than brain volume studies previously published in this population. We were unable to control for maternal smoking and Hepatitis C. Maternal smoking may be particularly relevant to brain development(52) and neurodevelopmental outcomes(53, 54) and future studies must be designed with the ability to control for this important variable. Although we had maternal report on alcohol use during pregnancy, we did not have any biomarkers of alcohol use, and a previous paper has suggested that opioid use and alcohol use disorder often coexist(55). Other potential confounders that we were unable to account for included maternal stress and maternal psychiatric disorders.
Conclusions
In this prospective imaging study of opioid-exposed infants and unexposed controls imaged between 40–48 weeks postmenstrual age, we found multiple differences in regional brain volumes between groups. More research is needed to understand how the type, timing, and duration of opioid exposure, as well as other confounding factors such as maternal smoking during pregnancy, affect the developing brain and subsequent neurodevelopment.
Supplementary Material
Impact statement:
Prenatal opioid exposure is associated with developmental and behavioral consequences, but the direct effects of opioids on the developing human brain are poorly understood
Prior small studies using MRI have shown smaller regional brain volumes in opioid-exposed infants and children
After controlling for covariates, infants with prenatal opioid exposure scanned at 40–48 weeks postmenstrual age had smaller brain volumes in multiple regions compared to controls, with two regions larger in the opioid-exposed group
This adds to the literature showing potential impact of prenatal opioid exposure on the developing brain
Funding source:
KL2 TR001426 (SM), R01 NS094200 (NP), R01 NS096037 (NP)
Statement of financial support: The authors have no financial relationships relevant to this article to disclose.
Footnotes
Disclosure statement: The authors have no conflicts of interest relevant to this article to disclose.
Category of study: Clinical Research
Consent statement: Consent was required from a parent/legal guardian for this study
References
- 1.Honein MA, Boyle C, Redfield RR Public Health Surveillance of Prenatal Opioid Exposure in Mothers and Infants. Pediatrics 143 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Concheiro M et al. Maternal buprenorphine dose, placenta buprenorphine, and metabolite concentrations and neonatal outcomes. Ther Drug Monit 32:206–215 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaves C, Remiao F, Cisternino S, Decleves X Opioids and the Blood-Brain Barrier: A Dynamic Interaction with Consequences on Drug Disposition in Brain. Curr Neuropharmacol 15:1156–1173 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeoh SL et al. Cognitive and Motor Outcomes of Children With Prenatal Opioid Exposure: A Systematic Review and Meta-analysis. JAMA Netw Open 2:e197025 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larson JJ et al. Cognitive and Behavioral Impact on Children Exposed to Opioids During Pregnancy. Pediatrics 144 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu VW, Mo Q, Yabe T, Schwartz JP, Robinson SE Perinatal opioids reduce striatal nerve growth factor content in rat striatum. Eur J Pharmacol 414:211–214 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Wu CC et al. Prenatal buprenorphine exposure decreases neurogenesis in rats. Toxicol Lett 225:92–101 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Vestal-Laborde AA, Eschenroeder AC, Bigbee JW, Robinson SE, Sato-Bigbee C The opioid system and brain development: effects of methadone on the oligodendrocyte lineage and the early stages of myelination. Dev Neurosci 36:409–421 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez ES, Bigbee JW, Fobbs W, Robinson SE, Sato-Bigbee C Opioid addiction and pregnancy: perinatal exposure to buprenorphine affects myelination in the developing brain. Glia 56:1017–1027 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology 42:829–836 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Younger JW et al. Prescription opioid analgesics rapidly change the human brain. Pain 152:1803–1810 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin JC et al. One Month of Oral Morphine Decreases Gray Matter Volume in the Right Amygdala of Individuals with Low Back Pain: Confirmation of Previously Reported Magnetic Resonance Imaging Results. Pain Med 17:1497–1504 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sirnes E et al. Brain morphology in school-aged children with prenatal opioid exposure: A structural MRI study. Early Hum Dev 106–107:33–39 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Walhovd KB et al. Volumetric cerebral characteristics of children exposed to opiates and other substances in utero. Neuroimage 36:1331–1344 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan Q et al. Do maternal opioids reduce neonatal regional brain volumes? A pilot study. J Perinatol 34:909–913 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Makropoulos A et al. The developing human connectome project: A minimal processing pipeline for neonatal cortical surface reconstruction. Neuroimage 173:88–112 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makropoulos A et al. Automatic whole brain MRI segmentation of the developing neonatal brain. IEEE Trans Med Imaging 33:1818–1831 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Kline JE, Illapani VSP, He L, Altaye M, Parikh NA. Retinopathy of Prematurity and Bronchopulmonary Dysplasia are Independent Antecedents of Cortical Maturational Abnormalities in Very Preterm Infants. Sci Rep 9:19679 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothman KJ No adjustments are needed for multiple comparisons. Epidemiology 1:43–46 (1990). [PubMed] [Google Scholar]
- 20.Cunha-Oliveira T, Rego AC, Oliveira CR Cellular and molecular mechanisms involved in the neurotoxicity of opioid and psychostimulant drugs. Brain Res Rev 58:192–208 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Brown GG, Jacobus J, McKenna B Structural imaging for addiction medicine: From neurostructure to neuroplasticity. Prog Brain Res 224:105–127 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tramullas M, Martínez-Cué C, Hurlé MA Chronic administration of heroin to mice produces up-regulation of brain apoptosis-related proteins and impairs spatial learning and memory. Neuropharmacology 54:640–652 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Han TZ Prenatal exposure to heroin in mice elicits memory deficits that can be attributed to neuronal apoptosis. Neuroscience 160:330–338 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci U S A 97:7579–7584 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao D, Lin H, Law PY, Loh HH Mu-opioid receptors modulate the stability of dendritic spines. Proc Natl Acad Sci U S A 102:1725–1730 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao D et al. Distinct effects of individual opioids on the morphology of spines depend upon the internalization of mu opioid receptors. Mol Cell Neurosci 35:456–469 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jantzie LL et al. Prenatal opioid exposure: The next neonatal neuroinflammatory disease. Brain Behav Immun 84:45–58 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wollman SC et al. White matter abnormalities in long-term heroin users: a preliminary neuroimaging meta-analysis. Am J Drug Alcohol Abuse 41:133–138 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Grandjean J et al. Chronic psychosocial stress in mice leads to changes in brain functional connectivity and metabolite levels comparable to human depression. Neuroimage 142:544–552 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Bierer LM et al. White matter abnormalities in Gulf War veterans with posttraumatic stress disorder: A pilot study. Psychoneuroendocrinology 51:567–576 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Monnelly VJ, et al. Prenatal methadone exposure is associated with altered neonatal brain development. Neuroimage Clin 18:9–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walhovd KB, Watts R, Amlien I, Woodward LJ Neural tract development of infants born to methadone-maintained mothers. Pediatr Neurol 47:1–6 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Towers CV et al. Neonatal Head Circumference in Newborns With Neonatal Abstinence Syndrome. Pediatrics 143 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Hytinantti T et al. Neonatal outcome of 58 infants exposed to maternal buprenorphine in utero. Acta Paediatr 97:1040–1044 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Garrison L et al. Fetal Growth Outcomes in a Cohort of Polydrug- and Opioid-Dependent Patients. J Reprod Med 61:311–319 (2016). [PMC free article] [PubMed] [Google Scholar]
- 36.Huang AS, Mitchell JA, Haber SN, Alia-Klein N, Goldstein RZ The thalamus in drug addiction: from rodents to humans. Philos Trans R Soc Lond B Biol Sci 373 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haber S, McFarland NR The place of the thalamus in frontal cortical-basal ganglia circuits. Neuroscientist 7:315–324 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Prosser J, London ED, Galynker II Sustained attention in patients receiving and abstinent following methadone maintenance treatment for opiate dependence: performance and neuroimaging results. Drug Alcohol Depend 104:228–240 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Wollman SC et al. Gray matter abnormalities in opioid-dependent patients: A neuroimaging meta-analysis. Am J Drug Alcohol Abuse 43:505–517 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Koob GF, Volkow ND Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3:760–773 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ting-A-Kee R, van der Kooy D The neurobiology of opiate motivation. Cold Spring Harb Perspect Med 2 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Droutman V, Read SJ, Bechara A Revisiting the role of the insula in addiction. Trends Cogn Sci 19:414–420 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott D, Hiroi N Deconstructing craving: dissociable cortical control of cue reactivity in nicotine addiction. Biol Psychiatry 69:1052–1059 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naqvi NH, Rudrauf D, Damasio H, Bechara A Damage to the insula disrupts addiction to cigarette smoking. Science 315:531–534 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lyoo IK et al. Prefrontal and temporal gray matter density decreases in opiate dependence. Psychopharmacology (Berl) 184:139–144 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Gardini S, Venneri A Reduced grey matter in the posterior insula as a structural vulnerability or diathesis to addiction. Brain Res Bull 87:205–211 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Goldstein RZ, Volkow ND Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–669 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takemura H et al. Occipital White Matter Tracts in Human and Macaque. Cereb Cortex 27:3346–3359 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamilton R et al. Ophthalmic, clinical and visual electrophysiological findings in children born to mothers prescribed substitute methadone in pregnancy. Br J Ophthalmol 94:696–700 (2010). [DOI] [PubMed] [Google Scholar]
- 50.McGlone L et al. Visual outcome in infants born to drug-misusing mothers prescribed methadone in pregnancy. Br J Ophthalmol 98:238–245 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Spiteri Cornish K, Hrabovsky M, Scott NW, Myerscough E, Reddy AR The short- and long-term effects on the visual system of children following exposure to maternal substance misuse in pregnancy. Am J Ophthalmol 156:190–194 (2013). [DOI] [PubMed] [Google Scholar]
- 52.El Marroun H et al. Prenatal tobacco exposure and brain morphology: a prospective study in young children. Neuropsychopharmacology 39:792–800 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nelson LF et al. Cognitive Outcomes of Young Children After Prenatal Exposure to Medications for Opioid Use Disorder: A Systematic Review and Meta-analysis. JAMA Netw Open 3:e201195 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holz NE et al. Effect of prenatal exposure to tobacco smoke on inhibitory control: neuroimaging results from a 25-year prospective study. JAMA Psychiatry 71:786–796 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Bakhireva LN et al. Prevalence of alcohol use in pregnant women with substance use disorder. Drug Alcohol Depend 187:305–310 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


