Abstract
Pathogenic invasion of Staphylococcus aureus is a major concern in patients with chronic skin diseases like atopic dermatitis (AD), epidermolysis bullosa (EB), or chronic diabetic foot and venous leg ulcers, and can result in persistent and life-threatening chronic non-healing wounds. Staphylococcus aureus is generally recognized as extracellular pathogens. However, S. aureus can also invade, hide and persist in skin cells to contribute to wound chronicity. The intracellular life cycle of S. aureus is currently incompletely understood, although published studies indicate that its intracellular escape strategies play an important role in persistent cutaneous infections. This review provides current scientific knowledge about the intracellular life cycle of S. aureus in skin cells, which can be classified into professional and non-professional antigen-presenting cells, and its strategies to escape adaptive defense mechanisms. First, we discuss phenotypic switch of S. aureus, which affects intracellular routing and degradation. This review also evaluates potential intracellular escape mechanism of S. aureus to avoid intracellular degradation and antigen presentation, preventing an immune response. Furthermore, we discuss potential drug targets that can interfere with the intracellular life cycle of S. aureus. Taken together, this review aimed to increase scientific understanding about the intracellular life cycle of S. aureus into skin cells and its strategies to evade the host immune response, information that is crucial to reduce pathogenic invasion and life-threatening persistence of S. aureus in chronic cutaneous infections.
Keywords: antigen-presenting cells, intracellular bacteria, skin, Staphylococcus aureus, wounds
1 |. INTRODUCTION
Staphylococcus aureus is a facultative anaerobic Gram-positive coccus. About 30% of the human population carries this commensal microorganism on their skin without any clinical manifestations.1 Cutaneous microbiome studies have also shown that S. aureus colonizes regions of the skin with higher humidity and temperature, such as the groyne, axillary vault and toe web.2 However, when the skin barrier is compromised, S. aureus can colonize the wound regardless of location and become pathogenic. Staphylococcus aureus infections can cause a range of illnesses from minor skin infections, such as pimples, impetigo and abscesses, to persistent chronic wounds to life-threatening diseases, such as pneumonia, endocarditis and sepsis.1,3,4 Infection often challenges the host’s immune system, and if the immune system fails to fight bacterial invasion, S. aureus can spread from the wound area to other tissues and ultimately lead to sepsis and death. Pathogenic invasion of S. aureus is, therefore, particularly a major concern in immune-compromised patients, such as elderly, diabetic and paediatric population, but also in patients with chronic skin diseases like atopic dermatitis (AD) and epidermolysis bullosa (EB).4
There is a great deal of genetic variation within the S. aureus species due to its fast-co-evolutionary adaptation skills.5 With molecular typing, at least 17 800 different strains of S. aureus have been identified of around the world, and a distribution map was generated of the most prevalent S. aureus protein A (spa, an immunoglobulin-binding protein) types in different continents.6 Furthermore, wounds, from EB patients can be colonized with multiple (ranging from 2–6) types of S. aureus, and the type of S. aureus mirrors the geographic location of the patient and can vary over time.1,7,8 Specific S. aureus strains have also found to be associated with non-healing diabetic foot ulcers.9 Nowadays, S. aureus–related infections are treated by a limited panel of antibiotics, but life-threatening resistance is rising, particularly towards pathogenic methicillin-resistant strains (MRSA).5 Therefore, there is an urgent need to develop alternative treatment options.
Generally, S. aureus is recognized as an extracellular pathogen, but increased evidence is rising that S. aureus can also invade, hide and persist into various types of cells, which can be classified into professional- and non-professional antigen-presenting host cells.10–12 The intracellular niche increases survival chances of S. aureus. However, host cells may also have the ability to kill intracellular bacteria and present antigens on the cell surface for the induction of an adaptive immune response.13 Currently, the intracellular life cycle of S. aureus is incompletely understood. The aim of this review was to discuss scientific knowledge on the intracellular life cycle of S. aureus in skin host cells and its strategies to evade the host immune system. This knowledge may be useful to help reduce S. aureus infections, reoccurrence and resistance to antibiotic treatment.
2 |. INTRACELLULAR LIFE CYCLE OF Staphylococcus aureus
2.1 |. Phenotypic switching of Staphylococcus aureus
Tight bacterial adherence to host structures is a crucial step in infection development. Staphylococcus aureus–derived fibronectin-binding proteins (FnBps) bind to host cell–derived integrin α5β1, inducing changes in the cytoskeleton that lead to the uptake of bacteria into phagosomes.10,14 Following invasion, S. aureus can induce a strong inflammatory and cytotoxic response or remain silent and hide intracellularly. The response mainly depends on the expression of the bacterial accessory gene regulator (agr) system. The agr system in S. aureus controls expression of many virulence factors, exoenzymes and other cytotoxic factors that kill the host cell.10,15 However, phenotypic switching within the agr system occurs in about 10% of the colonizing strains. These agr-deficient colonies, frequently associated with hospitalizations and persistent infections, exhibit a high rate of cellular internalization where they induce less toxicity within cells.10,12,16 S. aureus agr-deficient colonies are recognized as “small colony variants” (SCVs), which are characterized by small colony size, slow growth, reduced metabolism, downregulated virulence genes and low cytotoxicity (Figure 1), allowing S. aureus to escape the host’s immune system and reside in certain cell types. In line with this, studies have shown that SCVs can persist into host cells for several weeks.17 Furthermore, clinical cases have shown that S. aureus infections can remain dormant with relapses occurring months or years later after apparent successful antimicrobial treatment.18–20 Environmental factors, such as an increase in pH or exposure to nutrient-rich medium, can restore phenotype of SCVs back into a virulent and fast proliferating,10,17 suggesting that these variants may serve as a hidden source for persistent/reoccurring chronic infections. Interestingly, when host cells are infected with aggressive wild-type S. aureus, these bacteria induce inflammation and cytotoxicity in the intracellular location, which correlates with high agr expression (Figure 1A).10 Over the course of a couple of days, host cells activate certain degradation systems, which allow them to combat bacterial infection. Several weeks later, a small amount of S. aureus seems to be able to resist cellular degradation and persist inside host cells for long periods of time. A scheme of the difference between wild-type cytotoxic (agr-positive) and SCV-like dormant (agr-deficient) S. aureus strains is depicted in Figure 1A.
FIGURE 1.
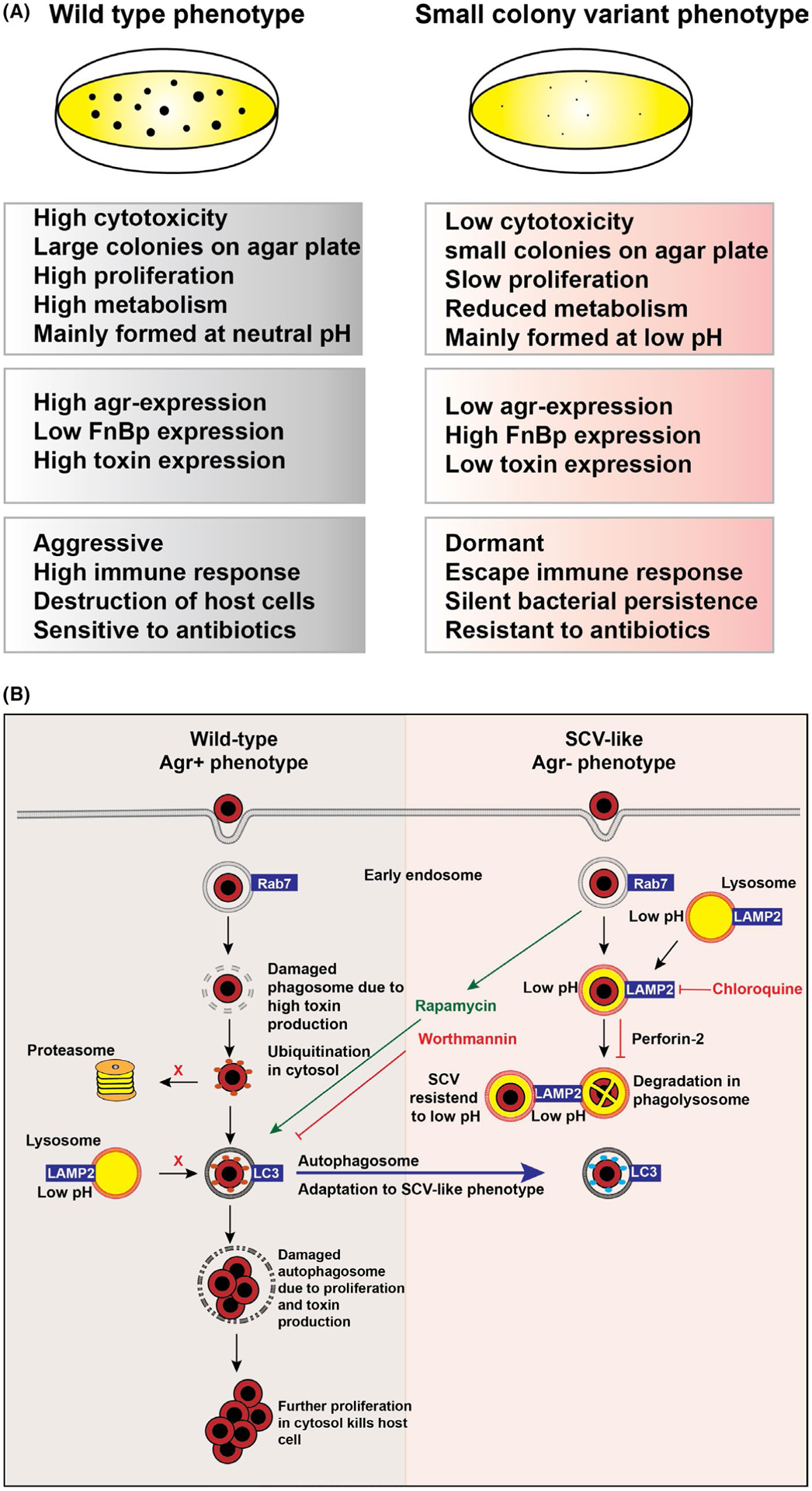
(A) Difference between wild-type cytotoxic agr-positive and small colony variants (SCV)-like dormant agr-deficient Staphylococcus aureus colonies. (B) Intracellular routing of S. aureus wild-type cytotoxic agr-positive and SCV-like dormant agr-deficient S. aureus colonies
2.2 |. Lysosomal pathway of Staphylococcus aureus
Skin cell types can be classified into professional and non-professional antigen-presenting cells (APCs). Professional APCs, such as macrophages, dendritic cells and B cells, are efficient in internalizing foreign antigen including S. aureus. After host cell internalization, bacteria generally route through the phagosomal degradation pathway that terminates into a highly degradative organelle with a low pH, the phagolysosome (Figure 1B). Via this vesicular pathway, many internalized microorganisms are efficiently eliminated by professional APC. Recently, research showed that the SCV-like (agr-deficient) S. aureus phenotype is induced by low pH. These low-pH–resistant bacteria are able to hijack the phagolysosome and remain dormant in these organelles. However, phenotypically switched SCV-like bacteria have the potential to revert back to fully aggressive wild-type phenotype by neutral pH.21 Therefore, treatment with lysosomal alkalizing agents, such as chloroquine together with antibiotics, may be a novel effective strategy to fight persistent chronic S. aureus infections.
2.3 |. Autophagy pathway of Staphylococcus aureus
Non-professional APCs, such as keratinocytes, endothelial cells and fibroblasts, also have the capacity to internalize bacteria.11,12,22,23 However, these cells do not have an optimal lysosomal capacity to degrade bacteria. Instead, non-professional antigen APCs combat intracellular pathogens by inducing autophagy.12
Autophagy is a conserved membrane trafficking pathway in eukaryotic cells that sequesters cytoplasmic contents by double-membrane autophagosomes and eventually delivers them to autophagolysosomes. Autophagy is regulated by the AKT pathway and can be pharmacologically induced by rapamycin, which is an inhibitor of the serine/threonine kinase Tor. In contrast, inhibition of type III phosphatidylinositol 3-kinase (PI3K) by the compound wortmannin inhibits autophagy.24 Rapamycin increases co-localization of agr-positive S. aureus with the autophagy marker LC3, while wortmannin drastically impairs bacterial co-localization with LC3 in non-professional APCs.25 Furthermore, rapamycin increases the intracellular growth of cytotoxic agr-positive S. aureus, ultimately leading to killing of host cells. This is in contrast to wortmannin, which drastically impairs the growth of intracellular S. aureus (Figure 1B). Interestingly, SCV-like/agr-deficient S. aureus did not localize in LC3-positive autophagosomes. However, when autophagy was induced by rapamycin, agr-deficient S. aureus were observed in autophagosomes enabling bacterial replication and killing of host cells.25 More recently, studies have shown that intracellular S. aureus is targeted by selective autophagy (also called xenophagy) involving ubiquitination and autophagy receptors such as SQSTM1 in non-professional APCs.26,27 Ubiquitination is also a protein modification used by host cells to tag proteins that are destined for proteasomal degradation (ubiquitin proteasome system [UPS]). Selective autophagy, however, is mediated by autophagy receptor molecules, like SQSTM1, that deliver ubiquitinated bacteria to autophagosomes.28 Therefore, inhibition of selective autophagy receptors might prevent S. aureus from routing through the autophagy machinery, and/or recruitment of proteasomes to these ubiquitin-surrounded bacteria might encourage cytosolic degradation.
2.4 |. Perforin-2 and Staphylococcus aureus
Perforin-2 is an innate antibacterial effector essential for elimination of intracellular bacteria in both professional and non-professional phagocytic skin cells (Figure 1B).11,23 First described in macrophages in vertebrates, it was initially named macrophage-expressed protein 1 (Mpeg1) before being renamed Perforin-2 due to its membrane attack complex/perforin (MACPF) domain.11,29 Perforin-2 is located within the membranes of endosomal vesicles which traffic to and fuse with the bacteria containing phagosome. Acidification triggers a conformational change in the MACPF and P-2 domains which allows membrane insertion and pore formation on bacterial surfaces, enabling reactive oxygen species, nitric oxide and lysozyme to enter and perform bacterial killing.11,29–32 Perforin-2 was shown to be critical for the clearance of a variety of Gram-positive and Gram-negative pathogens. Perforin-2–deficient mice are unable to clear S. aureus upon epicutaneous infection, highlighting its indispensable role in the host’s innate antimicrobial response.31,33 Multiple obligatory intracellular pathogens have developed mechanism to counteract Perforin-2,11,34 while recent data have shown S. aureus ability to suppress Perforin-2 in skin cells (Figure 1B),23 suggesting a novel mechanism of invasion and its possible role in chronic wounds in both elderly and paediatric EB population.
3 |. INDUCTION OF AN ADAPTIVE IMMUNE RESPONSE BY Staphylococcus aureus
Pathogens invading the host are first controlled by a rapid and broad targeting of the innate immune response. After a couple of days of fighting, the innate immune response is followed by a second line of defense, “the adaptive immune response.” During this response, APCs generate highly specific T and B cells that can recognize S. aureus peptides and kill pathogen-infected cells. After resolution of an immune response, antigen-specific memory T cells persist in many sites of the body, including the skin. Long-lived memory cells provide an effective quick first line of defense against a secondary infection of a particular S. aureus strain. Importantly, the majority of adults contain detectable levels of circulating antigen-specific memory T cells to S. aureus.33 This indicates that most adults have previously been exposed to S. aureus through either their commensal colonization or subclinical infection. Furthermore, this implies that S. aureus invades APCs where bacteria are proteo-lytically cleaved in phagolysosomes and peptides are loaded and presented on major histocompatibility complex (MHC) molecules for T-cell priming.
3.1 |. MHCI and MHCII vesicular transport
MHC class I molecules are expressed on both professional and non-professional APCs. For MHC class I peptide loading, intracellular cytosolic ubiquitinated non-self proteins, for example from viruses or mutated sequences, are destined for proteasomal degradation. Some of these trimmed peptides go into the endoplasmic reticulum via a peptide transporter called transporter associated with antigen processing (TAP). These peptides are loaded onto empty MHC class I molecules.35 Loaded MHC class I:peptide complexes are presented on the cell surface where it can activate cytotoxic CD8 T cells. These T cells have the potential to directly kill infected host cells. Importantly, most of the steps of the MHC class I pathway are not essential for the viability of cells. In line with this, viruses (eg, cytomegalovirus) and maybe also S. aureus hiding in the intracellular can be expected to interfere with this pathway so that they can escape an immune response.35 Therefore, S. aureus should be encouraged to leave vesicular compartments and go into the cytosol where it should be targeted to be ubiquitinated for proteasomal degradation and loaded onto MHC class I molecules for presentation (Figure 2).
FIGURE 2.
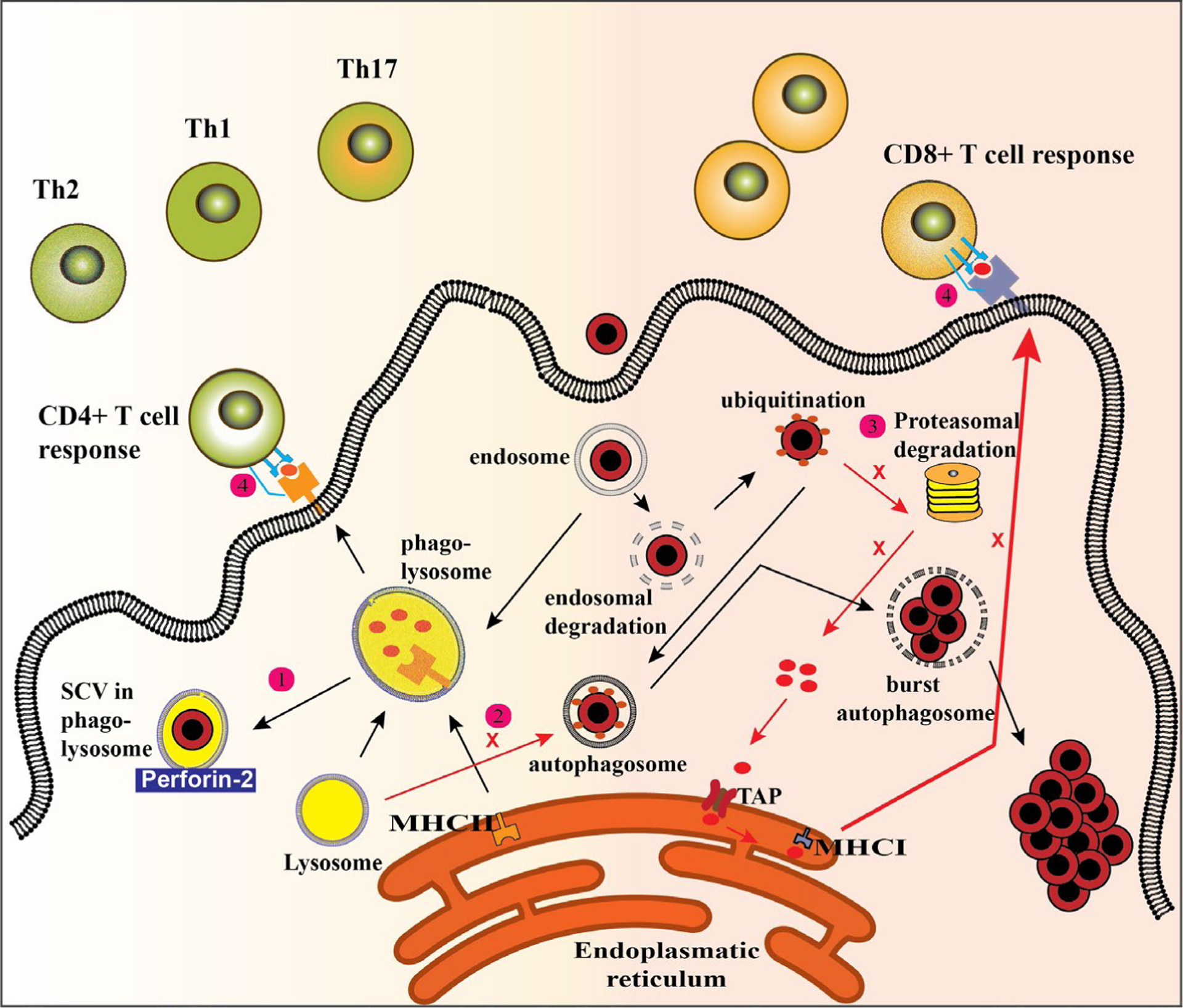
Activation of different T-cell populations through different pathways of antigen presentation. After host cell internalization, Staphylococcus aureus generally route through the phagosomal degradation pathway that, under normal circumstances, terminates into a highly degradative organelle with a low pH, the phagolysosome. 1) To circumvent lysosomal degradation, S. aureus can adapt to a low pH tolerant small colony variants (SCV)-like phenotype. 2) Staphylococcus aureus can also hide in autophagosomes where it prevents fusion with lysosomes preventing degradation and presentation on major histocompatibility complex (MHC) class II molecules. 3) Staphylococcus aureus that end up in the cytosol are ubiquitinated but not targeted for proteasomal degradation and subsequent MHC class I presentation escaping a cytotoxic T-cell response. 4) Staphylococcus aureus can also manipulate the expression of co-stimulatory molecules preventing an effective T-cell response
In contrast to MHC class I molecules, MHC class II molecules are generally expressed on professional APCs, which can present lysosomal degraded peptides and activate CD4 T cells.35 Binding of naïve CD4 T cells to loaded non-self peptide:MHC complexes results into rearrangement of T-cell receptors (TCR) and activation of CD4 T cells (Figure 2). Subsequently, primed CD4 T cells can activate B cells, allowing them to produce highly specific antimicrobial antibodies that specifically target pathogenic bacteria. Produced antibodies are helpful in the opsonization of bacteria and their engulfment by phagocytic cells, such as neutrophils and macrophages. CD4 T cells function mainly as helper cells and can be divided into Th1 and Th2 helper cells. Th1 cells produce IL-2 and interferon-gamma (IFN-y) and contribute to immune protection by helping macrophages, cytotoxic T cells, and B cells. Th2 cells, however, are characterized by IL-4 and IL-5 secretion and exacerbate disease by activating, mast cells, for example.36 CD4 T cell can also differentiate into Th17 cells. These cells secrete IL-17, which is a pro-inflammatory substance and crucial for neutrophil recruitment. Importantly, IL-17A/F knockout mice develop spontaneous S. aureus skin abscesses and humans with IL-17 defects are prone to S. aureus skin infections.37 In line with this, patients with a neutrophil disorder also suffer from an increased incidence of S. aureus infection.37 This suggests that induction of an effective Th17 response plays an essential role in protection against S. aureus infection. However, we have found that neutrophils accumulate in chronic non-healing wounds of EB patients (unpublished data) which are very likely colonized with S. aureus.8 Interestingly, IL-1β−/− mice, which have impaired neutrophil recruitment and host defense during an initial S. aureus infection, were found to be rescued during a secondary infection. Further mechanistic investigation showed that clonal expansion of TNF/IFN-γ–producing γσT cells induced by TLR2 signalling protected against additional S. aureus skin infections.38 Furthermore, activation of γσT cells by commensal Staphylococcus epidermidis resulted in upregulation of Perforin-2 and enhanced ability of cells to eliminate intracellular S. aureus, suggesting the protective role of beneficial bacteria against persistent cutaneous infections.39
In another immune evasion strategy to avoid intracellular host cell clearance, S. aureus can manipulate the expression of co-stimulatory molecules on the cell surface. Appropriate co-stimulation of APC-derived CD80/86 with CD28 on T cells is essential to activate T cells. Staphylococcus aureus, however, can inhibit expression of activating co-stimulatory molecules infected cells affecting T-cell activation (Figure 2).40 Additionally, S. aureus has been shown to induce programmed cell death 1 (PD-1) ligand expression in professional APCs.40,41 PD-L1 binds to PD-1–expressing T cells induces T-cell exhaustion, which is characterized by loss of its effector functions. Therefore, blockade of PD-1/PD-L1 interactions might be an effective treatment option for intracellular S. aureus–infected cells. In fact, PD-1/PD-L1 blockade has been shown to protect against S. aureus infection after skin burn in mice.41
4 |. INTRACELLULAR LIFE CYCLE IN PROFESSIONAL ANTIGEN-PRESENTING CELLS
4.1 |. Macrophages
Macrophages are professional phagocytes that can ingest death cells, cellular debris, microorganisms and aged neutrophils. Skin-resident macrophages are the most frequent resident immune cell type in the dermis (Figure 3).42 In response to local skin injury, resident macrophages and monocytes migrate to the affected site. Once arrived, monocytes differentiate into pro-inflammatory macrophages in response to local produced inflammatory cytokines. Macrophages play a key role in host defense by recognizing bacterial effectors and engulfing and phagocytosing them.42 Therefore, macrophages play a critical role in innate immunity as they have the potential to directly kill ingested S. aureus. When macrophages ingest bacteria, the bacteria become trapped in the phagosome, which then fuses with the lysosome. Within this organelle, enzymes and toxic peroxides digest and clear the pathogen (Figure 1B).43 However, macrophages have a limited capacity to degrade S. aureus and some bacteria can become resistant to this method of digestion and manage to prevent phagolysosomal maturation and acidification.27,44 Eventually, infected macrophages lyse and release viable bacteria, which can be phagocytosed by other macrophages. This continuous cycle of lysis and uptake maintains a viable pool of intracellular bacteria over time, leading to persistence of intracellular S. aureus.27
FIGURE 3.
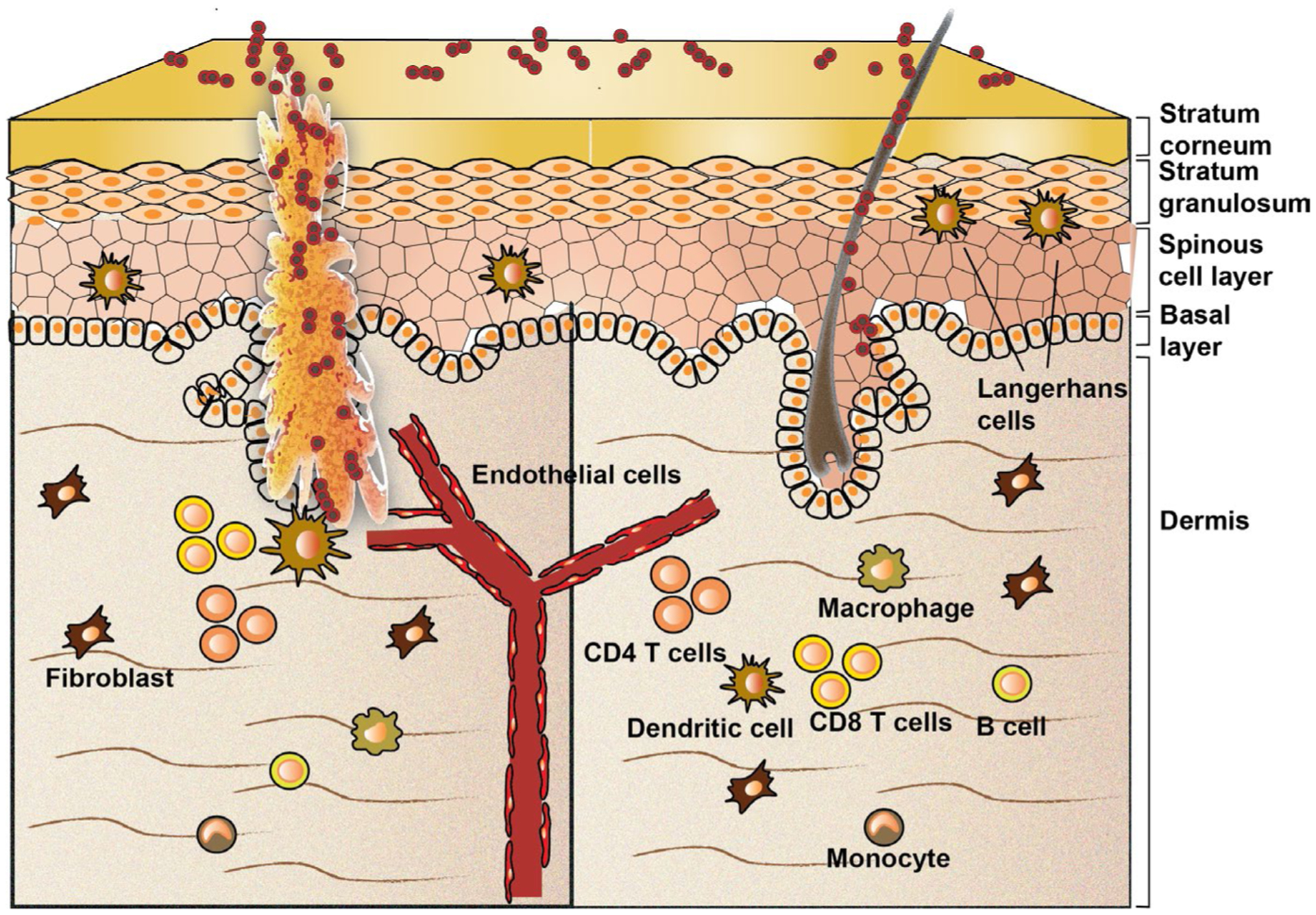
Potential antigen-presenting cells (APCs) that are present in the skin. When the skin barrier is disrupted, Staphylococcus aureus can colonize the open wound and become pathogenic. Professional APCs, such as macrophages, Langerhans cells, dendritic cells and B cells, can internalize and process S. aureus peptide fragments in lysosomes. Generated peptide fragments can be loaded onto major histocompatibility complex (MHC) class II molecules. Non-professional APCs, such as keratinocytes, fibroblasts and endothelial cells, also have the capacity to internalize and process peptide fragments of S. aureus. These cells activate their autophagy machinery as their lysosomal degradation capacity is limited. APC rely on Perforin-2–mediated kill of intracellular pathogens
Macrophages express high basal levels of the antibacterial molecule Perforin-2.11 Furthermore, it has been shown that Perforin-2–deficient macrophages infected with Listeria monocytogenes have increased acidification of vesicles resulting in bacterial replication and expression of virulence genes.34 This suggests that Perforin-2 works upstream of the lysosomal degradation system. Therefore, investigation towards Perforin-2 expression in macrophages and their subtypes, from early phases of healing and chronic non-healing wounds will be informative. Furthermore, induction of Perforin-2 expression may be an effective therapeutic strategy to combat antibiotic-resistant S. aureus infections. Therefore, it is important to gain more knowledge into pathways that can induce expression of this protein, such as type I and type II interferon signalling pathways,29 as well as Perforin-2 modulation by commensal microorganisms.39
Besides inducing an innate immune response, macrophages can also initiate an adaptive immune response and function as professional APCs. However, their antigen-presenting capacity is modest compared with dendritic cells. Furthermore, macrophages do not effectively migrate to skin draining lymph nodes.42 After digesting S. aureus within phagolysosomes, macrophages can load bacterial antigen on MHC molecules and present them to local T cells.45 Alternatively, macrophages may transfer antigen to dendritic cells, which can migrate and present antigen to highly organized lymphoid structures.45
When macrophages become dysregulated, wound healing complications and chronic infection may arise.46–48 For example, the skin of AD patients presents a significant imbalance of the microbiome with high colonization of S. aureus.14 Interestingly, macrophages from AD patients express significantly less Toll-like receptor 2 (TLR2) compared with healthy controls.49 Since TLR2 plays an important role in pathogen recognition and activation of the innate immune system, reduced expression in macrophages may contribute to enhanced susceptibility to S. aureus skin infection. Patients with diabetes who have hyperglycaemia due to a lack of insulin are also more prone to S. aureus infections compared with healthy individuals. In line with this, diabetic patients have macrophages with reduced apoptotic clearance activity because of the effects of hyperglycaemia.50 EB patients who develop mucocutaneous blisters after minimal trauma due to a genetic disorder are also prone to S. aureus infections.51 Importantly, we have recently found a loss of macrophages in chronic non-healing wounds of EB patients (unpublished data).
4.2 |. Dendritic cells and Langerhans cells
Dendritic cells differ from macrophages; in particular, because of their lower phagocytic capacity, they migrate more effectively to draining lymph nodes and have a better antigen-presenting capacity than macrophages.42 Upon detection of microbes, dendritic cells upregulate MHC molecules, activate co-stimulatory molecules, produce inflammatory cytokines and migrate to draining lymph nodes to activate CD4 T cells. In human skin, dendritic cells are sub-classified into Langerhans cells, which reside exclusively in the epidermis, and interstitial dermal dendritic cells (DDCs), which reside in the adjacent dermis (Figure 3). Importantly, compared with DDCs, Langerhans cells have a very low capacity to internalize and process bacteria like S. aureus. Furthermore, Langerhans cells have been shown to induce a very weak reactivation of memory CD4 T cells compared with DDCs.52 A possible explanation for the prevention of an antibacterial response by Langerhans cells is that the epidermal immune tolerance provides symbiotic relationship with commensal bacteria. However, when bacteria penetrate from the epidermis into the dermal layer, DDCs will be activated resulting in an effective inflammatory immune response. Recent research showed that Langerhans cells can directly interact with S. aureus through the pattern recognition receptor langerin (CD207), and this interaction influenced Langerhans cell pro-inflammatory cytokine production.53 In line with this, genome-wide association studies linked langerin to AD54 making C-type lectin langerin-dependent signalling an interesting therapeutic target for S. aureus–associated skin infections. Furthermore, reduced number of Langerhans cell was associated with non-healing diabetic foot ulcers,55 characterized with S. aureus colonization. Other studies followed engineered S. aureus–expressing zsgreen in DDCs that was colonized on a mouse skin. It was found that skin-derived migratory DC expressing high MHC molecules contained the majority of S. aureus antigen and that active CCR7-dependent transport of antigens to local lymph nodes is required for priming of bacteria-specific CD4+ T cells.56 Based on the in vivo model used in this study, it would be of importance to quantitatively compare ability of different skin cells subtypes to up take S. aureus–expressing zsgreen or fluorescent protein during the course of a skin wound infection.56–58
Like macrophages,49 AD skin–derived DDCs and Langerhans cells express less TLR2 compared with healthy skin.59 Impaired TLR2-mediated signalling results in a less effective immune response, which may contribute to a less effective clearance of S. aureus invasion. In vitro, treatment of human monocytes with anti-TLR2–blocking antibodies and S. aureus has been shown to induce MHC class II expression,39 demonstrating a link between TLR2 signalling and the presence of intracellular S. aureus during monocyte-derived dendritic cell maturation. Patients with diabetes have reduced DC maturation and migration to draining lymph nodes, likely due to hyperglycaemia60 which can contribute to high prevalence of S. aureus infections and associated complications.61,62
4.3 |. B cells
B-cell activation occurs in secondary lymphoid structures. Foreign proteins circulating through lymph fluid can activate B cells with help of T helper cells. Once a B cell receptor binds a foreign antigen, it is taken up by receptor-mediated endocytosis, degraded in lysosomes, and loaded onto MHC class II molecules for presentation to T cells (Figure 2). Subsequent T-cell receptor:MHC:peptide results in B-cell activation promoting proliferation and in antibody production inducing an humoral immune response.4 Highly specific antibodies will bind the antigen present of the surface of S. aureus, which can subsequently be cleared by either the complement system or phagocytosing cells (eg, neutrophils, macrophages). Most adults carriers and non-carriers for S. aureus have a broad spectrum of S. aureus–specific serum immunoglobulins (IgG) antibodies.37 Furthermore, the sera of EB patients with chronic non-healing wounds are colonized with higher levels of anti-S. aureus IgG than healthy controls. Additionally, EB patients who carry multiple S. aureus types have even higher levels of anti-S. aureus antibodies than EB patients who carry only a single type of S. aureus.63,64
Unfortunately, recovery from a particular S. aureus infection does not appear to guarantee immunity against subsequent infections.65 This may be explained by the highly polymorphic spa gene of S. aureus, of which currently more than 17 800 different types have been identified globally (SeqNet.org).6 The primary binding site of spa is the Fc region of IgG produced by B cells interfering with effective opsonization and subsequent phagocytosis of S. aureus.6 This increases the survival chance of S. aureus and circumvents B-cell memory. Furthermore, S. aureus produces various molecules with redundant functions, such that if one is eliminated (or targeted by an antibody), other S. aureus products may compensate for that loss of function.37 Importantly, although healthy individuals have high titres to S. aureus, patients with defects in humoral immunity are not particularly prone to S. aureus infections.37 Apparently, the lack of antibody against S. aureus is compensated by other adaptive immune mechanisms. This may explain why attempts to produce an effective S. aureus vaccine have failed.
5 |. INTRACELLULAR LIFE CYCLE IN NON-PROFESSIONAL ANTIGEN-PRESENTING CELLS
5.1 |. Keratinocytes
Healthy human skin acts as a physical barrier to prevent invasion of foreign pathogens while providing home to commensal microbiota. Structurally, the skin consists of several layers of keratinocytes, of which the outer horny layer (the epidermis) is constantly exposed to commensal bacteria, viruses and fungi (Figure 3). This top layer is composed of terminally differentiated, enucleated (squamous) keratinocytes that are chemically cross-linked to provide a barrier for the skin.3,66 However, when the skin barrier is broken or when the balance between commensals and pathogens is disturbed, or in the case of skin disease or systemic disease, S. aureus can invade to the stratum granulosum, which contains less differentiated keratinocytes, or to the basal layer of keratinocytes (stratum spinosum), which contains undifferentiated keratinocytes (Figure 3).3,66 Keratinocytes express several pattern recognition receptors (PRRs), which contribute to initial sensing of extracellular S. aureus.3 Binding of S. aureus to PRRs results in NF-κB activation and induction of proinflammatory cytokine production, such as TNF-α.67,68 Subsequently, an acute inflammatory response is initiated and inflammatory cells, such as professional APCs, are recruited.69
Keratinocytes are also able to phagocytose microorganisms, like S. aureus.68 Invasion of keratinocytes by S. aureus requires high-affinity FnBPs, which interact with cell surface integrin α5β70 and blockade of integrin α5β1; alternatively, disruption of FnBPs in S. aureus blocks internalization.71 Furthermore, heat-killed S. aureus have been shown to be internalized into Rab5- and Rab7-positive phagosomes of mouse keratinocytes.68 In HeLa cells, S. aureus has been shown to transit to LC3 positive autophagosomes where they are able replicate and inhibit fusion with lysosomes.25 Other studies have shown that S. aureus rather becomes targeted by selective autophagy through ubiquitination.26
Several studies have demonstrated that keratinocytes can also express MHC class II molecules both under homeostasis and in the context of inflammation. However, induction of MHC class II, by, for example, IFN-y, on keratinocytes has been shown to control T-cell responses.72,73 Moreover, it was recently shown that MHC class II–expressing keratinocytes control microbiota-induced Th1 cell responses.74 This indicates that MHC class II–expressing keratinocytes avoid a local inflammatory reaction to commensal microorganisms. This effect might favour S. aureus infection and spreading. However, other studies have shown that keratinocytes can process extracellular antigen and induce MHC class II and ICAM expression, which can subsequently activate CD4 T cells. Pretreatment of keratinocytes with chloroquine inhibited this effect (Figure 1B), suggesting that the endosomal pathway for antigen processing is involved.75 Whether intracellular S. aureus can induce or manipulate MHC class II expression and co-stimulatory molecules, such as CD80, CD86 or I-CAM, on keratinocytes is currently unknown.
It has been demonstrated that expression of antimicrobial Perforin-2 is induced during wound healing,23 suggesting its role in protection against intracellular pathogens during barrier restoration. In vitro, Perforin-2 is constitutively expressed in keratinocytes.11,29 However, Perforin-2 suppression was observed in S. aureus–infected wounds, while keratinocytes overexpressing Perforin-2 have demonstrated increased ability to eliminate intracellular S. aureus.23 This suggests that S. aureus is able to manipulate Perforin-2 expression to reside inside the keratinocytes. Therefore, it is important to gain further scientific knowledge on pathways involved in Perforin-2 regulation. Ongoing studies will reveal the role of Perforin-2 in chronic wound infections of different aetiologies.
Staphylococcus aureus strains isolated from AD skin, but not control S. aureus strains, have been demonstrated to internalize into keratinocytes and accumulate into lysosomes.76 This ability to internalize is very likely due to increased expression of FnBPs in AD-derived S. aureus strains as fibronectin antiserum partially inhibited uptake,76 suggesting an SCV-like phenotype (Figure 1A). Moreover, Th2 cytokine expression was shown to be increased in lesional skin biopsies of patients with AD.14 In line with this, dupilumab, which blocks Th2 signalling by blocking interleukin-4 treatment, has been shown to ameliorate dermatitis.77 This indicates that evolved SCV-like S. aureus strains from AD skin induce an imbalanced Th1/Th2 adaptive immune response resulting in a Th2-shifted immune response. In theory, a Th2 adaptive immune response is induced by MHC class II molecules that present peptides derived from lysosomal degradation (Figure 2). It might therefore be possible that intracellular (SCV-like) S. aureus can induce MHC class II expression and peptide presentation in keratinocytes as well as co-stimulatory molecules in AD lesions, but this needs to be confirmed in an experimental setting. Keratinocytes from AD patients do not express significantly less TLR2 compared with healthy controls. However, keratinocytes from AD patients have been shown to respond less to TLR-2 stimulation compared with healthy control keratinocytes.78
5.2 |. Endothelial cells
Endothelial cells are cells that line the interior surface of blood vessels (Figure 3) and lymphatic vessels, forming a passive barrier between blood or lymph in the lumen and the vessel wall. Endothelial cells are not only a passive barrier, but they also play an important role in antimicrobial defense. For example, they attract and activate leucocytes to an inflammatory site, and help in firm adhesion of leucocytes to the endothelium, eventually resulting transendothelial migration towards the infected location. However, S. aureus can also invade endothelial cells and migrate to the vascular system from local infection sites. Entering the bloodstream can lead to infection of other tissues/organs, which is associated with poor prognosis.79 Many S. aureus strains have been shown to be able to invade endothelial cells and, depending on virulence factors related to the agr system (Figure 1A,B), hide in endothelial cells in vitro.80 Furthermore, SCVs of S. aureus within endothelial cells were demonstrated to withstand lysosomal degradation.81 Additionally, Perforin-2 can be responsible for elimination of intracellular S. aureus from endothelial cells.11,23,82
5.3 |. Fibroblasts
The main cells in the dermis of the skin are fibroblasts (Figure 3), which produce collagen and elastin proteins that provide structure and elasticity to the skin. Dermal fibroblasts also communicate with each other and other cell types playing an essential role in maintaining skin physiology.42 Even though S. aureus has been demonstrated to invade fibroblasts in vitro,10,26 invasion into dermal fibroblasts has not been studied. In mouse embryonic fibroblasts (MEFs) and 3T3 fibroblast–like cells, S. aureus has been shown effectively invade, multiply and induce apoptosis.10 Specifically, in MEFs, S. aureus has been demonstrated to become targeted by selective autophagy through ubiquitination and the receptor proteins SQSTM1, OPTN and CALCOCO2 (Figure 2).26 It is therefore worthwhile to study to what extend S. aureus invades and hides in dermal skin fibroblasts in human in vivo. This could be studied by, for example, examining immunostainings of skin biopsies derived from confirmed S. aureus–infected patients. However, keratinocytes and fibroblasts have been also shown to have intracellular defense mechanisms to eliminate S. aureus for which Perforin-2 expression is critical.11,22 Like keratinocytes and endothelial cells, Perforin-2 expression in fibroblasts requires induction by interferons.11 Therefore, it would be informative to study the expression levels of Perforin-2 in fibroblasts derived from early, established and chronic EB wounds. Alternatively, engineered wild-type/agr-positive or SCV-like/agr-deficient strains of S. aureus–expressing zsgreen56 could be colonized on the back of a mouse skin and imaged over time.
5.4 |. Effect of conventional
antibiotics and antimicrobial peptides on intracellular Staphylococcus aureus
Treatment of intracellular S. aureus infections is a serious challenge, as conventional antibiotics tend to remain in the extracellular space. Most antibiotics, such as β-lactams and aminoglycosides, hardly penetrate into a eukaryotic cell.83,84 Other types of antibiotics have an intracellular short time retention (macrolides), inadequate cellular distribution and/or low intracellular concentration (fluoroquinolones and vancomycin).84 The use of carrier systems for delivery of antibiotics into the eukaryotic cells might be a way to kill intracellular S. aureus and studies towards this approach currently ongoing.83,84
Another serious treatment challenge is the phenotypic switching of S. aureus towards SCVs (Figure 1A,B). Recently, it has been shown that antibiotic pressure induces phenotypic switching of intracellular S. aureus towards non-growing persistent S. aureus in APC.85 Intracellular phenotypic reversion towards the rapid growing wild-type phenotype was observed after removal of the antibiotic pressure.85 This may explain the clinical observation of relapsing infections at the end of antibiotic therapy. It also indicates that persistence is highly reversible and it seems to correlate with the dose and duration of antibiotic exposure.86,87 Therefore, additional studies towards the intracellular life cycle of S. aureus into skin cells are urgently needed.
Antibacterial peptide antibiotics are promising alternative drug candidates, because of their potential to cross the eukaryotic cell membrane and maintaining intracellular activity against S. aureus.88 Several in vitro studies have shown good intracellular antibacterial activity against several S. aureus strains.89–93 Importantly, two short antimicrobial synthetic peptides, WR12 and D-IK8, have been shown to kill non-growing persistent S. aureus.90 Furthermore, topical application of these peptides on mice infected with MRSA was effective in reducing bacterial load.90 Therefore, additional studies are needed to determine whether antimicrobial peptides are also effective to eliminate intracellular SCVs in vivo and whether there is a synergistic effect in combination with antibiotics and/or upregulation of Perforin-2.
6 |. FUTURE PERSPECTIVES
Staphylococcus aureus skin infections remain a major challenge to the health of human societies around the world. Although most S. aureus strains are recognized as extracellular pathogens, S. aureus can also invade, hide and persist into various types of professional and non-professional APC hosts.10 Consequently, the adaptive immune response towards S. aureus is affected in patients with chronic skin diseases, which may result in life threatening chronic non-healing wounds. Many in vitro studies have revealed intracellular immune escape routes taken by S. aureus (Figures 1, 2 and 3), but additional research is needed to confirm these findings in patients and in vivo animal models. Since S. aureus interferes with the AKT pathway for its own survival,25,26 more clarification is needed about the role of this pathway and S. aureus persistence. To find novel manipulative S. aureus targets of the AKT pathway, a small interference RNA and a small molecule screen, as described for Salmonella typhimurium, might be utilized.94 Another major open research question is whether ubiquitination of S. aureus can be manipulated so that it will be targeted for proteasomal degradation, which may result in MHC class I antigen presentation and immune recognition. Considering the intracellular life cycle of S. aureus in professional APCs, an open research question is how S. aureus affects TLR2 expression, which seems to result in enhanced susceptibility to S. aureus skin infection. Furthermore, since Perforin-2 is critical for intracellular killing of S. aureus, further investigation towards signalling pathways affecting the expression of this protein is necessary. Next to that, it would be important to gain more knowledge about the crosstalk between the interferon and the AKT pathway and S. aureus persistence. Gaining more knowledge into these pathways might result in the identification of molecules able to induce Perforin-2 expression in chronic non-healing wounds. These novel therapeutic approaches could be used in combination with antibiotics to treat S. aureus–infected chronic non-healing wounds in both paediatric and adult population. Additionally, clarification is needed regarding which APC is used most by S. aureus to hide in vivo in different cutaneous pathologies utilizing in vivo preclinical models56–58 and patient-derived biopsies. Furthermore, early detection of SCV-like strains from S. aureus skin wounds may be clinically important to predict which individuals are prone develop reoccurrence after antibiotic treatment. This could be done, for example, by analysing agr, α-haemolysin (Hla) or FnBp gene expression from human samples. Taken together, chronic wound infections are one of the most frequent complications of chronic dermatologic diseases and the prevalence of antibiotic and antimicrobial resistance is rising. Therefore, interfering with one or more of the intracellular escape strategies of S. aureus in persistent cutaneous infections will provide novel more efficient therapeutic approaches.
ACKNOWLEDGEMENTS
This work was supported in part by the grants from Department of Defense (W81XWH1810628) to OI and National Institute of Health (NR015649) to MTC and IP.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- [1].van der Kooi-Pol MM, Duipmans JC, Jonkman MF, van Dijl JM. Host-pathogen interactions in epidermolysis bullosa patients colonized with Staphylococcus aureus. Int J Med Microbiol. 2014;304:195–203. [DOI] [PubMed] [Google Scholar]
- [2].Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Miller LS, Cho JS. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol. 2011;11:505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nowicka D, Grywalska E Staphylococcus aureus and host immunity in recurrent furunculosis. Dermatology. 2019;235:295–305. [DOI] [PubMed] [Google Scholar]
- [5].Gajdacs M The continuing threat of methicillin-resistant Staphylococcus aureus. Antibiotics (Basel). 2019;8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Asadollahi P, Farahani NN, Mirzaii M, et al. Distribution of the most prevalent spa types among clinical isolates of methicillin-resistant and -susceptible Staphylococcus aureus around the world: a review. Front Microbiol. 2018;9:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].van der Kooi-Pol MM, Sadaghian Sadabad M, Duipmans JC, et al. Topography of distinct Staphylococcus aureus types in chronic wounds of patients with epidermolysis bullosa. PLoS One. 2013;8:e67272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].van der Kooi-Pol MM, Veenstra-Kyuchukova YK, Duipmans JC, et al. High genetic diversity of Staphylococcus aureus strains colonizing patients with epidermolysis bullosa. Exp Dermatol. 2012;21:463–466. [DOI] [PubMed] [Google Scholar]
- [9].Kalan LR, Meisel JS, Loesche MA, et al. Strain- and species-level variation in the microbiome of diabetic wounds is associated with clinical outcomes and therapeutic efficacy. Cell Host Microbe. 2019;25:641–655.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Loffler B, Tuchscherr L, Niemann S, Peters G Staphylococcus aureus persistence in non-professional phagocytes. Int J Med Microbiol. 2014;304:170–176. [DOI] [PubMed] [Google Scholar]
- [11].McCormack RM, de Armas LR, Shiratsuchi M, et al. Perforin-2 is essential for intracellular defense of parenchymal cells and phagocytes against pathogenic bacteria. Elife. 2015;4:e06508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Soong G, Paulino F, Wachtel S, et al. Methicillin-resistant Staphylococcus aureus adaptation to human keratinocytes. MBio. 2015;6:e00289–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kahl BC, Becker K, Loffler B. Clinical significance and pathogenesis of staphylococcal small colony variants in persistent infections. Clin Microbiol Rev. 2016;29:401–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Iwamoto K, Moriwaki M, Miyake R, Hide M Staphylococcus aureus in atopic dermatitis: strain-specific cell wall proteins and skin immunity. Allergol Int. 2019;68:309–315. [DOI] [PubMed] [Google Scholar]
- [15].Wong Fong Lung T, Monk IR, Acker KP, et al. Staphylococcus aureus small colony variants impair host immunity by activating host cell glycolysis and inducing necroptosis. Nat Microbiol. 2020;5:141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Geisinger E, Muir TW, Novick RP. agr receptor mutants reveal distinct modes of inhibition by staphylococcal autoinducing peptide. Proc Natl Acad Sci U S A. 2009;106:1216–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tuchscherr L, Medina E, Hussain M, et al. Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol Med. 2011;3:129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kipp F, Ziebuhr W, Becker K, et al. Detection of Staphylococcus aureus by 16S rRNA directed in situ hybridisation in a patient with a brain abscess caused by small colony variants. J Neurol Neurosurg Psychiatry. 2003;74:1000–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Proctor RA, van Langevelde P, Kristjansson M, Maslow JN, Arbeit RD. Persistent and relapsing infections associated with small-colony variants of Staphylococcus aureus. Clin Infect Dis. 1995;20:95–102. [DOI] [PubMed] [Google Scholar]
- [20].von Eiff C, Bettin D, Proctor RA, et al. Recovery of small colony variants of Staphylococcus aureus following gentamicin bead placement for osteomyelitis. Clin Infect Dis. 1997;25:1250–1251. [DOI] [PubMed] [Google Scholar]
- [21].Leimer N, Rachmuhl C, Palheiros Marques M, et al. Nonstable Staphylococcus aureus small-colony variants are induced by low pH and sensitized to antimicrobial therapy by phagolysosomal alkalinization. J Infect Dis. 2016;213:305–313. [DOI] [PubMed] [Google Scholar]
- [22].McCormack R, de Armas LR, Shiratsuchi M, Ramos JE, Podack ER. Inhibition of intracellular bacterial replication in fibroblasts is dependent on the perforin-like protein (perforin-2) encoded by macrophage-expressed gene 1. J Innate Immun. 2013;5:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Strbo N, Pastar I, Romero L, et al. Single cell analyses reveal specific distribution of anti-bacterial molecule Perforin-2 in human skin and its modulation by wounding and Staphylococcus aureus infection. Exp Dermatol. 2019;28:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kirkegaard K, Taylor MP, Jackson WT. Cellular autophagy: surrender, avoidance and subversion by microorganisms. Nat Rev Microbiol. 2004;2:301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schnaith A, Kashkar H, Leggio SA, Addicks K, Kronke M, Krut O Staphylococcus aureus subvert autophagy for induction of caspase-independent host cell death. J Biol Chem. 2007;282:2695–2706. [DOI] [PubMed] [Google Scholar]
- [26].Neumann Y, Bruns SA, Rohde M, Prajsnar TK, Foster SJ, Schmitz I. Intracellular Staphylococcus aureus eludes selective autophagy by activating a host cell kinase. Autophagy. 2016;12:2069–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Horn J, Stelzner K, Rudel T, Fraunholz M. Inside job: Staphylococcus aureus host-pathogen interactions. Int J Med Microbiol. 2018;308:607–624. [DOI] [PubMed] [Google Scholar]
- [28].Sharma V, Verma S, Seranova E, Sarkar S, Kumar D. Selective autophagy and xenophagy in infection and disease. Front Cell Dev Biol. 2018;6:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].McCormack R, Podack ER. Perforin-2/Mpeg1 and other pore-forming proteins throughout evolution. J Leukoc Biol. 2015;98:761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bai F, McCormack RM, Hower S, Plano GV, Lichtenheld MG, Munson GP. Perforin-2 breaches the envelope of phagocytosed bacteria allowing antimicrobial effectors access to intracellular targets. J Immunol. 2018;201:2710–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ni T, Jiao F, Yu X, et al. Structure and mechanism of bactericidal mammalian perforin-2, an ancient agent of innate immunity. Sci Adv. 2020;6:eaax8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pang SS, Bayly-Jones C, Radjainia M, et al. The cryo-EM structure of the acid activatable pore-forming immune effector macrophage-expressed gene 1. Nat Commun. 2019;10:4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kolata JB, Kuhbandner I, Link C, et al. The fall of a dogma? Unexpected high T-cell memory response to Staphylococcus aureus in humans. J Infect Dis. 2015;212:830–838. [DOI] [PubMed] [Google Scholar]
- [34].McCormack R, Bahnan W, Shrestha N, et al. Perforin-2 protects host cells and mice by restricting the vacuole to cytosol transitioning of a bacterial pathogen. Infect Immun. 2016;84:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rock KL, Reits E, Neefjes J. Present yourself! By MHC class I and MHC class II molecules. Trends Immunol. 2016;37:724–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kaufmann SH. Role of T-cell subsets in bacterial infections. Curr Opin Immunol. 1991;3:465–470. [DOI] [PubMed] [Google Scholar]
- [37].Proctor RA. Challenges for a universal Staphylococcus aureus vaccine. Clin Infect Dis. 2012;54:1179–1186. [DOI] [PubMed] [Google Scholar]
- [38].Dillen CA, Pinsker BL, Marusina AI, et al. Clonally expanded γδ T cells protect against Staphylococcus aureus skin reinfection. J Clin Invest. 2018;128:1026–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pastar I, O’Neill K, Padula L, et al. Staphylococcus epidermidis boosts innate immune response by activation of gamma delta T cells and induction of perforin-2 in human skin. Front Immunol. 2020;11:550946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang J, Roderiquez G, Norcross MA. Control of adaptive immune responses by Staphylococcus aureus through IL-10, PD-L1, and TLR2. Sci Rep. 2012;2:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Patil NK, Luan L, Bohannon JK, Hernandez A, Guo Y, Sherwood ER. Frontline science: anti-PD-L1 protects against infection with common bacterial pathogens after burn injury. J Leukoc Biol. 2018;103:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Feuerstein R, Kolter J, Henneke P. Dynamic interactions between dermal macrophages and Staphylococcus aureus. J Leukoc Biol. 2017;101:99–106. [DOI] [PubMed] [Google Scholar]
- [43].Singh SK Staphylococcus aureus intracellular survival: a closer look in the process. Virulence. 2017;8:1506–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jubrail J, Morris P, Bewley MA, et al. Inability to sustain intraphagolysosomal killing of Staphylococcus aureus predisposes to bacterial persistence in macrophages. Cell Microbiol. 2016;18:80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Guerriero JL. Macrophages: their untold story in T cell activation and function. Int Rev Cell Mol Biol. 2019;342:73–93. [DOI] [PubMed] [Google Scholar]
- [46].Nassiri S, Zakeri I, Weingarten MS, Spiller KL. Relative expression of proinflammatory and antiinflammatory genes reveals differences between healing and nonhealing human chronic diabetic foot ulcers. J Invest Dermatol. 2015;35:1700–1703. [DOI] [PubMed] [Google Scholar]
- [47].Khanna S, Biswas S, Shang Y, et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010;4:e9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Deusenbery CB, Kalan L, Meisel JS, Gardner SE, Grice EA, Spiller KL. Human macrophage response to microbial supernatants from diabetic foot ulcers. Wound Repair Regen. 2019;27:598–608. [DOI] [PubMed] [Google Scholar]
- [49].Niebuhr M, Lutat C, Sigel S, Werfel T. Impaired TLR-2 expression and TLR-2-mediated cytokine secretion in macrophages from patients with atopic dermatitis. Allergy. 2009;64:1580–1587. [DOI] [PubMed] [Google Scholar]
- [50].Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol. 2018;9:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Brandling-Bennett HA, Morel KD. Common wound colonizers in patients with epidermolysis bullosa. Pediatr Dermatol. 2010;27:25–28. [DOI] [PubMed] [Google Scholar]
- [52].van der Aar AM, Picavet DI, Muller FJ, et al. Langerhans cells favor skin flora tolerance through limited presentation of bacterial antigens and induction of regulatory T cells. J Invest Dermatol. 2013;133:1240–1249. [DOI] [PubMed] [Google Scholar]
- [53].van Dalen R, De La Cruz Diaz JS, Rumpret M, et al. Langerhans cells sense Staphylococcus aureus wall teichoic acid through langerin to induce inflammatory responses. MBio. 2019;10:e00330–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Paternoster L, Standl M, Waage J, et al. Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat Genet. 2015;47:1449–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Stojadinovic O, Yin N, Lehmann J, Pastar I, Kirsner RS, Tomic-Canic M. Increased number of langerhans cells in the epidermis of diabetic foot ulcers correlates with healing outcome. Immunol Res. 2013;57:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Leech JM, Dhariwala MO, Lowe MM, et al. Toxin-triggered interleukin-1 receptor signaling enables early-life discrimination of pathogenic versus commensal skin bacteria. Cell Host Microbe. 2019;26:795–809.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Archer NK, Wang Y, Ortines RV, et al. Preclinical models and methodologies for monitoring Staphylococcus aureus infections using noninvasive optical imaging. Methods Mol Biol. 2020;2069:197–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Miller RJ, Crosby HA, Schilcher K, et al. Development of a Staphylococcus aureus reporter strain with click beetle red luciferase for enhanced in vivo imaging of experimental bacteremia and mixed infections. Sci Rep. 2019;9:16663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Iwamoto K, Numm TJ, Koch S, Herrmann N, Leib N, Bieber T. Langerhans and inflammatory dendritic epidermal cells in atopic dermatitis are tolerized toward TLR 2 activation. Allergy. 2018;73:2205–2213. [DOI] [PubMed] [Google Scholar]
- [60].Dejani NN, Brandt SL, Pineros A, et al. Topical prostaglandin E analog restores defective dendritic cell–mediated Th17 host defense against methicillin-resistant Staphylococcus aureus in the skin of diabetic mice. Diabetes. 2016;65:3718–3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gardner SE, Hillis SL, Heilmann K, Segre JA, Grice EA. The neuropathic diabetic foot ulcer microbiome is associated with clinical factors. Diabetes. 2013;62:923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].van Asten SA, La Fontaine J, Peters EJ, Bhavan K, Kim PJ, Lavery LA. The microbiome of diabetic foot osteomyelitis. Eur J Clin Microbiol Infect Dis. 2016;35:293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Romero Pastrana F, Neef J, Koedijk D, et al. Human antibody responses against non-covalently cell wall-bound Staphylococcus aureus proteins. Sci Rep. 2018;8:3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].van der Kooi-Pol MM, de Vogel CP, Westerhout-Pluister GN, et al. High anti-staphylococcal antibody titers in patients with epidermolysis bullosa relate to long-term colonization with alternating types of Staphylococcus aureus. In vivo and in vitro profiling of global interactions between Staphylococcus aureus and its human host. J Invest Dermatol. 2013;133:847–850. [DOI] [PubMed] [Google Scholar]
- [65].Schaffer AC, Lee JC. Staphylococcal vaccines and immunotherapies. Infect Dis Clin North Am. 2009;23:153–171. [DOI] [PubMed] [Google Scholar]
- [66].Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. 2018;16:143–155. [DOI] [PubMed] [Google Scholar]
- [67].Aufiero B, Guo M, Young C, et al. Staphylococcus aureus induces the expression of tumor necrosis factor-α in primary human keratinocytes. Int J Dermatol. 2007;46:687–694. [DOI] [PubMed] [Google Scholar]
- [68].Sayedyahossein S, Xu SX, Rudkouskaya A, McGavin MJ, McCormick JK, Dagnino L Staphylococcus aureus keratinocyte invasion is mediated by integrin-linked kinase and Rac1. FASEB J. 2015;29:711–723. [DOI] [PubMed] [Google Scholar]
- [69].Klicznik MM, Szenes-Nagy AB, Campbell DJ, Gratz IK. Taking the lead–how keratinocytes orchestrate skin T cell immunity. Immunol Lett. 2018;200:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Edwards AM, Potter U, Meenan NA, Potts JR, Massey RC Staphylococcus aureus keratinocyte invasion is dependent upon multiple high-affinity fibronectin-binding repeats within FnBPA. PLoS One. 2011;6:e18899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kintarak S, Whawell SA, Speight PM, Packer S, Nair SP. Internalization of Staphylococcus aureus by human keratinocytes. Infect Immun. 2004;72:5668–5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bal V, McIndoe A, Denton G, et al. Eur J Immunol. Antigen presentation by keratinocytes induces tolerance in human T cells. 1990;20:1893–1897. [DOI] [PubMed] [Google Scholar]
- [73].Gaspari AA, Jenkins MK, Katz SI. Class II MHC-bearing keratinocytes induce antigen-specific unresponsiveness in hapten-specific Th1 clones. J Immunol. 1988;141:2216–2220. [PubMed] [Google Scholar]
- [74].Tamoutounour S, Han SJ, Deckers J, et al. Keratinocyte-intrinsic MHCII expression controls microbiota-induced Th1 cell responses. Proc Natl Acad Sci U S A. 2019;116:23643–23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Black AP, Ardern-Jones MR, Kasprowicz V, et al. Human keratinocyte induction of rapid effector function in antigen-specific memory CD4+ and CD8+ T cells. Eur J Immunol. 2007;37:1485–1493. [DOI] [PubMed] [Google Scholar]
- [76].Moriwaki M, Iwamoto K, Niitsu Y, et al. Staphylococcus aureus from atopic dermatitis skin accumulates in the lysosomes of keratinocytes with induction of IL-1α secretion via TLR 9. Allergy. 2019;74:560–571. [DOI] [PubMed] [Google Scholar]
- [77].Beck LA, Thaci D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130–139. [DOI] [PubMed] [Google Scholar]
- [78].Niebuhr M, Heratizadeh A, Wichmann K, Satzger I, Werfel T. Intrinsic alterations of pro-inflammatory mediators in unstimulated and TLR-2 stimulated keratinocytes from atopic dermatitis patients. Exp Dermatol. 2011;20:468–472. [DOI] [PubMed] [Google Scholar]
- [79].Chavakis T, Wiechmann K, Preissner KT, Herrmann M Staphylococcus aureus interactions with the endothelium. Thromb Haemost. 2005;94:278–285. [DOI] [PubMed] [Google Scholar]
- [80].Grundmeier M, Tuchscherr L, Bruck M, et al. Staphylococcal strains vary greatly in their ability to induce an inflammatory response in endothelial cells. J Infect Dis. 2010;201:871–880. [DOI] [PubMed] [Google Scholar]
- [81].Schroder A, Kland R, Peschel A, von Eiff C, Aepfelbacher M. Live cell imaging of phagosome maturation in Staphylococcus aureus infected human endothelial cells: small colony variants are able to survive in lysosomes. Med Microbiol Immunol. 2006;195:185–194. [DOI] [PubMed] [Google Scholar]
- [82].Tomic-Canic M, Burgess JL, O’Neill K, Strbo N, Pastar I. Skin microbiota and its interplay with wound healing. Am J Clin Dermatol. 2020;21:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Briones E, Colino CI, Lanao JM. Delivery systems to increase the selectivity of antibiotics in phagocytic cells. J Control Release. 2008;125:210–227. [DOI] [PubMed] [Google Scholar]
- [84].Zhou K, Li C, Chen D, et al. A review on nanosystems as an effective approach against infections of Staphylococcus aureus. Int J Nanomedicine. 2018;13:7333–7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Peyrusson F, Varet H, Nguyen TK, et al. Intracellular Staphylococcus aureus persisters upon antibiotic exposure. Nat Commun. 2020;11:2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Barcia-Macay M, Seral C, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob Agents Chemother. 2006;50:841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Nguyen HA, Denis O, Vergison A, et al. Intracellular activity of antibiotics in a model of human THP-1 macrophages infected by a Staphylococcus aureus small-colony variant strain isolated from a cystic fibrosis patient: pharmacodynamic evaluation and comparison with isogenic normal-phenotype and revertant strains. Antimicrob Agents Chemother. 2009;53:1434–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Pfalzgraff A, Brandenburg K, Weindl G. Antimicrobial peptides and their therapeutic potential for bacterial skin infections and wounds. Front Pharmacol. 2018;9:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Brinch KS, Tulkens PM, Van Bambeke F, Frimodt-Moller N, Hoiby N, Kristensen HH. Intracellular activity of the peptide antibiotic NZ2114: studies with Staphylococcus aureus and human THP-1 monocytes, and comparison with daptomycin and vancomycin. J Antimicrob Chemother. 2010;65:1720–1724. [DOI] [PubMed] [Google Scholar]
- [90].Mohamed MF, Abdelkhalek A, Seleem MN. Evaluation of short synthetic antimicrobial peptides for treatment of drug-resistant and intracellular Staphylococcus aureus. Sci Rep. 2016;6:29707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Noore J, Noore A, Li B. Cationic antimicrobial peptide LL-37 is effective against both extra-and intracellular Staphylococcus aureus. Antimicrob Agents Chemother. 2013;57:1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wang X, Wang X, Teng D, et al. Increased intracellular activity of MP1102 and NZ2114 against Staphylococcus aureus in vitro and in vivo. Sci Rep. 2018;8:4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Zhang P, Wright JA, Tymon A, Nair SP. Bicarbonate induces high-level resistance to the human antimicrobial peptide LL-37 in Staphylococcus aureus small colony variants. J Antimicrob Chemother. 2018;73:615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Albers HM, Kuijl C, Bakker J, et al. Integrating chemical and genetic silencing strategies to identify host kinase-phosphatase inhibitor networks that control bacterial infection. ACS Chem Biol. 2014;9:414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]


