Abstract
The prevalence of rheumatic and musculoskeletal diseases (RMDs) including osteoarthritis (OA) and low back pain (LBP) in aging societies present significant cost burdens to health and social care systems. Intervertebral disc (IVD) degeneration, which is characterized by disc dehydration, anatomical alterations, and extensive changes in extracellular matrix (ECM) composition, is an important contributor to LBP. IVD cell homeostasis can be disrupted by mitochondrial dysfunction. Mitochondria are the main source of energy supply in IVD cells and a major contributor to the production of reactive oxygen species (ROS). Therefore, mitochondria represent a double-edged sword in IVD cells. Mitochondrial dysfunction results in oxidative stress, cell death, and premature cell senescence, which are all implicated in IVD degeneration. Considering the importance of optimal mitochondrial function for the preservation of IVD cell homeostasis, extensive studies have been done in recent years to evaluate the efficacy of small molecules targeting mitochondrial dysfunction. In this article, we review the pathogenesis of mitochondrial dysfunction, aiming to highlight the role of small molecules and a selected number of biological growth factors that regulate mitochondrial function and maintain IVD cell homeostasis. Furthermore, molecules that target mitochondria and their mechanisms of action and potential for IVD regeneration are identified. Finally, we discuss mitophagy as a key mediator of many cellular events and the small molecules regulating its function.
Keywords: Intervertebral disc (IVD), Degeneration, Mitochondrial dysfunction, Small molecule, Therapeutic, Mitophagy, Growth factor
Background
Low back pain (LBP) is one of the leading causes of disability worldwide and IVD degeneration is postulated to be the main pathogenic process involved [1, 2]. In a healthy IVD, the balance between anabolic and catabolic processes maintains ECM homeostasis; however, aging and persistent mechanical stress can disrupt IVD metabolism forcing an imbalance between the expression of catabolic factors (such as pro-inflammatory cytokines and matrix metalloproteinases), and anabolic mediators (such as growth factors), eventually leading to the loss of ECM homeostasis, destruction of macromolecules, and the subsequent development of IVD degeneration [3–5]. The pathophysiology of IVD degeneration is characterized by the loss of ECM components including collagen fibers and proteoglycans as well as the loss of cellularity [6].
Mitochondria perform crucial functions including energy production and intracellular calcium ion Ca2+ (homeostasis, regulation of apoptosis, and innate immunity). However, their primary function is related to energy production. They are the cell’s main powerhouse, providing metabolic energy in the form of adenosine triphosphate (ATP) molecules [7, 8]. Because of hypoxic conditions in the microenvironment of the IVD, ATP production mainly relies on glycolysis [9, 10]. Hypoxia-inducible factor 1-α (HIF-1α) is upregulated under hypoxia and mediates glycolysis-oxidative phosphorylation (OXPHOS) interplay to regulate metabolism [9]. Mechanical overload alters mitochondrial membrane potential (∆Ψm) in IVD cells. Mitochondria with disrupted membrane potential further mediate cell proliferation, induce apoptosis, and lead to IVD degeneration [11, 12]
Mitochondria are also the main producers of highly reactive radical species specifically reactive oxygen species (ROS) in cells. Accumulation of ROS alters the cellular metabolism, ATP production, and the synthesis of both nuclear and mitochondrial DNA encoded genes [13]. Accumulation of excessive ROS also leads to oxidative stress resulting in mitochondrial dysfunction [14]. Depolarized mitochondria are characterized by the loss of ∆Ψm that precedes intrinsic apoptosis [15]. Dysfunctional mitochondria fail to supply cellular energy homeostasis, which is an incident in many age-related and inflammatory joint diseases. Depolarized mitochondria are also susceptible to Ca2+ overload which contributes to programmed cell death such as apoptosis [16]. Reversing mitochondrial dysfunction can potentially counteract the oxidative stress and consequent cell death that can accelerate IVD degeneration [17]. Hence, the regulation of mitochondrial function is an emerging approach to delay IVD degeneration.
Mitochondria-driven ROS are mainly produced at If and IQ sites of complex I during electron transport to coenzyme Q (CoQ) and in a lower degree at QI site of complex III in the presence of ubisemiquinone [18, 19]. Electron transfer chain (ETC) is not always 100% efficient and up to 4% electron leakage happens before reaching the final electron acceptor in cytochrome c oxidase (complex IV) [20]. Superoxide as the proximal mitochondrial ROS is produced when the leaked electron is caught by an oxygen molecule. Superoxide is a highly reactive molecule with a short lifespan. It is dismutated to the more stable molecule, hydrogen peroxide (H2O2). Superoxide dismutase (SOD) enzyme isoforms, SOD1, SOD2, and SOD3 convert superoxide to hydrogen peroxide in cytosol, mitochondria, and ECM, respectively [21]. Hydrogen peroxide can later be decomposed to water by enzymes with anti-oxidant activity or turns to hydroxyl radical (OH°) and hydroxide ion (OH−) via the Fenton reaction [22]. Hydroxyl radical is found as the main ROS in cultured IVD cells [22]. Moreover, can react with nitroxide radical (NO°) and form the highly potent oxidant, peroxynitrite (ONOO−). Peroxynitrite is more potent than NO° as a degenerative signal in chondrocytes and progression of IVD degeneration [23, 24].
None of the clinically available LBP treatments including conservative treatments and surgical interventions addresses the regeneration of degenerated IVD though they may manage the pain and alleviate the symptoms [25–27]. Molecular therapy as a branch of regenerative medicine has been utilized to address the available treatment shortcomings [28]. The efficacy of various growth factor-based therapeutics as well as biopolymers for the treatment of degenerate and age-associated disc diseases has been extensively investigated. For instance, calcitonin, which is a thyroid gland-secreted polypeptide hormone, induces more vitamin D production and inhibits bone resorption [29, 30]. Calcitonin is thought to suppress IVD degeneration through maintaining matrix homeostasis in the IVD and inhibiting bone loss in the adjacent vertebral body [31–33]. Growth factors such as transforming growth factor-β1 (TGF-β1), bone morphogenetic proteins (BMPs), and basic fibroblast growth factor (bFGF) are other examples of proteins with pro-regenerative [34]. TGF-β1 can reverse mitochondrial dysfunction and abate oxidative stress in AF cells and decrease mitochondria-mediated apoptosis [35]. Insulin and insulin-like growth factor-1 (IGF-1) have been demonstrated to induce chondrogenic differentiation and reduce the progression of IVD degeneration in vitro and in vivo, and also in animal models of type 2 diabetes mellitus [36–39]. Growth factors possess therapeutic properties. However, there are a number of important shortcomings associated with their use and extensive discussion of the role of growth factors and small peptides is beyond the scope of this review article. Therefore, this review focuses on the role of small molecules as novel therapeutic agents for IVD degeneration.
Using small molecules may be a promising approach to potentially regulate intracellular signaling pathways and target mechanisms underlying common diseases [40]. Thus far, inflammation-targeting molecules and ROS scavenging therapeutic agents have been reviewed in the field of IVD degeneration [41, 42], aiming to provide a broader perspective of basic studies that have been done to evaluate the efficacy of small molecules regulating mitochondrial function and their underlying mechanisms to control IVD degeneration progression both in vitro and in vivo. Finally, the role of mitochondrial autophagy (mitophagy) in mitochondrial dysfunction and its regulatory molecules are discussed.
Mitochondrial dysfunction in IVD degeneration
Interplay between mitochondrial dysfunction-oxidative stress
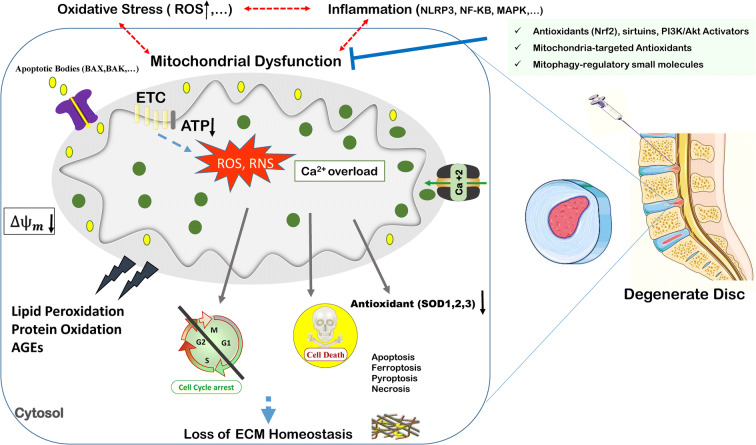
In addition to their pivotal role as energy providers, mitochondria are the main source of ROS generation in cells [43]. ROS including free radicals and non-radical molecules as well as reactive nitrogen species (RNS) are produced as byproducts of electron transfer in the mitochondrial respiratory chain (MRC) [18, 19]. Physiological levels of ROS and RNS are beneficial through mediating cellular redox signaling and homeostasis [44, 45]. Nonetheless, excessive production of ROS and RNS or impaired anti-oxidant defense mechanism induces oxidative stress. Oxidative stress can depolarize the mitochondrial membrane, leading to electron leakage, the release of apoptotic molecules, cellular Ca2+ overload, and cellular metabolism balance perturbation through ATP production imbalance [46–48]. Conversely, dysfunctional mitochondria with dissipated membrane potential exhibit exacerbated cellular stress and mediate multiple adverse cellular events including lipid peroxidation and protein oxidation, cell senescence, and cell death [49], indicating the interplay between mitochondrial dysfunction and induction of oxidative stress (Fig. 1). The level of malondialdehyde (MDA) and advanced oxidation protein products (AOPP) as lipid peroxidation and protein oxidation biomarkers has been demonstrated to be enhanced with age in IVD tissue of rats, while the level of SOD decreases [50]. Advanced glycation end products (AGEs) are a type of sugar-modified proteins, lipids, and nucleic acids, which are found in IVD. Among the identified AGEs, carboxymethyl lysine (CML), pentosidine, and methylglyoxal have been found in degenerate IVD [51–53]. Song et al. reported that AGE-induced oxidative stress targeted the mitochondrial sirtuin, sirtuin 3, impaired its function, and led to mitochondria-mediated apoptosis in human-derived NP cells. Mitochondrial redox homeostasis preservation via restoring sirtuin 3 function, nevertheless, inhibited apoptosis and alleviated IVD degeneration [54].
Fig. 1.
Consequences of intervertebral disc degeneration. The interplay between mitochondrial dysfunction, oxidative stress, and inflammation
Senescence is another pathophysiological consequence of oxidative stress and is implicated in mitochondrial dysfunction. Senescent cells are non-proliferative, apoptosis-resistant cells with dysregulated metabolic activity, arisen from persistent stress and cellular damage [55]. Many factors including telomere shortening, endogenous- and exogenous-driven stressors, and mitochondrial dysfunction are implicated in the cell entrance to irreversible growth arrest state or the so-called senescence [55]. The telomere-based P53-P21-PRB and stress-induced P16-PRB are the central pathways regulating cell senescence [55]. Aging correlates with mitochondrial dysfunction and accumulation of senescent cells. Nucleus pulposus (NP) samples from patients with degenerate IVD demonstrate more senescent cells with higher P53 and P21 expression and SA-β-gal activity, lower telomere length from old cases relative to younger patients [56]. Mitochondria directly control the P16-RPB pathway of senescence in a P38/MAPK (mitogen-activated protein kinase)-dependent manner and indirectly phosphorylate and activate P53 by inducing DNA damage response as well [57]. Given the significance of mitochondrial dysfunction in cell senescence, preserving ∆Ψm is an approach to protect mitochondrial function and decelerate cell senescence. ∆Ψm preservation inhibits excessive mitophagy and the loss of mitochondria [58]. Mitochondrial dysfunction and cellular senescence are implicated in the process of IVD aging [59]. ∆Ψm declination with age and proton leakage enhancement has been observed in IVD cells [47]. ATP production declines with aging in NP and annulus fibrosis (AF) cells [47]. Hartman et al. showed that mitochondria undergo morphological changes with aging as the number of mitochondria reduces while mean volume per mitochondria is enhanced in NP cells [47]. In contrast, enhancement of ATP synthase activity, ATP production, and mitochondrial numbers were reported in senescent NP cells [60]. Taken together, there is an interplay between mitochondrial dysfunction, aging, and cell senescence in the onset and progression of IVD degeneration; however, it is presently unclear as to which process comes first.
Mitochondrial dysfunction-inflammation cross talk—the role of NLRP3 inflammasome
IVD degeneration is characterized by high ROS content, impaired anti-oxidant activity, and inflammatory responses. Oxidative stress, especially driven from mitochondrial dysfunction, and inflammation are mechanistically linked and eventually lead to IVD degeneration [46, 49, 61] (Fig. 1). The inflammasome consists of a multiprotein complex which recognizes exogenous pathogenic factors including microorganisms, toxins, lipopolysaccharide (LPS), radiation, oxidative stress as well as endogenous danger signals, and mediates innate immune response [62, 63]. Nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain-containing protein (NLRPs) inflammasomes are a distinct sub-type of NOD-like receptors (NLRs) inflammasome, a cytoplasmic set of pattern-recognition receptors (PRRs), which recognize pathogen-associated molecular patterns/damage-associated molecular patterns (PAMPs/DAMPs) through toll-like receptors (TLRs) and stimulate immune response [62, 64, 65]. NLRPs comprise of fourteen isoforms, NLRP 1-14, in which the NLRP3 inflammasome is the most well-known NLRP [62]. NLRP3 inflammasome consists of an NLRP3 scaffold, a cysteine protease and proinflammatory caspase, procaspase 1, and apoptosis-associated speck-like protein, containing a caspase-recruitment domain (ASC) adaptor. NLRP3-ASC interaction and subsequent activation of procaspase 1 mediate activation and maturation of proinflammatory cytokines such as pro-IL-1β and pro-IL-18 which induce inflammatory response including NF-κB activation and lead to a type of cell death called “pyroptosis” [62, 63, 66]. Activation of the NLRP3 inflammasome requires two critical steps: priming and activation. Upon inflammatory, toxic, or microbial stimulus, NLRP3 priming occurs. Deubiquitylated NLRP3 and caspase1 make an association with mitochondria in a ROS-dependent manner and they independently bind to the externalized cardiolipin in the outer mitochondrial membrane (OMM). ASC recruitment to mitochondria and activation of NLRP3 inflammasome complex occurs in a Ca2+-dependent way [67, 68]. In addition to the pro-inflammatory signal, mtROS has been reported to be needed for NLRP3 priming [69]. Several mechanisms have been introduced for NLRP3 activation. NLRP3 may be activated in a ROS-dependent way by ROS producers such as ATP and silica and/or in a ROS-independent way by NLRP3 agonist, linezolid [70]. Thioredoxin-interacting protein (TXNIP) is the inhibitor of thioredoxin, a cytoprotective oxidoreductase with ROS scavenging activity. TXNIP expression leads to oxidative stress and is implicated in inflammation and mitochondrial damage [71]. NLRP3 binding of TXNIP led to inflammatory response, oxidative damage, and cell death in the hyperglycemic animal model [71]. In the normal condition, TXNIP is located in the nucleus. Upon oxidative stress, however, TXNIP translocates to cytosol and mitochondria and binds to TRX1 and TRX2, respectively. TXNIP facilitates NLRP3 inflammasome activation either by binding to NLRP3 or translocation to mitochondria and aggravates mitochondrial dysfunction. In the latter, the release of DAMPS to cytosol and recognition by NLRP3 is supposed to stimulate inflammatory response [72]. It has been reported that NLRP3 and TXNIP were activated in patients with degenerate disc [73]. TXNIP/NLRP3 activation led to increased apoptosis in NP cells, indicating the detrimental effect of the inflammatory pathway on IVD cell homeostasis [73]. In another study, NLRP3 inflammasome activation was seen in patients with LBP and the grade of cartilage endplate (CEP) degeneration was correlated with the level of inflammasome complex expression [74]. It was demonstrated that oxidative stress elevated mtROS content, MDA level, and activated TXNIP/NLRP3 signaling pathway as well [74, 75].
Small molecules targeting mitochondrial dysfunction in IVD degeneration
Phenolic compounds
Curcumin
Curcumin extracted from Curcuma longa exerts anti-inflammatory and anti-oxidative properties in different pathobiological states [76–78]. Curcumin can inhibit the NF-κB and MAPK pathways and has been shown to reduce the activity of pro-inflammatory cytokines in degenerate IVD [76, 77]. Posing the ROS scavenging property, curcumin protects mitochondrial homeostasis via inhibition of peroxidase formation and prevention of oxidative damage [78]. Curcumin exerts either pro-apoptotic or anti-apoptotic actions, depending on the context of its application and the cell type that is being studied [79, 80]. Curcumin has been suggested to support disc homeostasis and ameliorates its degeneration via protecting mitochondria and autophagic induction [14]. Kang et al. reported curcumin treatment (˂ 25 μM) reduced ROS production, restored mitochondrial ∆Ψm, and increased ATP production in stress-induced NP cells. Upregulation of Bcl-2 expression and downregulation of Bax, cytosolic cytochrome c, and caspase 9 expression were also shown, indicating the anti-apoptotic effect of curcumin on NP cells in a dose-dependent manner [14].
Tri-hydroxy stilbenes
Resveratrol (3,5,4’-trihydroxystilbene) is a naturally occurring phytoestrogen and hydroxylated derivative of stilbene [81, 82]. Resveratrol has been shown to reduce the expression of pro-inflammatory cytokines, TNF-α and IL-1β, in a radiculopathy pain model [83]. It also preserved cytoskeletal structure, scavenged ROS, and restored lost ∆Ψm which resulted in decreased apoptosis [84]. It was shown resveratrol prevented the loss of ∆Ψm, cellular ATP, and apoptosis against oxidative damage, and enhanced matrix biosynthesis in vivo [85]. The anti-degenerative property of resveratrol on NP cells has been attributed to the autophagy-inducing property of resveratrol through PI3K/Akt signaling pathway [86]. Different strategies have been applied to improve the metabolic stability and bioavailability of resveratrol using carriers and resveratrol derivatives [87]. Polydatin (piceid or resveratrol-3-O-d-glucoside) is a glucoside form of resveratrol with the same properties as well as improved resveratrol drawbacks [88, 89]. Polydatin exerts anti-oxidant and mitochondrial protection through sirtuin 1 upregulation and activation of nuclear factor-erythroid 2-related factor 2/anti-oxidant response element (NRF2/ARE) signaling pathway [90]. In vivo studies have demonstrated that polydatin attenuates the progression of IVD degeneration and enhances matrix biosynthesis and cell proliferation via Nrf2 signaling activation [49]. Restored ∆Ψm and decrease of ROS production and MDA level were also reported in polydatin-treated CEP chondrocytes [91].
Flavonoids
Naringenin and its glucoside derivative, naringin, belong to the flavanone subclass of flavonoids [92]. Tomatoes and citrus fruits including oranges and grapefruit are rich in naringenin and naringin [93]. These compounds exert anti-apoptotic and mitochondrial protective effects via activation of PI3K/Akt signaling pathway [94]. Nan et al. demonstrated that naringin can prevent mitochondrial damage and energy deprivation in stress-induced NP-derived mesenchymal stem cells which was attributed to PI3K/Akt signaling pathway [94]. Epigallocatechin-3-gallate (EGCG) is a catechin found abundantly in green tea (Camellia Sinensis) with negligible cytotoxicity when concentration is less than 50 μM [95]. EGCG protected ∆Ψm level against oxidative stress and acted as a pro-survival agent in NP cells via activation of PI3K/Akt signaling pathway [95]. Icariin, isolated from herba epimedii or horny goat weed, is a bioactive and peroxylated flavonol glycoside compound, which has been studied for the treatment of degenerative diseases of articular cartilage [96]. Icariin’s anti-oxidative and mitochondrial protective effects are attributed to the activation of PI3K/Akt and Nrf2 signaling pathways, resulting in ∆Ψm restoration, decrease of ROS production, and apoptosis in NP cells [96, 97]. Furthermore, it was supposed a probable cross talk between Nrf2 and PI3K/Akt for attenuating the mitochondrial damage and preserving the cellular redox homeostasis [97]. Nonetheless, because of rapid intestinal metabolism, icariin utilization is hindered by low bioavailability. Thus, controlled release systems have been introduced in recent years to deliver icariin more efficiently [98].
Other compounds with anti-oxidant activity
NAC
N-Acetyl-l-cysteine or N-acetylcysteine (NAC) is a thiol-containing anti-oxidant with anti-inflammatory, mucolytic, and acetaminophen detoxification activities [99, 100]. NAC exerts its anti-oxidant properties directly by scavenging free radicals such as hydroxyl radical through thiol interaction at a high rate. NAC as a precursor of cysteine enhances glutathione activity in the body, the master anti-oxidant in cells, and the anti-oxidant index, glutathione/oxidized glutathione (GSH/GSSG), is upregulated by NAC treatment [100]. The influence of NAC on cell senescence and apoptosis is controversial, depending on the cell type and the applied experimental conditions [101, 102]. NAC also counteracts mitochondrial dysfunction via enhancing ETC activity and ATP production [17]. The chondroprotective effects of NAC, as determined by the restoration of ∆Ψm, increase the GSH/GSSG ratio, which are lost in a harsh microenvironment characterized by hypoxia, low pH, and high levels of pro-inflammatory inducers [103]. NAC demonstrated cell proliferative and anti-senescent properties in stress-induced NP cells via regulating P16 and P53 expression [104, 105]. Though oral administration of NAC for evaluations in mouse models with degenerate IVD has been exploited, its oral bioavailability is low and is mostly eliminated from the body [106]. Therefore, utilization of vehicles for controlled release and stabilization of NAC has been suggested.
Melatonin
Melatonin is released from the pineal gland and regulates circadian rhythm [107]. Enzymatic production of melatonin concisely includes acetylation of serotonin in the presence of serotonin N-acetyl transferase and formation of melatonin via methylation of acetylated serotonin in the presence of hydroxindole-o-methyl transferase [108]. Melatonin deficiency is implicated in the mitochondrial dysfunction and aging [109, 110]. Because of its amphiphilic nature, melatonin can easily penetrate the plasma membrane and reach various subcellular organelles including the nucleus and mitochondria [111]. The proliferative, anti-apoptotic properties and autophagic induction of melatonin in AF cells have also been reported. Melatonin may act as a sirtuin 1 agonist and inhibits apoptosis and calcification in CEP chondrocytes [112]. Findings indicate that there is a correlation between aging, IVD degeneration, and reduction of melatonin secretion. It is hypothesized that melatonin retards the process of aging through its anti-oxidative and free radical scavenging properties as well as prevention of oxidative damage accumulation in cells [113]. Melatonin increased the activity of complexes I and IV of ETC and helped mitochondrial act properly to ameliorate oxidative stress in IVD cells via restoring mitochondrial ∆Ψm, ATP production enhancement, reducing cytochrome c leakage to the cytosol, and apoptosis subsequently [111, 114, 115]. He et al. demonstrated melatonin mitigated the apoptosis in stress-induced NP cells through restoring the lost ∆Ψm and inhibition of cytochrome c release from mitochondria [114]. Chen et al. obtained similar outcomes after melatonin treatment of oxidative-damaged cells. The level of ∆Ψm is reduced after tert-butyl hydroperoxide (TBHP) treatment; however, melatonin protected the mitochondrial function via upregulation of Bcl-2 and downregulation of Bax, cytosolic cytochrome c, and caspase 3 expression [115]. Melatonin inhibited mitochondrial-induced apoptosis, improved mitochondrial membrane potential, and enhanced the ECM synthesis in TBHP-treated NP cells. The mitochondrial protective effects of melatonin arise from mitophagy induction in a parkin-dependent manner. Conversely, either cyclosporine A (CsA), a mitophagy inhibitor, or parkin knockdown blunts the protective effects of melatonin [115]. Melatonin also showed the ability to prevent NLRP3 inflammasome priming and activation and inhibited IL-1β secretion [116]. In vitro IL-1β stimulation of NP cells increased the expression of NLRP3 and P20 and the level of mtROS and upregulated NF-κB signaling while the expression of SOD 2 decreased. Melatonin treatment, on the other hand, reduced the NP cells’ inflammatory response via disturbing NLRP3/IL-1β-positive loop, downregulation of NF-κB, and enhancing the anti-oxidant activity of mitochondria. In in vivo evaluations on AF-punctured rat models, melatonin treatment demonstrated the upper expression of collagen II and aggrecan and lower level of NLRP3, P20, and IL-1β in comparison to melatonin+ LPS (NLRP3 inflammasome activator) injection, indicating the anti-degenerative and anti-inflammatory response of melatonin against NLRP3 inflammasome priming and activation [116].
Alpha-lipoic acid
ALA is a mitochondrial compound which acts as a co-factor in enzymatic systems including pyruvate dehydrogenase and α-ketoglutarate dehydrogenase complexes in the Krebs cycle [117]. ALA is a thiol-containing anti-oxidant which scavenges ROS; elevates the level of other endogenous anti-oxidants including glutathione, vitamin C, and vitamin E; and reduces lipid peroxidation as well [117]. It acted as an anti-apoptotic agent via increasing mitochondrial ∆Ψm in high-glucose-induced mitochondrial damage in EPC cells [118].
Mitochondria-targeted anti-oxidants
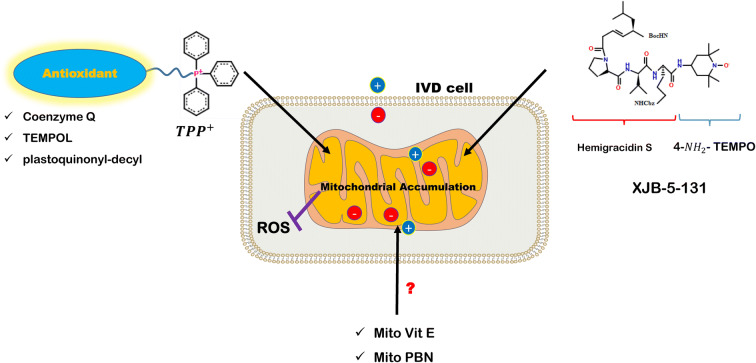
Many anti-oxidants may fail to permeate the cytoplasmic membrane to reach the cytosolic space and target damaged mitochondria. Also, high concentrations of most drugs cause cytotoxicity and cell death. To circumvent these limitations, novel and more objective delivery systems have been introduced for the precise targeting of mitochondria. Mitochondria-targeted moiety anti-oxidant conjugates which are referred to such systems are able to permeate the phospholipid bilayer of cytoplasm and accumulate in mitochondria [119]. Various strategies have been employed to target mitochondrion. In the tetraphenylphosphonium (TPP+)-linked ROS scavenger system, TPP+ penetrates the negatively charged lipophilic inner-membrane of the cell as a lipophilic and cation motif and delivers the anti-oxidant to mitochondria [119]. Mitoquinone (MitoQ), mitochondrial vitamin E (MitoVitE), (2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride (MitoTEMPO), mitochondrial alpha-phenyl-tert-butyl-nitrone (Mito PBN), Visomitin (also known as SkQs), and XJB-5-131 are all the compounds with a high potential to attack to the plasma membrane and accumulate in mitochondria multiple folds more than their unmodified counterparts [119, 120] (Fig. 2). Here, we review the mitochondria-targeted anti-oxidants, employed specifically for IVD application.
Fig. 2.
Mitochondria-targeted anti-oxidants using TPP+ and hemigracidin S as moieties highly accumulate in mitochondria relative to unmodified anti-oxidants
MitoQ
Ubiquinone or coenzyme Q, located in the inner mitochondrial membrane (IMM), is an endogenous anti-oxidant, which transports electrons from complexes I and II to complex IV. Along with CsA, MitoQ also inhibits mitochondrial membrane permeability transition pores (MPTP) opening and subsequent apoptosis in cells which is discussed in the “Other mitophagy- regulatory molecules” section. Previous studies have evaluated the mitochondrial protective effects of CoQ and its exogenous analogs; however, utilization of CoQ analogs is hindered by poor water solubility and auto-oxidation. Idebenone as a CoQ derivative could complement respiration in fibroblasts, while mitochondria-targeted CoQ derivative (MitoQ or ubiquinol) demonstrated to be 800-fold more potent than idebenone to prevent cell death [121]. Studying on compression-induced NP cells, MitoQ restored mitochondrial function and prevented the loss of mitochondrial ∆Ψm and mitochondria-mediated apoptosis [122]. MitoQ balanced mitochondrial dynamics via upregulation of mitochondrial fusion (Mfn) molecules Mfn1and Mfn2 as well as downregulation of fission molecule Drp1 to prevent cell death. It also activated Nrf2 and its downstream molecules SOD 2 and NQO-1. Ex vivo study on rat tail disc has convincingly demonstrated the in vitro outcomes that MitoQ could re-organize AF collagen fibers, restored the size of NP tissue, and alleviated disc degeneration [122]. Overall, MitoQ would be a potent mitochondrial specific anti-oxidant that preserves mitochondrial homeostasis in NP cells.
MitoTEMPO
Piperidine nitroxide radical or TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy) and its derivatives are anti-oxidants with reversible redox behavior that act as SOD mimetic and scavenger [123, 124]. To enhance the selective targeting to mitochondria, MitoTEMPO has been introduced that accumulates in mitochondria several hundredfolds more than untargeted TEMPOs [125]. Song et al. showed that AGE treatment activated NF-κB and NLRP3 inflammasome, promoted pro IL-1β expression, and enhanced mtROS production and mitochondrial membrane permeability in NP cells, while MitoTEMPO treatment alleviated inflammatory response and mitochondrial damage [126]. They reported that MitoTEMPO reduced apoptosis via rescuing mitochondrial ∆Ψm and inhibition of cytochrome c release. They claimed sirtuin 3 activation is implicated in the neutralization of AGE treatment. Nonetheless, they did not discuss about a probable MitoTEMPO contribution in activation of sirtuin 3.
SkQs
Plastoquinone is implicated in photosynthesis of plant cells as an electron carrier from photosystem II to cytochrome b6f of IMM [127]. Plastoquinone derivatives, SkQs (“Sk” from acclaimed Russian scholar, Skulachev and “Q” from quinone), are another class of TPP+-linked anti-oxidants [128]. SkQ1 (plastoquinonyl-decyl-triphenylphosphonium) is an outstanding SkQ compound, commercially available under the trade name “Visomitin” as eye drops [129]. In comparison to MitoQ, SkQ1 is more efficient to prevent lipid peroxidation, superoxide, and hydroxyl ion formation. SkQ1 has a higher transfer rate than MitoQ to cross the plasma membrane. Anti-oxidant properties of SkQ1 are attributed to (1) uncoupling cycling between cationic moiety (C12TPP) of SkQ1 and anionic fatty acid, resulting in reduced ROS level, (2) SkQ1 reaction with lipoperoxyls and reducing to SkQH2, and (3) inhibition of superoxide formation at the site If of complex I and site IQ of complex III and reducing to SkQH2 in a recycling way in which SkQ1 is regenerated again [130, 131]. In the AGE-treated NP cells, SkQ1 demonstrated an anti-apoptotic effect that partially reversed Bcl-2 level even more than MitoTEMPO [54]. It also restored mitochondrial ∆Ψm and reduced ROS level.
XJB-5-131
XJB-5-131 is a hemigracidin-(4-NH2-TEMPO) conjugate that functions as a mitochondria-targeted anti-oxidant. Hemigracidin as the cell-permeable moiety delivers 4-NH2-TEMPO to mitochondria [132]. XJB-5-131 prevents ferroptotic cell death and attenuated oxidative damage in aging HdhQ (150) Huntington’s disease mouse model as well [133, 134]. In the aging mouse model, ERCC1−/∆, XJB-5-131 improved matrix content in the NP section and cellularity in the CEP as well [59].
A list of small molecules targeting mitochondrial dysfunction and their biological effects are brought in Table 1.
Table 1.
Small molecules targeting mitochondrial dysfunction in IVD cells and their biological consequences
| Compound (molecular weight, g/mol) | Conditioning method | Biological consequences | References |
|---|---|---|---|
| Curcumin (368.38) | TBHP-treated NP cells | ∆Ψm preservation, increased ATP production, anti-apoptosis in a dose-dependent way (concentration < 25 μM) | [14] |
| Resveratrol (228.25) | Sodium nitroprusside-induced NP cells | Decreased intracellular ROS, ∆Ψm preservation, anti-apoptosis | [84] |
| H2O2-treated NP cells | ∆Ψm preservation via PI3K/Akt signaling pathway | [85] | |
| H2O2-treated NP cells | Autophagy induction via PI3K/Akt signaling pathway, anti-degenerative | [86] | |
| Polydatin (390.4) | TNF-α-treated NP cells, AF-punctured rat model | Inhibited cell senescence, activated Nrf2 both in vitro and in vivo, preserved ECM homeostasis, and alleviated IVD degeneration | [49] |
| H2O2-treated EPC | ∆Ψm preservation, decrease of mtROS, parkin activation, and Nrf2 upregulation | [91] | |
| Naringin (580.5) | H2O2-treated NPMSCs | ∆Ψm preservation, enhanced ATP level, decreased ROS content, anti-apoptosis (concentration < 100 μM) | [94] |
| Icariin (676.7) |
H2O2-treated NP cells AF-punctured rat model |
Activated Nrf2 and upregulated Nrf1 and TFAM, inhibited cytochrome C release from mitochondria, and apoptosis ameliorated IVD degeneration via Nrf2 upregulation | [97] |
| EGCC (458.4) | H2O2-treated IVD cells | ∆Ψm preservation, anti-apoptosis via PI3K/Akt signaling pathway (concentration < 50 μM) | [95] |
| NAC (163.2) | Mechanical-loaded NP cells | Downregulated P53 and 16 expressions and inhibited senescence | [104] |
| Hyper-osmolality-treated NP cells | Decreased intracellular ROS, and inhibited senescence | [105] | |
| Melatonin (232.28) | TBHP-treated EPC, AF-punctured rat model | Sirtuin 1 activation, inhibited EPC calcification | [112] |
| H2O2-treated NP cells | Inhibited mitochondrial injury by ∆Ψm preservation, inhibited mitochondria-mediated apoptosis | [114] | |
| TBHP-treated NP cells, AF-punctured rat model | Anti-apoptotic, preserved ECM homeostasis via mitophagy induction both in vitro and in vivo | [115] | |
| IL-1β-treated NP cells | Attenuated NLRP3 inflammasome activation, decreased mtROS level | [116] | |
| ALA (206.3) | High-glucose-treated EPC | Decreased ROS level, inhibited mitochondria-mediated apoptosis, ∆Ψm preservation | [118] |
| MitoQ (678.8) | Compression-loaded NP cells | Activated Nrf2 and upregulated downstream genes, SOD2 and NQO-1, regulated mitochondrial dynamic molecules, upregulation of Mfn1 and 2, and downregulation of Drp1, ∆Ψm preservation | [122] |
| MitoTEMPO (511) | AGE-treated NP cells | Alleviated mitochondrial membrane transition pore opening, decreased mtROS level, anti-apoptosis, ∆Ψm preservation | [126] |
| SkQ1 (617.6) | AGE-treated NP cells | Alleviated mitochondrial membrane transition pore opening, decreased mtROS level, anti-apoptosis | [54] |
| XJB-5-131 (959.2) | Accelerated aging ERCC1−/∆ mouse model | Ameliorated IVD degeneration, enhanced disc ECM content | [59] |
Mitophagy and its regulatory small molecules
Mitophagy (mitochondrial autophagy) is a type of selective autophagy occurring as a response to mitochondrial dysfunction which results from excessive ROS accumulation and/or loss of ∆Ψm.
Mitophagy is a homeostatic cellular process to remove excessive or dysfunctional mitochondria and preserve cellular energy metabolism. Pathological stimuli including oxidative stress as well as aging lead to impaired mitophagy in IVD. Impaired mitophagy leads to the loss of cellularity and apoptosis. The correlation between apoptosis and mitophagy has been extensively researched. Zhang et al. showed that parkin expression and mitophagy are upregulated as the IVD degenerates, and this upregulation has a direct relationship with the stage of degeneration. They reported that impaired mitophagy with accumulated P62 exacerbates apoptosis in NP cells, while salidroside improved autophagosome-lysosome fusion which consequently abated apoptosis and mitigated disc degeneration [135]. Conversely, Xu et al. reported that TBHP+ FCCP treatment resulted in more apoptosis relatively to only TBHP-treated NP cells, indicating the role of excessive mitophagy in apoptosis induction [136]. They argued that multiple factors including exposure time and concentration of stressors as well as the degree of degeneration determine the beneficial or detrimental role of mitophagy to suppress IVD degeneration. So far, the optimal level of mitophagy needed for IVD regeneration has not been determined. Nonetheless, it is obvious that oxidative stress induces mitophagy in the IVD, which is intensified with the degree of degeneration. Also, a regulated level of mitophagy might be useful through the elimination of depolarized mitochondria.
Regulated mitophagy can enhance cellular survival and delay aging by inducing cellular proliferation and inhibiting cellular senescence. In contrast, both excessive and insufficient mitophagy result in energy deprivation, ROS accumulation, and oxidative stress, which eventually lead to cell death. Understanding how mitophagy regulates IVD homeostasis, and targeting influential molecules and signaling pathways, has been considered in recent years. In mitophagy, the damaged mitochondrion is engulfed in the autophagosome and is degraded by lysosome [137]. This process takes place either in the canonical (parkin dependent) pathway or in the non-canonical (parkin independent) pathway (Fig. 3). Initiation of each pathway depends on the activation of various upstream molecules and signals which are described later.
Fig. 3.
Mitophagy pathways and regulatory small molecules for IVD cells: mitophagy in IVD cells takes place via two pathways: (i) Parkin-dependent: salidroside and FCCP as mitophagy activators enhance parkin level. Salidroside also downregulates P62 expression. Mito Q facilitates P62 degradation and leads to lysosomal digestion. (ii) Parkin independent: SP600125 (JNK inhibitor) inhibits JNK phosphorylation and LC3-II accumulation in oxidative stress-induced bone marrow-derived mesenchymal stem cells. Honokiol upregulates mitochondria dynamic molecules, Mfn-2 and Drp-1, expression, and activates the mitophagy receptor, BNIP3. Bafilomycin A1 inhibits lysosomal degradation of autophagosome and LC3-II accumulation. A PINK-1 phosphorylation and parkin ubiquitination, B, E mitophagy receptors activation, C, F autophagosome formation, D lysosomal digestion
The canonical pathway
In the canonical pathway, PINK-1 recruits parkin from the cytoplasm to OMM. Upon PINK-1 phosphorylation and parkin ubiquitination, mitophagy receptors such as P62 are activated, and mitophagosome is formed via interaction of mitophagy receptors and autophagy protein, LC3. Autophagosome is fused with the lysosome and is degraded [137]. Regulation of mitophagy is conducted through controlling the expression of parkin, PINK-1, and mitochondrial dynamics molecules [138].
PINK-1 silencing enhances NP cell senescence and ROS production either in a normal or oxidative stress-induced state, showing how mitophagy controls cell proliferation, aging, and perhaps IVD degeneration. Kang et al. observed that the impaired mitophagy in the compression-induced human NP cells was attributed to the decreased activity of lysosomal enzymes [122]. Parkin knockdown promotes apoptosis and mitochondrial impairment in NP cells [135]. Nevertheless, salidroside, a glucoside of tyrosol which is extracted from rhodiola, improves mitophagy through upregulation of parkin, inhibition of cytoplasmic accumulation of P62, and increasing the LC3-I to LC3-II conversion that is necessary for mitophagy initiation. Salidroside suppressed apoptosis by repairing the impaired mitochondria [135].
Non-canonical pathway
Mitophagy may be initiated through the parkin-independent pathway, non-canonical pathway, and occurs via various pathways including BNIP3/NIX, JNK/MAPK, HIF-1α, and PI3K/Akt/mTOR. Upon mitochondrial damage, various autophagy receptors such as FUNDC1, NIX, and BNIP3 are recruited in the outer mitochondrial membrane. Autophagosome is formed through the interaction of autophagy receptors and LC3 protein that finally will be removed via lysosomal destruction [137]. It has been found that honokiol upregulates Drp-1 and Mfn-2 and increases mitophagy in the sirtuin 3-dependent manner which was determined by upregulation of BNIP3 and the ratio of LC3-II to LC3-I [139].
Autophagy and mitophagy might be also activated in a JNK-dependent manner [140, 141]. JNK/MAPK pathway is initiated as a response to inflammation signals or oxidative stress [140, 142]. Wang et al. reported a non-canonical and JNK-dependent mitophagy activation in bone marrow-derived mesenchymal stem cells, which is a common cell source for cartilage and IVD regeneration [141]. They found that oxidative stress induction for a limited period of time (< 1 h) activates mitophagy in a JNK-dependent manner. To corroborate their findings, both mitophagy inhibitor (CsA) and JNK inhibitor (SP600125) increased apoptosis and catabolic activity. However, antimycin A constricted apoptotic cell death through mitophagy activation [141]. Urolithins are microbiota-derived gut metabolites, which are converted from ellagitannins available in pomegranate and some types of berries with anti-senescent and anti-degenerative properties in NP cells and IVD, respectively [143–145]. In particular, urolithin A has shown to be a potent mitophagy activator, being able to preserve IVD cell homeostasis [146]. In NP cells, urolithin A suppressed intrinsic apoptosis pathway and relatively restored ∆Ψm level which was lost by TBHP treatment. It was argued that urolithin A activates mitophagy in NP cells via the AMPK signaling pathway activation [146].
Other mitophagy regulatory molecules
Mitochondrial membrane permeability transition pore inhibitory molecules
Identification of molecules that are capable of inhibiting the opening of MPTP is another way to regulate mitophagy in IVD cells [147]. These molecules include CsA and CoQ that inhibit mitochondrial permeability and electron leakage to the cytosol. Adenine nucleotide translocate (ANT) that translocates AT/ADP across the mitochondrial membrane binds with CyP-D protein, and opens the MPTPs; however, the formation of this complex is inhibited in the presence of CsA [147]. CsA as a common mitophagy inhibitor has been widely exploited in IVD cells [115, 138, 141, 148].
The CoQ family is lipid-soluble small molecules with anti-oxidant and anti-inflammatory properties [149, 150]. MitoQ as a form of CoQ has been used for the repair of impaired mitophagy in the compression-induced human NP cells that was attributed to the decreased activity of lysosomal enzymes [122]. However, utilization of MitoQ counteracted the defective mitophagy flux by restoring the acidity of lysosome and consequently enhancement of P62 degradation. MitoQ also double activated the mitophagy via the PINK-1/Park signaling pathway.
Mfn-2
Mfn-2, a protein located in the mitochondrial outer membrane, is an indispensable factor for mitochondrial fusion. With increase of mitochondrial membrane permeability and parkin recruitment, Mfn-2 is ubiquitinated. Mfn-2 ubiquitination is associated with the activation of mitophagy and elimination of dysfunctional mitochondria. Mfn-2 acts in a PINK-1/parkin-dependent manner and activates mitophagy [151, 152]. Expression of Mfn-2 decreases as the degree of NP degeneration increases. Mfn-2 exerts an anti-apoptotic effect via ROS-dependent mitophagy and alleviates IVD degeneration. Recent findings indicate that Mfn-2 knockdown enhances apoptosis in NP cells and blocks autophagy, while Mfn-2 overexpression mitigates apoptosis through PINK1/parkin pathway and in a ROS-dependent manner [138].
NDUFA4L2
NDUFA4L2 is a mitochondrial gene, located in the complex I of ETC, and is implicated in the electron transport and mitochondrial respiratory system. NDUFA4L2 is activated in the response of HIF-1α upregulation, a crucial factor for cell proliferation and homeostasis of ECM in the hypoxic microenvironment of IVD [136, 153]. Under hypoxic conditions, HIF-1α signaling is activated and upregulates NDUFA4L2 expression, resulting in inhibition of complex I activity, reduction of ROS production, and enhancement of cellular survival [154, 155]. Studies on NP cells suggest that the anti-apoptotic effect of HIF-1α/NDUFA4L2 may be attributed to its mitophagy regulatory behavior [136]. In vivo studies by Xu et al. demonstrated that the expression levels of NDUFA4L2 and HIF-1α decrease as the progression of IVD degeneration increases. Conversely, injection of adeno-NDUFA4L2 lowered the levels of caspase 3 and parkin [136]. In addition, the mitophagy activator carbonyl cyanide 4-(trifluoromethoxy) FCCP led to excessive mitophagy and induced apoptosis [136]. In vitro evaluation of NP cells also determined that mitochondrial protective effect of NDUFA4L2 occurs via activation of HIF-1α signaling pathway [136]. Therefore, targeting NDUFA4L2 may be a promising approach to protect mitochondrial function and enhance NP cell survival.
Mitochondrial biogenic molecule PGC-1α
Peroxisome proliferator-activated receptor (PPAR)-ɣ co-activator 1α (PGC-1α) acts as a co-factor for thermogenesis and mitochondrial bioenergetics in cells. It is the master regulator of mitochondrial bioenergetics and preserves redox homeostasis via ROS detoxification. Findings in IVD cells have demonstrated that oxidative stress-induced mitophagy aggravates apoptosis and accelerates cellular senescence [54, 148]. Conversely, PGC-1α activation downregulates mitophagy and acts as an anti-apoptotic factor. Xu et al. found that PGC-1α overexpression mitigated the apoptosis upregulation via controlling the excessive mitophagy in a sirtuin 2-dependent manner. Song et al. also reported that PGC-1α alleviates apoptosis and mitophagy through the AMPK/PGC-1α/sirtuin 3 signaling pathway [54].
The list of small molecules employed to regulate mitophagy and their mechanism of action are brought in Table 2.
Table 2.
Mitophagy activators and their mechanism of action in IVD cells
| Small molecule (molecular weight, g/mol) | Concentration | Therapeutic target (molecule/signaling pathway) | Therapeutic outcome | Reference |
|---|---|---|---|---|
| MitoQ (678.8) | 200–500 nM | PINK1/parkin activation | Repair of impaired mitophagy (P62↓) | [122] |
| Honokiol (266.3) | < 10 μM | AMPK/PGC-1α/sirtuin 3 activation | Anti-oxidant activity (SOD)↑ | [139] |
| Cell senescence↓ | ||||
| Salidroside (300.3) | 200 μM | Parkin activation | Repair of impaired mitophagy (P62↓) | [135] |
| Urolithin A (228.2) | 0–20 μM | AMPK activation | Apoptosis↓, ∆Ψm restoration, regenerated degenerate disc | [146] |
Summary
In this review, we have discussed the role of mitochondrial dysfunction in IVD degeneration and explored the interplay with oxidative stress and inflammation. Consequences of oxidative stress such as apoptosis, cell senescence, and the production of MDA, AOPP, and AGEs are implicated in IVD cell mitochondrial dysfunction. ROS levels in mitochondria are associated with the inflammatory pathway and the NLRP-3 inflammasome. Targeting mitochondrial dysfunction with compounds such as NAC, melatonin, ALA, and phenolic and flavonoid compounds results in lowered ROS content, preserves ∆Ψm, and promotes ECM homeostasis in IVD cells. In addition, mitochondria permeable small molecules such as TPP+-linked anti-oxidants and XJB-5-131 have been shown to be efficient for mitigating mitochondrial dysfunction and alleviating IVD degeneration. Most of the in vivo studies done to date have concentrated on rodent models. However, the objective evaluation of these drugs should be carried out in more appropriate translational models that are closer to the human in vivo situation, including animal models that resemble the developmental aspects of the human IVD, particularly the non-persistence of notochordal cells. Molecules regulating mitophagy were also discussed, and it was concluded that insufficient mitophagy induces apoptosis and leads to IVD degeneration. It is also unclear whether small molecules are effective in humans. In conclusion, future clinical trials should focus on studying the safety and efficacy of compounds that promote mitophagy and alleviate mitochondrial dysfunction for treating IVD degeneration and preserving the structure and function of the IVD.
Acknowledgments
We would like to acknowledge our teams and collaborators for their support and encouragement.
Abbreviations
- AF
Annulus fibrosis
- AGEs
Advanced glycation end products
- ALA
Alpha-lipoic acid
- AOPP
Advanced oxidation protein products
- ARE
Anti-oxidant response element
- ASC
Apoptosis-associated speck-like protein, containing a caspase-recruitment domain
- ATG
Autophagy protein
- ATP
Adenosine triphosphate
- Bcl-2
B cell lymphoma
- bFGF
Basic fibroblast growth factor
- BMPs
Bone morphogenetic proteins
- CEP
Cartilage endplate
- CML
Carboxymethyllysine
- CsA
Cyclosporine A
- DAMPs
Damage-associated molecular patterns
- (∆Ψm)
Mitochondrial membrane potential
- ECM
Extracellular matrix
- EGCG
Epigallocatechin-3-gallate
- ETC
Electron transport chain
- FCCP
Carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone
- GSH
Glutathione
- GSSG
Oxidized glutathione
- GSH/GSSG
Glutathione/oxidized glutathione ratio
- GSk-3
Glycogen synthase kinase 3
- HIF-1α
Hypoxia inducible factor 1-α
- IL-1β
Interlukin-1beta
- IVD
Intervertebral disc
- Keap1
Kelch-like ECH-associated protein 1
- LBP
Low back pain
- LPS
Lipopolysaccharides
- MAPK
Mitogen-activated protein kinase
- Mito PBN
Mitochondrial alpha-phenyl-tert-butyl-nitrone
- MitoQ
Mitoquinone
- MitoTEMPO
2,2,6,6-Tetramethyl-4-[[2-(triphenylphosphonio)acetyl]amino]-1-piperidinyloxy,monochloride
- MitoVitE
Mitochondrial vitamin E
- MDA
Malondialdehyde
- MRC
Mitochondrial respiratory chain
- mtDNA
Mitochondrial DNA
- mtROS
Mitochondrial ROS
- NAC
N-Acetyl-l-cysteine or N-acetylcysteine
- NF-κB
Nuclear factor kappa B
- NLRP
Nucleotide-binding domain, leucine-rich repeat protein
- NP
Nucleus pulposus
- Nrf2
Nuclear factor-erythroid 2 related factor 2 (Nrf2)
- OMM
Outer mitochondrial membrane
- OXPHOS
Oxidative phosphorylation
- PAMPs
Pathogen-associated molecular patterns
- RAGE
Toll-like receptor (TLR)-receptor for AGE
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- RMDs
Rheumatic and musculoskeletal diseases
- SOD
Superoxide dismutase
- TBHP
Tert-butyl hydroperoxide
- TEMPO
2,2,6,6-Tetramethyl-1-piperidinyloxy
- TGF-β1
Transforming growth factor-β1
- TNF-α
Tumor necrosis factor-alpha
- TRX-1
Thioredoxin 1
- TRX-2
Thioredoxin 2
- TXNIP
Thioredoxin-interacting protein
Author contributions
Conceptualization: M.S., X.Z., and A.M: writing—original draft preparation, M.S.; writing—review and editing: M.S., X.Z., and A.M.
Funding
Open access funding provided by University of Oulu including Oulu University Hospital. A.M. has received funding from the following sources: The European Commission Framework 7 programme (EU FP7; HEALTH.2012.2.4.5-2, project number 305815; Novel Diagnostics and Biomarkers for Early Identification of Chronic Inflammatory Joint Diseases). The Innovative Medicines Initiative Joint Undertaking under grant agreement No. 115770, resources of which are composed of financial contribution from the European Union’s Seventh Framework programme (FP7/2007-2013) and EFPIA companies’ in-kind contribution. A.M. also wishes to acknowledge funding from the European Commission through a Marie Curie Intra-European Fellowship for Career Development grant (project number 625746; acronym: CHONDRION; FP7-PEOPLE-2013-IEF). A.M. also wishes to acknowledge financial support from the European Structural and Social Funds (ES Struktūrinės Paramos) through the Research Council of Lithuania (Lietuvos Mokslo Taryba) according to the activity “Improvement of researchers” qualification by implementing world-class R&D projects of Measure No. 09.3.3-LMT-K-712 (grant application code: 09.3.3-LMT-K-712-01-0157, agreement No. DOTSUT-215) and the new funding programme: Attracting Foreign Researchers for Research Implementation (2018-2022). X.Z. wishes to acknowledge funding from the Zhejiang Provincial Natural Science Foundation of China (LGF21H060011) and Lin He’s New Medicine and Clinical Translation Academician Workstation Research Fund.
Declaration
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaolei Zhang, Email: zhangxiaolei@wmu.edu.cn.
Ali Mobasheri, Email: ali.mobasheri@oulu.fi.
References
- 1.Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, Castel LD, Kalsbeek WD, Carey TS. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169(3):251–258. doi: 10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuesta-Vargas A, Farasyn A, Gabel CP, Luciano JV. The mechanical and inflammatory low back pain (MIL) index: development and validation. BMC Musculoskelet Disord. 2014;15(1):12. doi: 10.1186/1471-2474-15-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeiter S, Bishop N, Ito K. Significance of the mechanical environment during regeneration of the intervertebral disc. Eur Spine J. 2005;14(9):874–879. doi: 10.1007/s00586-005-0957-8. [DOI] [PubMed] [Google Scholar]
- 4.Sowa G, Vadalà G, Studer R, Kompel J, Iucu C, Georgescu H, Gilbertson L, Kang J. Characterization of intervertebral disc aging: longitudinal analysis of a rabbit model by magnetic resonance imaging, histology, and gene expression. Spine (Phila Pa 1976) 2008;33(17):1821–1828. doi: 10.1097/BRS.0b013e31817e2ce3. [DOI] [PubMed] [Google Scholar]
- 5.Zhao C-Q, Wang L-M, Jiang L-S, Dai L-Y. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev. 2007;6(3):247–261. doi: 10.1016/j.arr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Raj PP. Intervertebral Disc: Anatomy-Physiology-Pathophysiology-Treatment. Pain Pract. 2008;8(1):18–44. doi: 10.1111/j.1533-2500.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- 7.Wallace DC, Fan W, Procaccio V. Mitochondrial energetics and therapeutics. Annu Rev Pathol. 2010;5:297–348. doi: 10.1146/annurev.pathol.4.110807.092314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505(7483):335–343. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madhu V, Boneski PK, Silagi E, Qiu Y, Kurland I, Guntur AR, Shapiro IM, Risbud MV. Hypoxic regulation of mitochondrial metabolism and mitophagy in nucleus pulposus cells is dependent on HIF-1α-BNIP3 axis. J Bone Miner Res Off J Am Soc Bone Miner Res. 2020;35(8):1504–1524. doi: 10.1002/jbmr.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salvatierra JC, Yuan TY, Fernando H, et al. Difference in energy metabolism of annulus fibrosus and nucleus pulposus cells of the intervertebral disc. Cell Mol Bioeng. 2011;4(2):302–310. doi: 10.1007/s12195-011-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rannou F, Lee T-S, Zhou R-H, Chin J, Lotz JC, Mayoux-Benhamou MA, Barbet JP, Chevrot A, Shyy JYJ. Intervertebral disc degeneration: the role of the mitochondrial pathway in annulus fibrosus cell apoptosis induced by overload. Am J Pathol. 2004;164(3):915–924. doi: 10.1016/S0002-9440(10)63179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie M, Yang S, Win HL, Xiong L, Huang J, Zhou J. Rabbit annulus fibrosus cell apoptosis induced by mechanical overload via a mitochondrial apoptotic pathway. J Huazhong Univ Sci Technol Med Sci. 2010;30(3):379–384. doi: 10.1007/s11596-010-0361-4. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Slone J, Huang T. The role of mitochondrial-related nuclear genes in age-related common disease. Mitochondrion. 2020;53:38–47. doi: 10.1016/j.mito.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Kang L, Xiang Q, Zhan S, et al. Restoration of autophagic flux rescues oxidative damage and mitochondrial dysfunction to protect against intervertebral disc degeneration. Madamanchi N, ed. Oxid Med Cell Longev. 2019;2019:7810320. doi: 10.1155/2019/7810320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fearon U, Canavan M, Biniecka M, Veale DJ. Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis. Nat Rev Rheumatol. 2016;12(7):385–397. doi: 10.1038/nrrheum.2016.69. [DOI] [PubMed] [Google Scholar]
- 16.Giorgi C, Baldassari F, Bononi A, Bonora M, de Marchi E, Marchi S, Missiroli S, Patergnani S, Rimessi A, Suski JM, Wieckowski MR, Pinton P. Mitochondrial Ca(2+) and apoptosis. Cell Calcium. 2012;52(1):36–43. doi: 10.1016/j.ceca.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.González R, Ferrín G, Hidalgo AB, Ranchal I, López-Cillero P, Santos-Gónzalez M, López-Lluch G, Briceño J, Gómez MA, Poyato A, Villalba JM, Navas P, de la Mata M, Muntané J. N-acetylcysteine, coenzyme Q10 and superoxide dismutase mimetic prevent mitochondrial cell dysfunction and cell death induced by d-galactosamine in primary culture of human hepatocytes. Chem Biol Interact. 2009;181(1):95–106. doi: 10.1016/j.cbi.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Zhao RZ, Jiang S, Zhang L, Yu Z. Bin. Mitochondrial electron transport chain, ROS generation and uncoupling (Review) Int J Mol Med. 2019;44(1):3–15. doi: 10.3892/ijmm.2019.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanz A, Stefanatos R, McIlroy G. Production of reactive oxygen species by the mitochondrial electron transport chain in Drosophila melanogaster. J Bioenerg Biomembr. 2010;42(2):135–142. doi: 10.1007/s10863-010-9281-z. [DOI] [PubMed] [Google Scholar]
- 21.Peoples JN, Saraf A, Ghazal N, Pham TT, Kwong JQ. Mitochondrial dysfunction and oxidative stress in heart disease. Exp Mol Med. 2019;51(12):1–13. doi: 10.1038/s12276-019-0355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kudryavtseva AV, Krasnov GS, Dmitriev AA, et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget. 2016;7(29):44879–44905. doi: 10.18632/oncotarget.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abramson SB. Nitric oxide in inflammation and pain associated with osteoarthritis. Arthritis Res Ther. 2008;10 Suppl 2(Suppl 2):S2. doi: 10.1186/ar2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poveda L, Hottiger M, Boos N, Wuertz K. Peroxynitrite induces gene expression in intervertebral disc cells. Spine (Phila Pa 1976) 2009;34(11):1127–1133. doi: 10.1097/BRS.0b013e31819f2330. [DOI] [PubMed] [Google Scholar]
- 25.Errico TJ, Fardon DF, Lowell TD, Vaccaro A. Open discectomy as treatment for herniated nucleus pulposus of the lumbar spine. Spine J. 2003;3(3 SUPPL. 1):45–49. doi: 10.1016/S1529-9430(02)00561-2. [DOI] [PubMed] [Google Scholar]
- 26.Delitto A, Erhard RE, Bowling RW. A treatment-based classification approach to low back syndrome: identifying and staging patients for conservative treatment. Phys Ther. 1995;75(6):470–485. doi: 10.1093/ptj/75.6.470. [DOI] [PubMed] [Google Scholar]
- 27.Nelson DV, Turner JA, McCreary C. MMPI short forms as predictors of response to conservative treatment for low back pain. J Clin Psychol. 1991;47(4):533–537. doi: 10.1002/1097-4679(199107)47:4<533::AID-JCLP2270470410>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 28.Mócsai A, Kovács L, Gergely P. What is the future of targeted therapy in rheumatology: biologics or small molecules? BMC Med. 2014;12(1):43. doi: 10.1186/1741-7015-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacIntyre I, Blaschko HKF, Gregory RA, Harris GW, Kenner GW. Calcitonin: a review of its discovery and an account of purification and action. Proc R Soc Lond Ser B Biol Sci. 1968;170(1018):49–60. doi: 10.1098/rspb.1968.0023. [DOI] [PubMed] [Google Scholar]
- 30.Felsenfeld AJ, Levine BS. Calcitonin, the forgotten hormone: does it deserve to be forgotten? Clin Kidney J. 2015;8(2):180–187. doi: 10.1093/ckj/sfv011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang X, Tian F, Wang W, Yan J, Liu H, Liu B, et al. Effect of calcitonin pretreatment on naturally occurring intervertebral disc degeneration in guinea pig. Int J Clin Exp Med. 2015;8(7):10367–79 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4565210/. [PMC free article] [PubMed]
- 32.Ge J, Cheng X, Yan Q, Wu C, Wang Y, Yu H, Yang H, Zhou F, Zou J. Calcitonin inhibits intervertebral disc degeneration by regulating protein kinase C. J Cell Mol Med. 2020;24(15):8650–8661. doi: 10.1111/jcmm.15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo Y, Zhang L, Wang W-Y, Hu Q-F, Song H-P, Zhang Y-Z. The inhibitory effect of salmon calcitonin on intervertebral disc degeneration in an ovariectomized rat model. Eur spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2015;24(8):1691–1701. doi: 10.1007/s00586-014-3611-5. [DOI] [PubMed] [Google Scholar]
- 34.Kennon JC, Awad ME, Chutkan N, DeVine J, Fulzele S. Current insights on use of growth factors as therapy for intervertebral disc degeneration. Biomol Concepts. 9(1):43–52. 10.1515/bmc-2018-0003. [DOI] [PubMed]
- 35.Ni B, Shen H, Wang W, Lu H, Jiang L. TGF-β1 reduces the oxidative stress-induced autophagy and apoptosis in rat annulus fibrosus cells through the ERK signaling pathway. J Orthop Surg Res. 2019;14(1):241. doi: 10.1186/s13018-019-1260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Q, Li B, Zhao J, Pan W, Xu J, Chen S. IGF-I induces adipose derived mesenchymal cell chondrogenic differentiation in vitro and enhances chondrogenesis in vivo. In Vitro Cell Dev Biol Anim. 2016;52(3):356–364. doi: 10.1007/s11626-015-9969-9. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Liu X, Wang Y, Cao F, Chen Z, Hu Z, Yu B, Feng H, Ba Z, Liu T, Li H, Jiang B, Huang Y, Li L, Wu D. Intervertebral disc degeneration in mice with type II diabetes induced by leptin receptor deficiency. BMC Musculoskelet Disord. 2020;21(1):77. doi: 10.1186/s12891-020-3091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lambrechts M, Skrade AE, LePage EC, et al. 9. Insulin protects intervertebral discs stimulated with diabetes-related cytokines. Spine J. 2019;19(9, Supplement):S5. doi: 10.1016/j.spinee.2019.05.022. [DOI] [Google Scholar]
- 39.Phornphutkul C, Wu K-Y, Gruppuso PA. The role of insulin in chondrogenesis. Mol Cell Endocrinol. 2006;249(1-2):107–115. doi: 10.1016/j.mce.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Bade LK, Goldberg JE, DeHut HA, Hall MK, Schwertfeger KL. Mammary tumorigenesis induced by fibroblast growth factor receptor 1 requires activation of the epidermal growth factor receptor. J Cell Sci. 2011;124(18):3106–3117. doi: 10.1242/jcs.082651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wuertz K, Vo N, Kletsas D, Boos N. Inflammatory and catabolic signalling in intervertebral discs: the roles of NF-κB and MAP kinases. Eur Cells Mater. 2012;23(2012):102–120. doi: 10.22203/eCM.v023a08. [DOI] [PubMed] [Google Scholar]
- 42.Feng C, Yang M, Lan M, et al. ROS: crucial intermediators in the pathogenesis of intervertebral disc degeneration. Victor VM, ed. Oxid Med Cell Longev. 2017;2017:5601593. doi: 10.1155/2017/5601593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Starkov AA. Measurement of mitochondrial ROS production. Methods Mol Biol. 2010;648:245–255. doi: 10.1007/978-1-60761-756-3_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu H, Nan J, Sun Y, Zhu D, Xiao C, Wang Y, Zhu L, Wu Y, Zhao J, Wu R, Chen J, Yu H, Hu X, Zhu W, Wang J’. Electron leak from NDUFA13 within mitochondrial complex I attenuates ischemia-reperfusion injury via dimerized STAT3. Proc Natl Acad Sci U S A. 2017;114(45):11908–11913. doi: 10.1073/pnas.1704723114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teixeira CC, Ischiropoulos H, Leboy PS, Adams SL, Shapiro IM. Nitric oxide-nitric oxide synthase regulates key maturational events during chondrocyte terminal differentiation. Bone. 2005;37(1):37–45. doi: 10.1016/j.bone.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 46.Wu X, Song Y, Li S, Liu X, Hua W, Wang K, Liu W, Li S, Zhang Y, Shao Z, Yang C. Pramlintide regulation of extracellular matrix (ECM) and apoptosis through mitochondrial-dependent pathways in human nucleus pulposus cells. Int J Immunopathol Pharmacol. 2018;31:039463201774750. doi: 10.1177/0394632017747500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartman R, Patil P, Tisherman R, et al. Age-dependent changes in intervertebral disc cell mitochondria and bioenergetics. Eur Cells Mater. 2018;36:171–183. doi: 10.22203/eCM.v036a13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu S-S. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Physiol. 2004;287(4):C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Huang C, Lin Z, Pan X, Chen J, Zheng G, Tian N, Yan Y, Zhang Z, Hu J, Cheng P, Wang X, Zhang X. Polydatin suppresses nucleus pulposus cell senescence, promotes matrix homeostasis and attenuates intervertebral disc degeneration in rats. J Cell Mol Med. 2018;22(11):5720–5731. doi: 10.1111/jcmm.13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hou G, Lu H, Chen M, Yao H, Zhao H. Oxidative stress participates in age-related changes in rat lumbar intervertebral discs. Arch Gerontol Geriatr. 2014;59(3):665–669. doi: 10.1016/j.archger.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Illien-Junger S, Grosjean F, Laudier DM, Vlassara H, Striker GE, Iatridis JC. Combined anti-inflammatory and anti-AGE drug treatments have a protective effect on intervertebral discs in mice with diabetes. PLoS One. 2013;8(5):e64302. doi: 10.1371/journal.pone.0064302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Illien-Jünger S, Lu Y, Qureshi SA, Hecht AC, Cai W, Vlassara H, Striker GE, Iatridis JC. Chronic ingestion of advanced glycation end products induces degenerative spinal changes and hypertrophy in aging pre-diabetic mice. PLoS One. 2015;10(2):e0116625. doi: 10.1371/journal.pone.0116625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sivan SS, Tsitron E, Wachtel E, Roughley P, Sakkee N, van der Ham F, Degroot J, Maroudas A. Age-related accumulation of pentosidine in aggrecan and collagen from normal and degenerate human intervertebral discs. Biochem J. 2006;399(1):29–35. doi: 10.1042/BJ20060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song Y, Li S, Geng W, Luo R, Liu W, Tu J, Wang K, Kang L, Yin H, Wu X, Gao Y, Zhang Y, Yang C. Sirtuin 3-dependent mitochondrial redox homeostasis protects against AGEs-induced intervertebral disc degeneration. Redox Biol. 2018;19:339–353. doi: 10.1016/j.redox.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, Gil J, Hara E, Krizhanovsky V, Jurk D, Maier AB, Narita M, Niedernhofer L, Passos JF, Robbins PD, Schmitt CA, Sedivy J, Vougas K, von Zglinicki T, Zhou D, Serrano M, Demaria M. Cellular senescence: defining a path forward. Cell. 2019;179(4):813–827. doi: 10.1016/j.cell.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Kim K-W, Chung H-N, Ha K-Y, Lee J-S, Kim Y-Y. Senescence mechanisms of nucleus pulposus chondrocytes in human intervertebral discs. Spine J. 2009;9(8):658–666. doi: 10.1016/j.spinee.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 57.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509(7501):439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang D, Peng Y, Li Z, Chen S, Deng X, Shao Z, Ma K. Compression-induced senescence of nucleus pulposus cells by promoting mitophagy activation via the PINK1/PARKIN pathway. J Cell Mol Med. 2020;24(10):5850–5864. doi: 10.1111/jcmm.15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nasto LA, Robinson AR, Ngo K, Clauson CL, Dong Q, St. Croix C, Sowa G, Pola E, Robbins PD, Kang J, Niedernhofer LJ, Wipf P, Vo NV. Mitochondrial-derived reactive oxygen species (ROS) play a causal role in aging-related intervertebral disc degeneration. J Orthop Res. 2013;31(7):1150–1157. doi: 10.1002/jor.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patil P, Falabella M, Saeed A, Lee D, Kaufman B, Shiva S, Croix CS, van Houten B, Niedernhofer LJ, Robbins PD, Lee J, Gwendolyn S, Vo NV. Oxidative stress-induced senescence markedly increases disc cell bioenergetics. Mech Ageing Dev. 2019;180:97–106. doi: 10.1016/j.mad.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 61.Chu H, Yu H, Ren D, Zhu K, Huang H. Plumbagin exerts protective effects in nucleus pulposus cells by attenuating hydrogen peroxide-induced oxidative stress, inflammation and apoptosis through NF-B and Nrf-2. Int J Mol Med. 2016;37(6):1669–1676. doi: 10.3892/ijmm.2016.2564. [DOI] [PubMed] [Google Scholar]
- 62.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 63.Wang Z, Zhang S, Xiao Y, Zhang W, Wu S, Qin T, Yue Y, Qian W, Li L. NLRP3 inflammasome and inflammatory diseases. Oxidative Med Cell Longev. 2020;2020:4063562–4063511. doi: 10.1155/2020/4063562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liang L, Tan X, Zhou Q, Zhu Y, Tian Y, Yu H, Kijlstra A, Yang P. IL-1β triggered by peptidoglycan and lipopolysaccharide through TLR2/4 and ROS-NLRP3 inflammasome–dependent pathways is involved in ocular Behçet’s disease. Invest Ophthalmol Vis Sci. 2013;54(1):402–414. doi: 10.1167/iovs.12-11047. [DOI] [PubMed] [Google Scholar]
- 65.Huang Z, Zhuang X, Xie C, Hu X, Dong X, Guo Y, Li S, Liao X. Exogenous hydrogen sulfide attenuates high glucose-induced cardiotoxicity by inhibiting NLRP3 inflammasome activation by suppressing TLR4/NF-κB pathway in H9c2 cells. Cell Physiol Biochem. 2016;40(6):1578–1590. doi: 10.1159/000453208. [DOI] [PubMed] [Google Scholar]
- 66.Swanson KV, Deng M, Ting JP-Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elliott EI, Miller AN, Banoth B, Iyer SS, Stotland A, Weiss JP, Gottlieb RA, Sutterwala FS, Cassel SL. Cutting edge: mitochondrial assembly of the NLRP3 inflammasome complex is initiated at priming. J Immunol. 2018;200(9):3047–3052. doi: 10.4049/jimmunol.1701723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cassel SL, Elliott E, Iyer SS, Sutterwala F. Cardiolipin provides a platform for caspase-1 activation and NLRP3 inflammasome assembly. J Allergy Clin Immunol. 2016;137(2):AB72. doi: 10.1016/j.jaci.2015.12.244. [DOI] [Google Scholar]
- 69.Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, Alnemri ES. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem. 2012;287(43):36617–36622. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, Eisenbarth SC, Nauseef WM, Cassel SL, Sutterwala FS. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39(2):311–323. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wondafrash DZ, Nire’a AT, Tafere GG, Desta DM, Berhe DA, Zewdie KA. Thioredoxin-interacting protein as a novel potential therapeutic target in diabetes mellitus and its underlying complications. Diabetes Metab Syndr Obes. 2020;13:43–51. doi: 10.2147/DMSO.S232221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Devi TS, Lee I, Hüttemann M, Kumar A, Nantwi KD, Singh LP. TXNIP links innate host defense mechanisms to oxidative stress and inflammation in retinal Muller glia under chronic hyperglycemia: implications for diabetic retinopathy. Balasubramanyam M, ed. Exp Diabetes Res. 2012;2012:438238. doi: 10.1155/2012/438238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu L, Hao Y, Xu C, Zhu G, Cai Y. LINC00969 promotes the degeneration of intervertebral disk by sponging miR-335-3p and regulating NLRP3 inflammasome activation. IUBMB Life. 2019;71(5):611–618. doi: 10.1002/iub.1989. [DOI] [PubMed] [Google Scholar]
- 74.Tang P, Zhu R, Ji W-P, Wang JY, Chen S, Fan SW, Hu ZJ. The NLRP3/caspase-1/interleukin-1β axis is active in human lumbar cartilaginous endplate degeneration. Clin Orthop Relat Res. 2016;474(8):1818–1826. doi: 10.1007/s11999-016-4866-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tang P, Gu J-M, Xie Z-A, Gu Y, Jie ZW, Huang KM, Wang JY, Fan SW, Jiang XS, Hu ZJ. Honokiol alleviates the degeneration of intervertebral disc via suppressing the activation of TXNIP-NLRP3 inflammasome signal pathway. Free Radic Biol Med. 2018;120:368–379. doi: 10.1016/j.freeradbiomed.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 76.Yu Z, Xu N, Wang W, Pan S, Li K, Liu J. Interleukin-1 inhibits Sox9 and collagen type II expression via nuclear factor-kappaB in the cultured human intervertebral disc cells. Chin Med J (Engl). 2009;122(20):2483–8. https://journals.lww.com/cmj/fulltext/10.3760/cma.j.issn.0366-6999.2009.20.016; 10.3760/cma.j.issn.0366-6999.2009.20.016. [PubMed]
- 77.Klawitter M, Quero L, Klasen J, Gloess AN, Klopprogge B, Hausmann O, Boos N, Wuertz K. Curcuma DMSO extracts and curcumin exhibit an anti-inflammatory and anti-catabolic effect on human intervertebral disc cells, possibly by influencing TLR2 expression and JNK activity. J Inflamm (Lond) 2012;9(1):29. doi: 10.1186/1476-9255-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei Q-Y, Chen W-F, Zhou B, Yang L, Liu Z-L. Inhibition of lipid peroxidation and protein oxidation in rat liver mitochondria by curcumin and its analogues. Biochim Biophys Acta, Gen Subj. 2006;1760(1):70–77. doi: 10.1016/j.bbagen.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 79.Sen S, Sharma H, Singh N. Curcumin enhances vinorelbine mediated apoptosis in NSCLC cells by the mitochondrial pathway. Biochem Biophys Res Commun. 2005;331(4):1245–1252. doi: 10.1016/j.bbrc.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 80.Zhu Y, Chen X, Chen Z, Zeng YQ, Shi GB, Su YH, Peng X. Curcumin protects mitochondria from oxidative damage and attenuates apoptosis in cortical neurons. Acta Pharmacol Sin. 2004;25(12):1606–1612. [PubMed] [Google Scholar]
- 81.Bhat KPL, Lantvit D, Christov K, Mehta RG, Moon RC, Pezzuto JM. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res. 2001;61(20):7456–7463. [PubMed] [Google Scholar]
- 82.Matsuoka A, Takeshita K, Furuta A, Ozaki M, Fukuhara K, Miyata N. The 4’-hydroxy group is responsible for the in vitro cytogenetic activity of resveratrol. Mutat Res. 2002;521(1-2):29–35. doi: 10.1016/s1383-5718(02)00211-5. [DOI] [PubMed] [Google Scholar]
- 83.Lin B, Yu H, He Y, Xu Y, Zhang W, Lu C, Ao Q. Protective effects of resveratrol on autologous nucleus pulposus model of radiculopathy. Exp Ther Med. 2016;12(6):3917–3922. doi: 10.3892/etm.2016.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li K, Li Y, Mi J, Mao L, Han X, Zhao J. Resveratrol protects against sodium nitroprusside induced nucleus pulposus cell apoptosis by scavenging ROS. Int J Mol Med. 2018;41(5):2485–2492. doi: 10.3892/ijmm.2018.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang B, Xu L, Zhuo N, Shen J. Resveratrol protects against mitochondrial dysfunction through autophagy activation in human nucleus pulposus cells. Biochem Biophys Res Commun. 2017;493(1):373–381. doi: 10.1016/j.bbrc.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 86.Gao J, Zhang Q, Song L. Resveratrol enhances matrix biosynthesis of nucleus pulposus cells through activating autophagy via the PI3K/Akt pathway under oxidative damage. Biosci Rep. 2018;38(4). 10.1042/BSR20180544. [DOI] [PMC free article] [PubMed] [Retracted]
- 87.Amri A, Chaumeil JC, Sfar S, Charrueau C. Administration of resveratrol: what formulation solutions to bioavailability limitations? J Control Release. 2012;158(2):182–193. doi: 10.1016/j.jconrel.2011.09.083. [DOI] [PubMed] [Google Scholar]
- 88.Wang H-L, Gao J-P, Han Y-L, Xu X, Wu R, Gao Y, Cui XH. Comparative studies of polydatin and resveratrol on mutual transformation and antioxidative effect in vivo. Phytomedicine. 2015;22(5):553–559. doi: 10.1016/j.phymed.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 89.Di Benedetto A, Posa F, De Maria S, et al. Polydatin, natural precursor of resveratrol, promotes osteogenic differentiation of mesenchymal stem cells. Int J Med Sci. 2018;15(9):944–952. doi: 10.7150/ijms.24111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang K, Chen C, Hao J, Huang J, Wang S, Liu P, Huang H. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating Sirt1 to resist AGEs-induced upregulation of fibronetin and transforming growth factor-β1 in rat glomerular messangial cells. Mol Cell Endocrinol. 2015;399:178–189. doi: 10.1016/j.mce.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 91.Kang L, Liu S, Li J, Tian Y, Xue Y, Liu X. Parkin and Nrf2 prevent oxidative stress-induced apoptosis in intervertebral endplate chondrocytes via inducing mitophagy and anti-oxidant defenses. Life Sci. 2020;243:117244. doi: 10.1016/j.lfs.2019.117244. [DOI] [PubMed] [Google Scholar]
- 92.Silva LCRC, David JM, dos SQ BR, et al. Determination of flavanones in orange juices obtained from different sources by HPLC/DAD. Abdel-Rehim M, ed. J Anal Methods Chem. 2014;2014:296838. doi: 10.1155/2014/296838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alam MA, Subhan N, Rahman MM, Uddin SJ, Reza HM, Sarker SD. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv Nutr. 2014;5(4):404–417. doi: 10.3945/an.113.005603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nan LP, Wang F, Ran D, Zhou SF, Liu Y, Zhang Z, Huang ZN, Wang ZY, Wang JC, Feng XM, Zhang L. Naringin alleviates H2O2-induced apoptosis via the PI3K/Akt pathway in rat nucleus pulposus-derived mesenchymal stem cells. Connect Tissue Res. 2019;61:554–567. doi: 10.1080/03008207.2019.1631299. [DOI] [PubMed] [Google Scholar]
- 95.Krupkova O, Handa J, Hlavna M, et al. The natural polyphenol epigallocatechin gallate protects intervertebral disc cells from oxidative stress. 2016;2016:1–17. 10.1155/2016/7031397. [DOI] [PMC free article] [PubMed]
- 96.Chao Wei C, Qi Ping D, Tian You F, Yong Qiang C, Tao C. Icariin prevents cartilage and bone degradation in experimental models of arthritis. Valacchi G, ed. Mediators Inflamm. 2016;2016:9529630. doi: 10.1155/2016/9529630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hua W, Li S, Luo R, Wu X, Zhang Y, Liao Z, Song Y, Wang K, Zhao K, Yang S, Yang C. Icariin protects human nucleus pulposus cells from hydrogen peroxide-induced mitochondria-mediated apoptosis by activating nuclear factor erythroid 2-related factor 2. Biochim Biophys Acta Mol basis Dis. 1866;2020(1):165575. doi: 10.1016/j.bbadis.2019.165575. [DOI] [PubMed] [Google Scholar]
- 98.Sun X, Wei J, Lyu J, Bian T, Liu Z, Huang J, Pi F, Li C, Zhong Z. Bone-targeting drug delivery system of biomineral-binding liposomes loaded with icariin enhances the treatment for osteoporosis. J Nanobiotechnology. 2019;17(1):10. doi: 10.1186/s12951-019-0447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ezeriņa D, Takano Y, Hanaoka K, Urano Y, Dick TP. N-Acetyl cysteine functions as a fast-acting antioxidant by triggering intracellular H2S and sulfane sulfur production. Cell Chem Biol. 2018;25(4):447–459.e4. doi: 10.1016/j.chembiol.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tardiolo G, Bramanti P, Mazzon E. Overview on the effects of N-acetylcysteine in neurodegenerative diseases. Molecules. 2018;23(12). 10.3390/molecules23123305. [DOI] [PMC free article] [PubMed]
- 101.Chen L, Wang G, Wang Q, Liu Q, Sun Q, Chen L. N-acetylcysteine prevents orchiectomy-induced osteoporosis by inhibiting oxidative stress and osteocyte senescence. Am J Transl Res. 2019;11(7):4337–4347. [PMC free article] [PubMed] [Google Scholar]
- 102.Liu Y, Liu K, Wang N, Zhang H. N-acetylcysteine induces apoptosis via the mitochondria-dependent pathway but not via endoplasmic reticulum stress in H9c2 cells. Mol Med Rep. 2017;16(5):6626–6633. doi: 10.3892/mmr.2017.7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Collins JA, Moots RJ, Clegg PD, Milner PI. Resveratrol and N-acetylcysteine influence redox balance in equine articular chondrocytes under acidic and very low oxygen conditions. Free Radic Biol Med. 2015;86:57–64. doi: 10.1016/j.freeradbiomed.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li P, Hou G, Zhang R, Gan Y, Xu Y, Song L, Zhou Q. High-magnitude compression accelerates the premature senescence of nucleus pulposus cells via the p38 MAPK-ROS pathway. Arthritis Res Ther. 2017;19(1):209. doi: 10.1186/s13075-017-1384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu J, Li H, Yang K, et al. Hyper-osmolarity environment-induced oxidative stress injury promotes nucleus pulposus cell senescence in vitro. Biosci Rep. 2019;39(9). 10.1042/BSR20191711. [DOI] [PMC free article] [PubMed] [Retracted]
- 106.Lancheros R, Guerrero CA, Godoy-Silva RD. Improvement of N-acetylcysteine loaded in PLGA nanoparticles by nanoprecipitation method. Kumar B, ed. J Nanotechnol. 2018;2018:3620373. doi: 10.1155/2018/3620373. [DOI] [Google Scholar]
- 107.Maurizi C. Disorder of the pineal gland associated with depression, peptic ulcers, and sexual dysfunction. South Med J. 1984;77(12):1516–1518. doi: 10.1097/00007611-198412000-00010. [DOI] [PubMed] [Google Scholar]
- 108.Liu T, Borjigin J. N-acetyltransferase is not the rate-limiting enzyme of melatonin synthesis at night. J Pineal Res. 2005;39(1):91–96. doi: 10.1111/j.1600-079X.2005.00223.x. [DOI] [PubMed] [Google Scholar]
- 109.Srinivasan V, Spence DW, Pandi-Perumal SR, Brown GM, Cardinali DP. Melatonin in mitochondrial dysfunction and related disorders. Int J Alzheimers Dis. 2011;2011:1–16. doi: 10.4061/2011/326320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Zhou XJ, Xu B. Mitochondria: central organelles for melatonin’s antioxidant and anti-aging actions. Molecules. 2018;23(2):509. doi: 10.3390/molecules23020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Costa EJX, Lopes RH, Lamy-Freund MT. Permeability of pure lipid bilayers to melatonin. J Pineal Res. 1995;19(3):123–126. doi: 10.1111/j.1600-079X.1995.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 112.Zhang Z, Lin J, Tian N, Wu Y, Zhou Y, Wang C, Wang Q, Jin H, Chen T, Nisar M, Zheng G, Xu T, Gao W, Zhang X, Wang X. Melatonin protects vertebral endplate chondrocytes against apoptosis and calcification via the Sirt1-autophagy pathway. J Cell Mol Med. 2019;23(1):177–193. doi: 10.1111/jcmm.13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reiter RJ, Tan DX, Manchester LC, El-Sawi MR. Melatonin reduces oxidant damage and promotes mitochondrial respiration. Ann N Y Acad Sci. 2002;959(1):238–250. doi: 10.1111/j.1749-6632.2002.tb02096.x. [DOI] [PubMed] [Google Scholar]
- 114.He R, Cui M, Lin H, Zhao L, Wang J, Chen S, Shao Z. Melatonin resists oxidative stress-induced apoptosis in nucleus pulposus cells. Life Sci. 2018;199:122–130. doi: 10.1016/j.lfs.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 115.Chen Y, Wu Y, Shi H, Wang J, Zheng Z, Chen J, Chen X, Zhang Z, Xu D, Wang X, Xiao J. Melatonin ameliorates intervertebral disc degeneration via the potential mechanisms of mitophagy induction and apoptosis inhibition. J Cell Mol Med. 2019;23(3):2136–2148. doi: 10.1111/jcmm.14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen F, Jiang G, Liu H, Li Z, Pei Y, Wang H, Pan H, Cui H, Long J, Wang J, Zheng Z. Melatonin alleviates intervertebral disc degeneration by disrupting the IL-1β/NF-κB-NLRP3 inflammasome positive feedback loop. Bone Res. 2020;8(1):1–13. doi: 10.1038/s41413-020-0087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Salehi B, Berkay Yılmaz Y, Antika G, et al. Insights on the use of α-lipoic acid for therapeutic purposes. Biomolecules. 2019;9(8). 10.3390/biom9080356. [DOI] [PMC free article] [PubMed]
- 118.Jiang Z, Lu W, Zeng Q, Li D, Ding L, Wu J. High glucose-induced excessive reactive oxygen species promote apoptosis through mitochondrial damage in rat cartilage endplate cells. J Orthop Res. 2018;36(9):2476–2483. doi: 10.1002/jor.24016. [DOI] [PubMed] [Google Scholar]
- 119.Fink MP, Macias CA, Xiao J, Tyurina YY, Jiang J, Belikova N, Delude RL, Greenberger JS, Kagan VE, Wipf P. Hemigramicidin–TEMPO conjugates: novel mitochondria-targeted anti-oxidants. Biochem Pharmacol. 2007;74(6):801–809. doi: 10.1016/j.bcp.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 120.Jin H, Kanthasamy A, Ghosh A, Anantharam V, Kalyanaraman B, Kanthasamy AG. Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: preclinical and clinical outcomes. Biochim Biophys Acta Mol basis Dis. 2014;1842(8):1282–1294. doi: 10.1016/j.bbadis.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jauslin ML, Meier T, Smith RAJ, Murphy PM. Mitochondria-targeted antioxidants protect Friedreich ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J. 2003;17(13):1–10. doi: 10.1096/fj.03-0240fje. [DOI] [PubMed] [Google Scholar]
- 122.Kang L, Liu S, Li J, Tian Y, Xue Y, Liu X. The mitochondria-targeted anti-oxidant MitoQ protects against intervertebral disc degeneration by ameliorating mitochondrial dysfunction and redox imbalance. Cell Prolif. 2020;53:e12779. doi: 10.1111/cpr.12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Krishna MC, Russo A, Mitchell JB, Goldstein S, Dafni H, Samuni A. Do nitroxide antioxidants act as scavengers of O2-· or as SOD mimics? J Biol Chem. 1996;271(42):26026–26031. doi: 10.1074/jbc.271.42.26026. [DOI] [PubMed] [Google Scholar]
- 124.Zhao H, Wu J, Meng X, Zuo S, Wang W, Yuan H, Lan M. Novel piperidine nitroxide derivatives: synthesis, electrochemical and antioxidative evaluation. J Heterocyclic Chem. 2008;45(2):371–376. doi: 10.1002/jhet.5570450212. [DOI] [Google Scholar]
- 125.Trnka J, Blaikie FH, Smith RAJ, Murphy MP. A mitochondria-targeted nitroxide is reduced to its hydroxylamine by ubiquinol in mitochondria. Free Radic Biol Med. 2008;44(7):1406–1419. doi: 10.1016/j.freeradbiomed.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 126.Song Y, Wang Y, Zhang Y, Geng W, Liu W, Gao Y, Li S, Wang K, Wu X, Kang L, Yang C. Advanced glycation end products regulate anabolic and catabolic activities via NLRP3-inflammasome activation in human nucleus pulposus cells. J Cell Mol Med. 2017;21(7):1373–1387. doi: 10.1111/jcmm.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Van Eerden FJ, Melo MN, Frederix PWJM, Periole X, Marrink SJ. Exchange pathways of plastoquinone and plastoquinol in the photosystem II complex. Nat Commun. 2017;8:15214. doi: 10.1038/ncomms15214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Antonenko YN, Avetisyan AV, Bakeeva LE, Chernyak BV, Chertkov VA, Domnina LV, Ivanova OY, Izyumov DS, Khailova LS, Klishin SS, Korshunova GA, Lyamzaev KG, Muntyan MS, Nepryakhina OK, Pashkovskaya AA, Pletjushkina OY, Pustovidko AV, Roginsky VA, Rokitskaya TI, Ruuge EK, Saprunova VB, Severina II, Simonyan RA, Skulachev IV, Skulachev MV, Sumbatyan NV, Sviryaeva IV, Tashlitsky VN, Vassiliev JM, Vyssokikh MY, Yaguzhinsky LS, Zamyatnin AA, Jr, Skulachev VP. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1. Cationic plastoquinone derivatives: synthesis and in vitro studies. Biochem. 2008;73(12):1273–1287. doi: 10.1134/S0006297908120018. [DOI] [PubMed] [Google Scholar]
- 129.Novikova YP, Gancharova OS, Eichler OV, Philippov PP, Grigoryan EN. Preventive and therapeutic effects of SkQ1-containing Visomitin eye drops against light-induced retinal degeneration. Biochem. 2014;79(10):1101–1110. doi: 10.1134/S0006297914100113. [DOI] [PubMed] [Google Scholar]
- 130.Skulachev VP, Antonenko YN, Cherepanov DA, Chernyak BV, Izyumov DS, Khailova LS, Klishin SS, Korshunova GA, Lyamzaev KG, Pletjushkina OY, Roginsky VA, Rokitskaya TI, Severin FF, Severina II, Simonyan RA, Skulachev MV, Sumbatyan NV, Sukhanova EI, Tashlitsky VN, Trendeleva TA, Vyssokikh MY, Zvyagilskaya RA. Prevention of cardiolipin oxidation and fatty acid cycling as two antioxidant mechanisms of cationic derivatives of plastoquinone (SkQs) Biochim Biophys Acta Bioenerg. 2010;1797(6):878–889. doi: 10.1016/j.bbabio.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 131.Ježek J, Engstová H, Ježek P. Antioxidant mechanism of mitochondria-targeted plastoquinone SkQ1 is suppressed in aglycemic HepG2 cells dependent on oxidative phosphorylation. Biochim Biophys Acta Bioenerg. 2017;1858(9):750–762. doi: 10.1016/j.bbabio.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 132.Wipf P, Xiao J, Jiang J, Belikova NA, Tyurin VA, Fink MP, Kagan VE. Mitochondrial targeting of selective electron scavengers: synthesis and biological analysis of hemigramicidin-TEMPO conjugates. J Am Chem Soc. 2005;127(36):12460–12461. doi: 10.1021/ja053679l. [DOI] [PubMed] [Google Scholar]
- 133.Krainz T, Gaschler MM, Lim C, Sacher JR, Stockwell BR, Wipf P. A mitochondrial-targeted nitroxide is a potent inhibitor of ferroptosis. ACS Cent Sci. 2016;2(9):653–659. doi: 10.1021/acscentsci.6b00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Polyzos A, Holt A, Brown C, Cosme C, Wipf P, Gomez-Marin A, Castro MR, Ayala-Peña S, McMurray CT. Mitochondrial targeting of XJB-5-131 attenuates or improves pathophysiology in HdhQ150 animals with well-developed disease phenotypes. Hum Mol Genet. 2016;25(9):1792–1802. doi: 10.1093/hmg/ddw051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang Z, Xu T, Chen J, Shao Z, Wang K, Yan Y, Wu C, Lin J, Wang H, Gao W, Zhang X, Wang X. Parkin-mediated mitophagy as a potential therapeutic target for intervertebral disc degeneration. Cell Death Dis. 2018;9(10):1–16. doi: 10.1038/s41419-018-1024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xu WN, Zheng HL, Yang RZ, Liu T, Yu W, Zheng XF, Li B, Jiang SD, Jiang LS. Mitochondrial NDUFA4L2 attenuates the apoptosis of nucleus pulposus cells induced by oxidative stress via the inhibition of mitophagy. Exp Mol Med. 2019;51(11):1–16. doi: 10.1038/s12276-019-0331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Heo JW, No MH, Park DH, Kang JH, Seo DY, Han J, Neufer PD, Kwak HB. Effects of exercise on obesity-induced mitochondrial dysfunction in skeletal muscle. Korean J Physiol Pharmacol. 2017;21(6):567–577. doi: 10.4196/kjpp.2017.21.6.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen Y, Lin J, Chen J, Huang C, Zhang Z, Wang J, Wang K, Wang X. Mfn2 is involved in intervertebral disc degeneration through autophagy modulation. Osteoarthr Cartil. 2020;28(3):363–374. doi: 10.1016/j.joca.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 139.Wang J, Nisar M, Huang C, Pan X, Lin D, Zheng G, Jin H, Chen D, Tian N, Huang Q, Duan Y, Yan Y, Wang K, Wu C, Hu J, Zhang X, Wang X. Small molecule natural compound agonist of SIRT3 as a therapeutic target for the treatment of intervertebral disc degeneration. Exp Mol Med. 2018;50(11):1–14. doi: 10.1038/s12276-018-0173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li Z, Wang J, Deng X, Huang D, Shao Z, Ma K. Compression stress induces nucleus pulposus cell autophagy by inhibition of the PI3K/AKT/mTOR pathway and activation of the JNK pathway. Connect Tissue Res. 2020:1–13. 10.1080/03008207.2020.1736578. [DOI] [PubMed]
- 141.Fan P, Yu XY, Xie XH, Chen CH, Zhang P, Yang C, Peng X, Wang YT. Mitophagy is a protective response against oxidative damage in bone marrow mesenchymal stem cells. Life Sci. 2019;229:36–45. doi: 10.1016/j.lfs.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 142.Xu K, Chen W, Wang X, et al. Autophagy attenuates the catabolic effect during inflammatory conditions in nucleus pulposus cells, as sustained by NF-κB and JNK inhibition. Int J Mol Med. 2015;36(3):661–668. doi: 10.3892/ijmm.2015.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ryu D, Mouchiroud L, Andreuxlope A, et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. 2016. 10.1038/nm.4132. [DOI] [PubMed]
- 144.Andreux PA, Blanco-Bose W, Ryu D, Burdet F, Ibberson M, Aebischer P, Auwerx J, Singh A, Rinsch C. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat Metab. 2019;1(6):595–603. doi: 10.1038/s42255-019-0073-4. [DOI] [PubMed] [Google Scholar]
- 145.Liu H, Kang H, Song C, Lei Z, Li L, Guo J, Xu Y, Guan H, Fang Z, Li F. Urolithin A inhibits the catabolic effect of TNFα on nucleus pulposus cell and alleviates intervertebral disc degeneration in vivo. Front Pharmacol. 2018;9:1043. doi: 10.3389/fphar.2018.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lin J, Zhuge J, Zheng X, Wu Y, Zhang Z, Xu T, Meftah Z, Xu H, Wu Y, Tian N, Gao W, Zhou Y, Zhang X, Wang X. Urolithin A-induced mitophagy suppresses apoptosis and attenuates intervertebral disc degeneration via the AMPK signaling pathway. Free Radic Biol Med. 2020;150:109–119. doi: 10.1016/j.freeradbiomed.2020.02.024. [DOI] [PubMed] [Google Scholar]
- 147.Ding W-X, Yin X-M. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem. 393(7):547–64. 10.1515/hsz-2012-0119. [DOI] [PMC free article] [PubMed]
- 148.Xu WN, Yang RZ, Zheng HL, Yu W, Zheng XF, Li B, Jiang SD, Jiang LS. PGC-1α acts as an mediator of Sirtuin2 to protect annulus fibrosus from apoptosis induced by oxidative stress through restraining mitophagy. Int J Biol Macromol. 2019;136:1007–1017. doi: 10.1016/j.ijbiomac.2019.06.163. [DOI] [PubMed] [Google Scholar]
- 149.Cordero MD, Garrido-Maraver J, Oropesa-a M, et al. Secondary coenzyme Q 10 deficiency triggers mitochondria degradation by mitophagy in MELAS fibroblasts. FASEB J. 2011;25(8):2669–2687. doi: 10.1096/fj.10-165340. [DOI] [PubMed] [Google Scholar]
- 150.Bentinger M, Brismar K, Dallner G. The antioxidant role of coenzyme Q. Mitochondrion. 2007;7(Suppl):S41–S50. doi: 10.1016/j.mito.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 151.Poole AC, Thomas RE, Yu S, Vincow ES, Pallanck L. The mitochondrial fusion-promoting factor mitofusin is a substrate of the PINK1/parkin pathway. PLoS One. 2010;5(4):e10054. doi: 10.1371/journal.pone.0010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gegg ME, Cooper JM, Chau K-Y, Rojo M, Schapira AHV, Taanman J-W. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet. 2010;19(24):4861–4870. doi: 10.1093/hmg/ddq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Meng X, Zhuang L, Wang J, et al. Hypoxia-inducible factor (HIF)-1alpha knockout accelerates intervertebral disc degeneration in mice. Int J Clin Exp Pathol. 2018;11(2):548–557. [PMC free article] [PubMed] [Google Scholar]
- 154.Tello D, Balsa E, Acosta-Iborra B, Fuertes-Yebra E, Elorza A, Ordóñez Á, et al. Induction of the mitochondrial NDUFA4L2 protein by HIF-1α decreases oxygen consumption by inhibiting complex I activity. Cell Metab. 2011;14(6):768–79. 10.1016/j.cmet.2011.10.008. [DOI] [PubMed]
- 155.Zheng J, Zhang M, Weng H. Induction of the mitochondrial NDUFA4L2 protein by HIF-1a regulates heart regeneration by promoting the survival of cardiac stem cell. Biochem Biophys Res Commun. 2018;503(4):2226–2233. doi: 10.1016/j.bbrc.2018.06.142. [DOI] [PubMed] [Google Scholar]