Abstract
Background
COVID-19 has spread worldwide and become a pandemic. We report the epidemiological and clinical characteristics of cluster infections.
Methods
Data of clustered cases were retrieved from the public health emergency monitoring information system of China. We analyzed the incubation period, generation gap, secondary attack rate, and viral load in various grouped cases.
Results
A total of 60 COVID-19 infection clusters including 226 patients and 19 asymptomatic cases involving four generations were analyzed. With the increase of transmission generations, secondary attack rate decreased (P<0.001) and severity alleviated (P = 0.008). The median incubation period and intergenerational interval were 9 and 6 days, respectively. The secondary attack rate was 7.1% in the index cases, 5.0% in the first generation, 1.0% in the second generation, and 4.7% overall. Severe cases were seen more in the index (13, 65%) and first generation (7, 35%) ones, who had a significantly higher viral load than the mild and moderate ones.
Conclusions
With the increase of transmission generation, secondary infection rate and severity decreased. Severe patients had a higher virus load. Patients in the incubation period and asymptomatic carriers were potential infection sources who might play an important role in transmission.
Keywords: Coronavirus disease 19, SARS-CoV-2, Features, Cluster infection, Incubation period, Transmission
Introduction
COVID-19, a new contagious respiratory disease, occurred at the end of 2019, in Wuhan, China (Zu et al., 2020), and spread globally rapidly. Although various endeavors have been taken, the disease has not been well controlled except in a few countries. Up to now, COVID-19 has led to over millions of deaths, and become a pandemic and global public health crisis. The pathogen of COVID-19 was quickly confirmed to be SARS-CoV-2 (Schijns et al., 2020).
As a novel respiratory infectious disease, COVID-19 revealed some epidemiological features, which were like other coronavirus infections such as MERS and SARS to some extent. SARS-CoV-2 is commonly transmitted through infectious respiratory droplets and physical contact with contaminated persons (Cascella et al., 2020). Cluster infection is an identical transmission mode found to play an important role in the transmission of the disease (Chan et al., 2020, Huang Rui et al., 2020, Pan et al., 2020). According to the New Coronavirus Pneumonia Prevention and Control Protocol issued by the National Health Commission, China, cluster infections is defined as two or more confirmed cases or asymptomatic infections in a small area such as a family, a construction site, a work unit, etc., within 14 days. Although shared some common features with the other coronavirus diseases, COVID-19 demonstrated some epidemiological and clinical characteristics (Huang Chaolin et al., 2020, Li Q. et al., 2020, Wang et al., 2020). For example, the incubation period (interval between the time of a single exposure and onset of symptoms) of COVID-19 is shorter than that of SARS, and the proportion of severe cases is lower than that in SARS. However, the characteristics of cluster infection and inter-generational transmission of COVID-19 remain poorly investigated. Therefore, in this study, we analyzed the epidemiological feature of clustering infections of COVID-19 from all confirmed and asymptomatic cases of cluster infections of the novel coronavirus in Anhui Province, China, providing a clue for reasonable prevention and control of the disease.
Materials and methods
Biological diagnosis
The diagnosis of SARS-CoV-2 infection can be established by virus detection using real-time reverse-transcription–polymerase-chain-reaction (RT-PCR) assay (Huang Rui et al., 2020). Serum immunoglobulin M and immunoglobulin G antibody tests have also been used to confirm the diagnosis as complementary methods (Cascella et al., 2020). These contribute to the diagnosis and proper response to the epidemiological controlling of the disease. Herein, real-time PCR was performed routinely when a patient or a close contact was reported. Samples of throat swab were collected. Viral RNA was extracted using TANBead nucleic acid kit (Taiwan Advanced Nanotech Inc, Taiwan, China) in a bio-safety level-2 laboratory. The gene open reading frame 1ab (ORF1ab) and N (nucleocapsid protein) of SARS-CoV-2 in samples of throat swab collected from patients with COVID-19 were tested using 2019-nCoV dual fluorescent PCR kit (Shanghai Bio Germ Medical Biotechnology Co., Ltd, Shanghai, China and DAAN Gene Co., Ltd. Sun Yat-sen University, Guangdong, China). All experimental procedures were conducted according to the manufacturer’s guidelines.
Data Collection
In this paper, we statistically analyzed variables concentrating on the inter-generation transmission characteristics including the generation spacing, incubation period, recurrence rate, and viral load. The data of cluster infections of novel coronavirus pneumonia were obtained from the China Information System for Disease Control and Prevention (CISDCP). The diagnostic standards of suspected cases, confirmed cases, and asymptomatic infection of COVID-19 were defined in accordance with the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (5th or update version) sponsored by the National Health Commission of the People’s Republic of China. Generation gap was referred to the time interval between different transmission generations. The secondary attack rate was defined as the probability that an infection occurred among susceptible people within a specific group (household or close contacts) or positive SARS-CoV-2 nucleic acid rate of close contact of confirmed cases or asymptomatic carriers. The index case was defined as a confirmed case with a sojourn history in Wuhan or contacted a person returned from Wuhan 14 days before the onset of the disease. To investigate the influence of climate, geography, and social activity, the subjects were divided into three groups according to their residential region. Based on the boundary of the Yangtze River and Huai River basin, Anhui Province, which covers 14 square kilometers with 63,650,000 people, is divided into three regions as the southern region (the north of the Yangtze River), the middle (the north of the Yangtze River to the south of the Huai River basin) and the northern region (the north of the Huai River basin). Epidemiological features of subjects from different regions were compared. Only cases with a clear history of single exposure to a confirmed COVID-19 case were enrolled to calculate the incubation period. The original data were established when an asymptomatic or confirmed case of COVID-19 was reported by designated hospitals. A case study and interview was conducted by the epidemiological investigators of CDC in each district and county using a unified designed one-to-one epidemiological questionnaire. All investigators were pre-trained for the accuracy and effectiveness of information collection. Data were obtained from the China Information System for Disease Control and Prevention (CISDCP) and case studies.
Statistical analysis
Continuous variables were expressed as mean () and the median (IQR), while categorical variables were expressed as the frequency (n) and number (%). The analysis of variance, χ² test or Fisher’s exact test was used to find out statistical differences between groups. A two-sided α of less than 0.05 was considered statistically significant. Data were entered and sorted using Office software, and statistical analyses were performed using the SPSS software, version 21.0.
RESULTS
Four cluster infections of COVID-19
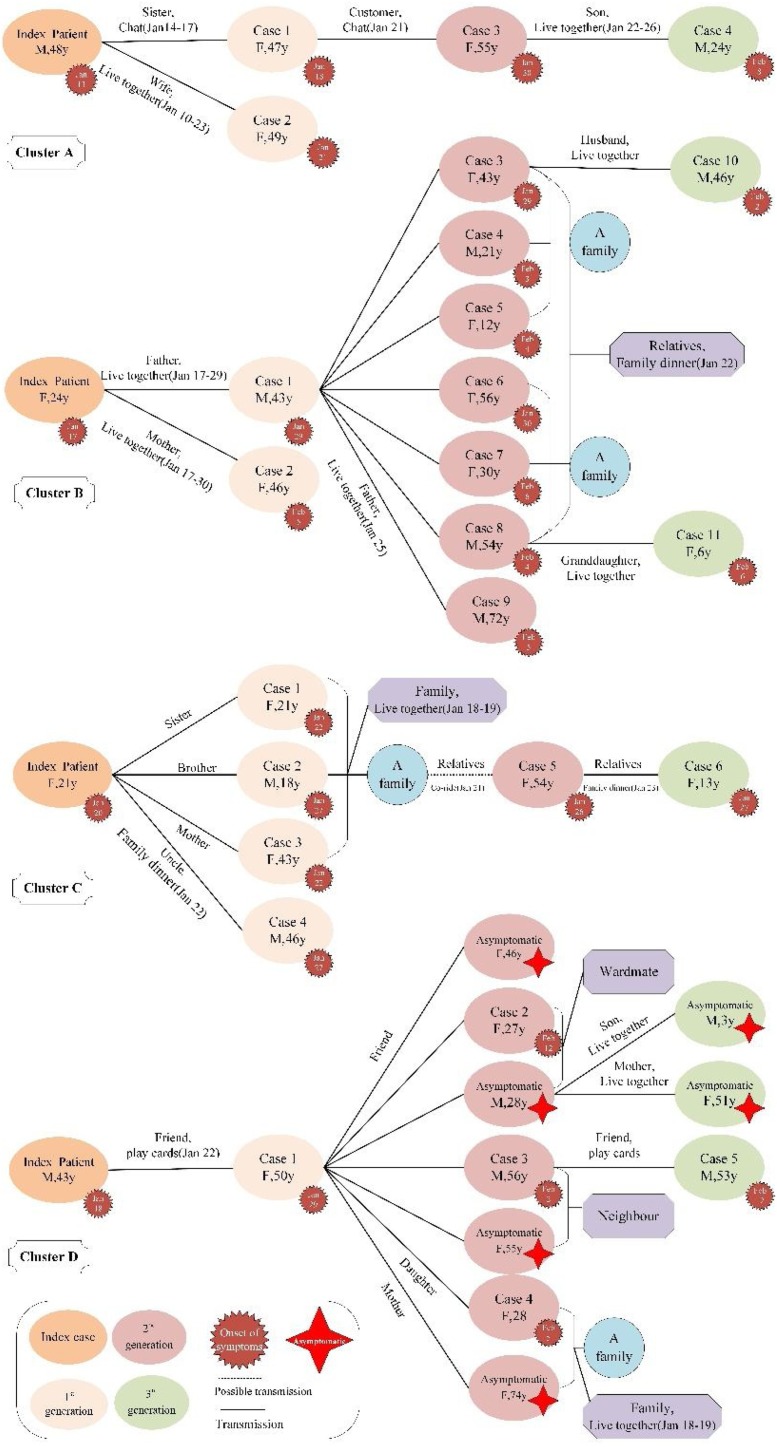
As shown in Figure 1 , there were four clusters of COVID-19 cases in Anhui Province. There were 36 individuals related to those four clusters, including 30 confirmed cases and 6 asymptomatic cases, referred to as cluster A (containing 5 cases), B (12 cases), C (7 cases), and D (12 cases containing 6 asymptomatic ones). Of these affected individuals, only four had a recent history of the sojourn in Wuhan 14 days before the onset of symptoms, of whom all were index cases interestingly. All of the four clusters involved four generation cases, and the clusters were spread mainly through living together, chatting, and dining together among family and friends.
Figure 1.
Transmission chains of four clustered COVID-19 cases in Anhui Province.
Demographic characteristics of the subjects
We analyzed a total of 245 patients from 60 cluster infections of novel coronavirus pneumonia. Demographic characteristics of the confirmed and asymptomatic cases were shown in Table 1 . The minimum age was 1 year, and the maximum was 87 years. The average age of all subjects was 28.6 years. There were no statistical differences in age distribution between different generations ( = 2.350, P = 0.503). 121 cases were male (49.4%) and 124 females (50.6%). Farmers, business people, and students were the top three occupations, and over half of the infected were farmers (57.6%). COVID-19 infection cases were more in the northern Anhui Province (64.1%) and less in the south (15.5%). According to the intergenerational criteria for transmission, the 60 cluster infections involved four generations of cases. There were 14 cluster infections of index cases (23.3%), 32 cluster infections in the first generation (53.4%), 9 in the second generation (15.0%), and 5 in the third generation (8.3%). Confirmed cases formed the majority (92.2%). Asymptomatic cases accounted for 7.8%. The difference in the proportion of asymptomatic and confirmed cases between different generations was statistically significant ( = 8.532, P = 0.027). The proportion of confirmed cases decreased, and asymptomatic cases were increased with the transmission algebra.
Table 1.
Demographic characteristics of clustered COVID-19 patients and asymptomatic cases
| Variables | Transmission generations, n (%) |
|||||
|---|---|---|---|---|---|---|
| Overall (n = 245) |
Index case (n = 92) |
1st generation (n = 95) |
2nd generation (n = 50) |
3rd generation (n = 8) |
P-value | |
| Number of clusters Age, mean ± SD |
60(100.0) 42.4 ± 17.2 |
14(23.3) 42.4 ± 13.7 |
32(53.4) 41.4 ± 19.2 |
9(15.0) 43.0 ± 17.9 |
5(8.3) 32.2 ± 22.3 |
0.503 |
| Gender | 0.369 | |||||
| Male | 121 (49.4) | 51 (55.4) | 46 (48.4) | 20 (40.0) | 4 (50.0) | |
| Female | 124 (50.6) | 41 (44.6) | 49 (51.6) | 30 (60.0) | 4 (50.0) | |
| Occupation | 0.002 | |||||
| Farmer | 141(57.6) | 51(55.4) | 53(55.8) | 32(64.0) | 5(62.5) | |
| Businessman | 32(13.1) | 21(22.8) | 9(9.5) | 2(4.0) | 0(0.0) | |
| Students | 28(11.4) | 2(2.2) | 17(17.9) | 7(14.0) | 2(25.0) | |
| Housework | 23(9.4) | 8(8.7) | 9(9.5) | 6(12.0) | 0(0.0) | |
| Others | 21(8.6) | 10(10.9) | 7(7.4) | 3(6.0) | 1(12.5) | |
| Region | 0.002 | |||||
| South of Anhui | 38 (15.5) | 15 (16.3) | 7 (7.4) | 12 (24.0) | 4 (50.0) | |
| Middle of Anhui | 50 (20.4) | 25 (27.2) | 20 (21.1) | 5 (10.0) | 0(0.0) | |
| North of Anhui | 157 (64.1) | 52 (56.5) | 68 (71.6) | 33 (66.0) | 4 (50.0) | |
| Category | 0.027 | |||||
| Asymptomatic case | 19(7.8) | 3(3.3) | 7(7.4) | 7(14.0) | 2(12.5) | |
| Confirmed case | 226(92.2) | 89(96.7) | 88(92.6) | 43(86.0) | 6(87.5) | |
Secondary attack rate and clinical category
The secondary attack rate was 7.1% in the index cases, 5.0% in the first generation, 1.0% in the second generation, and 4.7% in total, which suggested that the secondary attack rate decreased with the increase of propagation algebra (P<0.001), as shown in Table 2 . In severity analysis, data showed that most patients were mild and moderate in the second and third generations. More severe patients were seen in the first-generation patients (35.0%) and remarkably the index cases (65.0%). With the transmission generation increases, the risk of SARS-CoV-2 infection decreased, and there were no confirmed or asymptomatic cases in the third generation close contactors.
Table 2.
The secondary attack rate and severity category of cluster infection of COVID-19.
| Variables | Transmission generations, n (%) |
P-value | ||||
|---|---|---|---|---|---|---|
| Total | Index case | 1st generation | 2nd generation | 3rd generation | ||
| Secondary attack rate | <0.001 | |||||
| Confirmed and asymptomatic cases | 149 | 91 | 50 | 8 | 0 | |
| Number of close contacts | 3186 | 1282 | 1004 | 792 | 108 | |
| Secondary attack rate | 4.7 | 7.1 | 5.0 | 1.0 | 0.0 | |
| Clinical category | 0.008 | |||||
| Mild | 37(15.1) | 11(29.7) | 19(51.4) | 5(13.1) | 2(5.4) | |
| Moderate | 168(68.6) | 64(38.1) | 62(36.9) | 38(22.6) | 4(2.4) | |
| Severe | 20(8.2) | 13(65.0) | 7(35.0) | 0(0.0) | 0(0.0) | |
| Critical | 1(0.4) | 1(100.0) | 0(0.0) | 0(0.0) | 0(0.0) | |
Incubation period and generation gaps
The incubation period for a new coronavirus infection was 0-14 days, with an average of 3-7 days. The median incubation period was 9 days (0-17 days) based on the data of 39 cases with a clear history of single exposure. The median intergenerational interval of 119 cases was 6 days (2-15 days). There was no statistically significant difference between intergenerational incubation periods ( = 3.107, P = 0.212). A total of 119 cases had an intergenerational interval of 6.0 days, and no statistically significant difference among intergenerational transmission was found ( = 0.098, P = 0.952), as shown in Table 3 .
Table 3.
Incubation period of cluster COVID-19 infection and generation gap
| Transmission generations | Incubation period |
P-value | Generation gap |
P-value | ||
|---|---|---|---|---|---|---|
| N | M (Range) | n | M (Range) | |||
| From G0 to G1 | 20 | 8.5(3.0, 16.0) | 0.212 | 83 | 6.0(0.0, 15.0) | 0.952 |
| From G1 to G2 | 17 | 10.0(0.0, 17.0) | 36 | 6.0(-2, 14.0) | ||
| From G2 to G3 | 2 | 3.5(3.0, 4.0) | 6 | 5.5(1.0, 10.0) | ||
| Overall | 39 | 9.0(0.0, 17.0) | 119 | 6.0(-2, 15.0) | ||
G0, index case; G1, first generation; G2, second generation; G3, third generation; N, number of clusters; n, number of cluster infection case.
Viral load in COVID-19 patients
Specific rRT-PCR Ct values of SARS-CoV-2 gene ORF1ab and N in samples of throat swab collected from patients with COVID-19 were measured. The results showed that Ct values of gene ORFlab (n = 87) didn’t present significant differences between different clinical categories or transmission generations in those samples, and there was no significant difference between transmission generations either in Ct values of gene N. Nevertheless, the Ct values of gene N (n = 58) were significantly different among different clinical categories (P = 0.029), as shown in Table 4 . Further analysis by Q test revealed that severe patients had significantly lower Ct value of SARS-CoV-2 than the mild and moderate ones (P = 0.025).
Table 4.
Ct values of SARS-CoV-2 gene ORF1ab and N in samples of throat swab collected from patients with COVID-19.
| Variable | ORF1ab gene |
N gene |
||||
|---|---|---|---|---|---|---|
| n (%) | Ct value | P-value | n (%) | Ct value | P-value | |
| Clinical category | ||||||
| Mild | 61(70.1) | 28.40 ± 5.02 | 0.475 | 13(22.4) | 31.14 ± 3.33 | 0.029 |
| Moderate | 19(21.8) | 27.96 ± 4.78 | 41(70.7) | 28.72 ± 6.21 | ||
| Severe | 7(8.1) | 25.76 ± 9.55 | 4(6.9) | 22.12 ± 6.94 | ||
| Transmission generations | ||||||
| Index case | 43(49.4) | 27.40 ± 5.32 | 0.297 | 25(43.1) | 27.03 ± 4.59 | 0.091 |
| 1 st generation | 39(44.8) | 29.07 ± 5.09 | 29(50.0) | 29.77 ± 6.99 | ||
| 2nd + 3rd generation | 5(5.8) | 26.41 ± 8.12 | 4(6.9) | 32.90 ± 2.75 | ||
| Total | 87(100.0) | 28.09 ± 5.41 | 58(100.0) | 28.80 ± 6.02 | ||
Discussion
Epidemiological features are extremely important to control a contagious disease. Clarifying the transmission manner of COVID-19 is helpful for clinicians and public health workers in clinical practice and government to establish reasonable prevention policies. Herein, we demonstrated that COVID-19 could be transmitted to at least three generations and mostly clustered, and the severity could decrease during transmission while the incubation period prolonged. Moreover, this transmission could be blocked by stringent prevention and control measures.
Generally, cluster infection is common in respiratory tract contagious disease (Shen et al., 2020). It has been confirmed that SARS-COV-2 is transmitted from human-to-human primitively, particularly via respiratory droplets. (Lu et al., 2020, Tu et al., 2020). Therefore, it offers a significant possibility leading to interpersonal transmission and epidemic outbreak of cluster infections (Shen et al., 2020). From our research, it can be found that young and middle-aged people accounted for a large proportion in cluster infections, probably due to their more social activities than that of the elderly. In addition, strict quarantine measures (such as mandatory masks and community lockdowns) have been used to control the epidemic virus transmission in Wuhan and across the country, resulting in only a small proportion of third generation patients in this study.
In analysis of the influence of geographical factors, we found a significant difference in regions in COVID-19 morbidity. Over half of the cases (157, 64.1%) were reported in the northern region of Anhui Province, where the population density was higher and many people travelled for work to other regions including Wuhan, where the COVID-19 was epidemic at that time. Most of these infected people presented clinical symptoms, signs, and chest X-ray changes in this study. In addition, a certain proportion of asymptomatic cases (19, 7.8%) were also found.
In our study, the second and third generation patients accounted for only a small proportion of the COVID-19 cases, as manifested in Table 2. Besides, a significant difference was found in the secondary attack rate between different transmission generations, which was different from the reports of other scholars (Riou and Althaus, 2020). Our results also demonstrated that most patients were mild and moderate in severity in each of the generations, which was different from reports of other regions of China (Zhou et al., 2020). Severe cases were found in the index cases (65.0%) and first-generation cases (35.0%), but no severe or critical cases were found in the second or third generation patients. These implied that infectiousness and virulence might be weakened as the virus transmission continued. Nevertheless, this might also be the consequence of strict protection measures, change of behavior patterns, early virus nucleic acid detection, and other intervention measures in the close contactors.
The incubation period of the disease was reported to be 14 days on average, and even prolonged to 17.6 days (IQR 5-34 days) (Xie et al., 2020). In order to clarify the incubation period of COVID-19, we rigorously screened subjects who had a single contact with confirmed cases. Our results showed that the incubation period was 0-14 days, with an average of 3-7 days, and the median incubation period was 9 days, which were longer than the 8.4 and 7.6 days reported for SARS and MERS, respectively (Assiri et al., 2013, Lipsitch et al., 2003). Furthermore, we found that the intergenerational spacing in this study was shorter than the incubation period. In our exploration, two cases who closely contacted with a confirmed case developed COVID-19 earlier than the confirmed case. These results showed that some patients were contagious in the incubation period, which were consistent with other researches (Chen et al., 2020, Li Qun et al., 2020, Yang et al., 2020).
On the other hand, viral load is also an important factor for disease severity and potential transmission capability of a patient. Our results showed that Ct value of N gene was significantly different among different clinical categories. Severely ill patients had a significantly higher viral load. Therefore, severely ill patients with higher viral load might be correspondently higher transmission potentiality.
There were some limitations in this study. First, we did not present the characteristics of the asymptomatic cases due to technical difficulties. Secondly, the number of groups was not always the same in analyzing the data. For example, we analyzed the severity of 226 patients in different generations and Ct values of merely 87 cases between generations. Thirdly, as COVID-19 was an emergent novel epidemic disease, we did not collect more detailed data on the subjects. All these could possibly weaken the strength of our research conclusions and need to be concerned with in further studies.
In summary, cluster infection is the main mode of transmission of COVID-19. As the transmission continues, the secondary infection rate decreases and clinical manifestations become relatively mild. Severe patients, who have a significantly higher viral load, might play a considerable role in the transmission. Meanwhile, the incubation period and asymptomatic cases are still potential infection sources. In addition, for blocking the transmission of COVID-19 disease, protective measures such as quarantine, mandatory use of face masks, and community blockage were verified to be effective in our research.
Funding
This work was supported by the grant of Anhui Provincial Department of Science and Technology, Anhui Provincial Health Commission Emergency Research Project of Novel Coronavirus Infection. (Grant numbers 202004a07020002; 202004a07020004).
Ethical Approval
Data and sample collection from patients was a part of a routine surveillance and outbreak investigation and was therefore exempt from oversight by the institutional review board (IRB). The IRB of Anhui Provincial Center for Disease Control and Prevention reviewed this study.
Authors’ contribution
Zhirong Liu, Xuqin Jiang, Jiabing Wu, Xiuzhi Chen, Lei Gong, Shaohu Huo and Xuehuan Gao designed the study. Jiabing Wu, Xiuzhi Chen, Shuang Ni, Fang Chen and Sai Hou provided the study materials; Jiabing Wu, Dandan Song, Wanwan Ma and Xuqin Jiang collected and assembled the data; Jiabing Wu, Xiuzhi Chen, Lei Gong, Shaohu Huo and Xuehuan, Xuqin Jiang and Zhirong Liu analyzed and interpreted the data; Jiabing Wu and Xuqin Jiang wrote the manuscript and all the authors revised it. The fund supporter had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Consent for publication
Not applicable.
Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In: StatPearls. Treasure Island (FL): StatPearls Publishing; January 16, 2021.
Declaration of competing interest
There is no competing interest.
Acknowledgement
The authors would like to thank the local center for disease control and prevention for collection of epidemiological data and sample collection.
References
- Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A.T., et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369(5):407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Fan P., Liu Z., Pan R., Huang S., Li J., et al. A SARS-CoV-2 familial cluster infection reveals asymptomatic transmission to children. J Infect Public Health. 2020;13(6):883–886. doi: 10.1016/j.jiph.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitch M., Cohen T., Cooper B., Robins J.M., Ma S., James L., et al. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300(5627):1966–1970. doi: 10.1126/science.1086616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C.W., Liu X.F., Jia Z.F. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395(10224):e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Chen D., Xia Y., Wu X., Li T., Ou X., et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. The Lancet Infect Dis. 2020;20(4):410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou J., Althaus C.L. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Euro Surveill. 2020;25(4) doi: 10.2807/1560-7917.ES.2020.25.4.2000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Xu W., Li C., Handel A., Martinez L., Ling F., et al. A Cluster of Novel Coronavirus Disease 2019 Infections Indicating Person-to-Person Transmission Among Casual Contacts From Social Gatherings: An Outbreak Case-Contact Investigation. Open Forum Infect Dis. 2020;7(6) doi: 10.1093/ofid/ofaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Ju X.L., Xie F., Lu Y., Li F.Y., Huang H.H., et al. [Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China] Zhonghua Er Ke Za Zhi. 2020;58(4):269–274. doi: 10.3760/cma.j.cn112140-20200225-00138. [DOI] [PubMed] [Google Scholar]
- Xie S., Zhang G., Yu H., Wang J., Wang S., Tang G., et al. The epidemiologic and clinical features of suspected and confirmed cases of imported 2019 novel coronavirus pneumonia in north Shanghai, China. Ann Transl Med. 2020;8(10):637. doi: 10.21037/atm-20-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M.C., Hung P.P., Wu Y.K., Peng M.Y., Chao Y.C., Su W.L. A three-generation family cluster with COVID-19 infection: should quarantine be prolonged? Public Health. 2020;185:31–33. doi: 10.1016/j.puhe.2020.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Sun J., Cao Z., Wang W., Huang K., Zheng F., et al. Epidemiological and clinical features of 201 COVID-19 patients in Changsha city, Hunan. China. Medicine (Baltimore). 2020;99(34) doi: 10.1097/MD.0000000000021824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu Z.Y., Jiang M.D., Xu P.P., Chen W., Ni Q.Q., Lu G.M., et al. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology. 2020;296(2):e15–25. doi: 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]