Abstract
Physical frailty and sarcopenia (PF&S) is a prototypical geriatric condition characterized by reduced physical function and low muscle mass. The aim of the present study was to provide an initial selection of biomarkers for PF&S using a novel multivariate analytic strategy. Two-hundred community-dwellers, 100 with PF&S and 100 non-physically frail, non-sarcopenic (nonPF&S) controls aged 70 and older were enrolled as part of the BIOmarkers associated with Sarcopenia and Physical frailty in EldeRly pErsons (BIOSPHERE) study. A panel of 74 serum analytes involved in inflammation, muscle growth and remodeling, neuromuscular junction damage, and amino acid metabolism was assayed. Biomarker selection was accomplished through sequential and orthogonalized covariance selection (SO-CovSel) analysis. Separate SO-CovSel models were constructed for the whole study population and for the two genders. The model with the best prediction ability obtained with the smallest number of variables was built using seven biomolecules. This model allowed correct classification of 80.6 ± 5.3% PF&S participants and 79.9 ± 5.1% nonPF&S controls. The PF&S biomarker profile was characterized by higher serum levels of asparagine, aspartic acid, and citrulline. Higher serum concentrations of platelet-derived growth factor BB, heat shock protein 72 (Hsp72), myeloperoxidase, and α-aminobutyric acid defined the profile of nonPF&S participants. Gender-specific SO-CovSel models identified a “core” biomarker profile of PF&S, characterized by higher serum levels of aspartic acid and Hsp72 and lower concentrations of macrophage inflammatory protein 1β, with peculiar signatures in men and women.
SO-CovSel analysis allowed identifying a set of potential biomarkers for PF&S. The adoption of such an innovative multivariate approach could help address the complex pathophysiology of PF&S, translate biomarker discovery from bench to bedside, and unveil novel targets for interventions.
Keywords: Inflammation, Cytokines, Amino acids, Geroscience, Multivariate, Muscle
Introduction
Physical frailty and sarcopenia (PF&S) is a reversible risk condition for physical disability, characterized by the triad of weakness, slowness, and poor balance coupled with reduced appendicular lean mass (aLM) (Cesari et al. 2017a,b). PF&S has a multifaceted pathophysiology that recapitulates all major hallmarks of aging (López-Otín et al. 2013; Sierra 2016; Justice et al. 2018) and may therefore be envisioned as a prototypical geroscience condition (Sierra 2016). As a corollary to this, a single biomarker will hardly be able to capture the intrinsic complexity of PF&S (Cohen et al. 2018; Justice et al. 2018). The quest for biomarkers for PF&S, as for other complex geriatric conditions, is also hampered by the non-linear and often non-monotonic relationship between process-specific biomarkers, their strong interdependence, and their organization into complex regulatory networks (Cohen et al. 2018). Differences in aging biology between genders further hinders the identification of meaningful biomarkers for age-related conditions (Nakamura and Miyao 2008; Berghella et al. 2014). For instance, gender-specific inflammatory profiles have been described in several age-associated conditions (Ostan et al. 2016), including PF&S (Marzetti et al. 2019). Moreover, some circulating markers of sarcopenia show different patterns between genders (Drey et al. 2013; Chew et al. 2019).
To cope with the PF&S complexity, we have proposed innovative approaches for biomarker identification and validation, moving from the “one fits all” paradigm to multivariate methodologies (Calvani et al. 2015). In preliminary proof of principle works, we showed that patterns of circulating inflammatory cytokines were able to classify older persons with varying habitual walking speed (Marzetti et al. 2014b). Moreover, defined relationships among muscle imaging parameters, circulating biomolecules, and measures of functional status were found to characterize people of different ages and various physical performance levels (Calvani et al. 2017).
In the present study, we sought to add a new piece to this complex puzzle. After a thorough review of existing literature and based on previous evidence on similar populations (Lorenzi et al. 2016; Calvani et al. 2018 a,b; Marzetti et al. 2019; Picca et al. 2020), a comprehensive set of candidate biomarkers for PF&S linked to age-related decline of muscle mass and function was selected. Afterwards, a novel analytical approach was applied based on sequential and orthogonalized covariance selection (SO-CovSel) analysis (Biancolillo et al. 2020). This innovative analytical strategy is particularly suited for dealing with multi-block datasets, i.e., experimental settings in which variables are assayed using different platforms and/or at different time points (Biancolillo et al. 2020). SO-CovSel allowed selecting the variables of interest for PF&S among a large number of highly correlated candidate biomarkers. The existence of gender-specific biomarker profiles was also explored. A stringent cross-validation process was adopted to evaluate the classification ability of the identified biomarkers.
Methods
Participants
The study protocol was approved by the Ethics Committee of the Università Cattolica del Sacro Cuore (Rome, Italy; protocol number: 8498/15), and all procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Participants were recruited in the context of the BIOmarkers associated with Sarcopenia and Physical frailty in EldeRly pErsons (BIOSPHERE) project (Calvani et al. 2018a). BIOSPHERE was designed as a cross-sectional, case-control study, aimed at identifying and validating a panel of biomarkers for PF&S (Calvani et al. 2018a). The study protocol and the procedures adopted for participant recruitment are described in detail elsewhere (Calvani et al. 2018a; Marzetti et al. 2019). In brief, after obtaining written informed consent, 200 older persons, 100 community-dwellers with PF&S and 100 non-physically frail, non-sarcopenic (nonPF&S) controls aged 70 years and older were enrolled. Inclusion and exclusion criteria have been detailed previously (Calvani et al. 2018a). Participants were diagnosed with PF&S according to the operational definition elaborated within the Sarcopenia and Physical fRailty IN older people: multi-componenT Treatment strategies (SPRINTT) project (Marzetti et al. 2015, 2018). Candidate participants were enrolled in the PF&S group when showing (a) reduced physical performance, operationalized as a summary score on the short physical performance battery (SPPB) (Guralnik et al. 1994) between 3 and 9; (b) low aLM, according to the cut-points proposed by the Foundation for the National Institutes of Health (FNIH) sarcopenia project (Studenski et al. 2014); and (c) absence of major mobility disability (i.e., candidates had to be able to complete a 400-m walk test) (Newman et al. 2006).
Measurement of appendicular lean mass by dual X-ray absorptiometry
Whole-body dual X-ray absorptiometry (DXA) scans were acquired on a Hologic® Discovery A densitometer (Hologic, Inc., Bedford, MA, USA) according to the manufacturer’s directions. Criteria for low aLM were as follows: (a) aLM to body mass index (BMI) ratio (aLMBMI) < 0.789 and < 0.512 in men and women, respectively; or (b) absolute aLM < 19.75 kg in men and < 15.02 kg in women (Studenski et al. 2014).
Blood sample collection and processing
Fasting blood samples were collected by venipuncture of the median cubital vein using commercially available collection tubes (BD Vacutainer®; Becton, Dickinson and Co., Franklin Lakes, NJ, USA). Blood samples were left at room temperature for 20 min. Serum was then separated by centrifugation at 1000×g for 10 min at 4 °C. Serum aliquots were stored at −80 °C until analysis.
Measurement of candidate circulating biomarkers
After a thorough review of the literature, a panel of 74 candidate biomarkers was identified. Biomolecules were selected based on their involvement in pathways and processes related to PF&S pathophysiology (i.e., inflammation, muscle growth and remodeling, neuromuscular junction damage, and amino acid metabolism). A detailed description of the candidate biomarker selection process can be found elsewhere (Calvani et al. 2018a, b). A panel of 27 inflammatory markers, growth factors, and chemokines was measured simultaneously in serum through a magnetic bead-based immunoassay on a Bio-Plex® System with Luminex xMAP® Technology (Bio-Rad Laboratories Inc., Hercules, CA, USA), as previously described (Ponziani et al. 2018, 2019). The cytokine panel comprised interleukin (IL) 1-β, IL1 receptor agonist (IL1-ra), IL2, IL4, IL5, IL6, IL7, IL8, IL9, IL10, IL12, IL13, IL15, IL17, fibroblast growth factor (FGF) basic, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon (IFN) γ, C-C motif chemokine ligand (CCL) 2 [also known as monocyte chemoattractant protein 1 (MCP-1)], CCL3 [also known as macrophage inflammatory protein 1α (MIP-1α)], CCL4 (also known as MIP-1β), CCL5, CCL11, C-X-C motif chemokine ligand (CXCL) [also known as IFN-γ-induced protein 10 (IP-10)], platelet-derived growth factor (PDGF) BB, and tumor necrosis factor alpha (TNF-α).
Thirty-seven amino acids and derivatives (1-methylhistidine, 3-methylhistidine, 4-hydroxyproline, α-aminobutyric acid, β-alanine, β-aminobutyric acid, γ-aminobutyric acid, alanine, aminoadipic acid, anserine, arginine, asparagine, aspartic acid, carnosine, citrulline, cystathionine, cystine, ethanolamine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, ornithine, phenylalanine, phosphoethanolamine, phosphoserine, proline, sarcosine, serine, taurine, threonine, tryptophan, tyrosine, and valine) were measured in serum through an ultraperformance liquid chromatography/mass spectrometry (UPLC/MS) validated methodology, as described elsewhere (Calvani et al. 2018b).
The neuromuscular junction-derived peptide C-terminal agrin fragment (CAF), P-selectin, high-temperature requirement serine protease A1 (HtrA1), heat shock protein 72 (Hsp72), procollagen III N-terminal peptide (P3NP), and insulin-like growth factor 1 (IGF-1) were measured by commercial ELISA kits (CAF: Neurotune AG, Schlieren-Zurich, Switzerland; all other biomolecules: R&D Systems Inc., Minneapolis, MN, USA). Finally, C-reactive protein (CRP), myeloperoxidase (MPO), FGF21, and brain-derived neurotrophic factor (BDNF) were measured using commercially available kits on an ELLA™ automated immunoassay system (Bio-Techne, San Jose, CA, USA).
Sequential and orthogonalized covariance selection
Some datasets are “intrinsically” multi-block, since the experiments from which they are generated produce sets of different matrices. For instance, a number of variables may be measured on the same samples using different analytical platforms or at different time points (or both). In such circumstance, multi-block methodologies allow extraction of relevant information from all blocks at the same time, instead of building separate models for individual datasets (Westerhuis et al. 1998). In the present study, four matrices were populated based on the technology used for their determination. The multi-platform dataset was analyzed through a multi-block method called SO-CovSel coupled with linear discriminant analysis (LDA) to obtain the classification model. SO-CovSel is a multi-platform regression method developed to handle multi-block datasets like the one under investigation in the present study (Biancolillo et al. 2020). This approach was realized by combining some aspects of a well-known data fusion method, sequential and orthogonalized partial least squares (SO-PLS) (Naes et al. 2011; Biancolillo et al. 2015; Biancolillo and Naes 2019), with a feature regression approach called covariance selection (CovSel) (Roger et al. 2011). SO-PLS is a multi-block method where the information contained in each data matrix is sequentially incorporated into the regression model. SO-CovSel shares with SO-PLS the presence of an orthogonalization step prior to the inclusion of any data block, leading to the removal of redundancies across different datasets. CovSel, instead, is a feature selection approach conceived to identify which variables are the most relevant in a regression context. In other words, given a data matrix X used to predict the response Y, CovSel selects (through the estimation of covariances between each X-feature and the response) the X-variables contributing the most to Y description.
Starting from this scenario, SO-CovSel is a sequential regression method (like SO-PLS) where the information contained into distinct data blocks is extracted via CovSel. The SO-CovSel algorithm is quite easy from a computational point of view and does not pose any limit in terms of number of handled data blocks. The rationale behind this method is that the information is sequentially extracted by each data block and used to predict the response Y. CovSel selects the variables of interest from all blocks (which are orthogonalized with respect to one another, in order to remove possible redundant information). Eventually, the selected variables are used to predict the response Y. Among the different available multi-block methods, SO-CovSel was chosen for the present study because it ensures an extremely parsimonious selection of biomarkers, while retaining excellent prediction rates.
Due to the complex nature of the condition of interest and the substantial number of variables considered, the study population was not large enough for external validation of the models. All calculations were therefore run in a double cross validation (DCV) procedure and verified by randomization tests (Westerhuis et al. 2008). Model parameters (i.e., the order of blocks and the number of selected variables) were defined in a global strategy on the internal loop of the DCV procedure (Biancolillo et al. 2020). More in detail, at each DCV run, the order of blocks and the optimal model complexity (i.e., the number of variables to be selected from each block) were chosen as the ones leading to the lowest classification error on the inner cross-validation loop. However, if a model with a lower number of variables provided a classification error numerically slightly higher, but not statistically different than the minimum error, a more parsimonious solution would have been preferred. All analyses were performed using in-house routines running under MATLAB R2015b environment (The MathWorks, Natick, MA, USA). More details about the SO-CovSel algorithm may be found in specialized literature (Biancolillo et al. 2020).
Results
Study population
Two-hundred older community-dwellers, 100 participants with PF&S, and 100 nonPF&S controls were enrolled in the study. Table 1 reports the main characteristics of the study population according to PF&S status and gender. Men and women with PF&S were older and had higher BMI than their respective nonPF&S counterparts. As per the study design, the SPPB summary score was lower in people with PF&S than in controls. Likewise, aLM either absolute or adjusted by BMI was lower in PF&S participants compared with nonPF&S peers. No differences between groups were observed with regard to number of diseases and medications.
Table 1.
Main characteristics of study participants according to physical frailty and sarcopenia (PF&S) status and gender
| PF&S | NonPF&S | |||||
|---|---|---|---|---|---|---|
| All (n = 100) | Men (n = 25) | Women (n = 75) | All (n = 100) | Men (n = 50) | Women (n = 50) | |
| Age, years | 77.6 ± 5.1* | 79.6 ± 5.5* | 76.9 ± 4.8* | 74.8 ± 3.9 | 75.3 ± 4.0 | 74.4 ± 3.8 |
| BMI, kg/m2 | 30.0 ± 5.0* | 29.3 ± 4.5* | 30.2 ± 5.0* | 27.4 ± 2.9 | 27.1 ± 2.8 | 27.7 ± 3.0 |
| SPPB summary score | 7.2 ± 1.2* | 7.4 ± 1.0* | 7.1 ± 1.3* | 11.4 ± 0.8 | 11.5 ± 0.8 | 11.3 ± 0.8 |
| aLM, kg | 16.2 ± 3.0* | 20.1 ± 2.7* | 14.9 ± 2.0* | 20.1 ± 4.1 | 23.7 ± 2.6 | 16.7 ± 1.6 |
| aLMBMI | 0.54 ± 0.11* | 0.69 ± 0.08* | 0.49 ± 0.08* | 0.76 ± 0.19 | 0.88 ± 0.08 | 0.64 ± 0.20 |
| Number of disease conditions§ | 2.3 ± 1.8 | 2.1 ± 1.9 | 2.5 ± 2.0 | 2.1 ± 1.6 | 2.1 ± 1.6 | 2.0 ± 1.7 |
| Number of medications# | 3.6 ± 2.1 | 3.8 ± 2.2 | 3.5 ± 2.4 | 3.2 ± 2.0 | 3.3 ± 1.9 | 3.1 ± 2.4 |
Data are shown as mean ± standard deviation. *p < 0.05 vs. nonPF&S
§Includes hypertension, coronary artery disease, prior stroke, peripheral vascular disease, diabetes, chronic obstructive pulmonary disease, and osteoarthritis
#Includes prescription and over-the-counter medications
aLM, appendicular lean mass; aLMBMI, appendicular lean mass adjusted by body mass index (BMI); nonPF&S, non-physically frail, non-sarcopenic; SPPB, short physical performance battery
Biomarker selection through SO-CovSel analysis in the whole study sample
Serum concentrations of candidate biomarkers were organized into four matrices according to the analytic platform used for their measurement (Table 2). Several SO-CovSel models were built using different sequential combinations of the four data matrices. Among the SO-CovSel models tested, we selected the one that allowed the best classification performance [i.e., the one providing the lowest number of misclassifications (NMC)] with the smallest number of variables. This approach was elected to meet specific requirements for biomarkers to be used in the clinical setting (e.g., their determination must be reliable and feasible also from an economic point of view) (Sprott 2010; Justice et al. 2018). This “parsimonious” model was built using only seven markers (one from matrix 1, four from matrix 4, and one from matrices 2 and 3) (Table 3).
Table 2.
Composition of matrices used for sequential and orthogonalized covariance selection (SO-CovSel) analysis
| Data block | Number of variables | Variables |
|---|---|---|
| Matrix 1 | 27 | IL1-β, IL1-ra, IL2, IL4, IL5, IL6, IL7, IL8, IL9, IL10, IL12, IL13, IL15, IL17, FGF basic, G-CSF, GM-CSF, IFN-γ, MCP-1, MIP-1α, MIP-1β, CCL5, CCL11, IP-10, PDGF-BB, TNF-α |
| Matrix 2 | 4 | CRP, MPO, FGF-21, BDNF |
| Matrix 3 | 6 | CAF, P-Selectin, HtrA1, Hsp72, P3NP, IGF-1 |
| Matrix 4 | 37 | 1-methylhistidine, 3-methylhistidine, 4-hydroxyproline, α-aminobutyric acid, β-alanine, β-aminobutyric acid, γ-aminobutyric acid, alanine, aminoadipic acid, anserine, arginine, asparagine, aspartic acid, carnosine, citrulline, cystathionine, cystine, ethanolamine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, ornithine, phenylalanine, phosphoethanolamine, phosphoserine, proline, sarcosine, serine, taurine, threonine, tryptophan, tyrosine, valine |
BDNF, brain-derived neurotrophic factor; CAF, C-terminal agrin fragment; CCL, C-C motif chemokine ligand; CRP, C-reactive protein; FGF, fibroblast growth factor; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; Hsp72, heat shock protein 72; HtrA1, high-temperature requirement serine protease A; IFN, interferon; IGF-1, insulin-like growth factor 1; IL, interleukin; IL1-ra, IL1 receptor agonist; IP, IFN-γ-induced protein; MCP-1, monocyte chemoattractant protein 1; MIP-1α, macrophage inflammatory protein 1α; MIP-1β, macrophage inflammatory protein 1β; MPO, myeloperoxidase; P3NP, procollagen III N-terminal peptide; PDGF-BB, platelet-derived growth factor BB; TNF-α, tumor necrosis factor alpha
Table 3.
Serum concentrations of discriminant biomolecules identified by sequential and orthogonalized covariance selection (SO-CovSel) analysis in the whole study sample. Matrices are ordered according to the sequence in which they were entered in the model
| PF&S (n = 100) |
NonPF&S (n = 100) |
||
|---|---|---|---|
| Matrix 1 | PDGF-BB (ng/mL) | 2.8 (1.7) | 3.3 (2.0) |
| Matrix 4 | α-aminobutyric acid (μmol/L) | 18.8 (5.8) | 21.9 (7.4) |
| Asparagine (μmol/L) | 89.3 (19.15) | 77.7 (15.5) | |
| Aspartic acid (μmol/L) | 24.0 (7.4) | 16.4 (4.3) | |
| Citrulline (μmol/L) | 42.3 (19.3) | 37.6 (16.5) | |
| Matrix 3 | Hsp72 (ng/mL) | 1.3 (0.9) | 2.3 (1.8) |
| Matrix 2 | MPO (ng/mL) | 237.6 (204.4) | 367.3 (463.6) |
Data are shown as median and interquartile range
Hsp72, heat shock protein 72; MPO, myeloperoxidase; PDGF-BB, platelet-derived growth factor BB
Rates of correct classification were 80.6 ± 5.3% and 79.9 ± 5.1%, for PF&S and nonPF&S participants, respectively (80.3 ± 3.8% in the whole study population). The model showed that serum concentrations of three amino acids (asparagine, aspartic acid, and citrulline) were greater in older persons with PF&S, while higher circulating levels of PDGF-BB, Hsp72, MPO, and α-aminobutyric acid characterized nonPF&S participants (Table 3).
To rule out the possibility that age or BMI could confound biomarker selection, multivariate PLS regression models were built against age and BMI. No significant influence on biomarker selection was found for either age or BMI (Table 4). To evaluate possible associations between the identified biomarkers and PF&S severity, multivariate PLS regression models relating serum biomarker concentrations to SPPB scores, aLM, and aLMBMI were built and validated. PLS analyses showed that none of the candidate biomarkers selected by SO-CovSel was significantly associated with any of the variables considered (Table 4).
Table 4.
Results of partial least squares regression models relating SO-CovSel-selected biomarker concentrations in serum to age, anthropometry, body composition, and physical performance in the whole study sample and in the two genders
| R2 | Q2 | |
|---|---|---|
| Whole study sample | ||
| Age | 0.21 | 0.10 |
| BMI | 0.28 | 0.15 |
| SPPB summary score | 0.53 | 0.43 |
| aLM | 0.20 | 0.05 |
| aLMBMI | 0.25 | 0.15 |
| Men | ||
| Age | 0.37 | 0.04 |
| BMI | 0.35 | 0.08 |
| SPPB summary score | 0.48 | 0.28 |
| aLM | 0.31 | 0.07 |
| aLMBMI | 0.56 | 0.34 |
| Women | ||
| Age | 0.14 | 0.02 |
| BMI | 0.25 | 0.13 |
| SPPB summary score | 0.49 | 0.35 |
| aLM | 0.37 | 0.27 |
| aLMBMI | 0.22 | 0.08 |
aLM, appendicular lean mass; aLMBMI, appendicular lean mass adjusted by body mass index (BMI); SO-CovSel, sequential and orthogonalized covariance selection; SPPB, short physical performance battery
Gender-specific SO-CovSel models
Gender-specific differences in biomarker profiles between PF&S and nonPF&S participants were explored through separate SO-CovSel models for men and women. A common, gender-independent biomarker “core” was found in older adults with PF&S. This core included aspartic acid (higher in PF&S) and Hsp72 and MIP-1β (higher in nonPF&S) (Table 5).
Table 5.
Serum concentrations of discriminant biomolecules identified by gender-specific sequential and orthogonalized covariance selection (SO-CovSel) analysis
| Men (n = 75) | |||
|
PF&S (n = 25) |
NonPF&S (n = 50) |
||
| Matrix 1 | MIP-1β (ng/mL) | 144.0 (88.0) | 189.4 (58.4) |
| Matrix 4 | α-aminobutyric acid (μmol/L) | 18.7 (5.4) | 23.4 (6.1) |
| Asparagine (μmol/L) | 92.1 (14.3) | 76.6 (20.2) | |
| Aspartic acid (μmol/L) | 24.6 (14.3) | 16.4 (5.2) | |
| Phosphoetanolamine (μmol/L) | 0.9 (0.3) | 1.4 (0.8) | |
| Matrix 3 | CAF (ng/mL) | 0.2 (0.1) | 0.1 (0.1) |
| Hsp72 (ng/mL) | 1.3 (0.6) | 2.1 (1.5) | |
| Matrix 2 | FGF21 (ng/mL) | 535.2 (362.9) | 275.2 (340.9) |
| Women (n = 125) | |||
|
PF&S (n = 75) |
NonPF&S (n = 50) |
||
| Matrix 1 | PDGF-BB (ng/mL) | 3.1 (1.8) | 3.9 (2.5) |
| MIP-1β (ng/mL) | 175.6 (72.4) | 176.2 (76.7) | |
| Matrix 4 | Aspartic acid (μmol/L) | 23.9 (4.9) | 16.4 (3.6) |
| Matrix 3 | Hsp72 (ng/mL) | 1.4 (1.0) | 2.5 (1.7) |
| Matrix 2 | MPO (ng/mL) | 227.5 (182.3) | 667.3 (593.4) |
Matrices are ordered according to the sequence in which they were entered in the model. Bolded analytes are common to the two genders; gender-specific discriminant biomolecules are unbolded. Data are shown as median and interquartile range
CAF, C-terminal agrin fragment; FGF21, fibroblast growth factor 21; Hsp72, heat shock protein 72; MIP-1β, macrophage inflammatory protein 1β; MPO, myeloperoxidase; PDGF-BB, platelet-derived growth factor BB
The SO-CovSel model built for men correctly classified 64.5 ± 12.2% of PF&S participants and 66.9 ± 8.5% of nonPF&S controls. The rate of correct classification for the whole male sample was 65.7 ± 8.2%. The model included the three core biomolecules plus asparagine, CAF, and FGF21, which were higher in the PF&S group, and α-aminobutyric acid and phosphoetanolamine, which both were higher in nonPF&S participants (Table 5). In women, the best SO-CovSel model showed remarkable classification performance (86.9 ± 5.4% for PF&S, 79.7 ± 7.5% for nonPF&S, 84.1 ± 4.4% for the whole female sample). Besides the three shared biomolecules, SO-CovSel also selected PDGF-BB and MPO, which both were lower in PF&S participants (Table 5). The classification performance of gender-specific models was confirmed by DCV and by means of randomization tests (p < 0.05).
Multivariate PLS regression models relating serum biomarkers selected by gender-specific SO-CovSel models to potential confounders or to muscle-specific, and functional parameters were built and validated. PLS analyses showed that circulating levels of the selected biomarkers were not significantly associated with any of the variables considered in either gender (Table 4).
Discussion
The identification of reliable biomarkers of aging and age-related conditions has long been a chimera for biogerontologists (Justice et al. 2018). The main reason for the several hypes and falls in this task resides in the unquestioningly complex nature of aging itself. Indeed, during organismal aging, multiple overlapping processes co-occur, resulting in a multitude of different and stochastically determined phenotypes (Kirkwood 2011). Recently, the biological drivers of aging, the so-called hallmarks of aging, have been pinpointed (López-Otín et al. 2013). According to the geroscience hypothesis, perturbations in these mechanisms increase the susceptibility to most chronic diseases, functional loss, and eventually death (Sierra 2016). As a consequence, these biologic pillars represent ideal targets for interventions to foster healthy aging (Newman et al. 2016; Kaeberlein 2017). It follows that the biomarker discovery process should move from the traditional “one disease/one biomarker” approach to a more comprehensive, multivariable landscape that incorporates as many contributing factors as possible (Calvani et al. 2015; Jylhävä et al. 2017).
In the present study, we tested a new way in the uncharted waters of biomarker discovery in geroscience. To this aim, we selected a newly operationalized age-related condition, PF&S, which embodies in its complex pathophysiology all biological “pillars” of aging (Calvani et al. 2015). Notably, PF&S has received initial endorsement by the European Medicines Agency (EMA) as a prototypical geriatric/geroscience condition (Marzetti et al. 2018). Then, we applied an innovative analytical method, SO-CovSel, for biomarker selection. This approach allowed highlighting some distinctive features of PF&S as well as possible gender-specific pathways of interest (Fig. 1). Among the different SO-CovSel models tested, we selected those with the best classification performance using the smallest set of analytes. This choice was pursued to meet obvious requirements in biomarker implementation (e.g., economic feasibility) and to speed up biomarker use in the clinical arena (Sprott 2010; Justice et al. 2018).
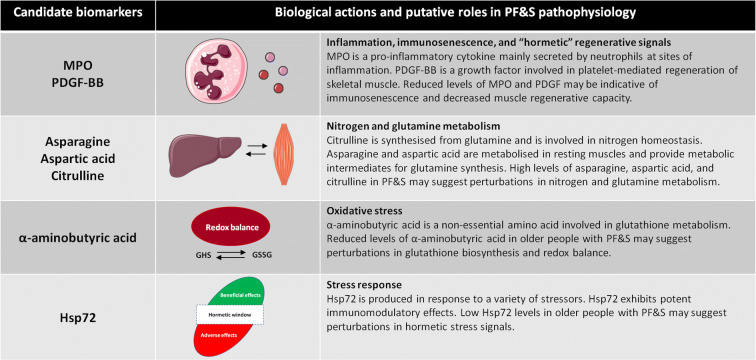
Fig. 1.
Candidate biomarkers for physical frailty and sarcopenia (PF&S) identified in the whole study sample through sequential and orthogonalized covariance selection (SO-CovSel) analysis and their putative roles in PF&S pathophysiology. Hsp72, heat shock protein 72; MPO, myeloperoxidase; PDGF-BB, platelet-derived growth factor BB. Marzetti (2020)
One major finding of our investigation was the definition of a reduced number of inflammatory biomolecules associated with PF&S. In the whole study sample, the inflammatory contribution may be recapitulated by only two mediators, MPO and PDGF-BB, which were both lower in participants with PF&S. In both gender-specific models, MIP-1β was also selected among the most relevant biomarkers for the discrimination between PF&S and control participants. Recently, we described a frailty “cytokinome” in older adults with PF&S (Marzetti et al. 2019). This “core” inflammatory profile was defined by higher serum levels of P-selectin, CRP, and IFN-γ-induced protein 10 (IP-10) and lower levels of MPO, IL8, MCP-1, MIP-1α, and PDGF-BB. SO-CovSel analysis allowed us further restricting the number of mediators required to describe the contribution of inflammation to PF&S. Pro-sarcopenic/pro-disability effects have traditionally been attributed to inflamm-aging (Bano et al. 2017; Wilson et al. 2017; Franceschi et al. 2018; Furman et al. 2019). Besides the increase in single canonical pro-inflammatory cytokines (e.g., CRP, IL6), alterations in anti-inflammatory pathways and in the composition/function of immune cell subsets have been associated with detrimental changes in body composition and reduced physical performance (Soysal et al. 2016; Wilson et al. 2017; Furman et al. 2019). In this context, the reduced circulating levels of MPO observed in PF&S participants may be interpreted as a specific immunosenescence marker for this condition. Indeed, among the perturbations of immune function that have been described in the context of frailty and sarcopenia, neutrophil dysfunction may lead to reduced MPO secretion (Butcher et al. 2000; Wilson et al. 2017). Decreased MPO levels may also be secondary to micronutrient inadequacies that are common in frail older people (Busti et al. 2014). Specifically, iron deficiency may impair MPO biosynthesis (Maggini et al. 2007).
Participants with PF&S also showed lower circulating levels of PDGF-BB and MIP-1β. PDGF-BB belongs to a family of growth factors that possess several biological functions, including regulation of proliferation, differentiation, and migration of cells of mesenchymal origin (Andrae et al. 2008). In particular, PDGF-BB plays a crucial role in platelet-mediated regeneration of skeletal muscle by stimulating proliferation of satellite cells and myoblasts (Scully et al. 2018, 2019). The reduced serum levels of PDGF-BB observed in older adults with PF&S may therefore suggest a defective muscle regenerative capacity as a possible contributing factor to PF&S development (Brzeszczyńska et al. 2018). This idea is also supported by the presence of MIP-1β among the selected biomarkers in both gender-specific models. MIP-1β is a member of CC family chemokines that directly regulate myoblast behavior in response to muscle injury (Yahiaoui et al. 2008). Lower circulating levels of MIP-1β may be indicative of perturbation in macrophage polarization, a process relevant for efficient skeletal muscle regeneration (De Santa et al. 2019).
Participants with PF&S were also characterized by specific amino acid fingerprints. In a preliminary analysis involving a smaller group of BIOSPHERE participants, we identified a distinct profile of circulating amino acids in older adults with PF&S (Calvani et al. 2018b). In particular, the combination of nine amino acids (i.e., α-aminobutyric acid, asparagine, aspartic acid, citrulline, ethanolamine, glutamic acid, methionine, sarcosine, and taurine) was able to correctly predict the presence of PF&S in about 75% of cases (Calvani et al. 2018b). The implementation of the SO-CovSel algorithm allowed us reducing the number of amino acids requested to distinguish older adults with PF&S from nonPF&S controls. A lower serum concentration of α-aminobutyric acid was the first feature that spotted the serum amino acid profile of PF&S. Alpha-aminobutyric acid is a non-essential amino acid deriving from the catabolism of methionine, threonine, and serine (Matsuo and Greenberg 1955). A role for α-aminobutyric has been demonstrated in the regulation of glutathione biosynthesis (Irino et al. 2016). Following oxidative stress, the glutathione biosynthetic pathway is stimulated to compensate for its increased consumption (Meister and Anderson 1983). The activation of glutathione synthesis initiates the parallel production of its analogue ophthalmic acid from α-aminobutyric (Soga et al. 2006). Alpha-aminobutyric acid was shown to modulate glutathione homeostasis in the myocardium, and its oral administration efficiently increased circulating and myocardial glutathione levels in mice (Irino et al. 2016). The reduced serum levels of α-aminobutyric acid found in PF&S participants may thus indicate increased oxidative stress and/or a perturbation in the glutathione biosynthetic pathway. Noticeably, glutathione metabolism shows sexual dimorphism in both rodents and humans (Wang et al. 2020), with males exhibiting more evident age-associated changes in glutathione content and oxidative damage than females in several tissues, including muscle (Pansarasa et al. 2000; Wang et al. 2003). These observations may, at least partly, explain the differential classification power of α-aminobutyric acid determined in gender-specific SO-CovSel analysis.
Collectively, the presence of asparagine, aspartic acid, and citrulline among the best predictors of PF&S may be indicative of disturbances in several muscle-specific and interorgan processes modulating whole-body energy metabolism, cellular anabolic pathways, and nitrogen/glutamine homeostasis. Asparagine and aspartic acid are among the amino acids that are metabolized in resting muscles (Wagenmakers 1998a,b). These amino acids provide both the amino groups and the carbon skeleton required for the synthesis of glutamine and tricarboxyl acid cycle intermediates (Owen et al. 2002). Notably, asparagine, that is structurally similar to glutamine, exerts several important functions in muscles. Indeed, asparagine may (a) be used as a preferred nitrogen source to support protein synthesis, (b) inhibit muscle protein degradation through its action on muscle protein kinase B and AMP-activated protein kinase, and (c) reduce the expression of muscle pro-inflammatory cytokines through toll-like receptor 4 and nucleotide-binding oligomerization domain protein signaling (Wang et al. 2016; Pavlova et al. 2018). Aspartic acid and citrulline are intermediates of the urea cycle, one of the major processes regulating whole-body nitrogen homeostasis. Alterations in urea cycle intermediates and nitrogen metabolic pathways have been described in aging and age-related chronic conditions, including frailty and type 2 diabetes (Prior et al. 1996; Mangoni et al. 2019; Calvani et al. 2020). Besides its role in nitrogen homeostasis, citrulline may has also stimulate muscle protein synthesis through its purported ability to activate the mechanistic target of rapamycin complex 1 and by reallocating mitochondrial fuel to the protein synthesis machinery (Le Plénier et al. 2012; Goron et al. 2019). For all these properties, citrulline has been suggested as a possible agent to contrast sarcopenia (Breuillard et al. 2015; Martone et al. 2015, Papadia et al. 2017). Serum citrulline levels may increase with aging due to the progressive decline of liver and renal function (Sarwar et al. 1991; Pitkänen et al. 2003; Kouchiwa et al. 2012; Chaleckis et al. 2016). However, kidney and liver function were not different between PF&S and nonPF&S participants.
In men, lower serum levels of phosphoethanolamine characterized the biomarker profile of PF&S. Phosphoethanolamine is an intermediate of the CDP-ethanolamine pathway, a main route in glycerophospholipid metabolism and biological membrane turnover (Patel and Witt 2017; van der Veen et al. 2017). Alterations in the CDP-ethanolamine pathway and skeletal muscle phospholipid metabolism have been associated with insulin resistance, myofiber damage, perturbed muscle mitochondrial integrity, and muscle wasting (Funai et al. 2016; Selathurai et al. 2019). In addition, we recently showed that this pathway may also be involved in the disabling cascade in frail older persons with type 2 diabetes mellitus (Calvani et al. 2020).
FGF21 was among the SO-CovSel-selected biomarkers in male-specific model. FGF21 is a myokine with pleiotropic functions that is secreted by muscles upon different stimuli, including fasting, endoplasmic reticulum stress, metabolic disorders, and mitochondrial dysfunction (Tezze et al. 2019). In rodents, FGF21 promotes muscle atrophy through (dys)regulation of mitophagy and anabolic/catabolic balance (Oost et al. 2019). In humans, circulating FGF21 levels increase with age and in the setting of various metabolic conditions, including obesity, insulin resistance, and type 2 diabetes mellitus (Zhang et al. 2008; Chavez et al. 2009; Conte et al. 2019). Though, in the present study, no relationships were found between FGF21 and age or anthropometric and functional parameters.
CAF is generated from the proteolytic cleavage of agrin, a critical component of the neuromuscular junction (Stephan et al. 2008). Increased CAF levels in the circulation have been associated with neuromuscular damage and sarcopenia in old multimorbid community-dwellers and in hip-fractured patients (Marzetti et al. 2014a; Landi et al. 2016). The relevance of CAF as a discriminant molecule in men with PF&S may reflect a gender-specific role for neuromuscular damage in the development of sarcopenia, as previously described by others (Drey et al. 2013).
The last relevant finding of the present study was the determination of lower serum Hsp72 levels in participants with PF&S compared with controls. Hsp72 is an almost ubiquitous protein that is upregulated in response to a variety of stressors (Johnson and Fleshner 2006). At the skeletal muscle level, Hsp72 induction preserves muscle architecture and function, increases mitochondrial number and oxidative capacity, reduces inflammation, and protects against obesity-induced insulin resistance (Chung et al. 2008; Gehrig et al. 2012; Henstridge et al. 2014). Circulating Hsp72 levels ramp up in response to several stress stimuli, including disease conditions such as renal and cardiovascular disease (Johnson and Fleshner 2006). Extracellular Hsp72 possesses potent protective modulatory effects on innate and acquired immunity (Johnson and Fleshner 2006). Recently, Hsp72 has been proposed as a sarcopenia biomarker, since its plasma concentrations were associated with low muscle mass and function in older adults (Ogawa et al. 2012). Moreover, circulating Hsp72 levels were reduced after a 16-week exercise program in older sarcopenic men, albeit no correlation among Hsp72 concentrations, muscle mass, or inflammatory markers was found (Perreault et al. 2016). Together with low levels of MPO, PDGF-BB and MIP-1β, reduced serum concentrations of Hsp72 in people with PF&S may result from perturbations in hormetic signals needed to preserve muscle mass and function in late life (Calabrese et al. 2015; Calabrese and Mattson 2017).
Some limitations of the present study should be acknowledged. First, although the study population was appropriately selected and characterized, the sample was relatively small and the number of variables analyzed was quite large. However, SO-CovSel is especially suited for handling matrices where strong interdependence is present among biomarkers and variables are organized in multi-block datasets (Biancolillo and Naes 2019; Biancolillo et al. 2020; Picca et al. 2020). Only Caucasian older people were enrolled in the present study; thus, our findings need to be validated in other ethnic groups. Both physical activity and nutritional habits may impact serum levels of several biomolecules assayed. Although only physically inactive, non-malnourished persons were enrolled in the study, physical activity levels and diet composition were not assessed. The investigation followed a cross-sectional design, which does not allow establishing definite mechanistic and temporal relationships between changes in circulating mediators and PF&S. Finally, although a large number of biomolecules was assayed, we could not analyze all possible mediators involved in PF&S. Hence, the presence of more powerful biomarkers of PF&S cannot be excluded.
In conclusion, SO-CovSel analysis allowed selecting and evaluating the classification performance of a panel of candidate biomarkers for PF&S as well as suggesting gender-specific fingerprints. As opposed to traditional, monodimensional biomarker discovery strategies, SO-CovSel is a promising tool to cope with the complex network of pathways underlying age-related phenomena (Calvani et al. 2015; Cohen et al. 2018; Justice et al. 2018, Picca et al. 2020). The longitudinal implementation of such a novel analytical framework could allow definite identification of biomarkers possessing the requirements for their application in the clinical arena (e.g., reliability, feasibility, predictive ability toward relevant health outcomes, responsiveness to interventions).
Author contributions
A.P., E.M., and R.C. conceived and designed the study and drafted the manuscript; G.O. and M.C. contributed to the study design and to the manuscript drafting; A.B. and F.M. analyzed the data; F.L. coordinated the participant recruitment; A.P., J.G., and S.P. designed and performed the experiments; H.J.C.-J. and M.B. were responsible for data curation; A.U. and R.B. acquired the funding and provided overall supervision.
Funding information
This study was supported by the Fondazione Roma (NCDs Call for Proposals 2013 to A.P., E.M., F.L., R.B., and R.C.), the Innovative Medicines Initiative – Joint Undertaking (IMI-JU 115621 to A.P., E.M., F.L., M.C., R.B., and R.C.), the nonprofit research foundation “Centro Studi Achille e Linda Lorenzon” (A.P, E.M., and R.C.), intramural research grants from the Università Cattolica del Sacro Cuore (Linea D3.2 2013 and D3.2 2015 to F.L.), and by a scholarship from the Brazilian federal government to H.J.C.-J. (Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior; 001).
Data availability
Data analyzed in the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Competing interests
A.P., E.M., F.L., M.C., and R.C. are partners of the SPRINTT consortium, which is partly funded by the European Federation of Pharmaceutical Industries and Associations (EFPIA).
E.M. served as a consultant for Abbott, Biophytis, Nutricia, and Nestlè. R.C. served as a consultant for Abbott and Nutricia. M.C. served as a consultant for and/or received honoraria for scientific presentations from Nestlé and received a research grant from Pfizer.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anna Picca, Email: anna.picca@guest.policlinicogemelli.it.
Roberto Bernabei, Email: roberto.bernabei@unicatt.it.
References
- Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bano G, Trevisan C, Carraro S, Solmi M, Luchini C, Stubbs B, Manzato E, Sergi G, Veronese N. Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas. 2017;96:10–15. doi: 10.1016/j.maturitas.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Berghella AM, Contasta I, Marulli G, D’Innocenzo C, Garofalo F, Gizzi F, Bartolomucci M, Laglia G, Valeri M, Gizzi M, Friscioni M, Barone M, del Beato T, Secinaro E, Pellegrini P. Ageing gender-specific “Biomarkers of Homeostasis”, to protect ourselves against the diseases of the old age. Immun Ageing. 2014;11:3. doi: 10.1186/1742-4933-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biancolillo A, Måge I, Næs T. Combining SO-PLS and linear discriminant analysis for multi-block classification. Chemom Intell Lab Syst. 2015;141:58–67. doi: 10.1016/J.CHEMOLAB.2014.12.001. [DOI] [Google Scholar]
- Biancolillo A, Marini F, Roger JM. SO-CovSel: a novel method for variable selection in a multi-block framework. J Chemom. 2020;34:e3120. doi: 10.1002/cem.3120. [DOI] [Google Scholar]
- Biancolillo A, Naes T. The sequential and orthogonalised PLS regression (SO-PLS) for multi-block regression: theory, examples and extensions. In: Cocchi M, editor. Data fusion methodology and applications. Amsterdam, Netherlands: Elsevier Inc.; 2019. pp. 157–177. [Google Scholar]
- Breuillard C, Cynober L, Moinard C. Citrulline and nitrogen homeostasis: an overview. Amino Acids. 2015;47:685–691. doi: 10.1007/s00726-015-1932-2. [DOI] [PubMed] [Google Scholar]
- Brzeszczyńska J, Meyer A, McGregor R, Schilb A, Degen S, Tadini V, Johns N, Langen R, Schols A, Glass DJ, Roubenoff R, Ross JA, Fearon KCH, Greig CA, Jacobi C. Alterations in the in vitro and in vivo regulation of muscle regeneration in healthy ageing and the influence of sarcopenia. J Cachexia Sarcopenia Muscle. 2018;9:93–105. doi: 10.1002/jcsm.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busti F, Campostrini N, Martinelli N, Girelli D. Iron deficiency in the elderly population, revisited in the hepcidin era. Front Pharmacol. 2014;5:83. doi: 10.3389/fphar.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher S, Chahel H, Lord JM. Review article: ageing and the neutrophil: no appetite for killing? Immunology. 2000;100:411–416. doi: 10.1046/J.1365-2567.2000.00079.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Dhawan G, Kapoor R, Iavicoli I, Calabrese V. What is hormesis and its relevance to healthy aging and longevity? Biogerontology. 2015;16:693–707. doi: 10.1007/s10522-015-9601-0. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Mattson MP. How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech Dis. 2017;3:13. doi: 10.1038/s41514-017-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvani R, Marini F, Cesari M, Tosato M, Anker SD, von Haehling S, Miller RR, Bernabei R, Landi F, Marzetti E, the SPRINTT consortium Biomarkers for physical frailty and sarcopenia: state of the science and future developments. J Cachexia Sarcopenia Muscle. 2015;6:278–286. doi: 10.1002/jcsm.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvani R, Marini F, Cesari M, Buford TW, Manini TM, Pahor M, Leeuwenburgh C, Bernabei R, Landi F, Marzetti E. Systemic inflammation, body composition, and physical performance in old community-dwellers. J Cachexia Sarcopenia Muscle. 2017;8:69–77. doi: 10.1002/jcsm.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvani R, Picca A, Marini F, Biancolillo A, Cesari M, Pesce V, Lezza AMS, Bossola M, Leeuwenburgh C, Bernabei R, Landi F, Marzetti E. The “BIOmarkers associated with Sarcopenia and PHysical frailty in EldeRly pErsons” (BIOSPHERE) study: rationale, design and methods. Eur J Intern Med. 2018;56:19–25. doi: 10.1016/j.ejim.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvani R, Picca A, Marini F, Biancolillo A, Gervasoni J, Persichilli S, Primiano A, Coelho-Junior H, Bossola M, Urbani A, Landi F, Bernabei R, Marzetti E. A distinct pattern of circulating amino acids characterizes older persons with physical frailty and sarcopenia: results from the BIOSPHERE study. Nutrients. 2018;10:1691. doi: 10.3390/nu10111691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvani R, Rodriguez-Mañas L, Picca A, Marini F, Biancolillo A, Laosa O, Pedraza L, Gervasoni J, Primiano A, Conta G, Bourdel-Marchasson I, Regueme SC, Bernabei R, Marzetti E, Sinclair AJ, Gambassi G. Identification of a circulating amino acid signature in frail older persons with type 2 diabetes mellitus: results from the MetaboFrail study. Nutrients. 2020;12:199. doi: 10.3390/nu12010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Landi F, Calvani R, et al. Rationale for a preliminary operational definition of physical frailty and sarcopenia in the SPRINTT trial. Aging Clin Exp Res. 2017;29:81–88. doi: 10.1007/s40520-016-0716-1. [DOI] [PubMed] [Google Scholar]
- Cesari M, Marzetti E, Calvani R, et al. The need of operational paradigms for frailty in older persons: the SPRINTT project. Aging Clin Exp Res. 2017;29:3–10. doi: 10.1007/s40520-016-0712-5. [DOI] [PubMed] [Google Scholar]
- Chaleckis R, Murakami I, Takada J, Kondoh H, Yanagida M. Individual variability in human blood metabolites identifies age-related differences. Proc Natl Acad Sci U S A. 2016;113:4252–4259. doi: 10.1073/pnas.1603023113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AO, Molina-Carrion M, Abdul-Ghani MA, Folli F, DeFronzo RA, Tripathy D. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care. 2009;32:1542–1546. doi: 10.2337/dc09-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew J, Tay L, Lim JP, Leung BP, Yeo A, Yew S, Ding YY, Lim WS. Serum myostatin and IGF-1 as gender-specific biomarkers of frailty and low muscle mass in community-dwelling older adults. J Nutr Health Aging. 2019;23:979–986. doi: 10.1007/s12603-019-1255-1. [DOI] [PubMed] [Google Scholar]
- Chung J, Nguyen A-K, Henstridge DC, Holmes AG, Chan MHS, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, Horvath I, Mestril R, Watt MJ, Hooper PL, Kingwell BA, Vigh L, Hevener A, Febbraio MA. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci. 2008;105:1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AA, Legault V, Fuellen G, Fülöp T, Fried LP, Ferrucci L. The risks of biomarker-based epidemiology: associations of circulating calcium levels with age, mortality, and frailty vary substantially across populations. Exp Gerontol. 2018;107:11–17. doi: 10.1016/j.exger.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte M, Ostan R, Fabbri C, Santoro A, Guidarelli G, Vitale G, Mari D, Sevini F, Capri M, Sandri M, Monti D, Franceschi C, Salvioli S. Human aging and longevity are characterized by high levels of mitokines. J Gerontol A Biol Sci Med Sci. 2019;74:600–607. doi: 10.1093/gerona/gly153. [DOI] [PubMed] [Google Scholar]
- De Santa F, Vitiello L, Torcinaro A, Ferraro E. The role of metabolic remodeling in macrophage polarization and its effect on skeletal muscle regeneration. Antioxid Redox Signal. 2019;30:1553–1598. doi: 10.1089/ars.2017.7420. [DOI] [PubMed] [Google Scholar]
- Drey M, Sieber CC, Bauer JM, Uter W, Dahinden P, Fariello RG, Vrijbloed JW. C-terminal Agrin Fragment as a potential marker for sarcopenia caused by degeneration of the neuromuscular junction. Exp Gerontol. 2013;48:76–80. doi: 10.1016/j.exger.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14:576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- Funai K, Lodhi IJ, Spears LD, Yin L, Song H, Klein S, Semenkovich CF. Skeletal muscle phospholipid metabolism regulates insulin sensitivity and contractile function. Diabetes. 2016;65:358–370. doi: 10.2337/db15-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrig SM, van der Poel C, Sayer TA, Schertzer JD, Henstridge DC, Church JE, Lamon S, Russell AP, Davies KE, Febbraio MA, Lynch GS. Hsp72 preserves muscle function and slows progression of severe muscular dystrophy. Nature. 2012;484:394–398. doi: 10.1038/nature10980. [DOI] [PubMed] [Google Scholar]
- Goron A, Lamarche F, Blanchet S, Delangle P, Schlattner U, Fontaine E, Moinard C. Citrulline stimulates muscle protein synthesis, by reallocating ATP consumption to muscle protein synthesis. J Cachexia Sarcopenia Muscle. 2019;10:919–928. doi: 10.1002/jcsm.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Henstridge DC, Bruce CR, Drew BG, Tory K, Kolonics A, Estevez E, Chung J, Watson N, Gardner T, Lee-Young RS, Connor T, Watt MJ, Carpenter K, Hargreaves M, McGee SL, Hevener AL, Febbraio MA. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes. 2014;63:1881–1894. doi: 10.2337/db13-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irino Y, Toh R, Nagao M, Mori T, Honjo T, Shinohara M, Tsuda S, Nakajima H, Satomi-Kobayashi S, Shinke T, Tanaka H, Ishida T, Miyata O, Hirata KI. 2-Aminobutyric acid modulates glutathione homeostasis in the myocardium. Sci Rep. 2016;6:36749. doi: 10.1038/srep36749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol. 2006;79:425–434. doi: 10.1189/jlb.0905523. [DOI] [PubMed] [Google Scholar]
- Justice JN, Ferrucci L, Newman AB, Aroda VR, Bahnson JL, Divers J, Espeland MA, Marcovina S, Pollak MN, Kritchevsky SB, Barzilai N, Kuchel GA. A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: report from the TAME Biomarkers Workgroup. GeroScience. 2018;40:419–436. doi: 10.1007/s11357-018-0042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jylhävä J, Pedersen NL, Hägg S. Biological age predictors. EBioMedicine. 2017;21:29–36. doi: 10.1016/J.EBIOM.2017.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M. Translational geroscience: a new paradigm for 21st century medicine. Transl Med Aging. 2017;1:1–4. doi: 10.1016/J.TMA.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood TBL. Systems biology of ageing and longevity. Philos Trans R Soc Lond Ser B Biol Sci. 2011;366:64–70. doi: 10.1098/rstb.2010.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchiwa T, Wada K, Uchiyama M, Kasezawa N, Niisato M, Murakami H, Fukuyama K, Yokogoshi H. Age-related changes in serum amino acids concentrations in healthy individuals. Clin Chem Lab Med. 2012;50:861–870. doi: 10.1515/cclm-2011-0846. [DOI] [PubMed] [Google Scholar]
- Landi F, Calvani R, Lorenzi M, Martone AM, Tosato M, Drey M, D'Angelo E, Capoluongo E, Russo A, Bernabei R, onder G, Marzetti E. Serum levels of C-terminal agrin fragment (CAF) are associated with sarcopenia in older multimorbid community-dwellers: results from the ilSIRENTE study. Exp Gerontol 2016. 2016;79:31–36. doi: 10.1016/j.exger.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Le Plénier S, Walrand S, Noirt R, et al. Effects of leucine and citrulline versus non-essential amino acids on muscle protein synthesis in fasted rat: a common activation pathway? Amino Acids. 2012;43:1171–1178. doi: 10.1007/s00726-011-1172-z. [DOI] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi M, Lorenzi T, Marzetti E, Landi F, Vetrano DL, Settanni S, Antocicco M, Bonassi S, Valdiglesias V, Bernabei R, onder G. Association of frailty with the serine protease HtrA1 in older adults. Exp Gerontol. 2016;81:8–12. doi: 10.1016/j.exger.2016.03.019. [DOI] [PubMed] [Google Scholar]
- Maggini S, Wintergerst ES, Beveridge S, Hornig DH. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr. 2007;98:S29–S35. doi: 10.1017/S0007114507832971. [DOI] [PubMed] [Google Scholar]
- Mangoni AA, Rodionov RN, Mcevoy M, et al. New horizons in arginine metabolism, ageing and chronic disease states. Age Ageing. 2019;48:776–782. doi: 10.1093/ageing/afz083. [DOI] [PubMed] [Google Scholar]
- Martone AM, Lattanzio F, Abbatecola AM, Carpia D, Tosato M, Marzetti E, Calvani R, onder G, Landi F. Treating sarcopenia in older and oldest old. Curr Pharm Des. 2015;21:1715–1722. doi: 10.2174/1381612821666150130122032. [DOI] [PubMed] [Google Scholar]
- Marzetti E. Identification of predictors of physical frailty and sarcopenia through a new multi‐marker approach. FASEB J. 2020;34:1–1. 10.1096/fasebj.2020.34.s1.09628.
- Marzetti E, Calvani R, Landi F, Hoogendijk EO, Fougère B, Vellas B, Pahor M, Bernabei R, Cesari M, SPRINTT Consortium Innovative medicines initiative: the SPRINTT project. J Frailty Aging. 2015;4:207–208. doi: 10.14283/jfa.2015.69. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Calvani R, Lorenzi M, Marini F, D'Angelo E, Martone AM, Celi M, Tosato M, Bernabei R, Landi F. Serum levels of C-terminal agrin fragment (CAF) are associated with sarcopenia in older hip fractured patients. Exp Gerontol. 2014;60:79–82. doi: 10.1016/j.exger.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Cesari M, Calvani R, Msihid J, Tosato M, Rodriguez-Mañas L, et al. The “Sarcopenia and Physical fRailty IN older people: multi-componenT Treatment strategies” (SPRINTT) randomized controlled trial: case finding, screening and characteristics of eligible participants. Exp Gerontol. 2018;113:48–57. 10.1016/j.exger.2018.09.017. [DOI] [PubMed]
- Marzetti E, Landi F, Marini F, Cesari M, Buford TW, Manini TM, onder G, Pahor M, Bernabei R, Leeuwenburgh C, Calvani R. Patterns of circulating inflammatory biomarkers in older persons with varying levels of physical performance: a partial least squares-discriminant analysis approach. Front Med. 2014;1:27. doi: 10.3389/fmed.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Picca A, Marini F, Biancolillo A, Coelho-Junior HJ, Gervasoni J, Bossola M, Cesari M, onder G, Landi F, Bernabei R, Calvani R. Inflammatory signatures in older persons with physical frailty and sarcopenia: the frailty “cytokinome” at its core. Exp Gerontol. 2019;122:129–138. doi: 10.1016/j.exger.2019.04.019. [DOI] [PubMed] [Google Scholar]
- Matsuo Y, Greenberg DM. Metabolic formation of homoserine and alpha-aminobutyric acid from methionine. J Biol Chem. 1955;215:547–554. doi: 10.1016/S0021-9258(18)65976-9. [DOI] [PubMed] [Google Scholar]
- Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Naes T, Tomic O, Mevik B-H, Martens H. Path modelling by sequential PLS regression. J Chemom. 2011;25:28–40. doi: 10.1002/cem.1357. [DOI] [Google Scholar]
- Nakamura E, Miyao K. Sex differences in human biological aging. J Gerontol A Biol Sci Med Sci. 2008;63:936–944. doi: 10.1093/gerona/63.9.936. [DOI] [PubMed] [Google Scholar]
- Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- Newman JC, Milman S, Hashmi SK, Austad SN, Kirkland JL, Halter JB, Barzilai N. Strategies and challenges in clinical trials targeting human aging. J Gerontol Ser A Biol Sci Med Sci. 2016;71:1424–1434. doi: 10.1093/gerona/glw149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Kim H, Shimizu T, Abe S, Shiga Y, Calderwood SK. Plasma heat shock protein 72 as a biomarker of sarcopenia in elderly people. Cell Stress Chaperones. 2012;17:349–359. doi: 10.1007/s12192-011-0310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oost LJ, Kustermann M, Armani A, Blaauw B, Romanello V. Fibroblast growth factor 21 controls mitophagy and muscle mass. J Cachexia Sarcopenia Muscle. 2019;10:630–642. doi: 10.1002/jcsm.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostan R, Monti D, Gueresi P, Bussolotto M, Franceschi C, Baggio G. Gender, aging and longevity in humans: an update of an intriguing/neglected scenario paving the way to a gender-specific medicine. Clin Sci (Lond) 2016;130:1711–1725. doi: 10.1042/CS20160004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem. 2002;277:30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- Pansarasa O, Castagna L, Colombi B, Vecchiet J, Felzani G, Marzatico F. Age and sex differences in human skeletal muscle: role of reactive oxygen species. Free Radic Res. 2000;33:287–293. doi: 10.1080/10715760000301451. [DOI] [PubMed] [Google Scholar]
- Papadia C, Osowska S, Cynober L, Forbes A (2017) Citrulline in health and disease. Review on human studies. Clin Nutr 37:1823–1828. 10.1016/j.clnu.2017.10.009. [DOI] [PubMed]
- Patel D, Witt SN. Ethanolamine and phosphatidylethanolamine: partners in health and disease. Oxidative Med Cell Longev. 2017;2017:4829180–4829118. doi: 10.1155/2017/4829180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova NN, Hui S, Ghergurovich JM et al (2018) As extracellular glutamine levels decline, asparagine becomes an essential amino acid. Cell Metab 27:428–438.e5. 10.1016/j.cmet.2017.12.006. [DOI] [PMC free article] [PubMed]
- Perreault K, Courchesne-Loyer A, Fortier M, Maltais M, Barsalani R, Riesco E, Dionne IJ. Sixteen weeks of resistance training decrease plasma heat shock protein 72 (eHSP72) and increase muscle mass without affecting high sensitivity inflammatory markers’ levels in sarcopenic men. Aging Clin Exp Res. 2016;28:207–214. doi: 10.1007/s40520-015-0411-7. [DOI] [PubMed] [Google Scholar]
- Picca A, Ponziani FR, Calvani R, Marini F, Biancolillo A, Coelho-Júnior HJ, Gervasoni J, Primiano A, Putignani L, del Chierico F, Reddel S, Gasbarrini A, Landi F, Bernabei R, Marzetti E. Gut microbial, inflammatory and metabolic signatures in older people with physical frailty and sarcopenia: results from the BIOSPHERE study. Nutrients. 2020;12:65. doi: 10.3390/nu12010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen HT, Oja SS, Kemppainen K, et al. Serum amino acid concentrations in aging men and women. Amino Acids. 2003;24:413–421. doi: 10.1007/s00726-002-0338-0. [DOI] [PubMed] [Google Scholar]
- Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, del Chierico F, Sanguinetti M, Morelli D, Paroni Sterbini F, Petito V, Reddel S, Calvani R, Camisaschi C, Picca A, Tuccitto A, Gasbarrini A, Pompili M, Mazzaferro V. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology. 2019;69:107–120. doi: 10.1002/hep.30036. [DOI] [PubMed] [Google Scholar]
- Ponziani FR, Putignani L, Parini Sterbini F, et al. Influence of hepatitis C virus eradication with direct-acting antivirals on the gut microbiota in patients with cirrhosis. Aliment Pharmacol Ther. 2018;48:1301–11. 10.1111/apt.15004. [DOI] [PubMed]
- Prior RL, Crim MC, Castaneda C, Lammi-Keefe C, Dawson-Hughes B, Rosen CJ, Spindler AA. Conditions altering plasma concentrations of urea cycle and other amino acids in elderly human subjects. J Am Coll Nutr. 1996;15:237–247. doi: 10.1080/07315724.1996.10718594. [DOI] [PubMed] [Google Scholar]
- Roger JM, Palagos B, Bertrand D, Fernandez-Ahumada E. CovSel: variable selection for highly multivariate and multi-response calibration: application to IR spectroscopy. Chemom Intell Lab Syst. 2011;106:216–223. doi: 10.1016/J.CHEMOLAB.2010.10.003. [DOI] [Google Scholar]
- Sarwar G, Botting HG, Collins M. A comparison of fasting serum amino acid profiles of young and elderly subjects. J Am Coll Nutr. 1991;10:668–674. doi: 10.1080/07315724.1991.10718185. [DOI] [PubMed] [Google Scholar]
- Scully D, Naseem KM, Matsakas A. Platelet biology in regenerative medicine of skeletal muscle. Acta Physiol. 2018;223:e13071. doi: 10.1111/apha.13071. [DOI] [PubMed] [Google Scholar]
- Scully D, Sfyri P, Verpoorten S, Papadopoulos P, Muñoz-Turrillas MC, Mitchell R, Aburima A, Patel K, Gutiérrez L, Naseem KM, Matsakas A. Platelet releasate promotes skeletal myogenesis by increasing muscle stem cell commitment to differentiation and accelerates muscle regeneration following acute injury. Acta Physiol. 2019;225:e13207. doi: 10.1111/apha.13207. [DOI] [PubMed] [Google Scholar]
- Selathurai A, Kowalski GM, Mason SA, Callahan DL, Foletta VC, Della Gatta PA, Lindsay A, Hamley S, Kaur G, Curtis AR, Burch ML, Ang T, McGee SL, Bruce CR. Phosphatidylserine decarboxylase is critical for the maintenance of skeletal muscle mitochondrial integrity and muscle mass. Mol Metab. 2019;27:33–46. doi: 10.1016/j.molmet.2019.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra F. The emergence of geroscience as an interdisciplinary approach to the enhancement of health span and life span. Cold Spring Harb Perspect Med. 2016;6:a025163. doi: 10.1101/cshperspect.a025163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soga T, Baran R, Suematsu M, Ueno Y, Ikeda S, Sakurakawa T, Kakazu Y, Ishikawa T, Robert M, Nishioka T, Tomita M. Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J Biol Chem. 2006;281:16768–16776. doi: 10.1074/jbc.M601876200. [DOI] [PubMed] [Google Scholar]
- Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, Sergi G, Isik AT, Manzato E, Maggi S, Maggio M, Prina AM, Cosco TD, Wu YT, Veronese N. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1–8. doi: 10.1016/j.arr.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Sprott RL. Biomarkers of aging and disease: introduction and definitions. Exp Gerontol. 2010;45:2–4. doi: 10.1016/j.exger.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Stephan A, Mateos JM, Kozlov SV, Cinelli P, Kistler AD, Hettwer S, Rülicke T, Streit P, Kunz B, Sonderegger P. Neurotrypsin cleaves agrin locally at the synapse. FASEB J. 2008;22:1861–1873. doi: 10.1096/fj.07-100008. [DOI] [PubMed] [Google Scholar]
- Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM, Kiel DP, Kritchevsky SB, Shardell MD, Dam TTL, Vassileva MT. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezze C, Romanello V, Sandri M. FGF21 as modulator of metabolism in health and disease. Front Physiol. 2019;10:419. doi: 10.3389/fphys.2019.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen JN, Kennelly JP, Wan S, Vance JE, Vance DE, Jacobs RL. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim Biophys Acta Biomembr. 2017;1859:1558–1572. doi: 10.1016/j.bbamem.2017.04.006. [DOI] [PubMed] [Google Scholar]
- Wagenmakers AJ. Protein and amino acid metabolism in human muscle. Adv Exp Med Biol. 1998a;441:307–19. 10.1007/978-1-4899-1928-1_28. [DOI] [PubMed]
- Wagenmakers AJ. Muscle amino acid metabolism at rest and during exercise: role in human physiology and metabolism. Exerc Sport Sci Rev. 1998;26:287–314. doi: 10.1249/00003677-199800260-00013. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu H, Liu RM. Gender difference in glutathione metabolism during aging in mice. Exp Gerontol. 2003;38:507–517. doi: 10.1016/s0531-5565(03)00036-6. [DOI] [PubMed] [Google Scholar]
- Wang L, Ahn YJ, Asmis R. Sexual dimorphism in glutathione metabolism and glutathione-dependent responses. Redox Biol. 2020;31:101410. 10.1016/j.redox.2019.101410. [DOI] [PMC free article] [PubMed]
- Wang X, Liu Y, Wang S, Pi D, Leng W, Zhu H, Zhang J, Shi H, Li S, Lin X, Odle J. Asparagine reduces the mRNA expression of muscle atrophy markers via regulating protein kinase B (Akt), AMP-activated protein kinase α, toll-like receptor 4 and nucleotide-binding oligomerisation domain protein signalling in weaning piglets after lipopolysaccharide challenge. Br J Nutr. 2016;116:1188–1198. doi: 10.1017/S000711451600297X. [DOI] [PubMed] [Google Scholar]
- Westerhuis JA, Hoefsloot HCJ, Smit S, Vis DJ, Smilde AK, van Velzen EJJ, van Duijnhoven JPM, van Dorsten FA. Assessment of PLSDA cross validation. Metabolomics. 2008;4:81–89. doi: 10.1007/s11306-007-0099-6. [DOI] [Google Scholar]
- Westerhuis JA, Kourti T, Macgregor JF. Analysis of multiblock and hierarchical PCA and PLS models. J Chemom. 1998;12:301–321. doi: 10.1002/(SICI)1099-128X(199809/10)12:53.0.CO;2-S. [DOI] [Google Scholar]
- Wilson D, Jackson T, Sapey E, Lord JM. Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res Rev. 2017;36:1–10. doi: 10.1016/J.ARR.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Yahiaoui L, Gvozdic D, Danialou G, Mack M, Petrof BJ. CC family chemokines directly regulate myoblast responses to skeletal muscle injury. J Physiol. 2008;586:3991–4004. doi: 10.1113/jphysiol.2008.152090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yeung DC, Karpisek M, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data analyzed in the current study are available from the corresponding author on reasonable request.