Abstract
Terpenes and their derivatives have been used conventionally as potential dietary supplements to boost the nutritional value of endless food products. Several plant-based complex terpenoid and their derivatives have been reported for a wide range of medicinal and nutritional properties. However, their simple counterparts, whose production is relatively easy, sustainable, and economic from food-grade microbial sources, have not been studied yet for any such biological activities. The present study aimed to investigate the longevity-promoting property and neuromodulatory effects of 3,3-dimethylallyl alcohol (Prenol), one of the simplest forms of terpenoid and a constituent of fruit aroma, in the animal model Caenorhabditis elegans. Prenol supplementation (0.25 mM) augmented the lifespan of wild-type nematodes by 22.8% over the non-treated worms. Moreover, a suspended amyloid-β induced paralysis and reduced α-synuclein aggregation were observed in Prenol-treated worms. The lifespan extending properties of Prenol were correlated with ameliorated physiological parameters and increased stress (heat and oxidative) tolerance in C. elegans. In silico and gene-specific mutant studies showed that pro-longevity transcription factors DAF-16, HSF-1, and SKN-1 were involved in the improved lifespan and health-span of Prenol-treated worms. Transgenic green fluorescent protein-reporter gene expression analysis and relative mRNA quantification (using real-time PCR) demonstrated an increase in the expression of DAF-16, HSF-1, and SKN-1 transcription factors and their downstream target genes in Prenol-treated worms. Together, the findings suggest that small molecules, like Prenol, could be explored as a potential alternate to develop therapeutics against aging and age-related ailments.
Electronic supplementary material
The online version of this article (10.1007/s11357-020-00241-w) contains supplementary material, which is available to authorized users.
Keywords: Prenol, Antiaging, C. elegans, Reactive oxygen species, Neuroprotection
Introduction
Recent advancement in medical technology together with easier availability of healthcare products has increased global average life expectancy, which is currently estimated to be 72 years (World Health Organization 2016). As a consequence, both developed and developing countries are exposed to threats that are associated with age-related disorders (Jimbo et al. 2009; Feng et al. 2018). The health care system in the developing nations, which are currently deploying strategies to harness the demographic dividend, would be in distress in near future due to rise in the population of elderly citizens. The rise in older population will bring with it a huge burden of age-associated disorder and other social challenges (Szeto et al. 2017; Gulia and Kumar 2018; United Nations, Department of Economic and Social Affairs 2019). Therefore, to enhance the illness-free period (health span) at the later stages of lifespan, the investigation of preventive medicine/therapeutics commands our prompt attention.
The use of terpenoid-rich extracts/formulations have been traditionally employed in several parts of the globe to enhance the nutritional value of food products and to fight against several age-associated ailments. Recent evidences, including our previous findings, have shown that the terpenoid-based compounds can extend healthy lifespan and stress tolerance (Shukla et al. 2012b; Asthana et al. 2015; Li et al. 2018). However, the majority of such compounds are plant-based and structurally complex. In addition to this, there are several environmental and economical concerns in the extraction of such compounds from their natural sources (Phulara et al. 2016). Furthermore, due to structural complexities, their production from engineered microbes is also a major bottleneck. Therefore, there is an immense urge to explore simple molecules for their antiaging potential, which are comparatively easy to produce in large quantities from microbial sources. The 3,3-dimethylallyl alcohol (Prenol) is an unsaturated prenyl alcohol and one of the simplest members of the terpenoid family (Fig. S1). It is present in citrus fruits, where it serves as an aroma constituent and also acts as a key precursor to several biological molecules (Lasekan 2017). Chemical industry utilizes it as an intermediate to synthesize various pharmaceuticals and aromatic products (Phulara et al. 2018a). Moreover, it can be produced in large amounts in microbial hosts without exerting any toxicity to microbial hosts (George et al. 2015). Recently, we have also successfully engineered a GRAS (generally regarded as safe) status microbe, Bacillus subtilis, for the non-natural production of Prenol for nutraceutical/pharmaceutical applications (Phulara et al. 2018b, a).
The free-living soil nematode, Caenorhabditis elegans, has established itself at the forefront for gerontological studies due to its remarkable properties. It has a simple, well-described, and fully annotated nervous system that contains 302 neurons (Bargmann 1998). In the past few decades, it has immensely contributed to upgrade our understanding about the mechanism underlying the aging and age-associated neurological disorders. Well-established transgenic strains expressing human amyloid-β (Aβ) and α-synuclein (αS) peptides offer a rapid in vivo investigation of the effects caused by the formation of plaques in the whole organism (Link 1995; van Ham et al. 2008). In addition, it has a short life-span, ease in culturing and maintenance, a high similarity with the aging mechanism in humans, and collection of genetic and epigenetic tools (Kenyon 2010). Together these remarkable features make this model organism a suitable host for performing a systematic and accurate investigation of the underlying mechanism behind aging and age-associated maladies in a short course of time.
The present study aimed to elucidate the lifespan- and health-promoting activity of Prenol using C. elegans. Various physiological and biochemical parameters were investigated in C. elegans after Prenol treatment. The gene-specific mutants and relative mRNA quantification were utilized to unveil the underlying mechanism behind the Prenol-mediated effects in C. elegans. To the best of our knowledge, it is one of the first reports evaluating the antiaging potential of Prenol in C. elegans.
Material and methodology
C. elegans strains
Escherichia coli OP50 and C. elegans strains, N2 Bristol (wild-type); GR1307, daf-16(mgDf50), EU 31 skn-1(zu135), PS3551 hsf-1(sy441), TK22, mev-1(kn1), TJ356 (zls356), daf-16::gfp; CF1553 muIs84[pAD76(sod-3::gfp)], CL2166 dvls19(gst-4∷gfp), NL5901 pkIs2386(unc-54p::α-synuclein::YFP + unc-119(+)), and CL4176 smg-1ts(myo-3::Aβ1–42 long 3′-UTR) were used to determine the neuroprotective and antiaging activities of Prenol. C. elegans is an invertebrate animal model; research protocols are well established and are not covered under any ethical demands.
Media and growth conditions
Routine culture and maintenance of the C. elegans strains were carried out at 20 °C on Nematode Growth Medium (NGM) spotted with E. coli OP50 bacterial lawn. The NGM plates were prepared by adding 3 mL molten NGM (~ 40–45 °C) in 30-mm culture plates. The plates were then allowed to solidify overnight at room temperature. To prepare bacterial lawn, 50 μL of overnight-grown E. coli OP50 culture was applied at the centre of the NGM plates using a micropipette. The plates were then kept at room temperature overnight so the bacterial lawn could grow and dry. For further usage, the seeded plates were placed in a refrigerator at 4 °C.
To provide Prenol (Sigma Aldrich, USA) exposure, the NGM plates were freshly spotted with desired doses of Prenol (0.1–0.5 mM final concentration) dissolved in 30 μL autoclaved MiliQ water. The days of adulthood were counted consequently as day 1, day 2, day 3, etc. after the four larval stages (L1–L4). Day 0 represents the L4 larval stage.
Toxicity assessment of Prenol on C. elegans
To evaluate the toxicity of Prenol in C. elegans, 0.1 mM, 0.5 mM, 1 mM, 2.5 mM, 5 mM, and 10 mM concentrations were used. For age-synchronization, eggs were isolated and allowed to hatch at 20 °C on NGM plates previously spotted with E. coli OP50 lawn. Toxicity assay was performed in 24-well plate using 500 μL liquid NGM supplied with different test concentrations of Prenol in triplicate. Age-synchronized (adult day 1) N2 worms were transferred to each well of the test concentration along with non-treated control worms and incubated at 20 °C. Survival of the worms was observed after every 2 h until 24 h. Nematodes were scored as dead if they were unresponsive to a gentle prod with a platinum wire (Liu et al. 2015).
Lifespan assay
To elucidate the effect of Prenol on the lifespan of C. elegans, 0.1 mM, 0.25 mM, and 0.5 mM concentrations were used. The wild-type N2 worms were age-synchronized on the NGM plates, spotted with the above-mentioned concentrations of Prenol on the OP50 lawn. The N2 worms, which were synchronized on untreated NGM plates, served as control. On day 0 of the adulthood, 30–40 L4 molts were moved to new NGM plates already treated with corresponding Prenol concentration. FUdR (2′-deoxy-5-fluorouridine; Sigma, USA) was also added onto the NGM plates at a final concentration of 50 μM to block progeny development. At every 2–3 days, worms from each test plate were shifted to fresh NGM plates of desired Prenol concentration to provide Prenol treatment throughout the experiment. The nematodes were scored daily for survival by observing their sensitivity to a mild poke with the help of a platinum wire (Liu et al. 2015). Additionally, to ensure that the bacteria are not mediating the effect of the Prenol, lifespan of worms on heat-killed E. coli OP50 (30 min at 65 °C) was also assayed as described by (Gruber et al. 2007).
Amyloid-β-mediated paralysis assays
Paralysis assays were carried out as mentioned previously with minor modifications (Ma et al. 2017). Briefly, C. elegans CL4176 worms were age-synchronized on NGM plates. When worms attained the L3 stage, they were transferred onto Prenol-treated (0.25 mM) and -untreated plates and placed in a 16 °C incubator. Following 48 h incubation, temperature was raised to 25 °C and incubated for an additional 36 h. Worms were then scored for paralysis at every 6 h interval until all animals in the control group were paralyzed. Worms failed to respond (insufficient body movement) against a gentle prod using a platinum loop were considered as paralyzed (Zhi et al. 2017).
Quantitative assay for α-Synuclein accumulation
Quantification of αS accumulation was carried out using C. elegans NL5901 mutants as described previously (Chalorak et al. 2017). The NL5901 worms were treated with 0.25 mM concentration of Prenol from the L1 larval stage. Worms without Prenol treatment served as control. On adult day 5, worms were photomicrographed under a fluorescent microscope (Carl Zeiss AxioScope A1) and fluorescence intensity of the accumulated αS was quantified by using ImageJ software.
ROS detection assay
Age-synchronized adult day 4 worms, treated with 0.1 mM and 0.25 mM Prenol, were homogenized, and the intracellular ROS was quantified using 2, 7-dichlorodihydrofluoresceindiacetate (H2DCF-DA) as described previously (Shukla et al. 2012b). An automatic microplate reader (Spectramax M2; Molecular Devices) was used to record fluorescence readings. The excitation was kept at 485 nm, and the emission was read at 525 nm. Data were recorded at every 20-min time interval for a consecutive period of 160 min.
Stress resistance assay
Effects of Prenol on the stress tolerance of wild-type nematodes were determined by investigating their survival under heat and oxidative stresses. Oxidative stress was provided to both treated (0.1 mM, 0.25 mM Prenol) and non-treated adult day 2 worms by exposing them to 250 μM juglone (5-Hydroxy-1,4-naphthoquinone, Sigma-Aldrich, USA) as described previously ((Senchuk et al. 2017)). Worms were constantly maintained on juglone treated plate for 8 h, and at the end of each hour, survival was recorded by the touch-provoked method (Wilson et al. 2006).
For thermo-tolerance assay, a mild heat shock at 37 °C for 4 h was given to treated (0.1 mM, 0.25 mM) and non-treated adult day 2 worms. Worms were then shifted to 20 °C incubator and scored daily for survival as mentioned above.
Pharyngeal pumping assay
Effect of Prenol treatment on the pharyngeal pumping of adult day 2, day 5, and day 10 wild-type N2 worms was observed by recording the movement of pharynx terminal bulb. Worms were treated with 0.1 mM and 0.25 mM Prenol from the L1 larval stage on NGM plates. The non-treated worms served as control. Pharyngeal pumping of the nematodes feeding on E. coli OP50 lawn was recorded for 30-s intervals at room temperature (RT).
Chemotaxis assay
Chemotaxis assay was carried out using adult day 5 Prenol-treated and non-treated groups according to Bargmann et al. 1993. Briefly, the N2 worms from both the groups were washed twice with M9 buffer before placing them onto the center of 90 mm agar plate. An aliquot (10 μL) of attractant (1 M sodium acetate) was spotted on one side of the plate and water on the other side. A 1 μL of 1 M sodium azide was also applied onto the both sides to restrict worms’ movement once they reached to either side. After 90-min incubation at 20 °C, the number of worms on each side was recorded and the chemotaxis index (CI) was calculated by employing the following formula:
where A is the number of worms present at the attractant side and B is the number of worms present at the control location,
Visualization and quantification of GFP
The C. elegans mutants CF1553 and CL2166 were used to quantify the expression of SOD-3 and GST-4, respectively, as described previously (Asthana et al. 2015). After 0.25 mM Prenol exposure at 20 °C, both the treated and non-treated adult day 2 worms were mounted onto 2% agarose pad and photomicrographed under a fluorescent microscope (Carl Zeiss AxioScope A1). Fluorescence images were captured using a × 20 objective lens at constant exposure (with excitation at 365 nm and emission at 420 nm). The exposure time was kept identical for each strain, while acquiring fluorescent images. For sod-3::gfp, fluorescence intensity for the head and tail region of the transgenic nematodes was recorded, while for gst-4::gfp the fluorescence intensity for the pharynx region was measured. The fluorescent intensity was quantified using ImageJ software (National Institute of Health, USA).
For visualizing DAF-16 localization, TJ356 worms were used. For the positive control of DAF-16 nuclear translocation, the untreated TJ356 nematodes were exposed to a short thermal stress at 37 °C for a 20-min time-span. Photomicrographs of the worms were taken under a fluorescent microscope (Carl Zeiss AxioScope A1) to investigate the subcellular localization of DAF-16 as described previously (Shukla et al. 2012b).
In silico analysis
To analyze the interaction between selected target proteins (DAF-16, HSF-1, and SKN-1) and Prenol, Schrodinger’s Maestro (Schrödinger Release: Maestro, version 10.5, Schrödinger, LLC, NY 2016-1) was used, which is capable of performing all the analysis required for in silico molecular docking. Since 3D structures of the proteins involved in the study were not available online, therefore, a homology modeling approach was employed using PRIME for modeling of the targeted proteins. Hence, the amino acid sequences (FASTA) of the target proteins were retrieved from UniProt. The template for sequences was generated using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and further subjected to structure generation wizard of Prime (Schrodinger). To determine the accuracy of the predicted homology modeled 3D structure, Protein Preparation Wizard (version 7.0, Schrödinger Release 2016–1) was used. Likewise, to rectify and stabilize the structure of ligand, the Ligprep (v3.7, Schrödinger Released 2016-1) application was used. SiteMap (version 3.8, Schrödinger Released 2016-1) was used to predict the binding site for the targeted proteins and Glide (version 7.0, Schrödinger Released 2016-1) was used to analyze the binding affinity and ligand–receptor interactions. The Receptor Grid Generation was used to predefine the size of the docking area according to the binding site present in respective protein structures.
Gene quantification through real-time PCR
The L4 Wild- N2 molts were grown on 0.25-mM Prenol-treated and untreated plates under standard laboratory conditions at 20 °C. After 72 h of incubation, total RNA was isolated from the worms using Trizol reagent (Invitrogen). The first-strand cDNA synthesis was performed from total RNA using Maxima H Minus M-MuLV reverse transcriptase (Thermo Scientific, USA) as per the kit-instruction manual. Expression levels of sod-1, sod-2, sod-3, gst-3, gst-4, hsp-16.2, hsp-70, daf-16, skn-1, and hsf-1 genes were observed by keeping β-actin (act-1) as internal control. Real-time PCR was carried out using 2X Brilliant III 235 SYBR® Green QPCR (Agilent Technologies, USA) on Stratagene Mx3000P (Agilent, USA). The experiment was performed in triplicates. The qRT-PCR cycling conditions were same as described previously (Tiwari et al. 2016), and primer sequences are available on request. Three independent trials were performed, and relative mRNA quantification of the desired genes was analyzed using the comparative CT (ΔΔ CT) method (Schmittgen and Livak 2008).
Statistical analysis
Each experiment was performed in minimum of two independent trials with comparable results, and data are represented as the mean of independent trials (unless otherwise stated). For plotting survival curves and comparing significant difference between control and treated groups in the lifespan assays, Aβ-induce toxicity assay, and thermal stress resistance assay, statistical calculations were performed by log-rank test using GraphPad Prism5 (GraphPad Software, Inc., La Jolla, CA). If the P values were less than 0.05 relative to control, the data were considered significant.
Statistical analyses for the experiments other than stated above assays were carried out by one-way ANOVA or by Student’s t tests using GraphPad Prism5. The results were presented as mean ± standard error (SE). The data were considered significant if P values were less than 0.05 relative to control.
Results
Prenol supplementation prolongs lifespan of the worms
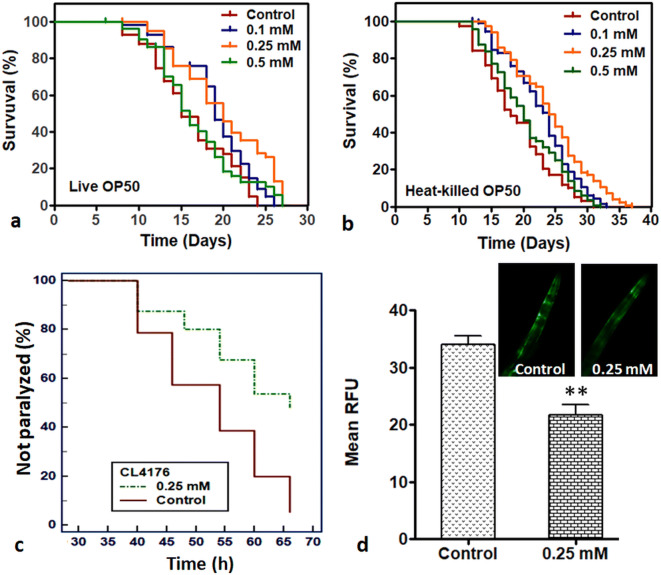
For the assessment of lifespan promoting effects of Prenol, an acute in vivo toxicity assay was carried out and non-toxic concentrations were determined. For this, age-synchronized adult day 1 wild-type nematodes were transferred to liquid NGM with different test concentrations of Prenol. After 24 h of continuous exposure, only 0.1 mM and 0.5 mM concentrations were found non-toxic to the wild-type N2 worms, while the rest of the concentrations were toxic to the nematodes in a dose-dependent manner (Fig. S2). The non-toxic concentrations of 0.1 mM, 0.5 mM, and an intermediate concentration of 0.25 mM were used to analyze the life-promoting activity of Prenol in C. elegans. On comparing the lifespan of the Prenol-treated worms with the control worms, it was found that Prenol at 0.1 mM (P = 0.0001) and 0.25 mM (P < 0.0001) concentrations increased the mean lifespan (Table 1; Fig. 1a) of wild-type nematodes. The C. elegans treated with 0.25 mM concentration of Prenol depicted a maximum (22.8%) increase in the mean lifespan, while 0.5 mM concentration failed (P = 0.27) to increase the mean lifespan of the wild-type N2 worms. Prenol supplementation not only increased the mean lifespan but also improved the maximum lifespan and delayed first death in treatment groups over the control.
Table 1.
Effect of Prenol on the lifespan of C. elegans at 20 °C
| Strains | Trial | Prenol treatment | No. of worms¥ | Mean lifespan ± SE | Median lifespan | Percent change | P value# | Max. lifespan ± SE | Min. lifespan ± SE |
|---|---|---|---|---|---|---|---|---|---|
| N2 (live OP50) | T1 | Control | 113 | 16.15 ± 0.24 | 15 | 23.66 ± 0.33 | 8.33 ± 0.33 | ||
| 0.1 mM | 110 | 18.88 ± 0.19 | 19 | 19.79 | < 0.0001 | 26.00 ± 0.00 | 10.00 ± 1 | ||
| 0.25 mM | 100 | 19.94 ± 0.15 | 20 | 23.40 | < 0.0001 | 27.33 ± 0.33 | 11.66 ± .066 | ||
| 0.5 mM | 81 | 16.53 ± 0.37 | 16 | 2.30 | = 0.275 | 26.00 ± 0.57 | 8.66 ± 0.66 | ||
| T2 | Control | 107 | 16.56 ± 0.15 | 15 | 24.00 ± 0 | 8.33 ± 0.33 | |||
| 0.1 mM | 108 | 19.19 ± 0.20 | 20 | 16.65 | = 0.0001 | 27.00 ± 1.22 | 10.00 ± 1 | ||
| 0.25 mM | 100 | 20.15 ± 0.25 | 20 | 22.52 | < 0.0001 | 28.66 ± .088 | 12.33 ± .066 | ||
| 0.5 mM | 101 | 17.01 ± 0.47 | 17 | 3.44 | = 0.55 | 26.00 ± 0.00 | 8.33 ± 0.66 | ||
| N2 (heat-killed OP50) | T1 | Control | 117 | 19.09 ± 0.30 | 18 | 30.33 ± 0.38 | 11.33 ± 0.38 | ||
| 0.1 mM | 112 | 22.79 ± 0.12 | 23 | 19.41 | < 0.0001 | 32 ± 0.57 | 14.33 ± 0.38 | ||
| 0.25 mM | 120 | 24.46 ± 0.28 | 24.5 | 27.16 | < 0.0001 | 35.66 ± 0.51 | 15 ± 0.33 | ||
| 0.5 mM | 116 | 20.46 ± 0.47 | 20 | 7.22 | = 0.12 | 31.33 ± 0.19 | 12.33 ± 0.19 | ||
| T2 | Control | 116 | 18.74 ± 0.46 | 18 | 29.66 ± 0.19 | 10.33 ± 0.19 | |||
| 0.1 mM | 113 | 22.03 ± 0.14 | 23 | 17.58 | < 0.0001 | 30 ± 0.66 | 14.00 ± 0.33 | ||
| 0.25 mM | 117 | 23.49 ± 0.21 | 24 | 25.35 | < 0.0001 | 36 ± 0.00 | 14.66 ± 0.19 | ||
| 0.5 mM | 114 | 20.15 ± 0.20 | 20 | 6.02 | = 0.056 | 29 ± 0.55 | 12.00 ± 0.33 | ||
| mev-1 | T1 | Control | 80 | 11.11 ± 0.36 | 11 | 7.00 ± 0.00 | 15.66 ± 0.33 | ||
| 0.25 mM | 86 | 13.34 ± 0.44 | 13 | 17.38 | = 0.0001 | 7.00 ± 0.00 | 19.00 ± 0.00 | ||
| T2 | Control | 88 | 10.60 | 11 | 7.00 ± 0.00 | 15.00 ± 0.00 | |||
| 0.25 mM | 72 | 12.84 | 13 | 21.18 | < 0.0001 | 8.33 ± 0.33 | 18.00 ± 0.66 | ||
| daf-16 | T1 | Control | 119 | 11.17 ± 0.65 | 12 | 4.50 ± 0.5 | 18.75 ± 0.65 | ||
| 0.25 mM | 112 | 11.67 ± 0.69 | 12 | 4.50 | = 0.409 | 5.00 ± 0.57 | 18.5 ± 0.29 | ||
| T2 | Control | 105 | 12.01 ± 0.56 | 12 | 5.75 ± 0.47 | 20.00 ± 0.57 | |||
| 0.25 mM | 126 | 11.64 ± 0.43 | 11 | −3.06 | = 0.70 | 5.75 ± 0.25 | 20.00 ± 0.5 | ||
| skn-1 | T1 | Control | 100 | 14.36 ± 0.27 | 14 | 8.5 ± 0.29 | 21.00 ± 0.00 | ||
| 0.25 mM | 111 | 14.78 ± 0.24 | 14 | 2.91 | = 0.419 | 9.00 ± 00 | 21.00 ± 0.00 | ||
| T2 | Control | 110 | 12.82 ± 0.44 | 14 | 7.5 ± 0.29 | 20.00 ± 0.00 | |||
| 0.25 mM | 103 | 13.41 ± 0.59 | 13 | 4.54 | = 0.37 | 7.5 ± 0.29 | 20.5 ± 0.24 | ||
| hsf-1 | T1 | Control | 118 | 11.97 ± 0.26 | 12 | 8.00 ± 0.00 | 15.25 ± 0.47 | ||
| 0.25 mM | 120 | 12.40 ± 0.23 | 12 | 2.11 | = 0.162 | 8.00 ± 0.21 | 16.75 ± 0.25 | ||
| T2 | Control | 117 | 12.11 ± 0.30 | 12 | 7.75 ± 0.00 | 17.00 ± 0.35 | |||
| 0.25 mM | 114 | 11.77 ± 22.5 | 12 | − 2.81 | = 0.31 | 8.00 ± 0.00 | 16.25 ± 0.21 |
¥Worms that died due to crawling on the wall of the plate, egg laying defects, and damage during transfer were excluded
#Statistical significance was determined by log-rank test using GraphPad Prism5
Fig. 1.
Prenol supplementation increased lifespan of wild-type C. elegans and reduced amyloid aggregation in transgenic C. elegans strains. a Effect of Prenol on lifespan of wild-type C. elegans fed on live OP50. b Effect of Prenol on lifespan of wild-type C. elegans fed on heat-killed OP50. The 0.25 mM (P < 0.0001) concentration enhanced the lifespan of wild-type N2 worms most significantly in both the conditions c Effect of Prenol supplementation on the amyloid-β induced toxicity in C. elegans. Prenol treatment significantly delayed the amyloid-β induced paralysis in transgenic CL4176 transgenic strain. GraphPad Prism5 was used to plot survival curves. d Effect of Prenol supplementation on the α-Synuclein aggregation in NL5901 transgenic strain its graphical presentation as measured by ImageJ software. Prenol supplementation reduced the α-Synuclein aggregation in NL5901 transgenic strains. Statistically significant at **P < 0.001
Further, to confirm that live E. coli OP50 did not mediate the lifespan extension effect of Prenol, lifespan of worms on heat-killed E. coli OP50 (30 min at 65 °C) was also observed. We found that 0.1 mM and 0.25 mM concentrations of Prenol (0.1 mM, 0.25 mM, and 0.5 mM) increased the mean lifespan of wild-type C. elegans (fed on heat-killed OP50). Similar to the observation with live food source, the 0.25 mM concentration of depicted a maximum (26.76%) increase in the mean lifespan of worms grown on heat-killed E. coli OP50 (Table 1; Fig. 1b). The results indicated that Prenol but not bacteria solely mediated the lifespan extension of C. elegans.
Prenol supplementation delays amyloid-β-induced paralysis and reduces α-synuclein aggregation in C. elegans
To identify the effects of the Prenol supplementation on the Aβ-induced paralysis, CL4176 transgenic worms were used, in which human Aβ1–42 is incorporated into its genome (Link 1995). A temperature upshift from 16 to 25 °C induces the expression of Aβ transgene in the muscles cell of this mutant that results into paralysis (Link et al. 2003). Worms were treated with the maximum lifespan promoting concentration (0.25 mM) of Prenol from L3 stage. The Prenol-treated nematodes showed a significant reduction (P < 0.001) in the Aβ-induced paralysis in comparison to the control worms (Fig. 1c).
Like Alzheimer’s disease (AD), the most widely accepted pathology for Parkinson’s disease (PD) is accumulations of αS plaques (van Ham et al. 2008). The C. elegans NL5901 is a transgenic strain, in which the human αS is fused with yellow fluorescent protein (YFP) that is constitutively expressed in the body wall of this transgenic strain (Liu et al. 2015). The αS-aggregation was investigated by observing the intensity of YFP after feeding the NL5901 worms with 0.25 mM Prenol. Prenol supplementation significantly (P < 0.001) reduced the αS aggregation in the mutant worms than the non-treated control worms (Fig. 1d).
Prenol attenuates the age-dependent decline in physiological and neurological behaviors of C. elegans
In C. elegans pharyngeal pumping regulates food intake and the alterations in the movement of the pharynx terminal bulb can induce dietary restriction (DR)-like effects (Powolny et al. 2011). The movement of pharynx terminal bulb of worms often linked with the longevity-promoting activity of dietary interventions (Rathor et al. 2017; Pandey et al. 2019b). Therefore, to observe the effects of prenol supplementation on the feeding behavior of the worms, pharyngeal pumping of the worms was recorded for 30 s. It was found that in wild-type nematodes, the introduction of Prenol did not cause any adverse effect on the pharyngeal pumping. However, a significant increase (P < 0.001 for day 2; P = 0.029 for day 5; P < 0.001 for day 10) in the rhythmic movement of pharynx was observed in nematodes treated with 0.25 mM Prenol (Fig. 2a). The results indicated that prenol supplementation improved feeding behavior and thus enhanced the health-span of the nematodes. The improved feeding behavior also diminished any possibility of DR-like effects after prenol treatment.
Fig. 2.
Prenol treatment augmented age-dependent physiological parameters and enhanced stress-tolerance ability in wild-type C. elegans. a Effect of Prenol treatment on the pharyngeal pumping of N2 worms. The experiment was repeated trice and the pharyngeal pumping was recorded for 30 s at room temperature. b Effect of Prenol supplementation on the chemotaxis index of the N2 worms (sodium acetate was used as attractant). c Effect of Prenol on the thermal stress tolerance of wild-type N2 worms. Prenol at 0.25 mM (P < 0.0001) concentration showed the most significant increase in the worms’ survival after heat shock. Survival plot was drawn GraphPad 5 using log-rank test. d Effect of Prenol on juglone-induced oxidative stress. Prenol treatment in all test concentrations significantly increased the survival of treated worms over the untreated control worms. The error bars represent the standard error of the mean. Statistically significant at *P < 0.01 and ** P < 0.001
To investigate the effect of Prenol supplementation on the capacity of C. elegans to sense and respond to the odorant, chemotaxis index (CI) of the Prenol-treated and non-treated adult day 1 and day 5 worms was determined. An aliquot (10 μL) of attractant (1 M sodium acetate) was spotted on the one side of the plate and water on the other side. Sodium azide, a chemical that induces paralysis in worms, was applied to both sides of the experimental plates to prevent the further movement of the worms once they reached on either side. CI was determined as fraction of the worms on the attractant side using the formula given in methodology. On day 1, there was no significant difference between the CI of treated and control groups. However, at the later stage of lifespan (day 5), CI of the 0.25 mM (p > 0.001) and 0.1 mM (p > 0.01) Prenol-treated worms were higher than the non-treated control worms, which indicated that Prenol-treated worms were more attracted to the attractant sodium acetate (Fig. 2b). Our results also divulged that Prenol treatment was able to augment the worms’ ability to sense the odorant in the later phase of the lifespan, which is correlated with improved health-span.
Prenol enhances stress tolerance in C. elegans
To investigate whether Prenol supplementation could improve stress tolerance in C. elegans, survival of the worms was observed under thermal and oxidative stress conditions. Prenol-treated worms demonstrated extended lifespan (P < 0.001 for 0.1 mM and P < 0.0001 for 0.25 mM) after exposure to 37 °C for 4 h than the non-treated control worms (Fig. 2c; Table S1). The 0.25 mM concentration showed a 32.53% increase in the mean lifespan of the worms under thermal stress over the non-treated control worms (Table S1).
Similarly, Prenol treatment also enhanced (P < 0.01 for 0.1 mM and P < 0.001 for 0.25 mM) juglone (250 μM)-induced oxidative stress tolerance in wild-type nematodes (Fig. S3). The N2 worms supplemented with 0.25 mM Prenol depicted ~ 46% more survival than the non-treated group after constant exposure to juglone for 8 h (Fig. 2d).
Prenol reduces ROS accumulation in C. elegans
To investigate the effects of Prenol on ROS accumulation in C. elegans, the intracellular ROS levels in Prenol-treated worms were compared with non-treated control worms on adult day 4. For this, the fluorescent readings of the reaction mixture containing worms’ lysate (prepared by equally timed homogenization and sonication) and H2DCF-DA (fluorescent probe) were read in a 96-well plate fluorescent-microplate-reader at every 20-min time interval for a consecutive period of 160 min (Shukla et al. 2012b). The experimental data revealed that Prenol supplementation significantly (P < 0.01 for 0.1 mM and P < 0.001 for 0.25 mM) reduced intracellular ROS accumulation in the wild-type nematodes (Fig. 3a).
Fig. 3.
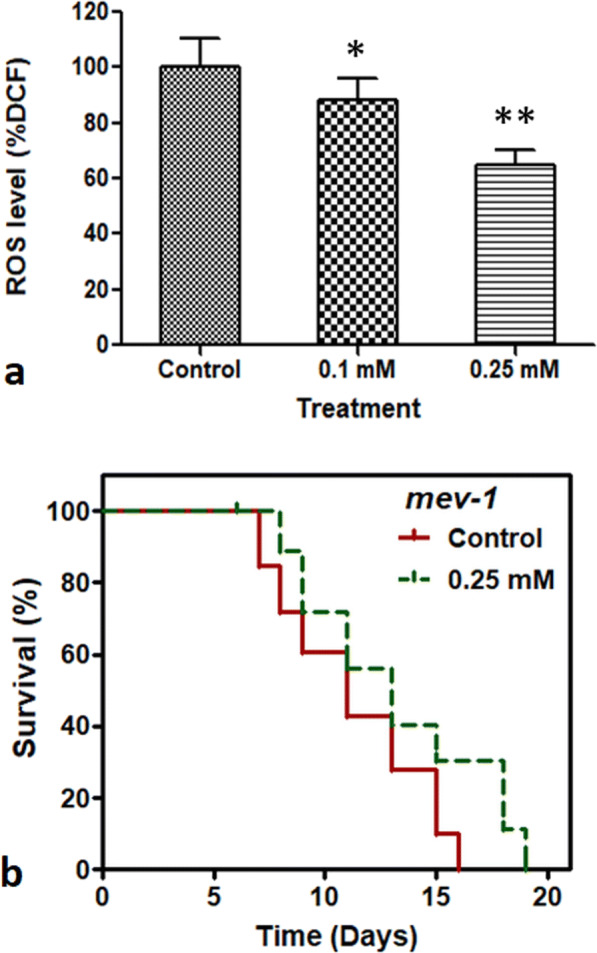
Prenol supplementation suppressed ROS accumulation in C. elegans. a Effect of Prenol on the ROS accumulation in wild-type C. elegans. Prenol treatment significantly reduced ROS generation in wild-type worms. Graph was plotted as the relative change in the intracellular ROS in treated worms compared with control at 100%. The error bars represent the standard error of the mean. Statistically significant at *P < 0.01 and **P < 0.001. b Effect of prenol on the lifespan mev-1. Prenol treatment significantly increased the lifespan of short-lived mutant (due to high ROS generation) strain of C. elegans. Survival plot was drawn GraphPad Prism5 using log-rank test.
The mev-1 mutants of C. elegans have mitochondrial dysfunction due to the mutation in the cytochrome b subunit of mitochondrial complex-II (Ishii et al. 1998). Due to mutation, these strains of C elegans have elevated levels of intracellular ROS and hence have a short lifespan (Ishii et al. 2011). Majority of the dietary supplements, which reduced ROS accumulation in wild-type worms, have also been shown to extend the lifespan of mev-1 mutant (Phulara et al. 2015; Zhang et al. 2016). Therefore, to support the results of intracellular ROS accumulation assay, the lifespan of mev-1 (kn1) mutant strain was observed after Prenol treatment. It was found that 0.25 mM Prenol increased the mean lifespan of mev-1(kn-1) by 17.4% over the non-treated control worms (P = 0.0001) (Table 1; Fig. 3b). The observed mean lifespan for the 0.25 mM Prenol-treated group was 13.04 days, whereas the mean lifespan of non-treated control group was 11.10 days (Table 1). This indicates that Prenol supplementation might rescue the mev-1 worms from elevated ROS levels and increased its lifespan.
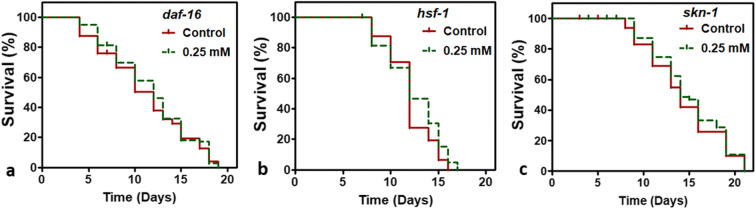
Prenol-mediated lifespan extension in C. elegans requires DAF-16, SKN-1, and HSF-1pathway
Previous studies have shown that dietary supplements could activate transcription factors (TFs) of insulin/insulin-like signaling (IIS) pathway, such as daf-16, skn-1, and hsf-1, to improve lifespan and to alleviate Aβ- and αS-induced toxicity in C. elegans (Cohen et al. 2006; Edwards et al. 2014; Govindan et al. 2018). To identify the genetic requirements for the Prenol-mediated lifespan extension in C. elegans, the lifespan of the null-mutants of daf-16, skn-1, and hsf-1 were observed. It was observed that Prenol supplementation did not increase the mean lifespan (P = 0.409 for daf-16, P = 0.162 for hsf-1, and P = 0.419 for skn-1 null mutants) of the tested null-mutants (Table 1; Fig. 4 a, b and c), suggesting the involvement of IIS pathway.
Fig. 4.
Genetic requirements for the Prenol mediated longevity in C. elegans. The figure shows the survival curves of the C. elegans mutants with and without Prenol treatment. Prenol treatment did not improve the lifespan of a daf-16 deletion mutant GR1307, daf-16(mgDf50) (P = 0.409), b hsf-1 deletion mutant PS3551 {hsf-1(sy441)} (P = 0.419), and c skn-1 deletion mutant, EU 31 {skn-1(zu135)} (P = 0.162). Experiments were performed in 2 independent trials for each mutant strain. GraphPad Prism5 was used to plot survival curves
The in silico study also predicted a strong interaction of Prenol with DAF-16 (− 3.114 kcal/mol), HSF-1 (− 3.447 kcal/mol), and SKN-1 (− 2.92 kcal/mol) proteins (Table 2; Fig. S4). On comparing binding energies, it was found that HSF-1 (emodel score: − 31.39) has better docking score and energy than DAF-16 (emodel score: − 18.83) and SKN-1 (emodel score: − 19.96) (Table 2).
Table 2.
Molecular docking interaction analysis between receptor and Prenol
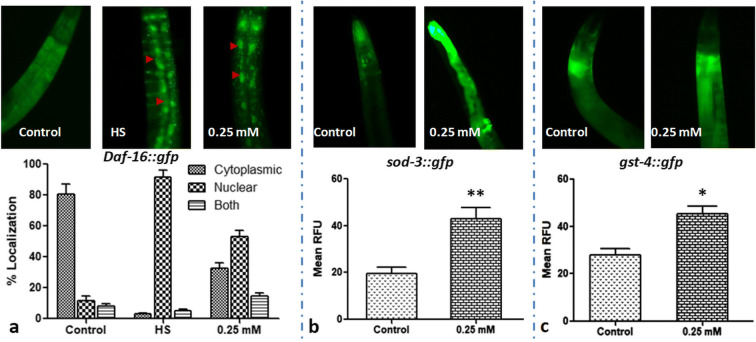
Prenol supplementation stimulates nuclear localization of DAF-16::GFP in C. elegans
To investigate the nuclear localization of DAF-16 after Prenol supplementation, TJ356 transgenic strain, carrying a daf-16∷gfp reporter gene, was used. The non-treated N2 worms without any heat-shock served as control, and the non-treated N2 worms with heat-shock served as positive control. The positive control worms depicted ~ 92% nuclear localization of DAF-16 after a heat shock at 37 °C for 20 min. Among Prenol-treated group, about 53% worms showed nuclear translocation, while 15% worms showed both cytoplasmic and nuclear localization of DAF-16 in C. elegans (Fig. 5a), indicating the requirement of DAF-16 for the Prenol-mediated neuroprotection and longevity in C. elegans.
Fig. 5.
Prenol supplementation promoted DAF-16 nuclear translocation and enhanced expression of sod3∷gfp and gst-4::gfp in transgenic C. elegans strains. a Prenol treatment–induced nuclear localization of DAF-16. Worms were mounted on a 2% agarose pad, and the intracellular localization of DAF-16 (nuclear, cytoplasmic, and both) was examined under a fluorescent microscope. Control worms (C; n = 94) showed cytosolic localization, whereas heat shock (HS; n = 102) and 0.5 mM Prenol-treated worms (n = 113) showed nuclear translocation of DAF-16. A significant increase in the fluorescent intensity was observed in 0.25 mM Prenol-treated b sod3∷gfp transgenic worms and c gst-4∷gfp transgenic worms than the non-treated control groups. GFP expression was quantified by ImageJ software (NIH). Data were statistically analyzed by GraphPad Prism5 software; the error bars represent the standard error of the mean. Statistically significant at *P < 0.01
Prenol treatment up-regulated the expression of SOD-3 and GST-4 proteins in GFP-tagged transgenic strains of C. elegans
The dietary supplements have been found to stimulate the nuclear translocation of transcription factors such as DAF-16 and SKN-1, which ultimately promote the expression of their downstream target genes such as sod-3 and gst-4, which are also associated to stress resistance (Powolny et al. 2011; Pandey et al. 2019a). Therefore, the effect of prenol supplementation on the expression of SOD-3 and GST-4 was observed by utilizing green fluorescent protein (GFP)-tagged transgenic strains. For this, C. elegans transgenic strains CL2166 and CF1553, in which GFP reporter is fused with GST-4 (glutathione S-transferases-4) and SOD-3 (superoxide dismutase-3) proteins, respectively, were explored to observe the effect of Prenol supplementation on the expression of the respective genes. On comparing the expression of SOD-3 and GST-4 in 0.25 mM Prenol-treated transgenic strains with the non-treated transgenic strains, it was observed that Prenol supplementation significantly increased the expression of both the proteins in C. elegans (Fig. 5b; P < 0.01, and Fig. 5c, P < 0.01). The results indicated that Prenol supplementation promoted the expression of DAF-16 and SKN-1 downstream genes and overexpression of these stress resistance-related genes (sod-3 and gst-4) might be responsible for worms’ survival under stress conditions.
Prenol augmented the expression on stress-related genes
To examine, whether the Prenol supplementation affected the expression of DAF-16, SKN-1, and HSF-1 TFs, we measured their change in mRNA level after Prenol treatment. Further, their transcriptional activities were analyzed by observing the expression levels of their downstream gene targets. For this, transcriptional expression of downstream targets of DAF-16 (sod-2 and sod-3) (Honda et al. 2008), HSF-1 (hsp-16.2 and hsp-70) (Brunquell et al. 2016), and SKN-1 (gst-3 and gst-4) (Rizki et al. 2012; Dues et al. 2017) was examined. We found that Prenol treatment increased the expression of daf-16, skn-1, and hsf-1 genes and their respective downstream test target genes (Fig. 6). Our results suggested that the Prenol supplementation enhanced the expression of DAF-16, SKN-1, and HSF-1 and promoted their nuclear translocation; as a result, the expression of downstream target genes also increased.
Fig. 6.
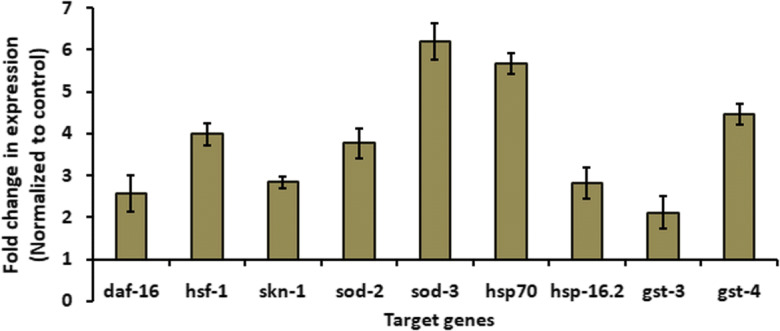
Prenol supplementation enhanced the expression of daf-16, hsf-16, and skn-1 and their downstream target genes. Expression levels of transcription factors’ genes (daf-16, hsf-16, and skn-1) and their downstream-target genes (sod-2, sod-3, hsp-70, hsp-16.2, gst-3, and gst-4) were determine using real-time PCR in worms treated 25 mM Prenol. Actin-1 was kept as an internal control. Data were presented as the averages of results obtained from three independent trials, and the error bars represent the standard error of the mean
Discussion
Over the centuries, terpenoids and their formulations have been utilized as a potent source of flavor, fragrance, and pharmaceuticals. Higher terpene compounds have shown several health benefits such as regulation of cation channels, suppression of tumor proliferation, and apoptosis induction (He et al. 1997; Roullet et al. 1997; Mo and Elson 1999). However, lifespan and health-span promoting effects of one of the simplest terpene, Prenol have been least studied yet. The present study, for the first time, demonstrates the lifespan, health-span, and stress resistance ameliorating ability of Prenol in C. elegans.
The study demonstrated that Prenol at 0.25 mM concentration increased the mean and maximum lifespan of the worms under standard laboratory conditions (Fig. 1a). Similar to higher mammals including humans, the aging process in C. elegans is associated with a decline in physiological and neurological behaviors (Ma et al. 2017). Pharyngeal pumping and chemotaxis are among such behaviors that falloff with aging in C. elegans (Shukla et al. 2012a). The rhythmic contractions and relaxations of the pharynx terminal bulb are associated with food intake in worms (Powolny et al. 2011). Recently, the association of pharyngeal pumping has been established with tonic and phasic signaling from the nervous system in C. elegans (Trojanowski et al. 2016). Increased rhythmic movement after Prenol supplementation in later stages of worms’ life indicated improved feeding behavior, constricted the occurrence of DR-like effects, and suggested that improved health-span (Fig. 2a) of the fraction of worms able to transform specific sensory stimuli into goal-directed motor responses.
CI is another physiological behavior, which is a measure of the fraction of worms, which are able to sense and response to an odorant (Bargmann et al. 1993). Any alteration in neuronal activity or progression of aging decline this worms’ ability to “transform a sensory stimuli into goal-directed motor response” in C. elegans (Brown et al. 2006; Wu et al. 2006). Previously, we have shown that terpenoid (Shukla et al. 2012a) and essential oil containing volatile terpenoids (Pandey et al. 2018) could reverse the age-dependent decline of CI in worms. In support of the previous findings, the present study also demonstrated the ability of Prenol to attenuate the age-dependent decline of CI in aged worms (Fig. 2b). The improved physiological and neurological behaviors that might be due to the neuroprotective activity of Prenol could be responsible for the increased lifespan and health-span of wild-type nematodes.
Alzheimer’s disease (AD) and Parkinson’s disease (PD) are the two most prominent age-associated, progressive, degenerative, and irreversible neurodegenerative disorders (NDs), which are becoming more common in the elderly population worldwide (Wei et al. 2017; Zhi et al. 2017). Despite intensive research, the pathologies of both the AD and PD are still unclear. Moreover, the available medication and surgery only alleviate the symptoms; however, currently, there is no cure for such ailments. The important hallmark and most accepted hypothesis for AD and PD are the aggregation of Aβ and αS peptides, respectively (Liu et al. 2015; Ahmad and Ebert 2017). The formation of Aβ and αS plaques leads to neurodegeneration and thereby disrupts neuronal functions. Reduction of generation and accumulation of the Aβ and αS are the primary goals of the anti-AD and anti-PD therapies, respectively. Several studies on C. elegans have shown that the food supplements including terpenoid that are not considered as essential foods can effectively attenuate the Aβ-induced toxicity and reduce αS accumulation (Wei et al. 2017; Ahmad and Ebert 2017). However, the effect of small molecules on Aβ and αS accumulation has been least studied in worms. We observed that Prenol treatment delayed Aβ-induced paralysis in CL4176 transgenic strain and also reduced αS aggregation in NL5901 transgenic strains (Fig. 1c, d). Expression of αS is also associated with reduction in physiological behaviors in C. elegans, such as pharyngeal pumping (Bodhicharla et al. 2012). It was found that Prenol supplementation (0.25 mM) attenuated the decline of pharyngeal pumping of aged NL5901 worms (data not shown). This suggests that the lower terpenoid alcohols might have similar neuroprotective effects as per their higher counterparts, which could be responsible for the longevity in wild-type worms.
The other pathologies that are associated with AD and PD are altered insulin metabolism, mitochondrial dysfunction, increased reactive oxygen species (ROS) level, and oxidative stress (Hwang 2013; Ma et al. 2017). In addition, association of longevity with the enhanced stress tolerance is well documented and we have demonstrated earlier that isoprenoid-based compounds, such as iridoid-glycosides that confer longevity in C. elegans also increase stress resistance in worms as well (Shukla et al. 2012a; Asthana et al. 2015). Like its complex counterparts, Prenol also improved thermal and oxidative stress tolerance ability in nematodes (Fig. 2c, d). In agreement with previous findings, our results also suggested a strong correlation between the improved age-associated parameters (such as the anti-amyloid plaques accumulating and lifespan promoting effects) and increased stress tolerance after Prenol supplementation in C. elegans.
As discussed earlier, increase ROS level and mitochondrial dysfunction are some other pathologies associated with neurodegenerative disorders (Hwang 2013; Ma et al. 2017). Reduced oxidative stress after dietary interventions has shown increased lifespan in wild-type as well as transgenes (Aβ or αS) expressing mutant strains of C. elegans (Shukla et al. 2012a; Liu et al. 2015; Ma et al. 2017). Our results demonstrated that the Prenol supplementation quenched intracellular ROS level in wild-type worms (Fig. 3a). An increased in the lifespan of short-lived (due to high ROS level) mitochondrial-dysfunction mutant mev-1(kn-1) (Fig. 3b) indicated the ability of small molecules like Prenol to reduce intracellular oxidative stress in worms. Altogether, the results indicated that the observed reduction in amyloid aggregation and oxidative stress is correlated to the intracellular ROS quenching activity of the Prenol.
The DAF-16, HSF-1, and SKN-1 are major transcription factors (TFs) in C. elegans, which are regulated by IIS. In worms, they are considered to put together longevity and stress resistance (Cohen et al. 2006; Tullet et al. 2008). In C. elegans, DAF-16 is a major TF of the IIS pathway, which is an ortholog of the FOXO transcription factor that conserved from lower invertebrate to higher mammals (Kenyon 2010). The HSF-1 is a TF that shares a common pathway with DAF-16 to regulate stress tolerance and several other physiological and neurological processes such as growth, aging, and degradation of amyloid plaques (Cohen et al. 2006). The SKN-1 is another major component of IIS that provokes phase-II detoxification response in C. elegans and is orthologous to mammalian Nrf proteins (Tullet et al. 2008). Activation of these TFs by dietary supplements has been correlated with improved lifespan and alleviation of Aβ- and αS-induced toxicity in C. elegans (Cohen et al. 2006; Edwards et al. 2014; Govindan et al. 2018). We observed that Prenol supplementation did not improve the mean lifespan of daf-16, hsf-1, and skn-1 mutants (Fig. 4), indicating the role of IIS in the Prenol mediated lifespan extension and stress tolerance. The in silico results also supported the findings of in-vivo studies, as Prenol showed strong binding ability with the DAF-16, HSF-1, and SKN-1 TFs (Table 2; Fig. S4). Previous studies have shown that the higher terpenoids, such as β-caryophyllene and ursolic acid, which increased lifespan in C. elegans through SKN-1 and JNK-1 signaling, respectively, also demonstrated strong binding with respective proteins in silico (Pant et al. 2014; Negi et al. 2016). Altogether, the in vivo and in silico findings explain how Prenol could significantly promote longevity in C. elegans, which might also be helpful in neuromodulation.
The nuclear localization of DAF-16 is essential to modulate the expression of several age- and stress-associated genes in C. elegans (Shukla et al. 2012a). It has been shown that longevity-promoting compounds including complex terpenoids promote nuclear translocation of DAF-16 in C. elegans (Shukla et al. 2012a; Pant et al. 2014). Our results showed that Prenol was also able to translocate DAF-16 into the nucleus (Fig. 5a) indicating role of DAF-16 in the lifespan extension and other attributes in C. elegans. It has been also reported that after activation the DAF-16, HSF-1, and SKN-1 TFs regulate the expression of stress-related proteins, such as SODs, HSPs, and GSTs, respectively (Honda et al. 2008; Blackwell et al. 2015; Brunquell et al. 2016). Previous studies have found that the terpenoids and their derivatives can increase the expression of these transcription factors and their downstream targets (both mRNA levels and GFP-tagged protein levels) (Pant et al. 2014; Asthana et al. 2015). We also observed that the supplementation of 0.25 mM Prenol significantly enhanced the expression SOD-3 and GST-4 in GFP-tagged transgenic strains of C. elegans (Fig. 5b, c). Additionally, mRNA expression levels of daf-16, hsf-1, and skn-1 TFs and their downstream test target genes were also enhanced after Prenol treatment (Fig. 6). Our results indicated that similar to the complex members of the terpenoid family, prenol supplementation not only increased the expression of these TFs but also promoted their transcriptional activities. Further, these investigations suggest that Prenol might act as an antagonist to IIS and provide stimulation to the nuclear translocation of longevity associated TFs, which might enhance stress tolerance and promote longevity in C. elegans.
The present study, for the first time, demonstrates the neuroprotective and longevity-promoting potential of a hemiterpene compound, Prenol, in C. elegans and unveils its underlying mechanism. The study reveals that Prenol rescues worms from the Aβ induced paralysis. It also reduces accumulation of αS in transgenic strains of C. elegans. Up-regulation of SOD-3 and GST-4 supports the findings of reduced oxidative stress after Prenol supplementation. Further, the study of mutants such as daf-16, skn-1, and hsf-1 demonstrates that the Prenol improves lifespan and health-span in IIS-dependent manner. Overall, the present study embarks the potential of the simplest form of terpenoids towards the development of economical and authentic therapeutics for the benefit to society.
Electronic supplementary material
(PDF 725 kb)
Acknowledgments
The authors are grateful to the Caenorhabditis elegans Genetics Center (Minneapolis, MN, USA) for providing nematode strains. Authors also heartily acknowledge Director, CSIR-National Botanical Research Institute, Lucknow, Uttar Pradesh, India and Director, National Institute of Technology, Raipur, Chhattisgahr, India for their kind support. Authors also show their sincere gratitude to Ms. Neetu Phulara, Child Development Project Officer, Uttarakhand Govetment, Chakrata, Dehradun, Uttarakhand, India for critically reading the manuscript and providing grammar check.
Abbreviations
- AD
Alzheimer’s disease
- Aβ
Amyloid-β
- BCP
β-caryophyllene
- BLAST
Basic local alignment search tool
- CI
Chemotaxis index
- DR
Dietary restriction
- FOXO
Forkhead box protein O
- FUdR
2′-Deoxy-5-fluorouridine
- GFP
Green fluorescent protein
- GRAS
Generally regarded as safe
- H2DCF-DA
7-dichlorodihydrofluoresceindiacetate
- IIS
Insulin/insulin like signaling
- NDs
Neurodegenerative disorders
- NGM
Nematode Growth Medium
- PD
Parkinson’s disease
- ROS
Reactive oxygen species
- TFs
Transcription factors
- YFP
Yellow fluorescent protein
- αS
α-Synuclein
Author contributions
SCP, SP, and VS conceived, designed, and performed in vivo and in vitro experiments; AJ performed in silico analysis; SCP, SP, and VS analyzed the data; PG and PSC contributed reagents/materials/analysis tools; SCP, SP, and AJ wrote the manuscript. PSC, VS, and PG critically reviewed and edited the manuscript. All authors have critically gone through the manuscript and approved it.
Funding information
SP was financially supported by Indian Council of Medical Research (ICMR), New Delhi, India (Grant Id- 45/11/2018-PHA/BMS/OL) through Senior Research Fellowship grant.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approvals
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Suresh Chandra Phulara and Swapnil Pandey contributed equally to this work.
Contributor Information
Pratima Gupta, Email: pguptabiotech@gmail.com.
Virendra Shukla, Email: shuklavirendra121@gmail.com.
References
- Ahmad W, Ebert PR. Metformin attenuates Aβ pathology mediated through levamisole sensitive nicotinic acetylcholine receptors in a C. elegans model of Alzheimer’s disease. Mol Neurobiol. 2017;54:5427–5439. doi: 10.1007/s12035-016-0085-y. [DOI] [PubMed] [Google Scholar]
- Asthana J, Yadav AK, Pant A, Pandey S, Gupta MM, Pandey R. Specioside ameliorates oxidative stress and promotes longevity in Caenorhabditis elegans. Comp Biochem Physiol Part - C Toxicol Pharmacol. 2015;169:25–34. doi: 10.1016/j.cbpc.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Bargmann CI (1998) Neurobiology of the Caenorhabditis elegans genome. Science (80- ) 282:2028–2033. [DOI] [PubMed]
- Bargmann CI, Hartwieg E, Horvitz HR. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–527. doi: 10.1016/0092-8674(93)80053-H. [DOI] [PubMed] [Google Scholar]
- Blackwell TK, Steinbaugh MJ, Hourihan JM, Ewald CY, Isik M. SKN-1/Nrf, stress responses, and aging in <i>Caenorhabditis elegans<i>. Free Radic Biol Med. 2015;88:290–301. doi: 10.1016/j.freeradbiomed.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhicharla R, Nagarajan A, Winter J, Adenle A, Nazir A, Brady D, Vere K, Richens J, O’Shea P, R. Bell D, de Pomerai D. Effects of α-synuclein overexpression in transgenic Caenorhabditis elegans strains. CNS Neurol Disord Drug Targets. 2012;11:965–975. doi: 10.2174/1871527311211080005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MK, Evans JL, Luo Y. Beneficial effects of natural antioxidants EGCG and α-lipoic acid on life span and age-dependent behavioral declines in Caenorhabditis elegans. Pharmacol Biochem Behav. 2006;85:620–628. doi: 10.1016/j.pbb.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Brunquell J, Morris S, Lu Y, Cheng F, Westerheide SD. The genome-wide role of HSF-1 in the regulation of gene expression in Caenorhabditis elegans. BMC Genomics. 2016;17:559. doi: 10.1186/s12864-016-2837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalorak P, Jattujan P, Nobsathian S, Poomtong T, Sobhon P, Meemon K. Holothuria scabra extracts exhibit anti-Parkinson potential in C. elegans: a model for anti-Parkinson testing. Nutr Neurosci. 2017;8305:1–12. doi: 10.1080/1028415X.2017.1299437. [DOI] [PubMed] [Google Scholar]
- Cohen E, Bieschke J, Perciavalle RM, et al (2006) Opposing activities protect against age-onset Proteotoxicitye. Science (80- ) 313:1604–1610. [DOI] [PubMed]
- Dues DJ, Schaar CE, Johnson BK, et al (2017) Uncoupling of oxidative stress resistance and lifespan in long-lived isp-1 mitochondrial mutants in Caenorhabditis elegans. Elsevier B.V. [DOI] [PMC free article] [PubMed]
- Edwards C, Canfield J, Copes N, et al. D-beta-hydroxybutyrate extends lifespan in C. elegans. Aging (Albany NY) 2014;6:621–644. doi: 10.18632/aging.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Cheng H, Xu Z, Yuan M, Huang Y, Liao J, Yang R, Zhou L, Ding C. Panax notoginseng polysaccharide increases stress resistance and extends lifespan in Caenorhabditis elegans. J Funct Foods. 2018;45:15–23. doi: 10.1016/j.jff.2018.03.034. [DOI] [Google Scholar]
- George KW, Thompson MG, Kang A, et al. Metabolic engineering for the high-yield production of isoprenoid-based C 5 alcohols in E . coli. Sci Rep. 2015;5:11128. doi: 10.1038/srep11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan S, Amirthalingam M, Duraisamy K, Govindhan T, Sundararaj N, Palanisamy S. Phytochemicals-induced hormesis protects Caenorhabditis elegans against α-synuclein protein aggregation and stress through modulating HSF-1 and SKN-1/Nrf2 signaling pathways. Biomed Pharmacother. 2018;102:812–822. doi: 10.1016/j.biopha.2018.03.128. [DOI] [PubMed] [Google Scholar]
- Gruber J, Soon YT, Halliwell B. Evidence for a trade-off between survival and fitness caused by resveratrol treatment of Caenorhabditis elegans. Ann N Y Acad Sci. 2007;1100:530–542. doi: 10.1196/annals.1395.059. [DOI] [PubMed] [Google Scholar]
- Gulia KK, Kumar VM. Sleep disorders in the elderly: a growing challenge. Psychogeriatrics. 2018;18:155–165. doi: 10.1111/psyg.12319. [DOI] [PubMed] [Google Scholar]
- He L, Mo H, Hadisusilo S, Qureshi AA, Elson CE. Isoprenoids suppress the growth of murine B16 melanomas in-vitro and in-vivo. J Nutr. 1997;127:668–674. doi: 10.1093/jn/127.5.668. [DOI] [PubMed] [Google Scholar]
- Honda Y, Tanaka M, Honda S. Modulation of longevity and diapause by redox regulation mechanisms under the insulin-like signaling control in Caenorhabditis elegans. Exp Gerontol. 2008;43:520–529. doi: 10.1016/j.exger.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Hwang O. Role of oxidative stress in Parkinson’s disease. Exp Neurobiol. 2013;22:11–17. doi: 10.5607/en.2013.22.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii N, Fujii M, Hartman PS, Tsuda M, Yasuda K, Senoo-Matsuda N, Yanase S, Ayusawa D, Suzuki K. A mutation in succinate dehydrogenase cytochrome b causes oxidative stress and ageing in nematodes. Nature. 1998;394:694–697. doi: 10.1038/29331. [DOI] [PubMed] [Google Scholar]
- Ishii T, Miyazawa M, Hartman PS, Ishii N. Mitochondrial superoxide anion (O2•-) inducible “mev-1” animal models for aging research. BMB Rep. 2011;44:298–305. doi: 10.5483/BMBRep.2011.44.5.298. [DOI] [PubMed] [Google Scholar]
- Jimbo D, Kimura Y, Taniguchi M, et al. Effect of aromatherapy on patients with Alzheimer’s disease. Psychogeriatrics. 2009;9:173–179. doi: 10.1111/j.1479-8301.2009.00299.x. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Lasekan O. Identification of the aroma compounds in Vitex doniana sweet: free and bound odorants. Chem Cent J. 2017;11:19. doi: 10.1186/s13065-017-0247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chotiko A, Chouljenko A, Gao C, Zheng J, Sathivel S (2018) Delivery of alpha-tocopherol through soluble dietary fibre-based nanofibres for improving the life span of Caenorhabditis elegans. Int J Food Sci Nutr 0:1–10. 10.1080/09637486.2018.1489785, 70. [DOI] [PubMed]
- Link CD. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1995;92:9368–9372. doi: 10.1073/PNAS.92.20.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link CD, Taft A, Kapulkin V, et al. Gene expression analysis in a transgenic Caenorhabditis elegans Alzheimer’s disease model. Neurobiol Aging. 2003;24:397–413. doi: 10.1016/S0197-4580(02)00224-5. [DOI] [PubMed] [Google Scholar]
- Liu J, Banskota AH, Critchley AT, Hafting J, Prithiviraj B. Neuroprotective effects of the cultivated Chondrus crispus in a C. elegans model of Parkinson’s disease. Mar Drugs. 2015;13:2250–2266. doi: 10.3390/md13042250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Cui X, Li J, Li C, Wang Z. Peptides from sesame cake reduce oxidative stress and amyloid-β-induced toxicity by upregulation of SKN-1 in a transgenic Caenorhabditis elegans model of Alzheimer’s disease. J Funct Foods. 2017;39:287–289. doi: 10.1016/j.jff.2017.10.032. [DOI] [Google Scholar]
- Mo H, Elson CE. Apoptosis and cell-cycle arrest in human and murine tumor cells are initiated by isoprenoids. J Nutr. 1999;129:804–813. doi: 10.1093/jn/129.4.804. [DOI] [PubMed] [Google Scholar]
- Negi H, Shukla A, Khan F, Pandey R. 3β-Hydroxy-urs-12-en-28-oic acid prolongs lifespan in C. elegans by modulating JNK-1. Biochem Biophys Res Commun. 2016;480:539–543. doi: 10.1016/j.bbrc.2016.10.073. [DOI] [PubMed] [Google Scholar]
- Pandey S, Tiwari S, Kumar A, Niranjan A, Chand J, Lehri A, Chauhan PS. Antioxidant and anti-aging potential of Juniper berry (Juniperus communis L.) essential oil in Caenorhabditis elegans model system. Ind Crop Prod. 2018;120:113–122. doi: 10.1016/j.indcrop.2018.04.066. [DOI] [Google Scholar]
- Pandey S, Phulara SC, Jha A, Chauhan PS, Gupta P, Shukla V. 3-Methyl-3-buten-1-ol (isoprenol) confers longevity and stress tolerance in Caenorhabditis elegans 3-methyl-3-buten-1-ol (isoprenol) confers longevity and stress tolerance in Caenorhabditis elegans. Int J Food Sci Nutr. 2019;70:595–602. doi: 10.1080/09637486.2018.1554031. [DOI] [PubMed] [Google Scholar]
- Pandey S, Phulara SC, Mishra SK, Bajpai R, Kumar A, Niranjan A, Lehri A, Upreti DK, Chauhan PS. Betula utilis extract prolongs life expectancy, protects against amyloid-β toxicity and reduces Alpha Synuclien in Caenorhabditis elegans via DAF-16 and SKN-1. Comp Biochem Physiol Part C Toxicol Pharmacol. 2019;228:108647. doi: 10.1016/J.CBPC.2019.108647. [DOI] [PubMed] [Google Scholar]
- Pant A, Saikia SK, Shukla V, et al. Beta-caryophyllene modulates expression of stress response genes and mediates longevity in Caenorhabditis elegans. Exp Gerontol. 2014;57:81–95. doi: 10.1016/j.exger.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Phulara SC, Shukla V, Tiwari S, Pandey R. Bacopa monnieri promotes longevity in Caenorhabditis elegans under stress conditions. Pharmacogn Mag. 2015;11:410–416. doi: 10.4103/0973-1296.153097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phulara SC, Chaturvedi P, Gupta P. Isoprenoid-based biofuels: homologous expression and heterologous expression in prokaryotes. Appl Environ Microbiol. 2016;82:5730–5740. doi: 10.1128/AEM.01192-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phulara SC, Chaturvedi P, Chaurasia D, Diwan B, Gupta P. Modulation of culture medium confers high-specificity production of isopentenol in Bacillus subtilis. J Biosci Bioeng. 2018;127:458–464. doi: 10.1016/j.jbiosc.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Phulara SC, Chaurasia D, Diwan B, Chaturvedi P, Gupta P. In-situ isopentenol production from Bacillus subtilis through genetic and culture condition modulation. Process Biochem. 2018;72:47–54. doi: 10.1016/j.procbio.2018.06.019. [DOI] [Google Scholar]
- Powolny AA, Singh SV, Melov S, Hubbard A, Fisher AL. The garlic constituent diallyl trisulfide increases the lifespan of C. elegans via skn-1 activation. Exp Gerontol. 2011;46:441–452. doi: 10.1016/j.exger.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathor L, Pant A, Awasthi H, Mani D, Pandey R. An antidiabetic polyherbal phytomedicine confers stress resistance and extends lifespan in Caenorhabditis elegans. Biogerontology. 2017;18:131–147. doi: 10.1007/s10522-016-9668-2. [DOI] [PubMed] [Google Scholar]
- Rizki G, Picard CL, Pereyra C, Lee SS. Host cell factor 1 inhibits SKN-1 to modulate oxidative stress responses in Caenorhabditis elegans. Aging Cell. 2012;11:717–721. doi: 10.1111/j.1474-9726.2012.00831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roullet JB, Luft UC, Xue H, Chapman J, Bychkov R, Roullet CM, Luft FC, Haller H, McCarron DA. Farnesol inhibits L-type Ca2+ channels in vascular smooth muscle cells. J Biol Chem. 1997;272:32240–32246. doi: 10.1074/JBC.272.51.32240. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Senchuk MM, Dues DJ, Van Raamsdonk JM (2017) Measuring oxidative stress in Caenorhabditis elegans: Paraquat and Juglone sensitivity assays. Bio-protocol 7: 10.21769/BIOPROTOC.2086 [DOI] [PMC free article] [PubMed]
- Shukla V, Phulara SC, Yadav D, Tiwari S, Kaur S, Gupta MM, Nazir A, Pandey R. Iridoid compound 10-O-trans-p-coumaroylcatalpol extends longevity and reduces α synuclein aggregation in Caenorhabditis elegans. CNS Neurol Disord Drug Targets. 2012;11:984–992. doi: 10.2174/1871527311211080007. [DOI] [PubMed] [Google Scholar]
- Shukla V, Yadav D, Phulara SC, Gupta MM, Saikia SK, Pandey R. Longevity-promoting effects of 4-hydroxy-E-globularinin in Caenorhabditis elegans. Free Radic Biol Med. 2012;53:1848–1856. doi: 10.1016/j.freeradbiomed.2012.08.594. [DOI] [PubMed] [Google Scholar]
- Szeto WY, Yang L, Wong RCP, Li YC, Wong SC. Spatio-temporal travel characteristics of the elderly in an ageing society. Travel Behav Soc. 2017;9:10–20. doi: 10.1016/j.tbs.2017.07.005. [DOI] [Google Scholar]
- Tiwari S, Lata C, Chauhan PS, Nautiyal CS. Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol Biochem. 2016;99:108–117. doi: 10.1016/j.plaphy.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Trojanowski NF, Raizen DM, Fang-Yen C. Pharyngeal pumping in Caenorhabditis elegans depends on tonic and phasic signaling from the nervous system. Sci Rep. 2016;6:22940. doi: 10.1038/srep22940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullet JMA, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations, Department of Economic and Social Affairs PD (2019) World Population Ageing 2019: Highlights (ST/ESA/SER.A/430).
- van Ham TJ, Thijssen KL, Breitling R, Hofstra RMW, Plasterk RHA, Nollen EAA. C. elegans model identifies genetic modifiers of α-synuclein inclusion formation during aging. PLoS Genet. 2008;4:e1000027. doi: 10.1371/journal.pgen.1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CC, Chang CH, Liao VHC. Anti-Parkinsonian effects of β-amyrin are regulated via LGG-1 involved autophagy pathway in Caenorhabditis elegans. Phytomedicine. 2017;36:118–125. doi: 10.1016/j.phymed.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Shukitt-Hale B, Kalt W, Ingram DK, Joseph JA, Wolkow CA. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell. 2006;5:59–68. doi: 10.1111/j.1474-9726.2006.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2016) Global Health Observatory (GHO) Data.
- Wu Y, Wu Z, Butko P, Christen Y, Lambert MP, Klein WL, Link CD, Luo Y. Amyloid-beta-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans. J Neurosci. 2006;26:13102–13113. doi: 10.1523/JNEUROSCI.3448-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Shi R, Li H, Xiang Y, Xiao L, Hu M, Ma F, Ma CW, Huang Z. Antioxidant and neuroprotective effects of Dictyophora indusiata polysaccharide in Caenorhabditis elegans. J Ethnopharmacol. 2016;192:413–422. doi: 10.1016/j.jep.2016.09.031. [DOI] [PubMed] [Google Scholar]
- Zhi D, Wang D, Yang W, et al. Dianxianning improved amyloid β-induced pathological characteristics partially through DAF-2/DAF-16 insulin like pathway in transgenic C. elegans. Sci Rep. 2017;7:11408. doi: 10.1038/s41598-017-11628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 725 kb)