Abstract
Osteosarcopenia is characterized by a progressive decline in muscle function and bone strength and associated with muscle fat accumulation. This study aimed to determine the effect of long-term high intensity resistance training (HIRT) on thigh muscle fat infiltration in older men with osteosarcopenia. Forty-three community-dwelling men (72 years and older) were randomly assigned to either an exercise group (EG, n = 21) or an inactive control group (CG, n = 22). EG participants performed a supervised single-set exercise training with high effort two times per week. Participants of both groups were individually provided with dietary protein to reach a cumulative intake of 1.5–1.6 g/kg/day or 1.2–1.3 g/kg/day (EG/CG), respectively, and Up to 10,000 IE/week of Vitamin-D were supplemented in participants with 25 OH Vitamin-D 3 levels below 100 nmol/l. Magnetic resonance (MR) imaging was performed to determine muscle and adipose tissue volume and fat fraction of the thigh. At baseline, there were no significant differences between the two groups. After 16 month,, there were significant training effects of 15% (p = 0.004) on intermuscular adipose tissue (IMAT) volume, which increased in the CG (p = 0.012) and was stable in the EG. In parallel, fat fraction within the deep fascia of the thigh (Baseline, EG: 18.2 vs CG: 15.5, p = 0.16) significantly differed between the groups (Changes, EG: 0.77% vs. CG: 7.7%, p = 0.009). The study confirms the role of fat infiltration of the muscles as an advanced imaging marker in osteosarcopenia and the favorable effects of HIRT on adipose tissue volume of the thigh, in men with osteosarcopenia.
Keywords: Aging, Intermuscular adipose tissue, IMAT, Osteoporosis, Sarcopenia, Magnetic resonance imaging, Exercise
Introduction
Osteosarcopenia, a combination of osteoporosis and sarcopenia, is characterized by a progressive decline in muscle function and bone strength [1], resulting in an increased risk of falls and eventually of fractures in older adults [2]. The term osteosarcopenia emphasizes the increasing understanding of bone muscle interactions and challenges the exclusive role of bone-related risk factors for osteoporotic fractures. Muscle-related risk factors may be equally important [3]. The interdependence of age-related changes of muscle, fat, and bone may be a key factor to develop effective therapies to target disability and subsequent mortality in older adults [4].
Muscle function declines with age but pathophysiological causes are not fully understood. Muscle size is only weakly associated with muscle function [5]. Recently, mechanisms of immobility and inflammation triggered fat accumulation have been suggested as leading causes for declining muscle quality, not only in osteosarcopenia but also in many other metabolic diseases [6–8]. Traditionally, intermuscular adipose tissue (IMAT) determined in T1-weighted magnetic resonance (MR) images and more recently muscle fat fraction (FF) determined in MR Dixon images have been used to quantify muscle fat infiltration.
Resistance exercise is an effective therapy to increase muscle strength and to reduce fractures in older adults [9]. Some initial studies have also shown beneficial effects of resistance exercises on muscle fat [10]. Several studies in postmenopausal women with increased risk for osteoporotic fractures focused on high intensity resistance training (HIRT) to improve muscle strength, balance, and to increase muscle mass [11]. Corresponding studies in males are rare and effects of HIRT in elderly males are not well known. Therefore, we have initiated the Franconian Osteopenia and Sarcopenia Trial (FrOST) to investigate effects of high intensity resistance training in older men with osteosarcopenia. The trial design and first results have been published earlier [12–14]. The aim of this study was to examine the effect of HIRT on thigh muscle and fat infiltration in the FrOST cohort. We hypothesized that long-term resistance exercise training has a positive effect on muscle quality and specifically on IMAT in older men with osteosarcopenia.
Material and methods
This randomized controlled trial was conducted by the Institute of Medical Physics, University of Erlangen-Nürnberg, Germany. The project was approved by the university ethics committee (numbers 67_15b and 4464b) and the federal bureau of radiation protection (BfS, number Z 5–2,246,212 - 2017-002). The project fully complied with the Helsinki Declaration [15]. All study participants gave their written informed consent after receiving detailed information. The study was performed between February 2018 and February 2020 and registered under ClinicalTrials.gov: NCT03453463.
Participants
Details of the recruitment procedure have been published previously in detail [13]. Briefly, 180 community-dwelling men 72 years and older of a previous study [16] were monitored. Applying the inclusion criteria (a) morphometric sarcopenia (skeletal muscle mass index (SMI) ≤ 7.26 kg/m2 [17, 18]) and (b) osteopenia or osteoporosis at the lumbar spine (LS) or total hip (tHip) [19]), and the exclusion criteria (a) secondary osteoporosis, (b) pharmacologic therapy or disease with impact on bone or muscle metabolism during the last 2 years, (c) hip fractures, (d) limitations or problems that prevent intense exercise (e) participation in any resistance training during the last 2 years, and (f) alcohol consumption > 60 g/d ethanol, in summary, 43 men were eligible and willing to participate. Men were randomly allocated to an exercise (EG, n = 21) or control (CG, n = 22) group; however, due to contraindications for MRI, the present analysis included 16 men of the EG and 20 men of the CG (Fig. 1).
Fig. 1.
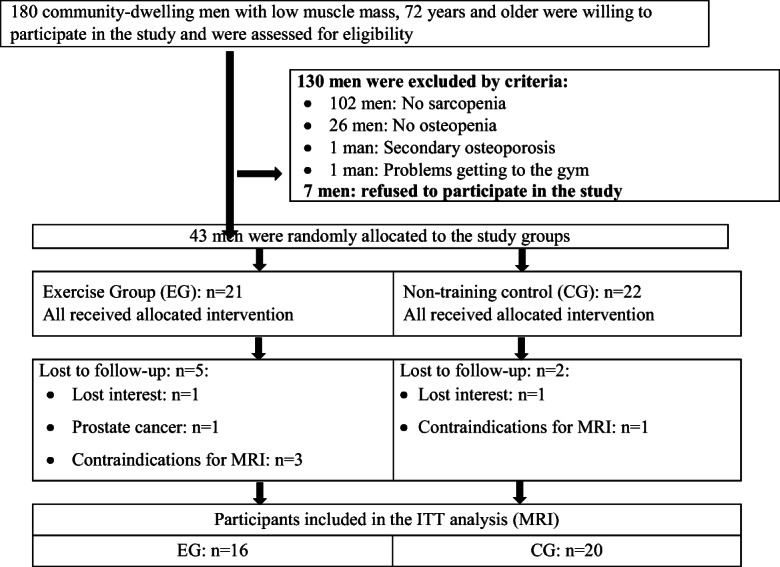
Participant flow through the study
Interventions
The EG attended two supervised exercise sessions per week for 16 months on machines in a well-equipped (MedX, Ocala, FL, USA), centrally located gym (Kieser Training, Erlangen, Germany). In intended or unintended cases of temporary inability (holidays, illness), subjects were allowed to visit a third session in the week before and/or after.
We applied a periodized HIRT defined as single-set exercise training with high effort; details have been published previously [13]. Our training strategy concentrated on short but intense bouts of resistance exercise performed on machines without any other types of exercises either in parallel or as a warm up. With few exceptions, all major and minor muscle groups were addressed by the various exercises periods.
The exercise intensity was consistently adopted by prescribing a range of repetitions (i.e., 5–7 or 8–10 reps) and the corresponding degree of work to failure (“effort”)1 [20–22]. We used a specific approach that classified set endpoints as “non-repetition maximum” (nRM), “(self-determined) repetition maximum” (RM), or (not applied during year one) “complete momentary muscular failure” [20]. In order to quantify the relative intensity (% 1RM) of a range of reps and the corresponding “effort” [20], we frequently conducted leg and bench press 1RM-maximum tests for according to the approach of Kraemer [23].
The 18 months intervention was structured into 5 periods (meso-cycles) with different aims and training protocols. The first training period focused on education and experience to select the adequate load. Eight exercises as single set and 4 exercises as double sets of 8–15 repetitions (reps), using nearly the full range of motion with 90–120 s rest, were prescribed. During the second period, participants performed the single set of 14 exercises with 90 s of rest. The programs designed to the repetition in reserve (RIR) approach [24]. The RM approach was introduced with the start of the third period, and with the exception of back extension, about one-third of the sets were conducted with an explosive movement in the concentric phase. The superset approach introduced at the start of the fourth period addressed the same or related muscle groups (e.g., knee extension and leg press), or agonist and antagonist (e.g., leg press and leg curls). At the end, we introduced drop-sets. That is, after work to RM (≤ 10 reps) or RM-1 rep, load was immediately decreased by 10–20% in order to complete more reps also conducted up to RM or RM-1 reps.
Supplements
All participants were provided with a supplementation of protein, cholecalciferol, and calcium according to recent recommendations [25, 26]. At baseline dietary protein intake was calculated based on 4-day dietary protocols (Freiburger Nutrition Record, nutri-science, Hausach, Germany). Results were carefully checked and discussed. In cases of unrealistic results (e.g., energy intake < 1000 kcal/d or > 3500 kcal/d), the participant was asked to complete another diet record based on more representative days.
All participants were provided with whey protein powder (Active PRO80, inkospor, Roth, Germany). According to present recommendations [25], the EG was supplied to reach a cumulative daily protein intake of 1.5–1.6 g/kg body-mass, while the CG was supplied to reach a cumulative daily protein intake of 1.2–1.3 g/kg body-mass/d. The chemical score of the protein product is 159, 100 g/d represented a caloric value of 360 kcal and contained 80 g of (whey) protein with a high L-leucine (9 g) and essential amino acid (57 g) component. Participants were asked to take the protein powder with low fat milk, doses of more than 40 g were split. There was no focus on intake at a specific time of the day.
Twenty-five OH vitamin D3 (25-OH D3) levels of all participants were determined at baseline and after 6 and 12 months. Independent of their group affiliation, participants with serum concentrations below 75 nmol/l (n = 37) were asked to ingest four cholecalciferol units (MYVITAMINS, Manchester, UK) of 2500 IE a week (i.e., 10,000 IE/week) and those with 76 to ≤ 100 nmol/l were requested to take two capsules of 2500 IE/d, a week (i.e., 5000 IE/week).
Based on dietary calcium questionnaires (Rheumaliga, Switzerland), each participant received an individual supplement (calcium capsules; Sankt Bernhard, Bad Dietzenbach, Germany) to ensure a cumulative calcium intake of 1000 mg/d.
Assessments
All assessments were highly standardized. The same research assistant consistently led, supervised, and/or analyzed a given assessment at about the same time of day (± 2 h). Tests were consistently conducted at the same location, in the identical order, and with the same calibrated devices. Participants were asked to maintain their dietary habits and lifestyle including physical activity and exercise routines outside the present study intervention. Before all experiments, participants completed a standardized questionnaire that asked for (a) demographic parameters, (b) diseases, pharmacologic therapy/dietary supplements, and operations, (c) physical limitations, (d) falls and injurious falls, (e) injuries and low trauma fractures within the last year, and (f) lifestyle, including physical activity and exercise [27, 28]. The questionnaires were carefully checked for consistency, completeness and accuracy in close interaction between the primary investigator and participants. The final assessments took place after the regeneration week of the last mesocycle. However, due to logistic reasons, baseline MR imaging assessments were conducted 5–6 weeks after the start of the intervention, i.e., 1–2 weeks after the conditioning and familiarization period. Final MR imaging assessments were performed during the regeneration week of the penultimate mesocycle (i.e., 4 weeks before intended study end).
Anthropometric measurements
Body height was assessed by a Holtain stadiometer (Crymych Dyfed, Great Britain). Body mass was determined via direct-segmental, multi-frequency Bio-Impedance-Analysis (DSM-BIA; InBody 770, Seoul, Korea); body composition was evaluated by Dual Energy X-Ray Absorptiometry (DEXA, QDR 4500a, Discovery-upgrade, Hologic Inc., Bedford, USA), according to the specification of the manufacturer.
MR imaging and analysis
MRI acquisition was performed using a 3T scanner (MAGNETOM Skyrafit, Siemens Healthineers AG, Erlangen, Germany) and an 18-channel body surface coil. The flexible coil was wrapped around the left mid-thigh. Thirty slices with a thickness of 3 mm covering a length of 9 cm were acquired. The protocol included a clinically common T1-weighted Turbo Spin Echo and a 6-point Dixon Gradient Echo Volumetric Interpolated Breath-hold Examination (Dixon) sequence to determine proton density fat fraction [29]. Intensities in the FF images are in a range of 0–1000 corresponding to a FF of 0.0–100.0%. By this sequence, a direct measurement of fat content in a specific voxel is possible.
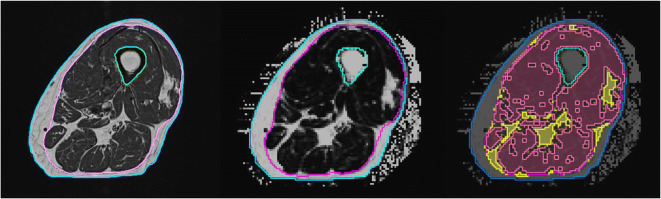
Image analysis was performed using MIAF (Medical Image Analysis Framework, University of Erlangen). The analysis started with a segmentation of the outer surface of the thigh, the femoral bone, and the fascia lata in the T1 images (Fig. 1). Segmentation was based on a multi-stage approach combining fuzzy c-means clustering, level sets, and 3D filtering of structures belonging to the fascia lata as described previously [30]. The analysis of the T1-weighted images resulted in total thigh volume, which was further divided in subcutaneous adipose tissue (SAT) and intra-fascia (IF) volume.
The segmentation masks obtained in the T1 images were 3D registered to quantitative Dixon FF images to directly measure the percentage of fat within IF. Based on the histogram of the FF values of all voxels within IF, a threshold was determined to separate IMAT from muscle tissue (Fig. 2). In order to avoid partial volume artifacts, voxels at the border between muscle tissue and IMAT were excluded from analysis. Subsequently volume of IMAT and muscle tissue and FF of muscle tissue were determined. In addition, ratios IMAT volume/intra fascia volume and muscle tissue volume/intra fascia volume were calculated.
Fig. 2.
Segmentation of magnetic resonance (MR) images. Left: T1-weighted image used for the segmentation of the fascia lata (magenta). Thigh volume: area inside blue contour; SAT (subcutaneous adipose tissue): area between blue and magenta contours. Intra fascia volume: area inside magenta contour excluding bone (green contour); Intermuscular adipose tissue (IMAT): all bright pixels inside the fascia lata; Center: Dixon fat fraction (FF) map. The poorer spatial resolution compared to the T1 image is obvious, Right: segmented Dixon FF map showing IMAT (yellow) and muscle tissue (red). Black voxels inside the fascia lata were not analyzed. They are located at the borders between IMAT and muscle tissue and their intensities are affected by partial volume artifacts
Statistical analysis
Multiple imputation (ITT) using R statistics software (R Development Core Team Vienna, Austria) in combination with Amelia II [31] was used to compare changes in MR based imaging parameters between EG and CG after 16 months. The full data set was used for ITT, and repeated imputation 100 times. As confirmed by over-imputation diagnostic plots (“observed versus imputed values”) provided by Amelia II, the imputation worked well in all cases. Normal distribution of the data was checked by statistical (Shapiro-Wilk test) and graphical (qq-plots) tests. All the study outcomes addressed here were analyzed by dependent t-tests, applying t-test comparisons with pooled SD. All tests were 2-tailed, significance was accepted at p < 0.05. ITT results were compared to a per protocol (PP) analysis in which outcomes were also analyzed by dependent t tests. Further, between group differences after 16 months were also analyzed by ANCOVA using group as covariate. As p values of the PP and the ANCOVA were very similar to the ITT analysis and significance did not change with one exception discussed below, results of the PP analysis are omitted.
Results
Three participants of the CG and two participants of the EG were lost to follow-up (Fig. 1). Two participants lost interest, one participant of the EG suffered from prostate cancer and withdrew from the study, and two other CG participants were unable to attend the final assessment due to a hip fracture and influenza infection.
Baseline characteristics
Baseline characteristics of anthropometric measurements and of variables determined from the questionnaires are presented in Table 1 (published previously [14]). There were no significant differences between exercise and control groups except for protein intake. Average dietary protein intake was very high in the CG and differed significantly from the EG.
Table 1.
Baseline characteristics of the participants of the exercise and control group
| Variable | EG (n = 21) | CG (n = 22) | p |
|---|---|---|---|
| Age [years] | 77.8 ± 3.6 | 79.2 ± 4.7 | .262 |
| Body mass index [kg/m2] | 25.0 ± 3.0 | 24.5 ± 1.9 | .515 |
| Total body fat [%] | 28.6 ± 5.8 | 30.5 ± 6.8 | .330 |
| Physical activity [index]a | 4.45 ± 1.32 | 4.15 ± 1.53 | .490 |
| Exercise per week [min] | 59 ± 56 | 46 ± 52 | .780 |
| Three (3) or more diseases [n]b | 10 | 12 | .826 |
| Hip and knee arthritis [n]b | 2 | 2 | .959 |
| Low back pain [n] | 3 | 4 | .527 |
| Diabetes mellitus type II [n] | 1 | 1 | .960 |
| Habitual gait velocity [m/s] | 1.25 ± 0.17 | 1.26 ± 0.15 | .703 |
| LLFDI [index] c | 1.51 ± 0.74 | 1.44 ± 0.53 | .727 |
| 25 (OH)D [nmol/l]d | 43.8 ± 17.5 | 54.0 ± 21.1 | .126 |
| Energy intake [MJ/d]e | 8.84 ± 1.71 | 9.39 ± 2.42 | .407 |
| Protein intake [g/kg/d]e | 1.10 ± 0.25 | 1.29 ± 0.34 | .043 |
| Calcium intake (mg/d)f | 802 ± 226 | 833 ± 282 | .636 |
| Smokers [n] | 3 | 4 | .959 |
CG control group; EG exercise group; significant p values are shown in bold
ascale from (1) “very low” to (7) “very high” [28]; b using the ICD-10 based disease cluster of Schäfer et al. [32]; c LLFDI (Late Life Function Disability Instrument) [33]: scale from (1) “no problem” to (5) “impossible”; d Roche Diagnostics, Mannheim, Germany; e as determined by a 4-day dietary record; f as determined by a Calcium Questionnaire provided by Rheumaliga, Switzerland)
MRI assessments
Results are summarized in Table 2. At baseline, no significant differences between control and exercise groups were observed for any of the variable, although numerical differences were in particular high for IMAT and total thigh volume.
Table 2.
Baseline and absolute and percentage changes after 16 months
| Baseline (SD) | ∆ Absolut (SD) | ∆ % | p* | ||
|---|---|---|---|---|---|
| Thigh volume [cm3] | CG | 1494 (122) | − 10.3 (52) | − 0.7 | .49 |
| EG | 1625 (243) | − 44.9 (75) | − 2.8 | .007 | |
| p** | .065 | .14 | – | ||
| SAT volume [cm3] | CG | 314 (72) | 1.6 (37) | 0.51 | .85 |
| EG | 358 (130) | − 14.7 (41) | − 4.1 | .15 | |
| p | .24 | .20 | |||
| Intra fascia volume [cm3] | CG | 1125 (99) | − 14.8 (38) | − 1.3 | .14 |
| EG | 1210 (164) | − 24.2 (50) | − 2.0 | .04 | |
| p | .08 | .54 | – | ||
| IMAT volume [cm3] | CG | 69.2 (27) | 7.1 (10) | 10.3 | .008 |
| EG | 104.4 (74) | − 4.8 (12) | − 4.6 | .09 | |
| p | .09 | .004 | – | ||
| Muscle tissue volume [cm3] | CG | 999 (91) | − 25.6 (38) | − 2.6 | .018 |
| EG | 1024 (113) | − 21.7 (53) | − 2.1 | .07 | |
| p | .49 | .81 | – | ||
| Intra fascia FF | CG | 15.5 (3.9) | 1.2 (0.97) | 7.7 | < .001 |
| EG | 18.2 (6.9) | 0.14 (1.2) | 0.77 | .60 | |
| p | .16 | .009 | – | ||
| Muscle tissue FF | CG | 7.7 (1.8) | 0.50 (0.33) | 6.5 | < .001 |
| EG | 8.3 (2.4) | 0.25 (0.40) | 3.0 | .007 | |
| p | .39 | .06 | – |
* Within-group differences (follow up vs baseline); ** Between-group differences (CG vs EG); CG control group; EG exercise group; ∆ change between baseline and 16 months measurements; FF fat fraction; IMAT intermuscular adipose tissue; SAT subcutaneous adipose tissue; SD standard deviation; significant p values are shown in bold
After 16 months thigh (− 2.8%) and intra-fascia volume (− 2.0%) decreased significantly in EG whereas for these parameters no significant changes were detected in CG. In contrast, CG IMAT volume (10.3%) significantly increased and muscle tissue volume (− 2.6%) significantly decreased, while no significant changes were observed in EG. Intra-fascia FF significantly increased in the CG and was stable in EG, while muscle tissue FF (CG: 6.5%; EG 3.0%) significantly increased in both groups after 16 months. The difference in increase (%) in muscle tissue FF between both groups was borderline significant. The only parameter were results of the ITT analysis (p = 0.57) and the Ancova (p = 0.02) differed with respect to significant–non significant.
Group differences after 16 months were significant for IMAT volume as well as for intra-fascia FF. IMAT volume and intra-fascia FF increased more in the CG than in EG.
Results of the derived MR assessments are summarized in Table 3. In CG, the ratio of IMAT to fascia volume significantly increased and relative muscle tissue volume significantly decreased after 16 months, while changes were not significant in the EG. Between groups differences after 16 months were significant for both parameters.
Table 3.
Mean relative value and changes of IMAT and muscle tissue in the exercise and control groups
| Baseline (SD) | ∆ Absolut (SD) | ∆% | p* | ||
|---|---|---|---|---|---|
| IMAT/fascia volume | CG | 0.059 (0.022) | 0.007 (0.008) | 11.9 | .002 |
| EG | 0.080 (0.044) | − 0.002 (0.012) | −2.5 | .49 | |
| p** | .11 | .014 | – | ||
| Muscle tissue/fascia volume | CG | 0.86 (0.05) | − 0.013 (0.011) | −1.5 | < .001 |
| EG | 0.82 (0.09) | − 0.003 (0.017) | −0.37 | .40 | |
| p | .19 | .056 | – | – |
* Within-group differences (follow up vs baseline); ** Between-group differences (CG vs EG); CG control group; EG exercise group; ∆ change between baseline and 16 months measurements; IMAT intermuscular adipose tissue; SD standard deviation; significant p values are shown in bold
Discussion
This study presents additional data of the randomized controlled FrOST trial, a study in elderly men with osteosarcopenia. In this study, we applied a time effective HIRT protocol, i.e., a single-set resistance exercise protocol with high strain magnitude, rate, and high effort which was recently recommended as a very effective training strategy [34]. Here we presented results of muscle fat infiltration of the thigh from 3D noninvasive MR imaging specifically focusing on intermuscular adipose tissue and fat fraction. Other aspects of the study such as the effect of HIRT on the sarcopenia Z-score [35], bone mineral density [14], and whole body composition [12] have been reported separately.
After 16 months, there was a significant increase of IMAT combined with a significant decrease in muscle tissue volume in the non-exercising compared to the exercising men. Results persisted after normalizing IMAT and muscle tissue volume to the fascia lata volume. In parallel, fat fraction within the fascia lata was significantly increased in CG relative to EG after 16 months. On the basis of these results, the HIRT prevented a further increase of IMAT and of muscle atrophy that was observed in the control group, possibly indicating that the exercise intervention was able to slow down or halt the process but did not reverse it. Interestingly, the dominant training effect was observed on IMAT volume and in parallel on intra fascia FF. The effect on FF of muscle tissue, which unexpectedly also increased in EG, was much lower and only borderline significant between the both groups.
The increase of the IMAT to fascia volume ratio in the control group implies an increase in muscle atrophy consistent with the results of a deceasing ratio of muscle tissue volume to fascia volume. The increase of IMAT and muscle atrophy in the control group is most likely normal age-related processes [10, 36]. Average protein levels of the control group observed at baseline were not changed during the course of the study; only three subjects of this group received any protein supplementation.
IMAT was not significantly reduced by exercise although a numerical decrease of 4.6% was observed. Other studies reported significant exercise related decreases in IMAT [10, 37, 38]. However, a comparison of exercise effects on IMAT across the few published studies is difficult. First, cohorts differ: elderly men with osteosarcopenia in our study, elderly men and women with different preconditions such as cancer, multiple sclerosis, or stroke in [10], and healthy young and older men and older women in [38] or obese elderly males and females [37]. Second, a non-exercising control group existed only in FrOST. Third, type of training, resistance versus aerobic, and duration of training differed. Further, MRI protocols differed. In our study, a 3D assessment of IMAT volume in cm3 and of FF in % was performed compared to the assessment of IMAT cross sectional area (CSA) in cm2 in the other two studies.
Two exercises studies, one in subjects with type 2 diabetes [39] and one in older adults with a history of falls [40] did not find any exercise effects on IMAT even when compared to a control group but training periods of just 3 months may have been too short. As detailed above, the majority of studies including FrOST showed positive effects of exercise on IMAT in elderly subjects across various diseases when comparing group differences. This is highly important as in contrast to SAT, IMAT of the thigh is associated with muscle function and mobility in older adults, either healthy or with comorbid conditions such as insulin resistance, type 2 diabetes, metabolic syndrome, stroke, spinal cord injury, or obesity [41–44]. Aging is related with an accumulation of IMAT [10, 45, 46] which contributes to osteosarcopenia [3]. Therefore, the prevention of fat infiltration has been suggested as a novel therapeutic method in osteosarcopenia [1].
A current limitation of studies assessing IMAT is the lack of standardization of the measurement. Even the term IMAT is used in different contexts. The traditional definition is based on T1-weighted MRI images and denotes the “white” pixels within the fascia lata [8], from which usually an IMAT CSA is measured. Sometimes IMAT CSA is averaged over multiple slices [10]. As an alternative, an IMAT volume as in our study can be measured, which also considers the slice thickness. The interpretation of absolute values such as area or volume may be problematic as they depend on body size. Thus, for comparison across studies IMAT should be normalized, for example, to the volume bounded by the fascia lata [30]. Some studies normalize IMAT area or volume also to the corresponding muscle area or volume [40, 43]. It must also be remembered that IMAT depends on the spatial resolution of the MRI dataset, the higher the resolution the higher IMAT [47]. Finally, in some publications [42] IMAT refers to intramuscular adipose tissue, which excludes all perimuscular adipose tissues. However, in in vivo imaging, this definition is problematic as in T1-weighted MRI images intramuscular adipose tissue would only comprise the visible white voxels within muscles, i.e., only an unknown portion of the total intramuscular adipose tissue.
It is a strength of our study that we presented IMAT volume, the ratio of IMAT to fascia volume and in addition FF obtained from an additional 6 pt Dixon sequence [48]. FF results confirmed the volumetric results obtained from the T1-weighted MR images. Another strength is the rather long study duration of 16 months. Limitations of the study were the relatively small number of subjects with respect to the MRI analysis. The FrOST study was powered for BMD, not for parameters of muscle fat infiltration. Nevertheless, significant results on IMAT were observed. Another limitation seems to be the relatively large although not significant baseline difference in IMAT volume between EG and CG. Also the ratio IMAT to fascia volume was comparable between the two groups.
Diagnosis of sarcopenia and also of sarcopenic obesity remains challenging due to the variability of key symptoms and cutoffs for functional deficits [49]. In addition, the variability of pathophysiological factors results in sometimes extreme complexity, mainly because there is a considerable influence of disuse in, e.g., cardiovascular and lung comorbidities and also of chronic inflammatory conditions in metabolic diseases including type 2 diabetes and obesity. IMAT and fatty muscle infiltration may be influenced by all the above conditions but may also develop as an endogenous problem based on chronic inflammatory tissue conditions like aging and cellular senescence [8]. Medical interventions such as SARMs and antagonists for myostatin and activin showed effects mainly on muscle mass but not on function, unless the respective clinical trials also included specific exercise interventions [50]. In contrast, a recent meta-analysis [51] showed that if exercise can be performed, physical training showed overall significant positive effects on muscle strength and physical performance but not on muscle mass. Exercise seems to halt the process of fatty infiltration as a read out of progressive disease and has positive effects on metabolic parameters and inflammation. This challenges questions as to which patients would get benefit from combined treatment strategies in order to reverse the inflammatory process that impairs continuous regeneration and functional performance. Future studies should be homogenized in design as recently recommended and should carefully evaluate the benefit of combined medical and physical treatments on functional performance, metabolic parameters including fatty muscle infiltration and healthy aging [52]. Chronic inflammatory processes also trigger fatty infiltration of bone marrow which impair mechanotransduction and consequently bone remodeling, one of the causes of osteoporosis [8]. Indeed, after 12 month of exercise in the FROST study, lumbar spine BMD as measured by quantitative computed tomography was maintained in the EG and decreased significantly by 4.1 in the CG, resulting in significant between-group differences [13].
Conclusion
In elderly men with osteosarcopenia, a high intensity resistance training prevented a further increase of muscle fat infiltration of the thigh determined as IMAT and FF which was observed in a non-exercising control group. In the CG, the increase in IMAT was accompanied by muscle atrophy. The combination of T1-weighted MR and Dixon sequences is an effective tool to investigate muscle fat infiltration, but outcome parameters must be further standardized to be better comparable across studies. The study confirms the value of fat infiltration as an advanced imaging marker in sarcopenia and its potential impact on osteoporosis. These data also demonstrate that the development of fatty infiltration of musculature is a rapidly progressive disease in these elderly males, which can be retarded but not substantially be reversed by HIRT at least with the time and intensity of exercise applied here. Whether combined HIRT exercise and sarcopenia medication can reverse IMAT in this elderly population remains to be documented in appropriate studies.
Acknowledgments
The present work was performed in partial fulfillment of the requirements for obtaining the PhD degree Dr. rer. biol. hum. at the Friedrich–Alexander University Erlangen-Nuremberg. We thank the Imaging Science Institute (Erlangen, Germany) for providing us with measurement time at the 3T MRI system. This work was in part supported by the Bavarian State Ministries for Science and Arts and for Economic Affairs, Regional Development and Energy through the European Regional Development Fund, within the “Center for Locomotion Research” Project granted to the Ludwig-Maximilians-University of Wuerzburg (project lead FJ).
Authors’ contributions
Study design: WK, FJ, and KE; Exercise training and related data collection: MG and WK; MR imaging: AN, MU, and OC; MR image analysis: OC and KE; Data analysis: MG and WK, Statistics: MK; Generation of manuscript: MG, KE, and WK; Review of manuscript: all.
Funding
The study was funded internally.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
E.g., maximum effort minus 1–3 reps; defined as non-repetition maximum: “Set endpoint when trainees completed a pre-determined number of repetitions despite the fact that further repetitions could be completed.”
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fatima M, Brennan-Olsen SL, Duque G. Therapeutic approaches to osteosarcopenia: insights for the clinician. Ther Adv Musculoskelet Dis. 2019;11:1759720X19867009. doi: 10.1177/1759720X19867009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sepulveda-Loyola W, Phu S, Bani Hassan E, Brennan-Olsen SL, Zanker J, Vogrin S, et al. The joint occurrence of osteoporosis and sarcopenia (Osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020;21(2):220–225. doi: 10.1016/j.jamda.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Hirschfeld HP, Kinsella R, Duque G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int. 2017;28(10):2781–2790. doi: 10.1007/s00198-017-4151-8. [DOI] [PubMed] [Google Scholar]
- 4.Dent E, Morley JE, Cruz-Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J, Bauer JM, Pahor M, Clark BC, Cesari M, Ruiz J, Sieber CC, Aubertin-Leheudre M, Waters DL, Visvanathan R, Landi F, Villareal DT, Fielding R, Won CW, Theou O, Martin FC, Dong B, Woo J, Flicker L, Ferrucci L, Merchant RA, Cao L, Cederholm T, Ribeiro SML, Rodríguez-Mañas L, Anker SD, Lundy J, Gutiérrez Robledo LM, Bautmans I, Aprahamian I, Schols JMGA, Izquierdo M, Vellas B. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148–1161. doi: 10.1007/s12603-018-1139-9. [DOI] [PubMed] [Google Scholar]
- 5.Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev. 2013;35:51–65. doi: 10.1093/epirev/mxs006. [DOI] [PubMed] [Google Scholar]
- 6.Wong AK, Chandrakumar A, Whyte R, Reitsma S, Gillick H, Pokhoy A, Papaioannou A, Adachi JD. Bone marrow and muscle fat infiltration are correlated among postmenopausal women with osteoporosis: the AMBERS cohort study. J Bone Miner Res. 2020;35(3):516–527. doi: 10.1002/jbmr.3910. [DOI] [PubMed] [Google Scholar]
- 7.Al Saedi A, Hassan EB, Duque GJJoL, Medicine P The diagnostic role of fat in osteosarcopenia 2019. 2019;4.
- 8.Herrmann M, Engelke K, Ebert R, Muller-Deubert S, Rudert M, Ziouti F, et al. Interactions between muscle and bone-where physics meets biology. Biomolecules. 2020;10(3). 10.3390/biom10030432. [DOI] [PMC free article] [PubMed]
- 9.Kemmler W, Haberle L, von Stengel S. Effects of exercise on fracture reduction in older adults : a systematic review and meta-analysis. Osteoporos Int. 2013;24(7):1937–1950. doi: 10.1007/s00198-012-2248-7. [DOI] [PubMed] [Google Scholar]
- 10.Marcus RL, Addison O, Kidde JP, Dibble LE, Lastayo PC. Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J Nutr Health Aging. 2010;14(5):362–366. doi: 10.1007/s12603-010-0081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shojaa M, von Stengel S, Kohl M, Schoene D, Kemmler W. Effects of dynamic resistance exercise on bone mineral density in postmenopausal women: a systematic review and meta-analysis with special emphasis on exercise parameters. Osteoporosis Int. 2020;31:1427–1444. doi: 10.1007/s00198-020-05441-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kemmler W, Kohl M, Frohlich M, Engelke K, von Stengel S, Schoene D. Effects of high-intensity resistance training on fitness and fatness in older men with Osteosarcopenia. Front Physiol. 2020;11:1014. doi: 10.3389/fphys.2020.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemmler W, Kohl M, Frohlich M, Jakob F, Engelke K, von Stengel S, et al. Effects of high-intensity resistance training on osteopenia and sarcopenia parameters in older men with Osteosarcopenia-one-year results of the randomized controlled Franconian osteopenia and sarcopenia trial (FrOST) J Bone Miner Res. 2020;35:1634–1644. doi: 10.1002/jbmr.4027. [DOI] [PubMed] [Google Scholar]
- 14.Lichtenberg T, von Stengel S, Sieber C, Kemmler W. The favorable effects of a high-intensity resistance training on sarcopenia in older community-dwelling men with osteosarcopenia: the randomized controlled FrOST study. Clin Interv Aging. 2019;14:2173–2186. doi: 10.2147/CIA.S225618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World_Medical_Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053. [DOI] [PubMed]
- 16.Kemmler W, Teschler M, Weissenfels A, Sieber C, Freiberger E, von Stengel S. Prevalence of sarcopenia and sarcopenic obesity in older German men using recognized definitions: high accordance but low overlap! Osteoporos Int. 2017;28(6):1881–1891. doi: 10.1007/s00198-017-3964-9. [DOI] [PubMed] [Google Scholar]
- 17.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 18.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Assessment of osteoporotic fracture risk and its application to screening for postmenopausal osteoporosis. Geneva: World Health Organization1994 1994. Report No.: Technical Report Series no. 843. [PubMed]
- 20.Steele J, Fisher J, Giessing J, Gentil P. Clarity in reporting terminology and definitions of set end points in resistance training. Muscle Nerve. 2017;368–374(3):368–374. doi: 10.1002/mus.25557. [DOI] [PubMed] [Google Scholar]
- 21.Kemmler W, Wittke A, Bebenek M, Fröhlich M, von Stengel S. High intensity resistance training methods with and without protein supplementation to fight cardiometabolic risk in middle-aged males a randomized controlled trial. Biomed Res Int. 2016;2016:1–9. doi: 10.1155/2016/9705287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kemmler W, Teschler M, Weissenfels A, Fröhlich M, Kohl M, von Stengel S. Ganzkörper-Elektromyostimulationst versus HIT-Krafttraining - Effekte auf Körperzusammensetzung und Muskelkraft. Dtsch Z Sportmed. 2015;66(12):321–327. doi: 10.5960/dzsm.2015.209. [DOI] [Google Scholar]
- 23.Kraemer WJ, Gordon SE, Fleck SJ, Marchitelli LJ, Mello R, Dziados JE, Friedl K, Harman E, Maresh C, Fry A. Endogenous anabolic hormonal and growth factor responses to heavy resistance exercise in males and females. Int J Sports Med. 1991;12(2):228–235. doi: 10.1055/s-2007-1024673. [DOI] [PubMed] [Google Scholar]
- 24.Zourdos MC, Klemp A, Dolan C, Quiles JM, Schau KA, Jo E, Helms E, Esgro B, Duncan S, Garcia Merino S, Blanco R. Novel resistance training-specific rating of perceived exertion scale measuring repetitions in reserve. J Strength Cond Res. 2016;30(1):267–275. doi: 10.1519/JSC.0000000000001049. [DOI] [PubMed] [Google Scholar]
- 25.Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, Phillips S, Sieber C, Stehle P, Teta D, Visvanathan R, Volpi E, Boirie Y. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE study group. J Am Med Dir Assoc. 2013;14(8):542–559. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 26.DVO. Prophylaxe, Diagnostik und Therapie der OSTEOPOROSE bei postmenopausalen Frauen und bei Männern Stuttgart: Schattauer; 2017.
- 27.Kemmler W, Lauber D, Weineck J, Hensen J, Kalender W, Engelke K. Benefits of 2 years of intense exercise on bone density, physical fitness, and blood lipids in early postmenopausal osteopenic women: results of the Erlangen Fitness Osteoporosis Prevention Study (EFOPS) Arch Intern Med. 2004;164(10):1084–1091. doi: 10.1001/archinte.164.10.1084/164/10/1084. [DOI] [PubMed] [Google Scholar]
- 28.Kemmler W, Weineck J, Kalender WA, Engelke K. The effect of habitual physical activity, non-athletic exercise, muscle strength, and VO2max on bone mineral density is rather low in early postmenopausal osteopenic women. J Musculoskelet Neuronal Interact. 2004;4(3):325–334. [PubMed] [Google Scholar]
- 29.Grimm A, Meyer H, Nickel MD, Nittka M, Raithel E, Chaudry O, Friedberger A, Uder M, Kemmler W, Engelke K, Quick HH. Repeatability of Dixon magnetic resonance imaging and magnetic resonance spectroscopy for quantitative muscle fat assessments in the thigh. J Cachexia Sarcopenia Muscle. 2018;9(6):1093–1100. doi: 10.1002/jcsm.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaudry O, Friedberger A, Grimm A, Uder M, Nagel AM, Kemmler W, et al. Segmentation of the fascia lata and reproducible quantification of intermuscular adipose tissue (IMAT) of the thigh. MAGMA. 2020. 10.1007/s10334-020-00878-w. [DOI] [PMC free article] [PubMed]
- 31.Honaker J, King G, Blackwell M. Amelia II: A program for missing data JSS 2011;45(7):1–47.
- 32.Schafer I, von Leitner EC, Schon G, Koller D, Hansen H, Kolonko T, et al. Multimorbidity patterns in the elderly: a new approach of disease clustering identifies complex interrelations between chronic conditions. PLoS One. 2010;5(12):e15941. doi: 10.1371/journal.pone.0015941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McAuley E, Konopack JF, Motl RW, Rosengren K, Morris KS. Measuring disability and function in older women: psychometric properties of the late-life function and disability instrument. J Gerontol A Biol Sci Med Sci. 2005;60(7):901–909. doi: 10.1093/gerona/60.7.901. [DOI] [PubMed] [Google Scholar]
- 34.Kemmler W, Shojaa M, Kohl M, von Stengel S. Exercise effects on bone mineral density in older men: a systematic review with special emphasis on study interventions. Osteoporosis Int. 2018;29(7):1493–1504. doi: 10.1007/s00198-018-4482-0. [DOI] [PubMed] [Google Scholar]
- 35.Kemmler W, Kohl M, Jakob F, Engelke K, von Stengel S. Effects of high intensity dynamic resistance exercise and whey protein supplements on osteosarcopenia in older men with low bone and muscle mass. Final Results of the Randomized Controlled FrOST Study. Nutrients. 2020;12(8):2341. doi: 10.3390/nu12082341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trappe TA, Lindquist DM, Carrithers JA. Muscle-specific atrophy of the quadriceps femoris with aging. J Appl Physiol. 2001;90(6):2070–2074. doi: 10.1152/jappl.2001.90.6.2070. [DOI] [PubMed] [Google Scholar]
- 37.Nicklas BJ, Chmelo E, Delbono O, Carr JJ, Lyles MF, Marsh AP. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: a randomized controlled trial. Am J Clin Nutr. 2015;101(5):991–9. 10.3945/ajcn.114.105270 The American Journal of Clinical Nutrition. [DOI] [PMC free article] [PubMed]
- 38.Konopka AR, Wolff CA, Suer MK, Harber MP. Relationship between intermuscular adipose tissue infiltration and myostatin before and after aerobic exercise training. Am J Physiol-Reg I. 2018;315(3):R461–R4R8. doi: 10.1152/ajpregu.00030.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ku YH, Han KA, Ahn H, Kwon H, Koo BK, Kim HC, Min KW. Resistance exercise did not alter intramuscular adipose tissue but reduced retinol-binding protein-4 concentration in individuals with type 2 diabetes mellitus. J Int Med Res. 2010;38(3):782–791. doi: 10.1177/147323001003800305. [DOI] [PubMed] [Google Scholar]
- 40.Jacobs JL, Marcus RL, Morrell G, LaStayo P. Resistance exercise with older fallers: its impact on intermuscular adipose tissue. Biomed Res Int. 2014;2014:398960–398967. doi: 10.1155/2014/398960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71(4):885–892. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 42.Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol. 2014;2014:309570–309511. doi: 10.1155/2014/309570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bang E, Tanabe K, Yokoyama N, Chijiki S, Kuno S. Relationship between thigh intermuscular adipose tissue accumulation and number of metabolic syndrome risk factors in middle-aged and older Japanese adults. Exp Gerontol. 2016;79:26–30. doi: 10.1016/j.exger.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Khan IM, Perrard XY, Brunner G, Lui H, Sparks LM, Smith SR, et al. Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration and insulin resistance. Int J Obes. 2015;39(11):1607–1618. doi: 10.1038/ijo.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hogrel JY, Barnouin Y, Azzabou N, Butler-Browne G, Voit T, Moraux A, Leroux G, Behin A, McPhee JS, Carlier PG. NMR imaging estimates of muscle volume and intramuscular fat infiltration in the thigh: variations with muscle, gender, and age. Age (Dordr) 2015;37(3):9798. doi: 10.1007/s11357-015-9798-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Power GA, Allen MD, Booth WJ, Thompson RT, Marsh GD, Rice CL. The influence on sarcopenia of muscle quality and quantity derived from magnetic resonance imaging and neuromuscular properties. Age (Dordr) 2014;36(3):9642. doi: 10.1007/s11357-014-9642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Positano V, Christiansen T, Santarelli MF, Ringgaard S, Landini L, Gastaldelli A. Accurate segmentation of subcutaneous and intermuscular adipose tissue from MR images of the thigh. J Magn Reson Imaging. 2009;29(3):677–684. doi: 10.1002/jmri.21699. [DOI] [PubMed] [Google Scholar]
- 48.Grimm A, Meyer H, Nickel MD, Nittka M, Raithel E, Chaudry O, et al. A Comparison between 6-point Dixon Mri and Mr spectroscopy to quantify muscle fat in the thigh of subjects with sarcopenia. J Frailty Aging. 2019;8(1):21–26. doi: 10.14283/jfa.2018.16. [DOI] [PubMed] [Google Scholar]
- 49.Donini LM, Busetto L, Bauer JM, Bischoff S, Boirie Y, Cederholm T, Cruz-Jentoft AJ, Dicker D, Frühbeck G, Giustina A, Gonzalez MC, Han HS, Heymsfield SB, Higashiguchi T, Laviano A, Lenzi A, Parrinello E, Poggiogalle E, Prado CM, Rodriguez JS, Rolland Y, Santini F, Siervo M, Tecilazich F, Vettor R, Yu J, Zamboni M, Barazzoni R. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin Nutr. 2020;39(8):2368–2388. doi: 10.1016/j.clnu.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 50.Kwak JY, Kwon KS. Pharmacological interventions for treatment of sarcopenia: current status of drug development for sarcopenia. Ann Geriatr Med Res. 2019;23(3):98–104. doi: 10.4235/agmr.19.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bao W, Sun Y, Zhang T, Zou L, Wu X, Wang D, et al. Exercise programs for muscle mass, muscle strength and physical performance in older adults with sarcopenia: a systematic review and meta-analysis. Aging Dis. 2020;11(4):863–873. doi: 10.14336/AD.2019.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reginster JY, Beaudart C, Al-Daghri N, Avouac B, Bauer J, Bere N, et al. Update on the ESCEO recommendation for the conduct of clinical trials for drugs aiming at the treatment of sarcopenia in older adults. Aging Clin Exp Res. 2020. 10.1007/s40520-020-01663-4. [DOI] [PMC free article] [PubMed]