Abstract
Loss of protein homeostasis is a hallmark of the aging process. We and others have previously shown that maintenance of proteostasis is a shared characteristic of slowed-aging models. Rapamycin (Rap) exerts sex-specific effects on murine lifespan, but the combination of Rap with the anti-hyperglycemic drug metformin (Rap + Met) equally increases male and female mouse median lifespan. In the current investigation, we compare the effects of short-term (8 weeks) Rap and Rap + Met treatments on bulk and individual protein synthesis in two key metabolic organs (the liver and skeletal muscle) of young genetically heterogeneous mice using deuterium oxide. We report for the first time distinct effects of Rap and Rap + Met treatments on bulk and individual protein synthesis in young mice. Although there were decreases in protein synthesis as assessed by bulk measurements, individual protein synthesis analyses demonstrate there were nearly as many proteins that increased synthesis as decreased synthesis rates. While we observed the established sex- and tissue-specific effects of Rap on protein synthesis, adding Met yielded more uniform effects between tissue and sex. These data offer mechanistic insight as to how Rap + Met may extend lifespan in both sexes while Rap does not.
Electronic supplementary material
The online version of this article (10.1007/s11357-020-00243-8) contains supplementary material, which is available to authorized users.
Keywords: Proteostasis, Skeletal muscle, Deuterium oxide, Proteomics
Introduction
Protein homeostasis (proteostasis) requires the integration of complex processes to maintain a functional proteome. Loss of cellular proteostatic regulation is a hallmark of aging [23, 33, 58] and associated chronic diseases [25, 38]. This loss of proteostatic regulation leads to the accumulation of damaged proteins, which contributes to cellular/organismal dysfunction. Numerous studies have investigated how protein degradation machinery (e.g., autophagy and/or proteasomal degradation) contributes to proteostasis [55], but the importance of protein synthesis as a mechanism of proteostatic regulation is often overlooked.
Rapamycin (Rap) treatment extends murine lifespan in a sex- and dose-dependent manner [33]. Specifically, Rap treatment improves the median lifespan more in females, though this might be due to higher circulating Rap levels in females when administered in the diet [31, 33]. Both short- and long-term Rap treatments exert beneficial effects on age-related tissue and organismal function. Rap treatment slows the age-related decline in skeletal muscle function [2, 48], reverses age-related cardiovascular deficits [7], and improves cognitive function [27]. Because Rap treatment can induce hepatic insulin resistance [26], the NIA Interventions Testing Program (ITP) added co-treatment with metformin (Met) to improve glucose regulation [44] for potential additional improvements in lifespan. This combination (Rap + Met) increased median lifespan (23% for both sexes; [54]), more than Rap alone (13% for males and 21% for females; R. A. [31, 58]), although no direct statistical comparisons could be made between the Rap + Met–treated and Rap-treated animals [54]. Moreover, Met treatment alone did not improve the median or maximal lifespan of genetically heterogeneous mice [54].
Skeletal muscle and liver are critically important to healthy aging. Skeletal muscle is the largest organ in the body, contributing to locomotion as well as playing an integral role in systemic metabolic health by regulating energy balance [60], insulin sensitivity [9], and immune function [45] to name a few. Similarly, the liver contributes to systemic metabolism [51] and insulin sensitivity [36] to maintain organismal function. Thus, age-related decrements in muscle or liver function negatively impact health.
Few studies have investigated the mechanisms accounting for differential effects of Rap and Rap + Met treatments on lifespan [49, 57, 61]. Weiss et al. [57] demonstrated that the addition Met to Rap abolished the glucose intolerance found in HET3 mice that were treated with Rap only, which indicates a differential treatment effect to the liver, muscle, or both. We previously reported that both treatments decrease protein synthesis in cultured C2C12 skeletal muscle myotubes [61], whereas Rap + Met, but not Rap, treatment decreases mitochondrial protein synthesis rates in brain tissue from female mice [49]. When accounting for the independent effect of cell proliferation, Rap increased mitochondrial protein synthesis in C2C12 myotubes, but Rap + Met did not [61]. These data suggest that Rap and Rap + Met treatments may not always affect the maintenance of proteostasis in a similar manner and outcomes may be tissue-specific.
With mTOR inhibition, there is a selective translation of some transcripts, such as those for mitochondrial proteins, while others are transcriptionally repressed [7, 22, 64]. Data from our laboratory show that 12 weeks of Rap treatment maintained mitochondrial protein synthesis in the skeletal muscle when protein synthesis rates in other tissue fractions (e.g., cytosolic) were reduced [10], which was consistent with our findings in vitro [61]. In studies of cardiac muscle from the Rabinovitch lab, 10 weeks of Rap treatment increased the average half-life of individual mitochondrial proteins, implying that both protein synthesis and protein breakdown were slowed [4, 7, 22]. Importantly, although these investigations report increased protein half-life, nearly half of the mitochondrial proteins reported in those investigations had a decreased half-life (i.e., increased synthesis). Therefore, assessing both individual and bulk protein synthesis rates can provide more complete insight into the specific effects of treatments on proteostatic maintenance.
Here, we examine how protein synthesis affects proteome remodeling, and thus proteostasis, during Rap or Rap + Met treatment in liver and skeletal muscle. We compared the effects of Rap versus Rap + Met treatments on bulk and individual protein synthesis in the liver and skeletal muscle of young male and female mice. We hypothesized that (1) the addition of Met to Rap would change the synthesis rates of individual proteins in both liver and skeletal muscle compared with Rap treatment alone, (2) there would be sex-specific effects of Rap treatment, but not Rap + Met treatment, on individual or bulk protein synthesis rates in both liver and skeletal muscle, and (3) there would be a selective translation of mitochondrial proteins with Rap that is suppressed with the addition of Met.
Methods
Animals and experimental design
All procedures and conditions for the housing and care of the animals were approved by the Animal Care and Use Committee at the University of Michigan. Ninety (45 male and 45 female), 4-month UM-HET3 mice were housed on a 12-h light-dark cycle. The UM-HET3 genetically heterogeneous mice were crossbred from two different F1 strains: (BALB/cByJ x C57BL/6J) F1 females (JAX stock 10,009) and (C3H/HeJ x DBA/2J) F1 males (JAX stock 10,004), as previously described [17, 49]. Thirty mice were randomized into control diet (Purina 5LG6, CON), rapamycin encapsulated into control diet (14 ppm (ppm)) or metformin (Met) + Rap encapsulated into control diet (1000 ppm Met and 14 ppm Rap), as in the Interventions Testing Program [54]. Rap and Rap + Met groups were treated for 8 weeks (56 days). To measure protein synthesis over time, and to reduce the bias of highly abundant, rapidly synthesized proteins [34], mice were randomly assigned to a time course of labeling including 1, 4, 8, 15, and 32 days of D2O across the 3 treatments (Fig. 1(A)). All mice received exactly 8 weeks of treatment and labeling was started such that all mice were euthanized at the same age. At the indicated D2O time point, mice were given an initial i.p. injection of 99% D2O, followed by 8% in the animals drinking water until euthanasia. Animals were euthanized using carbon dioxide following a 12 h fast. At each time point, 6 mice (3 male and 3 female) were sacrificed from each treatment (Con, Rap, Rap + Met) group. We collected the liver and gastrocnemius from each animal to assess isotope incorporation into proteins and plasma assess precursor enrichment (described in detail below).
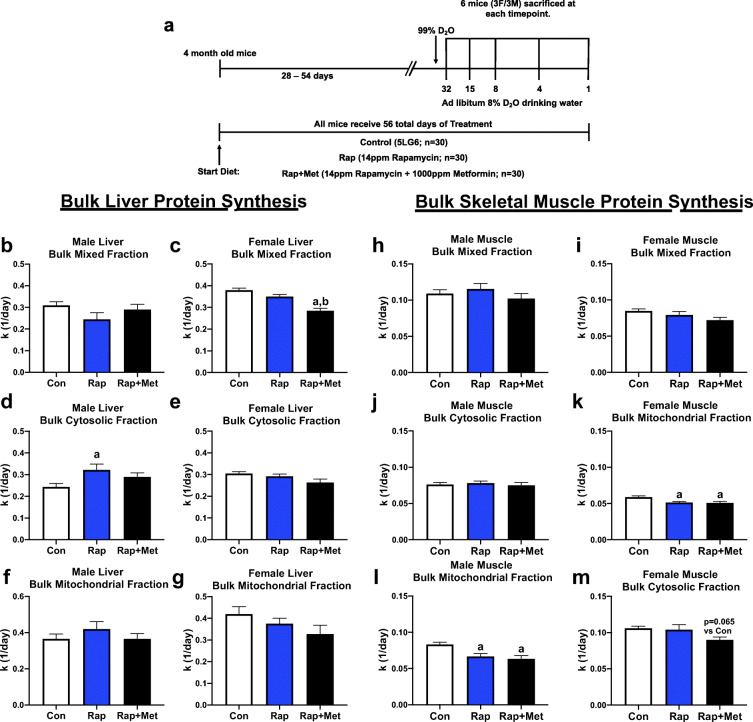
Fig. 1.
Experimental design and effects of Rap and Rap + Met treatments on bulk protein turnover. (A) Experimental design, young (4 months old) male and female UM-HET3 mice underwent 56 total days of the experimental treatment. Effects of Con, Rap, and Rap + Met treatments on liver bulk protein synthesis rates in mixed (B and C), cytosolic (D and E), and mitochondrial (F and G) fractions of male and female mice. Effects of Con, Rap, and Rap + Met treatments on skeletal muscle bulk protein synthesis rates in mixed (H and I), cytosolic (J and K), and mitochondrial (L and M) fractions of male and female mice a = different than Con, b = different than Rap, P < 0.05
D2O and treatment time course
After 24 days of treatment, the first subset of mice received an intraperitoneal injection of 99% deuterium oxide (D2O) and subsequently had access to drinking water enriched with 8% D2O. Those mice continued treatment for another 32 days (32d) at which point they were sacrificed. At 41 days of treatment, a second subset of mice received a 99% D2O injection with subsequent access to drinking water enriched with 8% D2O, and those mice were sacrificed after 15 days of labeling (15d). At 48 days of treatment, a third subset of mice received a 99% D2O injection with subsequent access to drinking water enriched with 8% D2O, and those mice were sacrificed after 8 days of labeling (8d). At 52 days of treatment, a fourth subset of mice received a 99% D2O injection with subsequent access to drinking water enriched with 8% D2O, and those mice were sacrificed after 4 days of labeling (4d). At 55 days of treatment, a final subset of mice received a 99% D2O injection with subsequent access to drinking water enriched with 8% D2O, and those mice were sacrificed after 1 day of labeling (1d). Therefore, all mice received 8 full weeks of treatment and were sacrificed at the same age.
Assessments of subcellular fraction protein synthesis
Excised skeletal muscle and liver samples were snap-frozen in liquid nitrogen and subsequently powdered in liquid nitrogen. Powdered samples were homogenized using zirconium oxide beads in a bullet blender with mitochondrial isolation buffer (100 mM KCl, 40 mM Tris HCl, 10 mM Tris Base, 5 mM MgCl2, 1 mM EDTA, 1 mM ATP, pH = 7.5) as previously described in detail [3, 10, 49]. Following subcellular protein fraction isolation, hydrolyzed subcellular fraction aliquots underwent cation exchange chromatography prior to derivatization. Dried derivatives were resuspended in 700 μl of ethyl acetate and analyzed using a 7890A gas chromatograph coupled to a 5975C mass spectrometer with a DB-5MS GC column (30 m × 0.25 mm × 0.25 μm; all from Agilent).
Measurement of deuterium enrichment in body water
Deuterium enrichment of the total body water pool was used to estimate the alanine precursor enrichment based upon mass isotopomer distribution analysis [18]. Body water enrichment was measured in 125 μl of evaporated plasma samples from each animal as previously described [10, 49]. Briefly, plasma samples were placed onto inverted plastic microcentrifuge caps with a rubber o-ring. Tubes were then sealed and placed onto a heat block at 80 °C overnight. Evaporated water from the plasma was captured at the top of the tubes. The evaporated water then underwent proton exchange with 10 M NaOH and acetone overnight. Finally, proton exchanged samples were extracted in hexanes into anhydrous sodium sulfate before being transferred into chromatography vials for analysis on a 7890A gas chromatograph coupled to a 5975C mass spectrometer using a DB-17 Column (Agilent, Santa Clara, CA).
Protein synthesis calculations
Bulk protein synthesis of subcellular fractions was calculated based on the precursor-product relationship. Briefly, protein synthesis was calculated from the precursor enrichment that was determined from the deuterium enrichment in the body water pool and then adjusted using mass isotopomer distribution analysis [18]. The fraction of new proteins for each time point (1, 4, 8, 15, or 32 days) was plotted as a function of time.
Kinetic and quantitative proteomics sample prep
Samples were prepared for protein mass spectrometry using modified filter-aided sample preparation [59] with modifications as we described previously [29]. Briefly, approximately 25 mg of liver or gastrocnemius muscle samples were pulverized under liquid nitrogen. Powdered samples were homogenized using zirconium oxide beads in a bullet blender with mitochondrial isolation buffer (100 mM KCl, 40 mM Tris HCl, 10 mM Tris Base, 5 mM MgCl2, 1 mM EDTA, 1 mM ATP, pH = 7.5) as previously described in detail [3, 10, 49]. Differential centrifugation was then performed to obtain a cytosolic-/mitochondrial-enriched protein pool for liver homogenates and mitochondrial- and cytosolic-enriched protein pool for muscle as previously described by us [3, 10, 49]. The pellets were solubilized in 4% SDS, and then 50 mg, determined by a BCA assay (Pierce, Rockford, IL), was added to a new tube containing 100 mM Tris HCl, pH 8.5. Samples were then reduced within 5 mM dithiothreitol (DTT) for 15 min at 55 °C, then alkylated with 15 mM iodoacetamide for 1 h at room temperature. The entire sample was then transferred to a spin filter (VWR no. 29300-622, Radnor, PA, USA) and spun at 14,000g for 10 min at room temperature. Samples were then denatured and washed (3 times) with 100 μl 8 M urea at 14,000g for 6 min per spin. Next, samples were washed (3 times) with 100 μl of 25 mM ammonium bicarbonate and spun at 14,000g for 6 min. The collection tube filtrant was discarded as needed and thoroughly rinsed in Milli-Q water following the final spin. A 1:50 (2 ml of 0.5 mg/ml) dilution of MS grade trypsin (Promega) was added to each spin filter with an additional 50 ml of 25 mM ammonium bicarbonate added to aid in moisture retention. Samples were then incubated at 37 °C with gentle shaking (300 rpm) overnight. Trypsin digestion was quenched by adding phenylmethanesulfonyl fluoride (1 mM final concentration). Spin filters were then centrifuged at 14,000g for 30 min at room temperature. An additional elution was performed with 100 ml of 25 mM ammonium bicarbonate and repeating the 14,000g for 30 min spin. The total resulting filtrant was transferred to 11 mm plastic snap top autosampler vials (Thermo Fisher Scientific no. C4011-13, Waltham, MA, USA) and capped (Thermo Fisher Scientific no. C4011-55R) until further LC-MS/MS analysis.
LC-MS data acquisition
Mass spectrometry data were collected using an EASY-nLC 1200 liquid chromatography (LC) pump (Thermo Fisher Scientific) coupled to an Orbitrap Fusion Lumos mass spectrometer (Thermo Fisher Scientific). A capillary RSLC column (EASY-spray column pepMap ® RSLC, C18, 2 μm, 100 Å, 75 μm × 15 cm) was used for separation of peptides. The mobile phase was comprised of buffer A (0.1% formic acid in optima water) and buffer B (optima water and 0.1% formic acid in 80% acetonitrile). The peptides were eluted at 300 nl/min with the following gradients over 2 h: 3–25% B for 80 min; 25–35% B for 20 min; 35–45% B for 8 min; 45–95% B for 2 min; and 95% for 8 min. Peptide sequence data were acquired using the top speed method (3 s cycle). A full scan MS at a resolution of 120,000 at 200 m/z mass was acquired in the Orbitrap with a target value of 4e5 and a maximum injection time of 60 ms. Peptides with charge states of 2–4 were selected from the top abundant peaks by the quadrupole for high-energy collisional dissociation (HCD with normalized energy 29) MS/MS, and the fragment ions were detected in the linear ion trap with target AGC value of 1e4 and a maximum injection time of 250 ms. The dynamic exclusion time was set at 40 s. Precursor ions with ambiguous charge states were not fragmented.
Kinetics data acquisitions were performed in MS-only mode and collected at 60,000 m/z resolution. These settings increase the signal intensity, improve signal-to-noise, and give more scan points per elution chromatogram, greatly enhancing kinetic analysis accuracy [40]. Raw kinetic proteomic data is included for the liver (Table S1) and muscle (Table S2).
Peptide identification
PEAKS Studio software (version 8.5) was used for de novo sequencing and database searching to identify proteins in our raw MS data as well as to quantify, filter (quality control), and normalize our quantitation data for each protein [63]. Peptides were identified from MS/MS spectra by searching against the Swiss-Prot mouse (downloaded January 2018) with a reverse sequence decoy database concatenated. Variables for the search were as follows: enzyme was set as trypsin with one missed cleavage site. Carbamidomethylation of cysteine was set as a fixed modification while N-terminal acetylation and methionine oxidation were set as variable modifications. A false-positive rate of 0.01 was required for peptides and proteins. The minimum length of the peptide was set to 7 amino acids. At least 2 peptides were required for protein identification. The precursor mass error of 20 ppm was set for the precursor mass, and the mass error was set as 0.3 Da for the MSMS. Label-free quantitation was enabled with MS1 tolerance ± 20 ppm and a MS2 tolerance ± 50 ppm, and carbamidomethylation of cysteine was set as a fixed modification, while N-terminal acetylation and methionine oxidation were set as variable modifications. Peptide assignments with a false discovery rate of less than 1% were included in comparative quantitative analyses and used to generate protein identification files for the quantitative and kinetic analyses.
Quantitative proteomics data analysis
Protein quantitation was done on the cytosolic/mitochondrial liver protein pool only (i.e., not muscle) using label-free quantitation (LFQ) in the PEAKS software package (Version 8.5). To test our hypotheses that mitochondrial proteins would be preferentially synthesized in the Rap and Rap + Met–treated animals compared with that in the control, we employed differential centrifugation to enrich muscle and liver homogenates in mitochondrial proteins. For the liver, we performed a low-speed spin and only analyzed the mixed protein supernatant which contains both cytosolic and mitochondrial proteins. Thus, we had high confidence in LFQ of the liver supernatant because no further processing of the supernatant was done and all proteins in the supernatant were assumed to be the same across samples. For the muscle, we performed the same low-speed spin, but then further processed the mixed protein supernatant to enrich in either a cytosolic or mitochondrial protein pool. Because of the further processing, we did not have enough confidence to perform LFQ because we could not accurately determine how many proteins were separated into either the cytosolic- or mitochondrial-enriched fractions. For the liver cytosolic-/mitochondrial-enriched protein samples, after the removal of contaminants, low scoring peptides, and reverse matches, the LFQ values for the top three peptides were summed for each protein. The biological replicates were grouped by gender and treatment. Only proteins with at least three valid LFQ values in each group were used for quantification. Individual protein quantitation data is available for the liver (Table S1).
Kinetic proteomics data analysis
MS-only isotope distribution data was analyzed on the mixed liver-enriched protein pool, and the cytosolic and mitochondrial muscle-enriched protein pools as previously described [40]. Neutromer [53] intensities and spacing values for each peptide were extracted from the RAW data files based on peptide identification from MSMS acquisition using m/z (± 12 ppm) and retention time alignment (± 0.8 min). Briefly, neutromer peaks M0–M4 were normalized against the sum of the signal intensity, then compared with theoretical calculations based on percentage D2O enrichment to determine fraction deuterium-enriched (new) peptide [29, 40]. Theoretical changes were calculated using the eMASS algorithm [50] and based on the number of possible deuterium incorporation sites per amino acid [40]. The theoretical changes in abundance of each neutromer peak M0–M4 were compared against experimental changes at each time point in order to determine a time-dependent percentage of newly synthesized peptide reported for each isotope peak. Thus, for each peptide, there were up to 9 semi-independent measurements of the peptide turnover (measuring M0–M4 results in 5 intensity and 4 spacing metrics), as previously described [29, 40]. We used the standard deviation between these measurements as a metric of the measurement precision for that peptide. If peptide precision was low (i.e., standard deviation exceeded 0.1), the data point was removed from the downstream analysis [29]. Additional filters were also applied to remove peptides with total relevant intensity below 20,000 counts and a retention time deviation greater than 0.5 min.
The median percent new peptide was calculated at each point and outliers (defined as greater than 1.4 the median absolute standard deviation) were removed from the calculation of the protein percent new. All peptide measurements for an individual time point that passed these filters were weighted equally in the calculation of the percent new protein at that time point. As described previously, the fraction new measurement of the above-mentioned peptides was combined and fit using a non-linear least squares regression based on first-order rate kinetic equations [29, 40]. The proteins with high precision data for at least two peptides at 3 or more time points were fit according to first-order rate kinetics. We required 3 or more labeled time points in order to increase the confidence of the rate constant. For the regression fit, time point zero was set to 0% new and was given a standard deviation of 0.05 based on the accuracy during the long-term performance of the instrument. A coefficient of variation (CV) was calculated to assess analytical and biological variability for each protein as the ratio of standard deviation reported for the regression over the rate constant [46]. Proteins that had a CV of more than 20% for the fit in either Con, Rap, or Rap + Met were removed from the data set.
Calculation of absolute synthesis rate
In the kinetic labeling experiment, we calculated the turnover rate constant (k) from the change in f over time using the relationship f = 1 − e(−kt). We calculated the absolute synthesis rates of each specific protein pool. Protein absolute synthesis was calculated as the turnover rate constant (k) multiplied by the protein pool size (V): Flux = kV. For this calculation, we assumed steady state in protein concentrations in these 4-month-old mice during the ~ 1-month labeling period (i.e., although V might be different between Con, Rap, and Rap + Met, we assumed it to be constant over the duration of the experiment). For our calculations, we normalized all concentration measurements to the Con pool size. This normalization resulted in a unitless (relative mass per day) synthesis rate that was directly comparable between experimental groups, as described previously by us [46].
Proteomic cellular compartment and pathway analysis
Proteomics data from the mixed liver-enriched protein pool, and the cytosolic and mitochondrial muscle–enriched protein pools were analyzed for protein distribution into subcellular components using the Database for Annotation, Visualization and Integrated Discovery (DAVID), v6.8, from the National Institute of Allergy and Infectious Diseases at the National Institutes of Health [19].
Analysis and statistics
GraphPad Prism 8.3 was used to complete statistical analyses for this study. To assess the increase in the fraction of new proteins over time, we applied a one-phase association non-linear regression model to the data. The slope of the lines represents the protein synthesis rate constant (k; 1/day). Differences in bulk protein synthesis were assessed by two-way analysis of variance (ANOVA) tests with a Tukey post hoc test. For kinetic and quantitative proteomics analyses, sex was treated independently due to the large variances in proteins identified between the sexes (Fig. S1a–c), so only comparisons within a sex were made. For proteomics analyses, either a one-way ANOVA for Con, Rap, or Rap + Met comparisons or multiple t tests for Rap and Rap + Met were used with a two-stage step-up method of Benjamini, Krieger, and Yekutieli for multiple comparisons to control for the false discovery rate. Canonical pathway analyses were performed using Python and basic data processing [30, 56]. An F statistic was computed for the protein synthesis (k values) data to identify proteins with any significant alterations between sexes and treatments. This was followed by paired post hoc tests to identify between-group differences. Proteins with significant differences between pairs were then used to compute activation scores for downstream pathways (Ingenuity Pathway Analysis, Qiagen, Inc.). The pathways with the greatest difference in activation between groups were plotted, with pathway activation Z-scores hierarchically clustered to identify similar patterns. Significance was accepted as P < 0.05 for these experiments. All data are presented as (mean ± SEM).
Results
Bulk protein synthesis in the liver and skeletal muscle
To compare the effects of Rap and Rap + Met over 8 weeks of treatment (Fig. 1(A)), we measured bulk protein synthesis rates in the liver separated into cytosolic protein, mixed (i.e., structural and nuclear) protein, and mitochondrial-enriched protein fractions. In males, Rap treatment had no effect on mixed or mitochondrial protein synthesis (Fig. 1(B, F)), although Rap-treated animals had greater cytosolic protein synthesis rates than Con (Fig. 1(D)). In contrast, the liver from female mice had lower rates of mixed protein synthesis in Rap + Met–treated compared with both Rap and Con (Fig. 1(C)), with no differences in bulk cytosolic or mitochondrial fractions (Fig. 1(E, G)).
In skeletal muscle, Rap and Rap + Met treatments did not change bulk protein synthesis rates in either the mixed or cytosolic fractions of the gastrocnemius from male or female mice compared with Con (Fig. 1(H–K)). However, both Rap and Rap + Met treatments had significantly lower bulk mitochondrial protein synthesis compared with Con in both male and female mice (Fig. 1(L, M)).
After 8 weeks of treatment, Rap and Rap + Met treatments resulted in a lower RPS6 phosphorylation in the livers of female mice compared with Con (Fig. 2(A)), while only Rap + Met had lower 4EBP1 phosphorylation compared with Con (Fig. 2(B)). Rap- and Rap + Met–treated female mice had lower ACC phosphorylation compared with Con (Fig. 2(C)), whereas Rap + Met–treated male mice displayed a greater ACC phosphorylation compared with Rap and Con (Fig. 2(C)). In skeletal muscle, there were minimal differences of Rap and Rap + Met treatments on mTOR and AMPK signaling targets, although Rap + Met decreased ACC phosphorylation in female mice compared with Rap and Con animals (Fig. 2(E–H)).
Fig. 2.
Tissue-, sex-, and treatment-specific effects of Rap and Rap + Met Treatments on AMPK/mTORC1 signaling. Representative western blot images and quantification of phosphorylated (p) to total (t) protein densities for RPS6, 4EBP1, ACC, and AMPK in the muscle (A–D) and liver (E–H), respectively, from Con, Rap, and Rap + Met treatments in both male and females. a = different than Con, b = different than Rap, P < 0.05
Individual protein synthesis and content in the liver
For individual cytosolic/mitochondrial protein synthesis analyses in the liver, we identified 694 and 774 proteins in males and females (Fig. S2a), respectively, that were present across all 3 treatments and met our inclusion criteria of a coefficient of variation (CV) less than 20%. There was a large difference in the proteins identified across the three treatments in the liver for males and females (Fig. S2a). Therefore, in order to present a like-to-like comparison of averaged rates of individual proteins, we only used proteins that were present across every treatment. Distribution analysis of the change in individual protein synthesis rates compared with Con during Rap and Rap + Met treatments showed hundreds of proteins with faster or slower synthesis rates (Fig. 3(A, B)). In the liver of Rap-treated male mice, 128 proteins exhibited slower and 457 proteins exhibited faster synthesis rates compared with Con. In Rap + Met–treated male mice, 199 proteins exhibited slower synthesis rates and 378 proteins exhibited faster synthesis rates compared with Con. In Rap-treated female mice, 218 proteins exhibited slower and 211 proteins exhibited faster synthesis rates compared with Con. In Rap + Met–treated females, 291 proteins exhibited slower and 160 proteins exhibited faster synthesis rates compared with Con. These results demonstrate the variation in responses to Rap and Rap + Met treatments within protein fractions and between individual proteins (Fig. 3(A, B)).
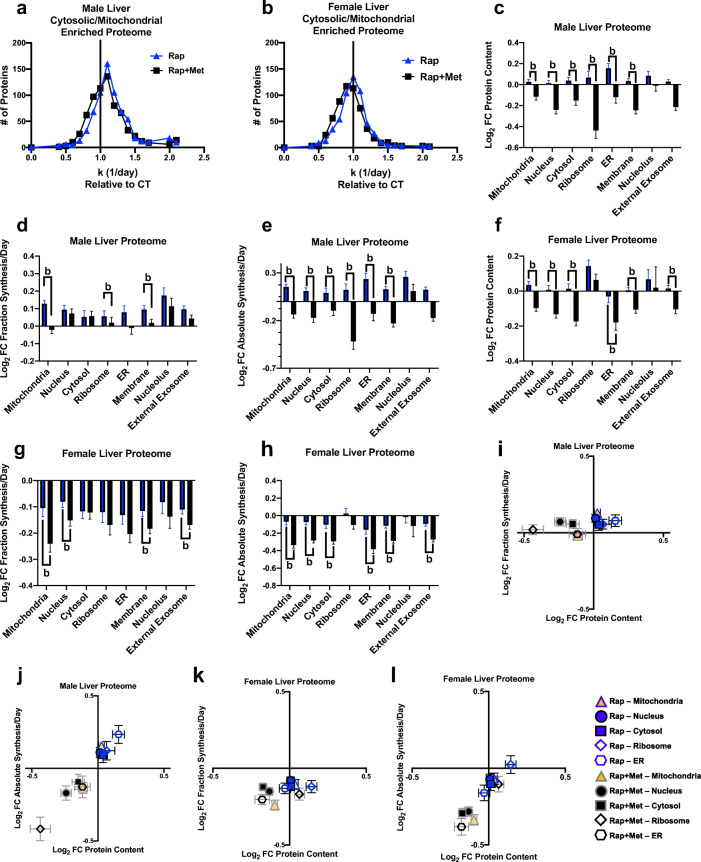
Fig. 3.
Distinct effects of Rap and Rap + Met treatments on liver proteome remodeling. Frequency distribution of individual liver protein synthesis rates relative to control for male (A) and female (B) mice. Effects of Rap and Rap + Met treatments on log2-fold change (FC) from control in protein content (C and F), fractional protein synthesis (D and G), and absolute protein synthesis (E and H) in specific cellular compartments from male (C, D, and E, respectively) and female (F, G, and H, respectively) liver cytosolic-/mitochondrial-enriched proteome. Also, plots of Rap and Rap + Met treatments on log2-fold change (FC) from control in fractional protein synthesis rates (I and K) or absolute synthesis rates (J and L) by the log2-fold change (FC) from control in abundance of specific cellular compartments of the liver proteome in males (I and J) and females (K and L). b = significantly different than Rap, P < 0.05
Next, we grouped proteins by subcellular compartment from GO terms (DAVID, v6.8; [19] to compare protein synthesis rates (Fig. S3) and fold change compared with Con for protein content, fractional synthesis (fraction of the total proteins), and absolute synthesis (content multiplied by fractional synthesis). In the liver of males, Rap + Met treatment had lower protein content in mitochondrial, nuclear, cytosolic, ribosomal, endoplasmic reticulum (ER), and membrane compartments (Fig. 3(C)) compared with Rap, which was also true for absolute synthesis rates (Fig. 3(E)). Compared with Rap, Rap + Met treatment in females had a lower protein content of mitochondrial, nuclear, cytosolic, ER, membrane, and external exosome compartments (Fig. 3(F)), which was also the case for absolute synthesis rates (Fig. 3(H)). When comparing Rap + Met to Rap, male mice had slower protein synthesis (k, 1/day) in mitochondria, ribosome, and membrane compartments (Fig. 3(D)), while female mice had slower rates in mitochondria, nuclear, membrane, and external exosome compartments (Fig. 3(G)). Of note is the interesting response of ribosomal proteins compared with the other subcellular protein groups.
In Fig. 3(I–L), we show the mean log2-fold change in content (y-axis) and fractional or absolute synthesis (x-axis) for males and females (Fig. 3(I–L)). These figures demonstrate how the rates of protein synthesis influence changes in content for specific subcellular compartments; upper right quadrant—higher protein synthesis and greater content; lower right quadrant—lower protein content despite elevated synthesis (i.e., increased breakdown); lower left quadrant—lower protein content and reduced synthesis rates; and upper left quadrant—greater protein content despite lower synthesis (i.e., lowered breakdown). When looking at absolute rates (Fig. 3(J, L)), there are clear differences between the sexes in how the liver proteome is maintained. Furthermore, there are differences in Rap versus Rap + Met, although these differences were similar between the sexes.
We have previously reported that maintenance of mitochondrial proteostasis is a shared characteristic of slowed-aging models [16]; thus, we examined the liver mitochondrial proteome (Fig. 4(A–F)). When compared with Con for absolute synthesis rates, there were differences between Rap and Rap + Met, with Rap + Met always lower (Fig. 4(E, F)). The Rap and Rap + Met treatment effects were largely confined to respiratory complexes I, IV, and V (Figs. S3 and S4). The pattern of the changes was also different between sexes where males had positive changes with Rap that were not apparent in females (Fig. 4(E, F)).
Fig. 4.
Sex- and treatment-specific effects of Rap and Rap + Met treatments on mitochondrial proteome turnover in the liver. Effects of Rap and Rap + Met treatments on log2-fold change (FC) from control in protein content (A and B), fractional protein synthesis (C and D), and absolute protein synthesis (E and F) in specific mitochondrial components from male (A, C, and E, respectively) and female (B, D, and F, respectively). Sex-independent top significantly upregulated or downregulated enriched canonical pathways of individual proteins with significantly different synthesis rates from Con in Rap, Rap + Met, or Rap + Met versus Rap in the liver (G). b = significantly different than Rap, P < 0.05
To examine relationships between the proteins that had changed protein synthesis rates with treatment, but independent of sex, we performed pathway analyses (IPA, Qiagen, Germantown, MD) and present the top 10 pathways most altered in response to Rap and Rap + Met treatments compared with Con (Log2-FC) (Fig. 4(G)). These analyses show that the synthesis rates of proteins for gluconeogenesis, glycolysis, and PKA signaling were higher following Rap treatment compared with Con. On the other hand, following Rap + Met treatment, there were lower synthesis rates of proteins for triacylglycerol degradation, PPARα activation, and glycine betaine degradation. When comparing Rap + Met to Rap, proteins associated with EIF2 signaling and gluconeogenesis had lower synthesis rates following Rap + Met treatment, and proteins associated with NRF2 response had higher synthesis rates following Rap + Met treatment compared with Rap.
Effects of Rap and Rap + Met treatments on protein synthesis in skeletal muscle
In a tissue fraction enriched for muscle mitochondrial (Fig. S2b) and cytosolic (Fig. S2c) proteins, we identified 764 and 697 proteins, in males and females, respectively, that were identified in all 3 treatments and met our inclusion criteria of a CV less than 20%. The distribution of proteins with faster and slower synthesis rates compared with Con was leftward shifted with Rap + Met in both sexes, whereas Rap had a more even distribution (Fig. 5(A, B)). In skeletal muscle of female mice, the synthesis rates of 153 proteins were slower following Rap treatment, and the synthesis rates of 139 proteins were higher following Rap treatment compared with Con. Following Rap + Met treatment, 240 proteins had lower synthesis rates and 53 proteins had higher synthesis rates compared with Con. In skeletal muscle of male mice, following Rap treatment, 159 proteins had lower synthesis rates compared with Con and 163 proteins had higher protein synthesis rates compared with Con. Following Rap + Met treatment, 258 proteins had lower synthesis rates than Con, and 63 proteins had higher synthesis rates compared with Con (Fig. 5(A, B)).
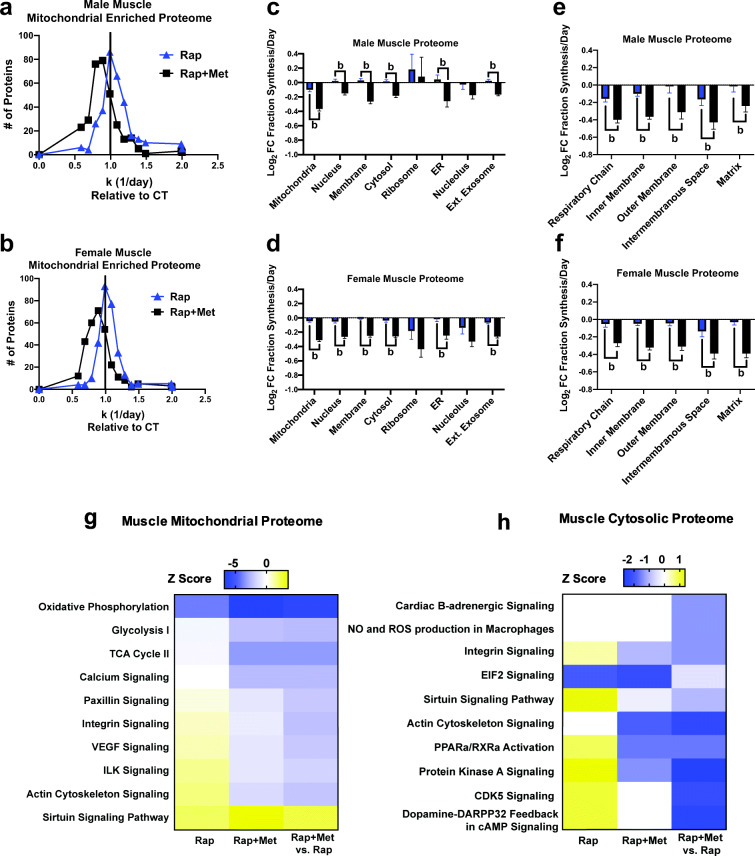
Fig. 5.
Distinct effects of Rap and Rap + Met treatments on skeletal muscle proteome remodeling. Frequency distribution of individual skeletal muscle protein synthesis rates relative to control for male (A) and female (B) mice. Effects of Rap and Rap + Met treatments on log2-fold change (FC) from control in fractional protein synthesis in specific cellular compartments from male (C) and female (D) skeletal muscle proteome. Effects of Rap and Rap + Met treatments on log2-fold change (FC) from control in fractional protein synthesis in mitochondrial compartments from male (E) and female (F) skeletal muscle proteome. Also, the sex-independent top significantly upregulated or downregulated enriched canonical pathways of individual proteins with significantly different synthesis rates from control in Rap, Rap + Met, and Rap + Met versus Rap from muscle mitochondrial (G) or cytosolic (H) proteome. a = different than Con, b = different than Rap, P < 0.05
We then examined skeletal muscle for the effect of Rap and Rap + Met treatments on protein synthesis rates compared with Con when proteins were grouped by subcellular compartments. There was a pattern in which the response in Rap + Met was significantly different from Rap (Fig. 5(C, D)), with the exception of ribosomal and nucleolus proteins. This finding was similar between both sexes. We further compared the mitochondrial proteome and again, the response of Rap + Met compared with Con was significantly different than Rap compared with Con (Fig. 5(E, F)). We also found that Rap + Met treatment had significantly lower average individual protein synthesis rates of skeletal muscle mitochondrial complexes I, IV, and V compared with Rap in both males and females (Figs. S4 and S5). We then identified the 10 pathways most altered in response to Rap and Rap + Met treatment in the mitochondrial- and cytosolic-enriched proteomes (Fig. 5(G, H)). Similar to that in the liver, Rap-treated animals had higher synthesis rates of proteins associated with PKA signaling in the muscle cytosol but also lower synthesis rates of proteins associated with EIF2 signaling. The higher synthesis rate of PKA proteins following Rap treatment was not present and was lower following Rap + Met treatment. In the mitochondria, Rap-treated animals had lower synthesis rates of oxidative phosphorylation proteins and higher synthesis rates of sirtuin signaling proteins. Rap + Met also leads to higher protein synthesis rates for sirtuin signaling and lowered synthesis rates of oxidative phosphorylation, TCA cycle, and calcium signaling proteins.
Effects of Rap and Rap + Met treatments on mitochondrial translation
As we hypothesized that mitochondrial proteins would be selectively translated, we analyzed the synthesis rates of mitochondrial proteins grouped by GO terms across treatments. In the liver, we identified 336 and 309 GO-identified mitochondrial proteins from females and males, respectively (Fig. S6a). We further separated these proteins by the number of mitochondrial proteins that did not change from Con (0.95–1.05), the number of protein with at least a 5% faster synthesis rate than Con (i.e., FC from Con > 1.05), and the number of proteins that were at least 5% slower (< 0.95) in Rap and Rap + Met (Fig. 6(A)). Compared with Con, the liver of male mice had a greater number of mitochondrial proteins with faster synthesis rates than slower synthesis rates (Fig. 6(A)). This greater number of mitochondrial proteins with faster synthesis rates was diminished in female mice, with Rap + Met, and in skeletal muscle. Interestingly, in skeletal muscle, the pattern was quite different when compared with the liver and between the sexes (Fig. 6(B)). Biological processes pathway analyses (DAVID) of the 5+% upregulated mitochondrial proteins revealed that in both male and female liver samples, Rap treatment had a greater number and more significantly enriched pathways related to mitochondria compared with Rap + Met (Table S3). Similar to the liver, muscle of Rap-treated males and females exhibited a greater number and more significantly enriched pathways related to mitochondria than Rap + Met (Table S3).
Fig. 6.

Selective translation of mitochondrial proteins is greater in Rap compared with Rap + Met treatment in liver and skeletal muscle. Rap and Rap + Met treatment effects on the fold change (FC) from control changes in synthesis rates of mitochondrial GO-identified proteins in the liver (a) and muscle (b) from both sexes
Discussion
This study is the first to compare sex- and tissue-specific changes in protein synthesis from Con with Rap and Rap + Met treatments. This study expands on our studies of bulk protein synthesis [10, 49, 61] and those done by others with Rap alone [4, 7, 22, 48] to understand protein synthesis as a proteostatic mechanism. The main findings of the study are that when not accounting for changes in cellular proliferation: (1) even when there is a lowering of bulk protein synthesis in some subcellular protein fractions with Rap or Rap + Met, there are many individual proteins synthesized at faster rates, (2) when compared with Con, Rap and Rap + Met changed protein synthesis rates differently with a greater suppression of some individual proteins with Rap + Met, (3) the addition of Met minimized the differences in protein synthesis rates between sexes, and (4) like sex, differences between tissues were more apparent with Rap than Rap + Met.
Treatment-specific effects on proteome remodeling as a potential mediator of lifespan
The addition of Met to Rap eliminates sex differences in lifespan extension and may increase median lifespan when compared with Rap alone [54]. In the current study, we looked at differences in individual protein synthesis rates between the two treatments, without considering how changes in cell proliferation rates affected these rates. We know from our previous studies that cell proliferation is slowed with Rap and Rap + Met in the liver, heart, skeletal muscle, and cultured skeletal myotubes [10, 61], and decreased proliferation by itself decreases protein synthesis rates [32]. However, it is technically challenging to account for the independent effect of cell proliferation on protein synthesis rates of individual proteins. Given our previous findings showing decreased cell proliferation, it was not surprising to us that bulk measurements of protein synthesis with Rap and Rap + Met showed no difference or reduced synthesis rates across the hepatic and skeletal muscle proteomes. The differences between Rap and Rap + Met from Con in subcellular protein synthesis rates between tissues did not have a clear pattern. What was more enlightening was the number of individual proteins that increased synthesis rates compared with Con (Fig. 3(A, B) and Fig. 5(A, B)). These data indicate that although Rap and Rap + Met are mTOR inhibitors, and thus expected to slow the cell cycle and diminish global protein synthesis, there are still proteins in both tissues that have increased protein synthesis rates because they either escape global suppression or are selectively translated.
The rationale of the ITP to add Met to Rap alone was to correct the glucose intolerance that develops with long-term Rap treatment [54]. Our pathway analysis showed that Rap upregulated pathways related to hepatic gluconeogenesis and glycolysis (in both tissues) whereas Rap + Met treatment did not. The increase in protein synthesis of gluconeogenic proteins confirms previous findings of increased expression of gluconeogenic genes during Rap treatment [26] and supports the insulin-sensitizing effect of Met [28, 57]. Therefore, our data support the idea that the addition of Met to Rap may correct the glucose intolerance with Rap alone [57]. This potentially improved glucoregulation following the addition of Met suggest that systemic metabolic changes may confer the increased median lifespan following Rap + Met compared with Rap treatment.
The general consensus is that Rap, and other treatments that extend lifespan, slow translation, and improve protein fidelity [5, 43, 52]. Prior to our previous study in the brain [49], there was no such data with Rap + Met. With Rap, improvements in proteostasis are thought to contribute to improved insulin sensitivity [2], increased muscle function [2, 62], decreased ectopic lipid storage [8], and improved metabolic flux [14, 58]. However, this generalization of the decreased translation does not adequately address tissue-specificity or the heterogeneity of proteostatic maintenance. Although studies have demonstrated overall suppression of synthesis rates [20], which we have at least partially attributed to decreased cell proliferation [10], there is great heterogeneity in the synthesis rates of proteins with some increasing and some decreasing [7, 22]. In line with the positive effects of Rap treatment on insulin sensitivity, muscle function, lipid storage, and metabolic flux, our previous studies indicate that treatments that extend lifespan selectively translate mitochondrial proteins [10–12, 32]. In our study in the brain of the mice used in this study [49], there was an indication that a selective translation of mitochondrial proteins might not be uniform throughout all tissues, and may differ by treatment and sex. As discussed below, the selective translation of mitochondrial proteins also differs by sex and treatment in liver and skeletal muscle.
Rap and Rap + Met treatments induce sex-specific proteome remodeling
More often than not, there are sex differences in response to treatments that extend lifespan [39]. An exception is that Rap + Met treatment equally increases the lifespan of both male and female mice [54]. We therefore hypothesized that Rap + Met treatment would induce similar protein synthesis responses in male and female mice, whereas Rap would be different between the sexes. In support of our hypothesis are the similar changes in absolute synthesis rates in the subcellular compartments of the liver with Rap and Rap + Met, which were different between sexes with Rap only. This sex-specificity is illustrated in the liver in Fig. 3(I, L) where there is a similar grouping in the lower left quadrant for both sexes with Rap + Met, but the clustering is different by sex with Rap. In skeletal muscle, Rap + Met and Rap have similar responses within a treatment between the two sexes, although Rap + Met reduces protein synthesis rates more so than Rap. The addition of Met to Rap may therefore have an effect that normalizes the sex differences apparent with Rap alone. It is not yet clear if the sex differences of Rap are due to differences in circulating levels of the drug [33], but it is interesting that the addition of Met equalizes the responses nonetheless.
There was one subcellular compartment that was particularly noteworthy in its change with treatment and sex, the ribosomal proteins (Figs. 3 and 5). In the male liver, we found that Rap + Met, but not Rap treatment, reduced ribosomal protein content, whereas both treatments lowered ribosome protein content and synthesis rates to the same extent in female liver samples. Like other proteins, it is expected that both rates of protein turnover and cell proliferation would affect the measured ribosomal protein synthesis rates. If we only considered cell proliferation, we would expect that both treatments would decrease ribosomal protein synthesis in both sexes. However, we do not see this pattern. A previous study using caloric restriction demonstrated an increase in ribosomal protein turnover relative to cell proliferation [29] but only looked at male mice. The study of Mathis et al. speculated that this was a mechanism to maintain translational fidelity. The similar reduction in ribosome protein content in male and female mice following Rap + Met treatment and sexually distinct effects of Rap treatment on ribosome protein content in the liver suggest the control of ribosome turnover, and potentially translational fidelity could contribute to sex differences in both lifespan and the cellular response to lifespan-extending interventions. However, although it has been speculated that reduced translational machinery, and protein synthesis, improves proteostasis by reducing error rates and facilitating co-translational folding [52], this proposed mechanism has some inconsistencies. With our treatments, there were many proteins that have greater protein synthesis than control. In fact, in the case of Rap, there is nearly an equal number that have greater protein synthesis rates compared with that in the control as less than. Therefore, decreasing ribosomal content with Rap and Rap + Met does not universally slow protein synthesis and in fact speeds translation of many proteins. Further studies are needed to understand the relationship between ribosomes, selective translation, and treatments that slow aging.
Tissue-specific proteome remodeling with Rap and Rap + Met
As expected, we showed that there were differences in protein synthetic responses between liver and skeletal muscle. These tissue-specific effects on protein synthesis are consistent with the tissue-specific regulation of proteostasis in short-term calorically restricted mice [35]. We note that although in both the liver and muscle, the responses of subcellular proteins to Rap + Met are almost always to reduce protein synthesis compared with Con, Rap either increased, decreased, or did not change relative to Con, and this varied by tissue.
When performing pathway analysis in both tissues, there were some similarities in the top 10 most enriched pathways. Of particular note was that both Rap and Rap + Met treatments increased the synthesis of proteins associated with the sirtuin signaling pathway in both tissues. Sirtuins are NAD-dependent deacetylases implicated in longevity [15, 42]. In the muscle, but not the liver, Rap + Met increased the synthesis rates of sirtuin pathway proteins to a greater extent than Rap alone. The effect of Rap treatment on activation of the sirtuin pathway has not been previously reported. However, recent data suggest that Met can directly bind to SIRT1 [6]. Upregulation of the sirtuin pathway increases mitochondrial protein quality control and mitochondrial function, as well as cellular stress resistance to contribute to longevity [37]. Our finding that both Rap and Rap + Met treatments increase the synthesis of sirtuin pathway proteins and that Rap + Met led to a further increase in the synthesis of the sirtuin proteins compared with Rap indicate that upregulation of sirtuin protein synthesis may partially contribute to the positive effects of Rap and Rap + Met to longevity.
Selective translation of mitochondrial proteins
The question of whether Rap and Rap + Met resulted in the selective translation of mitochondrial proteins is difficult to answer. When we grouped proteins using GO terms by mitochondrial, mitochondrial compartment, or individual complexes, it is clear that Rap + Met does not have greater mitochondrial protein synthesis rates compared with Con. These data are supported by pathway analysis indicating a strong downregulation of oxidative phosphorylation proteins with Rap + Met in skeletal muscle. With Rap, the data are not as clear. In the liver, it appears that mitochondrial protein synthesis rates are at minimum maintained in both sexes, and even increased in males suggesting selective translation of mitochondrial proteins may be a specific mechanism through which Rap treatment, but not Rap + Met treatment, influences lifespan. Most importantly, these results demonstrate that there is heterogeneity in response to treatments between tissues and even between proteins within mitochondria. As for the striking difference between Rap versus Rap + Met, the addition of Met may shift cellular priorities. The inefficiency in mitochondrial respiration with Met [1, 24] may provide a greater energetic challenge to cells compared with Rap alone. Although we would predict that these energetic constraints would further stimulate mitochondrial protein synthesis, there may be other competing cellular demands that have priority. Subjecting the mice to other physiological stressors during treatment could help understand these shifts.
Limitations and conclusions
This is the first study to examine the effects of Rap and Rap + Met treatments on bulk and individual protein synthesis in the liver and skeletal muscle of male and female mice. There were a couple of limitations to our current study. First, we only investigated two tissues and other tissues likely have different responses to treatment. Measuring the content and synthesis of individual proteins is technically challenging; thus, we narrowed our choice to two tissues—one that had proliferative capacity (the liver) and one that was largely post-mitotic (the skeletal muscle). Although cardiac tissue could have fit the latter requirement, we ultimately settled on skeletal muscle because it collaborates with the liver to regulate energy metabolism [47] and impacts other important age-related conditions such as mobility [21]. Moreover, age-related decrements in the liver [13] or skeletal muscle [41] function are associated with increased mortality making them ideal targets of study for the current investigation. Second, we were not able to measure protein content in skeletal muscle, as we did for the liver. The reason for this is that we chose to fractionate the muscle prior to analysis to minimize the highly abundant contractile proteins and to enrich for mitochondrial and cytosolic proteins. We were concerned that potential variability in the fractionation procedure would affect protein concentrations. Second, as noted, we do not report cellular proliferation rates. Although we know from our previous studies [10, 49, 61] that these rates are changed with Rap and Rap + Met treatments and that they have a large effect on protein synthesis rates, we were not sure how best to normalize the synthesis rates of individual proteins to cell proliferation rates. We also worked with tissue homogenates, which are a collection of cell types. There are likely cell type–specific changes within a given tissue dependent on sex and on drug treatment. Finally, we acknowledge the potential limitation of not including a Met only group. However, the purpose of this investigation was to compare treatments that are known to extend lifespan, and Met treatment does not increase the lifespan of the UM-HET3 mice used in the current study [54].
The current study highlights the differences between Rap and Rap + Met treatments on proteome turnover. Our findings offer insight as to why the addition of Met to Rap normalizes sex differences in lifespan seen with Rap only. Rap + Met creates a uniform protein synthesis response in males and females, compared with sex-specific changes in protein synthesis rates following Rap treatment. These data offer insight into potential protein translational responses that confer increases in longevity for both male and female mice. We further highlight the large heterogenic responses between proteins indicating that there is a selective translation of many proteins despite global suppression of protein synthesis. Finally, we discuss some key differences by sex and treatment that represent some key unanswered questions related to slowing the aging process.
Electronic supplementary material
(PDF 866 kb)
(PDF 726 kb)
(PDF 36 kb)
(PDF 38 kb)
(PDF 36 kb)
(PDF 473 kb)
(XLSX 2044 kb)
(XLSX 1328 kb)
(PDF 36 kb)
Acknowledgments
The authors would like to thank the members of the Translational Research on Aging and Chronic Disease Laboratory. The authors are also grateful for the assistance of Dr. Rich Miller, MD, PhD, for animal housing, tissue collection, and critical feedback on the manuscript.
Author contributions
The following authors contributed to the following: the design of the work (CAW, MML, KLH, BFM), data collection (CAW, MML, JJR, JLL, RVM, MAL, QZ, FFP, JCP), or analysis (CAW, MML, HP, FFP, JDW, JCP, KLH, BFM), drafting (CAW, MML, HP, KLH, BFM), critically revising (CAW, MML, HP, RVM, JDW, JCP, KLH, BFM), and final approval with an agreement to be accountable for all aspects of the work (CAW, MML, HP, JJR, JLL, RVM, MAL, QZ, FFP, JDW, JCP, KLH, BFM).
Funding information
This research was supported by a grant funded by NIH grants 1R01AG042569 (BFM and KLH), NIA Training Grant T32AG052363 (MML and HP), and a gift from the Fritz B Burns foundation to JCP and the Fritz B. Burns Biological Mass spectrometry facility.
Data availability
Raw data will be uploaded to the PRIDE database following the publication of this manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All animal procedures and protocols were approved by the University of Michigan Institutional Animal Care and Use Committee (IACUC) prior to the initiation of this study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Christopher A. Wolff and Marcus M. Lawrence are co-first authors.
Karyn L. Hamilton and Benjamin F. Miller are co-principal investigators.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Andrzejewski S, Gravel S-P, Pollak M, St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab. 2014;2:12. doi: 10.1186/2049-3002-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bitto A, Ito TK, Pineda VV, LeTexier NJ, Huang HZ, Sutlief E, et al. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. Elife. 2016:5. 10.7554/eLife.16351. [DOI] [PMC free article] [PubMed]
- 3.Bruns DR, Ehrlicher SE, Khademi S, Biela LM, Peelor FF, III, Miller BF, Hamilton KL. Differential effects of vitamin C or protandim on skeletal muscle adaptation to exercise. J Appl Physiol. 2018;125:661–671. doi: 10.1152/japplphysiol.00277.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiao YA, Kolwicz SC, Basisty N, Gagnidze A, Zhang J, Gu H, Djukovic D, Beyer RP, Raftery D, MacCoss M, Tian R, Rabinovitch PS. Rapamycin transiently induces mitochondrial remodeling to reprogram energy metabolism in old hearts. Aging (Albany NY) 2016;8:314–327. doi: 10.18632/aging.100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conn CS, Qian S-B. Nutrient signaling in protein homeostasis: an increase in quantity at the expense of quality. Sci Signal. 2013;6:ra24–ra24. doi: 10.1126/scisignal.2003520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuyàs E, Verdura S, Llorach-Parés L, Fernández-Arroyo S, Joven J, Martin-Castillo B, et al. Metformin is a direct SIRT1-activating compound: computational modeling and experimental validation. Front Endocrinol (Lausanne). 2018;9. 10.3389/fendo.2018.00657. [DOI] [PMC free article] [PubMed]
- 7.Dai DF, Karunadharma PP, Chiao YA, Basisty N, Crispin D, Hsieh EJ, Chen T, Gu H, Djukovic D, Raftery D, Beyer RP, MacCoss MJ, Rabinovitch PS. Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell. 2014;13:529–539. doi: 10.1111/acel.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deblon N, Bourgoin L, Veyrat-Durebex C, Peyrou M, Vinciguerra M, Caillon A, Maeder C, Fournier M, Montet X, Rohner-Jeanrenaud F, Foti M. Chronic mTOR inhibition by rapamycin induces muscle insulin resistance despite weight loss in rats. Br J Pharmacol. 2012;165:2325–2340. doi: 10.1111/j.1476-5381.2011.01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drake JC, Peelor FF, Biela LM, et al. Assessment of mitochondrial biogenesis and mtorc1 signaling during chronic rapamycin feeding in male and female mice. Journals Gerontol - Ser A Biol Sci Med Sci. 2013;68:1493–1501. doi: 10.1093/gerona/glt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drake JC, Bruns DR, Peelor FF, et al. Long-lived crowded-litter mice have an age-dependent increase in protein synthesis to DNA synthesis ratio and mTORC1 substrate phosphorylation. Am J Physiol - Endocrinol Metab. 2014;307:E813–E821. doi: 10.1152/ajpendo.00256.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drake JC, Bruns DR, Peelor FF, et al. Long-lived Snell dwarf mice display increased proteostatic mechanisms that are not dependent on decreased mTORC1 activity. Aging Cell. 2015;14:474–482. doi: 10.1111/acel.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Kamary SS, Jhaveri R, Shardell MD. All-cause, liver-related, and non-liver-related mortality among hcv-infected individuals in the general US population. Clin Infect Dis. 2011;53:150–157. doi: 10.1093/cid/cir306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang Y, Westbrook R, Hill C, Boparai RK, Arum O, Spong A, Wang F, Javors MA, Chen J, Sun LY, Bartke A. Duration of rapamycin treatment has differential effects on metabolism in mice. Cell Metab. 2013;17:456–462. doi: 10.1016/j.cmet.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guarente L. Sirtuins, aging, and metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:81–90. doi: 10.1101/sqb.2011.76.010629. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton KL, Miller BF. Mitochondrial proteostasis as a shared characteristic of slowed aging: the importance of considering cell proliferation. J Physiol. 2017;595:6401–6407. doi: 10.1113/JP274335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellerstein MK, Neese RA. Mass isotopomer distribution analysis at eight years: theoretical, analytic, and experimental considerations. Am J Physiol - Endocrinol Metab. 1999. [DOI] [PubMed]
- 19.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 20.Huo Y, Iadevaia V, Yao Z, Kelly I, Cosulich S, Guichard S, Foster LJ, Proud CG. Stable isotope-labelling analysis of the impact of inhibition of the mammalian target of rapamycin on protein synthesis. Biochem J. 2012;444:141–151. doi: 10.1042/BJ20112107. [DOI] [PubMed] [Google Scholar]
- 21.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 22.Karunadharma PP, Basisty N, Dai DF, Chiao YA, Quarles EK, Hsieh EJ, Crispin D, Bielas JH, Ericson NG, Beyer RP, MacKay VL, MacCoss MJ, Rabinovitch PS. Subacute calorie restriction and rapamycin discordantly alter mouse liver proteome homeostasis and reverse aging effects. Aging Cell. 2015;14:547–557. doi: 10.1111/acel.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konopka AR, Laurin JL, Schoenberg HM, Reid JJ, Castor WM, Wolff CA, et al. Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell. 2019:e12880. 10.1111/acel.12880. [DOI] [PMC free article] [PubMed]
- 25.Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu Rev Biochem. 2015;84:435–464. doi: 10.1146/annurev-biochem-060614-033955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamming DW, Ye L, Katajisto P, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science (80- ) 2012;335:1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majumder S, Caccamo A, Medina DX, Benavides AD, Javors MA, Kraig E, Strong R, Richardson A, Oddo S. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1β and enhancing NMDA signaling. Aging Cell. 2012;11:326–335. doi: 10.1111/j.1474-9726.2011.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathis AD, Naylor BC, Carson RH, Evans E, Harwell J, Knecht J, Hexem E, Peelor FF, III, Miller BF, Hamilton KL, Transtrum MK, Bikman BT, Price JC. Mechanisms of in vivo ribosome maintenance change in response to nutrient signals. Mol Cell Proteomics. 2017;16:243–254. doi: 10.1074/mcp.M116.063255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKinney W. Data structures for statistical computing in Python. Proc 9th Python Sci Conf. 2010.
- 31.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. Journals Gerontol - Ser A Biol Sci Med Sci. 2011;66A:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller BF, Drake JC, Naylor B, Price JC, Hamilton KL. The measurement of protein synthesis for assessing proteostasis in studies of slowed aging. Ageing Res Rev. 2014;18:106–111. doi: 10.1016/j.arr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, Pletcher S, Salmon AB, Sharp ZD, van Roekel S, Winkleman L, Strong R. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13:468–477. doi: 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller BF, Wolff CA, Peelor FF, et al. Modeling the contribution of individual proteins to mixed skeletal muscle protein synthetic rates over increasing periods of label incorporation. J Appl Physiol. 2015;118:655–661. doi: 10.1152/japplphysiol.00987.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller BF, Pharaoh GA, Hamilton KL, Peelor FF, III, Kirkland JL, Freeman WM, Mann SN, Kinter M, Price JC, Stout MB. Short-term calorie restriction and 17α-estradiol administration elicit divergent effects on proteostatic processes and protein content in metabolically active tissues. J Gerontol A Biol Sci Med Sci. 2019;75:849–857. doi: 10.1093/gerona/glz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Misu H, Takamura T, Takayama H, Hayashi H, Matsuzawa-Nagata N, Kurita S, Ishikura K, Ando H, Takeshita Y, Ota T, Sakurai M, Yamashita T, Mizukoshi E, Yamashita T, Honda M, Miyamoto KI, Kubota T, Kubota N, Kadowaki T, Kim HJ, Lee IK, Minokoshi Y, Saito Y, Takahashi K, Yamada Y, Takakura N, Kaneko S. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 2010;12:483–495. doi: 10.1016/j.cmet.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Cantó C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, Guarente L, Auwerx J. XThe NAD+/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Musci RV, Hamilton KL, Miller BF. Targeting mitochondrial function and proteostasis to mitigate dynapenia. Eur J Appl Physiol. 2018;118:1–9. doi: 10.1007/s00421-017-3730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nadon NL, Strong R, Miller RA, Harrison DE. NIA interventions testing program: investigating putative aging intervention agents in a genetically heterogeneous mouse model. EBioMedicine. 2017;21:3–4. doi: 10.1016/j.ebiom.2016.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naylor BC, Porter MT, Wilson E, Herring A, Lofthouse S, Hannemann A, et al. DeuteRater: a tool for quantifying peptide isotope precision and kinetic proteomics. Bioinformatics. 2017:btx009. 10.1093/bioinformatics/btx009. [DOI] [PubMed]
- 41.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB, on Behalf of the Health, Aging and Body Composition Study Investigators Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 42.O’Callaghan C, Vassilopoulos A. Sirtuins at the crossroads of stemness, aging, and cancer. Aging Cell. 2017;16:1208–1218. doi: 10.1111/acel.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan KZ, Palter JE, Rogers AN, Olsen A, Chen D, Lithgow GJ, Kapahi P. Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans. Aging Cell. 2007;6:111–119. doi: 10.1111/j.1474-9726.2006.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. 2017;13:572–587. doi: 10.1038/nrendo.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pillon NJ, Bilan PJ, Fink LN, Klip A. Cross-talk between skeletal muscle and immune cells: muscle-derived mediators and metabolic implications. Am J Physiol-Endocrinol Metab. 2013. [DOI] [PubMed]
- 46.Price JC, Khambatta CF, Li KW, Bruss MD, Shankaran M, Dalidd M, Floreani NA, Roberts LS, Turner SM, Holmes WE, Hellerstein MK. The effect of long term calorie restriction on in vivo hepatic proteostatis: a novel combination of dynamic and quantitative proteomics. Mol Cell Proteomics. 2012;11:1801–1814. doi: 10.1074/mcp.M112.021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Priest C, Tontonoz P. Inter-organ cross-talk in metabolic syndrome. Nat Metab. 2019;1:1177–1188. doi: 10.1038/s42255-019-0145-5. [DOI] [PubMed] [Google Scholar]
- 48.Ramos FJ, Chen SC, Garelick MG, et al. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med. 2012;4:144ra103. doi: 10.1126/scitranslmed.3003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reid JJ, Linden MA, Peelor FF, et al. Brain protein synthesis rates in the UM-HET3 mouse following treatment with rapamycin or rapamycin with metformin. Journals Gerontol Ser A. 2020;75:40–49. doi: 10.1093/gerona/glz069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rockwood AL, Haimi P. Efficient calculation of accurate masses of isotopic peaks. J Am Soc Mass Spectrom. 2006;17:415–419. doi: 10.1016/j.jasms.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 51.Rui L. Energy metabolism in the liver. Compr Physiol. 2014. 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed]
- 52.Sherman MY, Qian SB. Less is more: improving proteostasis by translation slow down. Trends Biochem Sci. 2013;38:585–591. doi: 10.1016/j.tibs.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Smith R, Taylor RM, Prince JT. Current controlled vocabularies are insufficient to uniquely map molecular entities to mass spectrometry signal. BMC Bioinformatics. 2015;16:S2. doi: 10.1186/1471-2105-16-S7-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, Fernandez E, Flurkey K, Hamilton KL, Lamming DW, Javors MA, de Magalhães JP, Martinez PA, McCord JM, Miller BF, Müller M, Nelson JF, Ndukum J, Rainger GE, Richardson A, Sabatini DM, Salmon AB, Simpkins JW, Steegenga WT, Nadon NL, Harrison DE. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15:872–884. doi: 10.1111/acel.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vilchez D, Saez I, Dillin A. The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat Commun. 2014. [DOI] [PubMed]
- 56.Waskom M, Botvinnik O, Hobson P, et al. Seaborn: v0.5.0 (November 2014). 2014. 10.5281/ZENODO.12710.
- 57.Weiss R, Fernandez E, Liu Y, Strong R, Salmon AB. Metformin reduces glucose intolerance caused by rapamycin treatment in genetically heterogeneous female mice. Aging (Albany NY) 2018;10:386–401. doi: 10.18632/aging.101401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, Woodward MA, Miller RA. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 60.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–482. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 61.Wolff CA, Reid JJ, Musci RV, Bruns DR, Linden MA, Konopka AR, Peelor FF, III, Miller BF, Hamilton KL. Differential effects of rapamycin and metformin in combination with rapamycin on mechanisms of proteostasis in cultured skeletal myotubes. Journals Gerontol Ser A. 2020;75:32–39. doi: 10.1093/gerona/glz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye L, Widlund AL, Sims CA, Lamming DW, Guan Y, Davis JG, Sabatini DM, Harrison DE, Vang O, Baur JA. Rapamycin doses sufficient to extend lifespan do not compromise muscle mitochondrial content or endurance. Aging (Albany NY) 2013;5:539–550. doi: 10.18632/aging.100576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J, Xin L, Shan B, Chen W, Xie M, Yuen D, Zhang W, Zhang Z, Lajoie GA, Ma B. PEAKS DB: de novo sequencing assisted database search for sensitive and accurate peptide identification. Mol Cell Proteomics. 2012;11:M111.010587. doi: 10.1074/mcp.M111.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in drosophila. Cell. 2009;139:149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 866 kb)
(PDF 726 kb)
(PDF 36 kb)
(PDF 38 kb)
(PDF 36 kb)
(PDF 473 kb)
(XLSX 2044 kb)
(XLSX 1328 kb)
(PDF 36 kb)
Data Availability Statement
Raw data will be uploaded to the PRIDE database following the publication of this manuscript.