Abstract
5′-Hydroxy-6, 7, 8, 3′, 4′-pentamethoxyflavone (5-HPF), a polymethoxyflavone compound found in dikamali gum, has been shown to exert a range of beneficial effects on health. We have previously reported that 5-HPF improves the cholinergic dysfunction and also possesses antioxidant properties in Caenorhabditis elegans. In this study, we have identified the effect of 5-HPF on the worm lifespan and its underlying molecular mechanisms. Out of the five tested pharmacological doses of 5-HPF, viz. 6.25, 12.5, 25, 50, and 100 μM, the 50 μM dose maximally extended the mean life of C. elegans by 28%. The present study revealed that 5-HPF supplementation leads to dietary restriction (DR)-like effects in the worms without altering bacterial metabolism. The analysis of mutant animals fed with 5-HPF suggested that the extended lifespan of C. elegans depends upon multiple DR-related signaling pathways, with NRF2 and FOXA being critical factors. Further investigation into the mechanistic aspects indicated that 5-HPF utilizes autophagy pathway induced by DR through the upregulation of autophagy genes bec-1 and lgg-1, evident from the increase in autophagic puncta in the seam cells of lgg-1::gfp tagged worms. This study identifies the longevity-promoting activity of 5-HPF in C. elegans regulated by oxidative stress-resistance genes and DR-induced autophagy pathway.
Electronic supplementary material
The online version of this article (10.1007/s11357-020-00229-6) contains supplementary material, which is available to authorized users.
Keywords: 5′-Hydroxy-6, 7, 8, 3′, 4′-pentamethoxyflavone; Dietary restriction; C. elegans; Aging; Gardenia lucida; Reactive oxygen species
Introduction
Aging is marked with progressive deterioration of physiological functions resulting from the cellular impairment. This stimulates the progression of major ailments such as diabetes, cardiovascular disease, cancer, osteoporosis, arthritis, and neurodegenerative disorders (Franceschi et al. 2018). Major molecular mechanisms regulating the aging process have been distinguished in lower invertebrates, and the same mechanisms are often noted in mammals, providing the evidence of highly conserved aging genes and signaling pathways (Smith et al. 2008). Among all pathways, dietary restriction (DR) is the most extensively studied and established phenomenon of longevity from yeast to mammals (Kennedy et al. 2007). DR prolongs lifespan by curtailing nutrient uptake, altering several nutrient processing pathways. In Caenorhabditis elegans, DR-mediated longevity is governed by two transcription factors PHA-4/FOXA and SKN-1/NRF2 (Panowski et al. 2007). Specifically, PHA-4/FOXA enhances the lifespan of C. elegans in food-deprived conditions and regulates the expression level of stress modulatory genes (Panowski et al. 2007). PHA-4/FOXA is the primary mediator that regulates DR in worms, encodes for the pharynx and foregut development in C. elegans (Panowski et al. 2007). SKN-1/NRF2 activates a wide range of genes involved in cellular repair, detoxification, and stress resistance (Oliveira et al. 2009). In C. elegans, SKN-1/NRF2 is required for the response to oxidative stress and starvation (An et al. 2005). The expression of SKN-1/NRF2 in ASI neurons has also been reported in lifespan extension (Bishop and Guarente 2007). SKN-1/NRF2 and PHA-4/FOXA both contribute to DR-regulated longevity. The autophagic response induced by LET-363/ MTOR inhibition requires PHA-4 activity. LET-363/MTOR and SKN-1/NRF2 function through a feedback circle for the activation of stress-responsive genes (Hansen et al. 2008; Robida-Stubbs et al. 2012).
The modulation of ribosome biogenesis and autophagy is regulated by target of rapamycin (TOR) (Wullschleger et al. 2006). In C. elegans, the components of MTOR, TOR kinase (let-363 (Long et al. 2002)) and Raptor (daf-15 (Jia et al. 2004)), regulate growth, protein synthesis, aging, and autophagy (Hansen et al. 2007; Vellai et al. 2003). DR induced by eat-2 mutation has also been influenced by let-363, under the regulation of PHA-4/FOXA (Hansen et al. 2007; Meissner et al. 2004). DR-mediated longevity depends on the nutrient sensing and energy availability, which controls the synthesis and degradation of cellular macromolecules and organelles through a recycling mechanism, “autophagy” (Hansen et al. 2008). In C. elegans, longevity is also regulated by autophagy genes unc-51/ULK1, bec-1/Beclin1, and lgg-1/LC3 (Melendez and Levine 2009). Principally, autophagy or self-eating entails scavenging of damaged cellular components, which serve as a major source of reactive oxygen species. The reactive oxygen species (ROS) produced from the aerobic metabolism of an organism causes oxidative stress (Cadet and Wagner 2013). Oxidative stress has been known to possess a crucial role in aging and age-related degenerative diseases (Finkel and Holbrook 2000; Lin and Beal 2006). The characteristic exogenous and endogenous cell defense system tackles free radical formation by several enzymatic and non-enzymatic strategies (Sies 1997). Plant-derived bioactive molecules/extracts have been widely explored for their efficacy in treating different diseases and restorative properties. Previously, phytomolecules have been shown to participate in the regulation of various metabolic processes and have lifespan-extending properties in animal models (Brown et al. 2006; Kampkötter et al. 2008; Koch et al. 2014; Saul et al. 2009). They have also been shown to improve diseases such as cancer, neurodegeneration, and metabolic syndrome. Flavonoids, consisting of mainly polymethoxyflavones (PMFs), have been shown to exhibit a broad spectrum of biological properties including antitumor (Miyata et al. 2008), anti-carcinogenic (Li et al. 2007), anti-inflammatory (Ho et al. 2012), and antioxidant (Li et al. 2007). Hence, with the reported efficacy of PMFs, we undertook the present study to elucidate the effect of 5′-hydroxy-6, 7, 8, 3′, 4′-pentamethoxyflavone (5-HPF) on lifespan. Earlier, we have reported the extraction and identification of 5-HPF and its role in the improvement of cholinergic function and oxidative stress tolerance in C. elegans (Trivedi et al. 2017). The systematic evaluation of adequacy, wellbeing, and mechanism of phytomolecule in a mammalian model is expensive and tedious. C. elegans is the most favored model inferable from its short life expectancy and life cycle, simple research maintenance, and high homology to mammalian particularly human biochemical and hereditary pathways (Kenyon 2010). The present study highlights the beneficial effect of 5′-hydroxy-6, 7, 8, 3′, 4′-pentamethoxyflavone (5-HPF) on lifespan in the C. elegans model system.
Methods
Culture and maintenance of strains
C. elegans strains, Bristol N2; DA1116, eat-2 (ad116); GR1307, daf-16 (mgDf50); CB1370, daf-2 (e1370); EU-31, skn-1 (zu131); SM190, smg-1/pha-4; VC424, bec-1; BE150, unc-51 (e369); KR344, let-363 (h98) dpy-5 (e61) unc-13(e450); DA2123,lgg-1::gfp; SM481, pha-4::gfp; and Escherichia coli OP50 were procured from Caenorhabditis Genetics Center (University of Minnesota, MN, USA) and grown on Nematode growth medium (NGM) and cultured at 20 °C. A synchronized population of worms was obtained by sodium hypochlorite treatment (Porta-de-la-Riva et al. 2012). Worms were maintained on media seeded with E. coli OP50 bacteria using established standard protocol (Brenner 1974).
Lifespan assay
Lifespan assays were performed at 20 °C as described previously (Srivastava et al. 2017). Briefly, age-synchronized eggs were added to NGM plates seeded with E. coli OP50 along with different concentrations of test compound 5-HPF and 0.05% DMSO serves as vehicle control. At L4 stage, worms (n = 60–70 per 6 cm Petri plate) were transferred to new treatment plates supplemented with 50 μM 5-fluoro-2′-deoxyuridine (FUdR) (Sigma-Aldrich) to inhibit the progeny. Worms were observed daily for survival, scoring until the last worm survived. Worms were transferred to fresh treatment plates, every 48 h to avoid bacterial contamination and maintain a specific concentration of 5-HPF. Any worms desiccated or damaged were excluded from the study.
Measurement of brood size and reproductive span
For reproduction assay, age-synchronized N2 worms at the L1 stage were transferred to pre-treated NGM plates as described in the lifespan assay without FUdR. Individual L4-stage worm was transferred to fresh NGM plates (n = 5) treated with and without 5-HPF each day until the worms ceased to lay eggs. Numbers of hatched worms per plate were counted daily (Pant et al. 2014).
Lipid staining assay by Nile Red
The effect of 5-HPF treatment on lipid levels in worms was measured using Nile Red (a fluorescent dye used to stain intracellular lipid droplets) staining. A 0.5-mg/ml stock solution of Nile Red was prepared in acetone, further diluted with OP50 in a ratio of 1:250 and spotted onto NGM plates along with or without 5-HPF treatment. Thereafter, age-synchronized L1 worms were transferred to treatment plates and incubated at 20 °C. After 72 h, worms were transferred to 3 different treatment plates according to the day of observation under a microscope. At the day of imaging (day 3/6/12), worms were washed off from the plates using M9 buffer. Worms were anesthetized using 100 mM sodium azide, mounted onto slides, and were observed using rhodamine filter. The fluorescence intensity was calculated semi-quantitatively using ImageJ (Ashrafi et al. 2003).
Lipofuscin assay
Lipofuscin comprises highly oxidized and cross-linked proteins, which are considered as prominent “biomarkers of aging”. The intestinal accumulation of auto-fluorescent lipofuscin was quantified in treated/control worms. For lipofuscin assay, treated and control day 3/6/12 worms (n = 30) were washed off and monitored on DAPI filter using a fluorescent microscope as previously described (Berdichevsky et al. 2010; Lee et al. 2015a). Briefly, age-synchronized L1 worms were transferred to treatment plates and incubated at 20 °C. After 72 h, worms were transferred to three different treatment plates according to the day of observation under microscope. At the day of imaging (day 3/6/12), the worms were washed off from the plates using M9 buffer. The worms were anesthetized using 100 mM sodium azide, mounted onto slides, and were observed under the fluorescent microscope (excitation/emission 358 nm/461 nm). The fluorescence intensity was calculated semi-quantitatively using ImageJ and represented in terms of normalized values of corrected total cell fluorescence (CTCF)(CTCF = Integrated Density − (Area of selected cell × Mean fluorescence of background readings)).
Stress resistance assay through juglone exposure in C. elegans
The accumulation of oxidized toxic products and free radicals may cause oxidative stress which leads to impaired mitochondrial function (López-Otín et al. 2013). Thus, sensitivity to oxidative stress can be measured by quantifying the survival of worms following exposure to free radical generating compounds like juglone and paraquat. To analyze the oxidative stress tolerance, control and 50 μM 5-HPF-treated worms were subjected to 250 μM concentration of juglone (5-Hydroxy-1, 4-naphthoquinone). The survivals of the worms were scored after 6 h (Cong et al. 2015).
Measurement of intracellular ROS in C. elegans
Intracellular ROS levels were quantified using the H2DCF-DA method (Labuschagne and Brenkman 2013). Adult worms were washed thrice using M9 buffer and finally collected in 300 μl of 0.1% PBST buffer and transferred to a 96well black plate (Thermo Scientific). Fifty micromolar working concentration of H2DCF-DA was added to each well. Fluorescence was measured using a microplate reader (BMG polarstar Omega) at 485 nm excitation and 520 nm emission. Observations were recorded for 120 min with intervals of 20 min each at 37 °C.
GFP reporter assay
Protein reporter assay was performed using transgenic strains expressing LGG-1 and PHA-4 tagged with GFP. Age-synchronized L1 worms were transferred to NGM plates treated with or without 5-HPF and incubated for 3 days at 20 °C allowed to egg lay and hatch. Second-generation worms were washed off at the L3 stage from the plates using M9 buffer. Worms were anesthetized using 100 mM sodium azide, mounted onto slides, and were observed using GFP filter. GFP-autophagy puncta and localization of PHA-4 were analyzed using × 20 and × 100 magnification by confocal laser scanning microscopy (CLSM) using a Zeiss Confocal LSM700 microscope equipped with Plan-Apochromat × 20/0.8 M27 and Plan-Apochromat × 100/1.40 Oil DIC M27 objectives. Acquisitions were realized in a plane scan mode, with excitation at 488 nm and emission at 530 nm. Images were processed with the Zeiss ZEN 2 software, in which background was reduced using brightness and contrast adjustments applied to the full set of images and finally exported as TIFF files.
RNA isolation, cDNA synthesis, and quantitative qPCR
Total RNA was extracted from adult worms using RNAzol reagent (Molecular Research Centre Inc., Cat. No. RN190) according to the manufacturer’s protocol. cDNA synthesis was done from 1 μg of total C. elegans RNA in a 96-well thermal cycler using a cDNA Synthesis Kit (Thermo Scientific) according to the manufacturer’s protocol. qRT-PCR studies were done using the QuantStudio 3 and 5 Real-Time PCR Systems (Applied biosystem, USA). Differential expression was calculated by the 2-∆∆CT method. gpd-1 was used as housekeeping control. Primers were procured from Eurofins.
Statistical analysis
Statistical analysis and Graphical representation of data were done using GraphPad Prism version 5. Analysis of variance and independent t test was used to calculate statistical significance where ever applicable. Significant differences between the lifespan of treated and control worms were determined using the Kaplan–Meier survival assay. All the experiments were performed thrice independently, and the results were plotted as mean ± SEM (standard error of the mean).
Results
5-HPF enhances longevity and reduces the aging biomarkers
We evaluated the lifespan extension potential of 5-HPF in wild-type N2. The population of worms was treated with different concentrations (6.25, 12.5, 25, 50, and 100) μM of 5-HPF and control (0.05% DMSO) at the early embryonic stage on NGM plates. Subsequently, we observed a significant increase in mean lifespan of 5-HPF-treated worms at 6.25 μM (18.05 ± 0.42, p ≤ 0.01), 12.5 μM (18.50 ± 0.28, p ≤ 0.001), 25 μM (18.98 ± 0.38, p ≤ 0.001), 50 μM (20.76 ± 0.49, p ≤ 0.001), and 100 μM (17.67 ± 0.41, p ≤ 0.05), as compared with control (16.18 ± 0.34) (Fig. 1 a–c, Table S1). The maximum longevity was observed at 50 μM 5-HPF treatment (28.30%). We also evaluated the effect of control and treated 50 μM 5-HPF treatment starting from adult day 1 stage; 5-HPF significantly increased the mean lifespan by 31% as compared to control (Fig. S3 b). The result suggested that the effect is fully penetrant whether the treatment is initiated at any stage of life. Next, we examined aging biomarker, lipofuscin (an intestinal auto florescent age pigment), and found that 5-HPF treatment delays the accumulation of lipofuscin at 50 μM as compared with control (Fig. 1d, e and Fig. S5 a-b,). Fifty micromolars 5-HPF treatment also alleviated the total neutral lipid levels in Fig. 1f, g (Table S3) and specifically triglycerides (Fig.S5 c-e,) in comparison to their respective controls.
Fig. 1.
5-HPF extends the mean lifespan and modulates aging parameters in C. elegans. a Survival curves of animal control and treated with different doses (5-HPF treated worms at 6.25 μM, 12.5 μM, 25 μM, 50 μM, 100 μM) of 5-HPF. 5-HPF significantly increased the mean lifespan of treated worms. b 50 μM 5-HPF significantly increased the mean lifespan by 28% as compared to control. c Bar graph representing mean lifespan treated with different concentration of 5-HPF. d Representative images of lipofuscin levels in control and treatment group, respectively. e 50 μM 5-HPF treatment alleviates relative lipofuscin levels. f Representative images of lipid levels in control and treatment group, respectively. g 5-HPF significantly reduces lipid levels in 50 μM 5-HPF-treated worms. The images were quantified by determining the average pixel intensity in each worm using ImageJ software (NIH). The data were analyzed using the Kaplan–Meier survival analysis and independent t test wherever applicable in GraphPad Prism version 5. Differences between the data were considered significant at p ≤ .05 (*p value < 0.05 and *** p value < 0.001, Scale bar = 200 μm, N = 30/group)
5-HPF enhances oxidative stress tolerance and reduces intracellular ROS levels in wild-type worms
Oxidative stress in terms of reactive oxygen species (ROS) is a by-product of normal metabolism and is known to cause deleterious cellular damage leading to instability of lipids and proteins, resulting in the normal aging phenomenon. To ascertain the antioxidant potential of 5-HPF, we performed oxidative stress tolerance assay. 5-HPF treatment was found to enhance the percentage survival of worms under juglone-induced oxidative stress (Fig. 2a). The outcome suggested increased percentage survival in 5-HPF-treated worms at 50 μM dose of 5-HPF (88.33 ± 3.19, p ≤ 0.001) as compared to control (51.66 ± 1.67).
Fig. 2.

5-HPF modulates oxidative stress resistance and reduces ROS levels in C. elegans. a Pre-treatment of 50 μM 5-HPF significantly increases resistance against juglone-induced oxidative stress. b 50 μM 5-HPF-treated worms show a significant reduction in the intracellular ROS level. c Pre-treatment of 50 μM 5-HPF significantly reduces intracellular ROS level against juglone-induced oxidative stress. The data were analyzed using the ANOVA and independent t test wherever applicable in GraphPad Prism version 5. Differences between the data were considered significant at p ≤ .05(***p value < 0.001)
We also studied the effect of 5-HPF on intracellular ROS levels using a cell-permeant dye, H2DCFDA. 5-HPF alleviates the ROS levels at 50 μM by 59.20% as compared to control (Fig. 2b). Moreover, to further validate the result, we have determined the ROS level under juglone exposure. Interestingly, 5-HPF curtailed the ROS levels against juglone-exposed condition significantly (Fig. 2c). The augmentation of oxidative stress tolerance and decline in ROS levels in 5-HPF-treated worms implies considerable ROS scavenging properties of 5-HPF.
5-HPF works independently of IS/ILS signaling
IS/ILS signaling is an established mechanism of lifespan extension in C. elegans (Altintas et al. 2016). To elucidate the molecular mechanism underlying the 5-HPF-mediated lifespan extension, we started screening for IS/ILS signaling pathway. DAF-16/FOXO is the major transcription factor that regulates lifespan extension by upregulating the genes responsible for cell survival and maintenance (Sun et al. 2017). 5-HPF extended the mean lifespan of DAF-2/IGF1R mutant significantly by 26.80% (31.50 ± 0.72, p ≤ 0.001) at 50 μM concentration as compared with control (24.83 ± 0.68) (Fig. 3a). In addition to daf-2 mutant, 50 μM 5-HPF treatment also enhanced the mean lifespan of daf-16 mutant by 26.72% (18.19 ± 0.46, p ≤ 0.001) compared to control (14.36 ± 0.41) (Fig. 3c). These data show that 5-HPF asserts longevity independent of the IS/ILS pathway.
Fig. 3.
5-HPF does not modulate IS/ILS pathway. a Survival curves of the daf-2 mutant in the presence and absence of 50 μM 5-HPF. b Survival curves of the skn-1 mutant in the presence and absence of 50 μM 5-HPF. No significant augmentation in the mean lifespan of the mutant was observed in skn-1 (EU-31) mutant (1.74%, p = 0.528). c Survival curves of the daf-16 mutant in the presence and absence of 50 μM 5-HPF. d 5-HPF treatment significantly upregulated the fold change in the mRNA expression level of daf-2, daf-16, and skn-1 and its downstream target genes daf-9, gcs-1, and gst-7. The data were analyzed using the Kaplan–Meier survival analysis and two-way ANOVA wherever applicable in GraphPad Prism version 5. Differences between the data were considered significant at p ≤ .05(*p value < 0.05, **p value < 0.01, and ***p value < 0.001)
5-HPF mimics DR and regulates autophagy
5-HPF was found to act independently of the IS/ILS pathway, so we next examined other longevity pathways. To examine the connection of the DR pathway, we studied the effect of 5-HPF in eat-2 mutants (Fig. 4b) and found that 50 μM 5-HPF failed to enhance the mean lifespan of eat-2 (23.63 ± 0.46, p = 0.660) compared to the control (23.66 ± 0.67). We also observed that 50 μM 5-HP- treated wild-type worms possess reduced brood size (270.8 ± 3.6) in comparison to control (289.8 ± 7.3) (Fig. 4 c and d).
Fig. 4.
Effect of 5-HPF on dietary restriction. a Survival curves of the let-363 mutant in the presence and absence of 50 μM 5-HPF; 5-HPF significantly failed to extend the mean lifespan. b 50 μM 5-HPF-treated eat-2 (DA1116) mutant (− 0.12%, p = 0.660) failed to extend the mean lifespan of worm in comparison with the control group. c 50 μM 5-HPF treatment exhibits a substantial reduction in brood size and also d prolongs reproductive span. The data were analyzed using the Kaplan–Meier survival analysis and independent t test wherever applicable in GraphPad Prism version 5. Differences between the data were considered significant at p ≤ .05(*p value < 0.05)
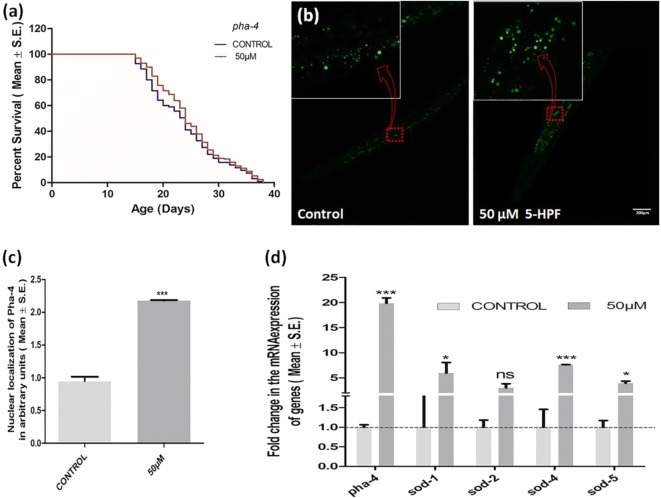
PHA-4/FOXA transcription factor is vital for DR-mediated lifespan extension, and its activity is independent of the IS/ILS pathway. So next, we evaluated the effect of 5-HPF treatment on pha-4 mutant worms. Fifty micromolars 5-HPF was unable to enhance the mean lifespan of pha-4 (13.88 ± 0.326, p = 0.761) compared to the control (13.98 ± 0.3) (Fig. 5a). Therefore, like eat-2 mutant, 5-HPF also requires PHA-4 for longevity. Besides, we also found increased nuclear localization of pha-4 in intestinal cells of pha-4::gfp-tagged worms upon treatment with 50 μM 5-HPF (2.17 ± 0.10, p ≤ 0.001) in comparison to the normalized value of control (0.94 ± 0.068) (Fig. 5 b and c). Moreover, we have also evaluated the 5-HPF treatment on let-363 (ortholog of human MTOR (mechanistic target of rapamycin kinase)) mutant worms. Fifty micromolar 5-HPF failed to extend the mean lifespan of let-363 (16.07 ± 0.25, p = 0.89) compared to the control (16.45 ± 0.46). Besides pha-4, SKN-1/NRF2 transcription factor also regulates DR-induced longevity in C. elegans. So next, we studied the role of skn-1 in 5-HPF-mediated longevity. It was found that 50 μM 5-HPF treatment failed to enhance the lifespan of the skn-1 mutant (13.97 ± 0.27) compared to control (13.73 ± 0.26) (Fig. 3b). In order to further validate our results, we performed drug uptake studies in various genetic backgrounds (N2, eat-2, skn-1, pha-4) by using the absorption spectrometry. Insignificant alterations were observed in the drug uptake studies in mutants (eat-2, skn-1, pha-4) as compared to wild type (Fig. S6a-d; Table S4). We further cross-checked our results by calculating bioconcentration factor and insignificant changes were observed in different genetic backgrounds (Fig. S6e; Table S4).
Fig. 5.
5-HPF mimics dietary restriction in the regulation of pha-4. a Survival curves of the pha-4 mutant in the presence and absence of 50 μM 5-HPF. No significant augmentation in the mean lifespan of the mutant was observed in pha-4 (SM190) mutant (0.71%, p = 0.761). b Representative image of nuclear localization of pha-4 using PHA-4::GFP in intestinal cells treated with 50 μM 5-HPF and control. c Graphical representation of nuclear localization of pha-4 using PHA-4::GFP in intestinal cells treated with 50 μM 5-HPFand control. d 5-HPF treatment significantly upregulated the fold change in the mRNA expression level of pha-4 and its downstream target genes sod-1, sod-4, and sod-5. The images were quantified by determining the average pixel intensity in each worm using ImageJ software (NIH). The data were analyzed using the Kaplan–Meier survival analysis, two-way ANOVA, and independent t test wherever applicable in GraphPad Prism version 5. Differences between the data were considered significant at p ≤ .05(*p value < 0.05 and ***p value < 0.001; scale bar = 200 μm, N = 30/group)
These evidences suggest that 5-HPF treatment exhibits longevity effects similar to DR. We also examined sir-2.1 for its role in 5-HPF-mediated longevity and observed that 50 μM 5-HPF significantly enhances the mean lifespan by 18.61% (20.01 ± 0.501, p ≤ 0. 001) (Fig. S1b-c) as compared with control (16.87 ± 0.47). SIR-2.1 stimulates activation of AMPK pathway member AAK-2 that contributes to longevity (Mansfeld 2015). So next, we evaluated the effect of 5-HPF treatment on aak-2 (an ortholog of human PRKAA1 (protein kinase AMP-activated catalytic subunit alpha 1) and PRKAA2 (protein kinase AMP-activated catalytic subunit alpha 2) mutant worms. Fifty micromolars 5-HPF significantly enhanced the mean lifespan of aak-2 by 22% (18.32 ± 0.68, p ≤ 0. 001) compared to the control (14.96 ± 0.39) (Fig. S1a-c). Although 5-HPF significantly increases the lifespan in both sir-2.1 and aak-2 mutants (Fig. S1a-c), it is not to the same level as wild type, i.e., the increased lifespan of sir-2.1 and aak-2 mutants treated with 5-HPF was found significantly less than that of wild-type worms (Fig. S1d) Therefore, this result indicates that both sir-2.1 and aak-2 are partially involved in 5-HPF-mediated longevity.
Further, the role of autophagy genes bec-1 and unc-51 was also studied. We observed that 5-HPF treatment (50 μM) failed to extend the mean lifespan of bec-1 mutant (18.88 ± 0.25, p = 0.864) as well as unc-51 mutant (10.79 ± 0.37, p = 0.446) as compared to respective control (18.95 ± 0.23; 10.56 ± 0.34) (Fig. 6 a and b). Besides, we also found increased levels of autophagy puncta in seam cells of lgg-1::gfp in 50 μM 5-HPF-treated worms (1.94 ± 0.13, p ≤ 0.001) over the normalized value of control (1.00 ± 0.18) (Fig. 6 c and d).
Fig. 6.
5-HPF extends the mean lifespan of C. elegans by altering the autophagy pathway. a No significant augmentation in the mean lifespan of the mutant was observed in unc-51 (BE150) mutant (2.17%, p = 0.446). b 50 μM 5-HPF-treated bec-1 (VC424) mutant (− 0.36%, p = 0.864) failed to extend the mean lifespan of worm in comparison with the control group. c Representative image of localization of lgg-1::gfp puncta in autophagic membranes treated with 50 μM 5-HPF and control. d Graphical representation of localization of lgg-1::gfp puncta in autophagic membranes treated with 50 μM 5-HPF and control. e 5-HPF treatment significantly upregulated the fold change in the mRNA expression level of genes bec-1 and lgg-1. The images were quantified by determining the average pixel intensity in each worm using ImageJ software (NIH). The data were analyzed using the Kaplan–Meier survival analysis, two-way ANOVA, and independent t test wherever applicable in GraphPad Prism version 5. Differences between the data were considered significant at p ≤ .05 (*p value < 0.05 and ***p value < 0.001; scale bar = 200 μm, N = 30/group)
5-HPF regulates the expression of skn-1 and pha-4 target genes
5-HPF treatment significantly upregulated the fold change in the mRNA expression level of insulin signaling pathway genes daf-2 (6.77-fold) and daf-16 (5.15-fold) (Fig. 3d). Downstream targets of SKN-1 coordinately affect the phenomena of longevity in the entire cellular system. We observed a significant upregulation of skn-1 (4.12-fold), gst-7 (11.91-fold), and gcs-1 (8.08-fold). This justifies the observed oxidative stress resistance along with the upregulation of daf-9 (4.59-fold) which is crucial for xenobiotic detoxification (Lindblom and Dodd 2006) (Fig. 3d). In addition to this, we also observed an increase in mRNA transcript levels of pha-4 (1.34-fold), and its downstream target genes sod-1 (5.95-fold), sod-4 (7.59-fold), and sod-5 (3.94-fold) (Fig. 5d). Moreover, 5-HPF treatment significantly upregulated the fold change in the mRNA expression level of genes bec-1 (2.34-fold) and lgg-1 (2.73-fold) confirming the role of 5-HPF in autophagy mediated by DR (Fig. 6e).
Discussion
5-HPF is a polymethoxyflavone, found in Gardenia lucida, Citrus sp., Mentha piperita, and Thymus sp. (Akao et al. 2008; Georgiou et al. 2015; Mimica-Dukic and Bozin 2008; Trivedi et al. 2017). 5-HPF exhibits several bioactivities, such as antibacterial, antioxidant, anti-inflammatory, antihypertension, antiobesity, and neuroprotection activities (Maurya et al. 2017; Trivedi et al. 2017). We have previously reported that 5-HPF improves cholinergic function and oxidative stress tolerance in C. elegans (Trivedi et al. 2017). In the present study, we showed that 5-HPF enhances the lifespan of nematode C. elegans in a dose-dependent manner (Fig. 1 a–c, Table S1). Notably, 50 μM 5-HPF treatment enhanced the mean lifespan by 28% to an extent higher than several phytomolecules within the same chemical group (Kampkötter et al. 2008; Yao et al. 2012; Yang et al. 2020). Lifespan extension by 5-HPF is likely to involve DR-related nutrient-sensing pathways. Our data show that 5-HPF extended the lifespan of C. elegans, curtailed the age-related biomarkers, and improved the resistance to oxidative stress. The free radical theory of aging is a widely accepted hypothesis that describes reactive oxygen species (ROS) as tremendously reactive molecules that contribute to aging and age-related manifestations (Gladyshev 2014; Harman 1992). Excess ROS generated during cellular metabolism and damage to DNA, proteins, and lipids contribute to aging (Goh et al. 2019). Polymethoxyflavones exert indirect antioxidant potential by the modulation of antioxidant defense systems (Büchter et al. 2013; Yao et al. 2012). The ROS scavenging ability of polymethoxyflavones has previously been reported (Finkel and Holbrook 2000). The results showed a decrease in total ROS, supporting our hypothesis about the mitigated ROS-mediated lifespan extension (Fig. 2b). Previous reports suggest the role of oxidative stress resistance in the lifespan extension (Grünz et al. 2012; Nohara et al. 2019). Consequently, in the present study, 5-HPF exhibited enhanced oxidative stress resistance against exposure to a ROS inducer, 5-hydroxy-1, 4-naphthalenedione (Fig. 2 a and c).
There is an inverse correlation between longevity and fecundity or brood size in C. elegans (Berman and Kenyon 2006). Kirkwood has reported that lifespan extension is accompanied by a reduced reproduction rate (Liu et al. 2013). Notably, germline ablation has been reported to increase lifespan in C. elegans (Berman and Kenyon 2006). As expected, we observed a decrease in brood size, along with an increase in the reproductive span in treated worms. These results imply that 5-HPF contribute to lifespan extension in a manner similar to DR. Notably, curtailed brood size is a characteristic feature of DR-mediated lifespan extension. Increased reproductive span correlates with the extended lifespan in treated worms (Fig. 4 c and d). Accumulation of age pigment, lipofuscin, with the advancement in age is pathologically connected with various age-associated disorders (Skoczyńska et al. 2017). It has been reported that an increased level of intracellular ROS contributes to the aggregation of lipofuscin (König et al. 2017). The reduced levels of lipofuscin in 5-HPF treated worms (Fig. 1 d and e) correlate with mitigated levels of free radicals. Previous studies have linked altered lipid metabolism with aging (Dexter et al. 1994; (Ackerman and Gems 2012). Due to their methylation potential, polymethoxyflavones possess a greater degree of lipid-solubilizing activity (Yang et al. 2020). We observed a significant reduction in triglyceride levels upon treatment with 5-HPF (Fig. S5c-e). Besides, a significant decrease in lipid levels (Fig. 1 f and g) suggested the fat-solubilizing potential of 5-HPF.
After the efficacy of 5-HPF against aging endpoints was established, the study was furthered to identify underlying longevity pathways using mutants and transcriptional profiling. The insulin/IGF-1 signaling pathway is highly conserved among species, and its major transcription factor DAF-16 plays a significant role in growth, lifespan, and detoxification mechanism (Sun et al. 2017). Moreover, it regulates aging, stress resistance, and immunity in C. elegans. It has been reported that DAF-16 is responsible for the activation of major antioxidant machinery (Klotz et al. 2015). Also, daf-16 mutants lack resistance to oxidative stress suggesting the vital role of transcriptional targets of DAF-16 in conferring stress resistance (Honda and Honda 1999). In consensus to this, the lifespan-extending property of 5-HPF is independent of the IIS pathway-related genes daf-16 and daf-2. Lifespan extension as a result of 5-HPF treatment remained unaltered in daf-16 and daf-2 mutant worms (Fig. 3 a and c). The NAD+/Sirtuin signaling pathway interacts with the DAF-16 transcription factor to contain oxidative stress. SIR-2.1 and AMPK both are evolutionarily conserved energy sensors that respond against augmented levels of cellular AMP and NAD+ concentrations. Moreover, SIR-2.1 stimulates the activation of AMPK that has been reported to reduce intracellular ROS level against any ROS generator. The results from the mutant study indicated that 5-HPF significantly increases the lifespan in both sir-2.1 and aak-2 mutants (Fig. S1a-c). However, the increased lifespan was significantly lesser in comparison to wild-type worms (Fig. S1d). Thus, the results reveal the partial involvement of sir-2.1 and aak-2 in 5-HPF-mediated longevity.
SKN-1 plays a diverse role in the enhancement of life expectancy during oxidative and ER stress, dietary restriction, proteasome action, and lowered translation (Ogawa et al. 2016). In the present study, 5-HPF failed to augment the lifespan of skn-1 mutants (Fig. 3b). 5-HPF was found to upregulate the mRNA expression of skn-1 and its downstream target gcs-1, gst-7, and daf-9 in wild-type worms (Fig. 3d). Mutant and qPCR studies identified that skn-1 was critical to 5-HPF-mediated lifespan extension. In C. elegans, daf-9 is equivalent to cytochrome P450 that regulates phase-I–induced longevity that might depend on the dietary restriction (DR) pathway (Baumeister et al. 2006). Various plant-derived molecules have been reported to show antimicrobial activity. Considering the relation between antimicrobial activity, pharyngeal pumping, and DR activity, worms were devoid of any alteration in the pharyngeal pumping of wild-type N2 worm (Fig. S4) and no bacterial zone of inhibition was observed. Hence, we concluded that DR did not result due to any bactericidal activity (Fig. S3 a and b). Previous studies reported that besides SKN-1/NRF2, PHA-4/FOXA is also necessary for DR-induced lifespan extension in C. elegans (Smith-Vikos et al. 2014). Reduced TOR signaling triggers autophagy by activating PHA-4/FOXA and reduces translation by downregulating ribosomal S6 kinase 1 (RSKS-1) (Lee et al. 2015b). DR induced by TOR signaling has also been influenced by eat-2, under the regulation of PHA-4/FOXA (Hansen et al. 2007; Meissner et al. 2004).The eat-2 mutant is feeding defective and exhibits DR independent of DAF-16. Surprisingly, we observed that 5-HPF failed to augment the lifespan in let-363 and eat-2 mutants (Fig. 4a, b). It also affects the PHA-4 expression (Fig. 5 b and c) in pharyngeal and intestinal cells in PHA-4::GFP reporter strain and failed to enhance the mean lifespan of pha-4 mutant worms (Fig. 5a). This was further validated by qPCR studies, which exhibited upregulated expression of pha-4 and its target genes sod-1, sod-4, and sod-5. The C. elegans genome has five SOD genes including sod-1, sod-4, and sod-5 (Lee et al. 2003). The sod-1, sod-4, and sod-5 are cytoplasmic Cu/Zn superoxide dismutases that have consensus PHA-4-binding sites. These genes are known to protect against oxidative damage and are also required for redox maintenance (Fukai and Ushio-Fukai 2011). The response to dietary restriction involves the PHA-4-dependent expression of sod-1, sod-2, sod-4, and sod-5 (Panowski et al. 2007). Hence, the 5-HPF-related longevity is majorly dependent on dietary restriction pathway. Moreover, the vital role of autophagy in DR-induced longevity in C. elegans has also been reported. In C. elegans, unc-51 is involved in autophagy. ULK-1 (unc-51 like autophagy activating kinase 1) and ULK-2 (unc-51 like autophagy activating kinase 2) are human orthologs of unc-51(Ogura et al. 1994). Similarly, bec-1 is the ortholog of the human tumor suppressor gene Beclin1 (Ruck et al. 2011). This gene collaborates with class III PI3 kinase VPS-34 and is critical for autophagy, endocytosis, and membrane trafficking (Gelino et al. 2016; Melendez and Levine 2009). Recent findings suggest that bec-1 and daf-16 act in parallel pathways, augmenting lifespan in daf-2 mutants (Lionaki et al. 2013). Enhanced expression of bec-1 triggers autophagy, decreases α-synuclein toxicity, and alleviates related neuritic alterations. Involvement of the autophagy was confirmed through studies on bec-1 and unc-51 mutants. 5-HPF failed to extend the mean lifespan of bec-1 and unc-51 mutants (Fig. 6 a and b), identifying the underlying role of bec-1 and unc-51 in 5-HPF-mediated lifespan extension.
Enhanced autophagy was further confirmed through transgenic strain DA2123, expressing lgg-1::gfp. C. elegans, gene lgg-1, is the homolog of yeast Atg8 and mammalian MAP-LC3, which is vital for the degradation of cellular components through autophagy. It has been reported that lgg-1 enhances normal Dauer formation and lifespan (Alberti et al. 2010). On the other hand, in extended lifespan showing worms, LGG-1 localizes at hypodermal seam cells. It enhances the number of pre-autophagosomal and autophagosomal structures that are observed as punctate structures. The result showed a slight increase in punctate structures of LGG-1 representing augmented autophagy in 5-HPF-treated worm (Fig. 6 d and e). In the present study, 5-HPF significantly augments the mRNA expression of lgg-1 and bec-1 in wild-type worms (Fig. 6c) that supports the previous finding performed with DA2123 strain. Hence, this augmented mRNA expression level showed that 5-HPF leads to lgg-1-mediated autophagy in wild-type worms.
The above studies for the first time identified the stress-reducing and longevity-promoting potential of 5-HPF in the C. elegans model system. The study suggests that 5-HPF extends the lifespan by modulating the DR pathway, through activation of autophagy in eat-2/let-363/pha-4-dependent manner. Our study advocates the supplementation of 5-HPF in the development of useful therapeutic interventions that unravel a novel protective strategy for aging and age-linked diseases.
Electronic supplementary material
(DOCX 3.64 mb)
Acknowledgments
The authors are grateful to Director CSIR-CIMAP, Lucknow. Nematode strains and E. coli OP50 were provided by the C. elegans Genetics Center (CGC) University of Minnesota, MN, USA, funded by the NIH National Center for Research Resources (NCRR). ST is highly thankful to Dr. Shreesh Raj Sammi, School of Health Sciences and Purdue Institute for Integrative Neurosciences, Purdue University, and West Lafayette, Indiana, for his editorial help and suggestions during the revision of the paper. The authors are also thankful to Ms. Shruti Sharma, CSIR-CIMAP, Lucknow, for her help during experiments.
Funding information
ST received financial support from the University Grants Commission (UGC), New Delhi, India.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ackerman D, Gems D. The mystery of C. elegans aging: an emerging role for fat: distant parallels between C. elegans aging and metabolic syndrome? Bioessays. 2012;34:466–471. doi: 10.1002/bies.201100189. [DOI] [PubMed] [Google Scholar]
- Akao Y, Ohguchi K, Iinuma M, Nozawa Y. Interactive effects of polymethoxyflavones from Citrus on cell growth inhibition in human neuroblastoma SH-SY5Y cells. Bioorg Med Chem. 2008;16:2803–2810. doi: 10.1016/j.bmc.2008.01.058. [DOI] [PubMed] [Google Scholar]
- Alberti A, Michelet X, Djeddi A, Legouis R. The autophagosomal protein LGG-2 acts synergistically with LGG-1 in Dauer formation and longevity in C. elegans. Autophagy. 2010;6:622–633. doi: 10.4161/auto.6.5.12252. [DOI] [PubMed] [Google Scholar]
- Altintas O, Park S, Lee S-JV. The role of insulin/IGF-1 signaling in the longevity of model invertebrates, C. elegans and D. melanogaster. BMB Rep. 2016;49:81. doi: 10.5483/BMBRep.2016.49.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An JH, Vranas K, Lucke M, Inoue H, Hisamoto N, Matsumoto K, Blackwell TK. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proc Natl Acad Sci. 2005;102:16275–16280. doi: 10.1073/pnas.0508105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421:268. doi: 10.1038/nature01279. [DOI] [PubMed] [Google Scholar]
- Baumeister R, Schaffitzel E, Hertweck M. Endocrine signaling in Caenorhabditis elegans controls stress response and longevity. J Endocrinol. 2006;190:191–202. doi: 10.1677/joe.1.06856. [DOI] [PubMed] [Google Scholar]
- Berdichevsky A, Nedelcu S, Boulias K, Bishop NA, Guarente L, Horvitz HR. 3-Ketoacyl thiolase delays aging of Caenorhabditis elegans and is required for lifespan extension mediated by sir-2.1. Proc Natl Acad Sci. 2010;107:18927–18932. doi: 10.1073/pnas.1013854107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JR, Kenyon C. Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell. 2006;124:1055–1068. doi: 10.1016/j.cell.2006.01.039. [DOI] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MK, Evans JL, Luo Y. Beneficial effects of natural antioxidants EGCG and α-lipoic acid on life span and age-dependent behavioral declines in Caenorhabditis elegans. Pharmacol Biochem Behav. 2006;85:620–628. doi: 10.1016/j.pbb.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Büchter C, Ackermann D, Havermann S, Honnen S, Chovolou Y, Fritz G, Kampkötter A, Wätjen W. Myricetin-mediated lifespan extension in Caenorhabditis elegans is modulated by DAF-16. Int J Mol Sci. 2013;14:11895–11914. doi: 10.3390/ijms140611895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet J, Wagner JR. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb Perspect Biol. 2013;5:a012559. doi: 10.1101/cshperspect.a012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong W, Wang P, Qu Y, Tang J, Bai R, Zhao Y, Chunying Chen, Bi X. Evaluation of the influence of fullerenol on aging and stress resistance using Caenorhabditis elegans. Biomaterials. 2015;42:78–86. doi: 10.1016/j.biomaterials.2014.11.048. [DOI] [PubMed] [Google Scholar]
- Dexter DT, et al. Increased levels of lipid hydroperoxides in the parkinsonian substantia nigra: an HPLC and ESR study. Mov Disord. 1994;9:92–97. doi: 10.1002/mds.870090115. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Garagnani P, Morsiani C, Conte M, Santoro A, Grignolio A, Monti D, Capri M, Salvioli S. The continuum of aging and age-related diseases: common mechanisms but different rates. Front Med. 2018;5:61. doi: 10.3389/fmed.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelino S, Chang JT, Kumsta C, She X, Davis A, Nguyen C, Panowski S, Hansen M. Intestinal autophagy improves healthspan and longevity in C. elegans during dietary restriction. PLoS Genet. 2016;12:e1006135. doi: 10.1371/journal.pgen.1006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou D, Djeddi S, Skaltsa H. Secondary metabolites from Thymus numidicus Poiret. Biochem Syst Ecol. 2015;59:104e106. [Google Scholar]
- Gladyshev VN. The free radical theory of aging is dead. Long live the damage theory! Antioxid Redox Signal. 2014;20:727–731. doi: 10.1089/ars.2013.5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JXH, Tan LT-H, Goh JK, Chan KG, Pusparajah P, Lee L-H, Goh B-H. Nobiletin and derivatives: functional compounds from citrus fruit peel for colon cancer chemoprevention. Cancers. 2019;11:867. doi: 10.3390/cancers11060867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünz G, Haas K, Soukup S, Klingenspor M, Kulling SE, Daniel H, Spanier B. Structural features and bioavailability of four flavonoids and their implications for lifespan-extending and antioxidant actions in C. elegans. Mech Ageing Dev. 2012;133:1–10. doi: 10.1016/j.mad.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Hansen M, Taubert S, Crawford D, Libina L, Lee S-J, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6(1):95–110 [DOI] [PubMed]
- Hansen M, Chandra A, Mitic LL, Onken B, Driscoll M, Kenyon C. A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet. 2008:4. [DOI] [PMC free article] [PubMed]
- Harman D. Free radical theory of aging. Mutat Res/DNAging. 1992;275:257–266. doi: 10.1016/0921-8734(92)90030-s. [DOI] [PubMed] [Google Scholar]
- Ho C, Pan M, Lai C, Li S. Polymethoxyflavones as food factors for the management of inflammatory diseases neuropathology. 2012;20:337–41.
- Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- Kampkötter A, Timpel C, Zurawski RF, Ruhl S, Chovolou Y, Proksch P, Wätjen W. Increase of stress resistance and lifespan of Caenorhabditis elegans by quercetin. Comp Biochem Physiol B Biochem Mol Biol. 2008;149:314–323. doi: 10.1016/j.cbpb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Kennedy B, Steffen K, Kaeberlein M. Ruminations on dietary restriction and aging. Cell Mol Life Sci. 2007;64:1323–1328. doi: 10.1007/s00018-007-6470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Klotz L-O, Sánchez-Ramos C, Prieto-Arroyo I, Urbánek P, Steinbrenner H, Monsalve M. Redox regulation of FoxO transcription factors. Redox Biol. 2015;6:51–72. doi: 10.1016/j.redox.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K, Havermann S, Büchter C, Wätjen W. Caenorhabditis elegans as model system in pharmacology and toxicology: effects of flavonoids on redox-sensitive signalling pathways and ageing. Sci World J. 2014:2014. [DOI] [PMC free article] [PubMed]
- König J, Ott C, Hugo M, Jung T, Bulteau A-L, Grune T, Höhn A. Mitochondrial contribution to lipofuscin formation. Redox Biol. 2017;11:673–681. doi: 10.1016/j.redox.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne CF, Brenkman AB. Current methods in quantifying ROS and oxidative damage in Caenorhabditis elegans and other model organism of aging. Ageing Res Rev. 2013;12:918–930. doi: 10.1016/j.arr.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Lee SS, Lee RY, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet. 2003;33:40–48. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- Lee J, Kwon G, Lim Y-H. Elucidating the mechanism of Weissella-dependent lifespan extension in Caenorhabditis elegans. Sci Rep. 2015;5:17128. doi: 10.1038/srep17128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, An SWA, Artan M, Seo M, Hwang AB, Jeong D-E, et al. Genes and pathways that influence longevity in Caenorhabditis elegans. Aging Mech (Springer). 2015b:123–69.
- Li S, Pan M-H, Lai C-S, Lo C-Y, Dushenkov S, Ho C-T. Isolation and syntheses of polymethoxyflavones and hydroxylated polymethoxyflavones as inhibitors of HL-60 cell lines. Bioorg Med Chem. 2007;15:3381–3389. doi: 10.1016/j.bmc.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lindblom TH, Dodd AK. Xenobiotic detoxification in the nematode Caenorhabditis elegans. J Exp Zool A Comp Exp Biol. 2006;305:720–730. doi: 10.1002/jez.a.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionaki E, Markaki M, Tavernarakis N. Autophagy and ageing: insights from invertebrate model organisms. Ageing Res Rev. 2013;12:413–428. doi: 10.1016/j.arr.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Liu E-H, Zhao P, Duan L, Zheng G-D, Guo L, Yang H, Li P. Simultaneous determination of six bioactive flavonoids in Citri Reticulatae Pericarpium by rapid resolution liquid chromatography coupled with triple quadrupole electrospray tandem mass spectrometry. Food Chem. 2013;141:3977–3983. doi: 10.1016/j.foodchem.2013.06.077. [DOI] [PubMed] [Google Scholar]
- Long X, Spycher C, Han ZS, Rose AM, Müller F, Avruch J. TOR deficiency in C. elegans causes developmental arrest and intestinal atrophy by inhibition of mRNA translation. Curr Biol. 2002;12:1448–1461. doi: 10.1016/s0960-9822(02)01091-6. [DOI] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfeld J. Regulation of health and lifespan by activation of energy and nutrient sensors in the model organism Caenorhabditis elegans. Diss. ETH Zurich. 2015
- Maurya P, Singh S, Gupta MM, Luqman S. Characterization of bioactive constituents from the gum resin of Gardenia lucida and its pharmacological potential. Biomed Pharmacother. 2017;85:444–456. doi: 10.1016/j.biopha.2016.11.049. [DOI] [PubMed] [Google Scholar]
- Meissner B, Boll M, Daniel H, Baumeister R. Deletion of the intestinal peptide transporter affects insulin and TOR signaling in Caenorhabditis elegans. J Biol Chem. 2004;279:36739–36745. doi: 10.1074/jbc.M403415200. [DOI] [PubMed] [Google Scholar]
- Melendez A, Levine B. Autophagy in C. elegans. WormBook. 2009;24:1–26. doi: 10.1895/wormbook.1.147.1. [DOI] [PubMed] [Google Scholar]
- Mimica-Dukic N, Bozin B. Mentha L. species (Lamiaceae) as promising sources of bioactive secondary metabolites. Curr Pharm Des. 2008;14:3141–3150. doi: 10.2174/138161208786404245. [DOI] [PubMed] [Google Scholar]
- Miyata Y, Sato T, Imada K, Dobashi A, Yano M, Ito A. A citrus polymethoxyflavonoid, nobiletin, is a novel MEK inhibitor that exhibits antitumor metastasis in human fibrosarcoma HT-1080 cells. Biochem Biophys Res Commun. 2008;366:168–173. doi: 10.1016/j.bbrc.2007.11.100. [DOI] [PubMed] [Google Scholar]
- Nohara K, Nemkov T, D’Alessandro A, Yoo S-H, Chen Z. Coordinate regulation of cholesterol and bile acid metabolism by the clock modifier nobiletin in metabolically challenged old mice. Int J Mol Sci. 2019;20:4281. doi: 10.3390/ijms20174281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Kodera Y, Hirata D, Blackwell TK, Mizunuma M. Natural thioallyl compounds increase oxidative stress resistance and lifespan in Caenorhabditis elegans by modulating SKN-1/Nrf. Sci Rep. 2016;6:21611. doi: 10.1038/srep21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura K-I, Wicky C, Magnenat L, Tobler H, Mori I, Müller F, Ohshima Y. Caenorhabditis elegans unc-51 gene required for axonal elongation encodes a novel serine/threonine kinase. Genes Dev. 1994;8:2389–2400. doi: 10.1101/gad.8.20.2389. [DOI] [PubMed] [Google Scholar]
- Oliveira RP, Abate JP, Dilks K, Landis J, Ashraf J, Murphy CT, Blackwell TK. Condition-adapted stress and longevity gene regulation by Caenorhabditis elegans SKN-1/Nrf. Aging Cell. 2009;8:524–541. doi: 10.1111/j.1474-9726.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panowski SH, Wolff S, Aguilaniu H, Durieux J, Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007;447:550. doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- Pant A, Saikia SK, Shukla V, Asthana J, Akhoon BA, Pandey R. Beta-caryophyllene modulates expression of stress response genes and mediates longevity in Caenorhabditis elegans. Exp Gerontol. 2014;57:81–95. doi: 10.1016/j.exger.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Porta-de-la-Riva M, Fontrodona L, Villanueva A, Cerón J. Basic Caenorhabditis elegans methods: synchronization and observation. J Vis Exp. 2012:e4019. [DOI] [PMC free article] [PubMed]
- Robida-Stubbs S, Glover-Cutter K, Lamming DW, Mizunuma M, Narasimhan SD, Neumann-Haefelin E, Sabatini DM, Blackwell TK. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 2012;15:713–724. doi: 10.1016/j.cmet.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruck A, Attonito J, Garces KT, Nuñez L, Palmisano NJ, Rubel Z, et al. The Atg6/Vps30/Beclin 1 ortholog BEC-1 mediates endocytic retrograde transport in addition to autophagy in. Autophagy. 2014;7(4):386–400 [DOI] [PMC free article] [PubMed]
- Saul N, Pietsch K, Menzel R, Stürzenbaum SR, Steinberg CE. Catechin induced longevity in C. elegans: from key regulator genes to disposable soma. Mech Ageing Dev. 2009;130:477–486. doi: 10.1016/j.mad.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol Transl Integr. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- Skoczyńska A, Budzisz E, Trznadel-Grodzka E, Rotsztejn H. Melanin and lipofuscin as hallmarks of skin aging. Adv Dermatol Allergol/Postȩpy Dermatologii i Alergologii. 2017;34:97. doi: 10.5114/ada.2017.67070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ED, Tsuchiya M, Fox LA, Dang N, Hu D, Kerr EO, Johnston ED, Tchao BN, Pak DN, Welton KL. Quantitative evidence for conserved longevity pathways between divergent eukaryotic species. Genome Res. 2008;18:564–570. doi: 10.1101/gr.074724.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Vikos T, de Lencastre A, Inukai S, Shlomchik M, Holtrup B, Slack FJ. MicroRNAs mediate dietary-restriction-induced longevity through PHA-4/FOXA and SKN-1/Nrf transcription factors. Curr Biol. 2014;24:2238–2246. doi: 10.1016/j.cub.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, et al. Silymarin promotes longevity and alleviates Parkinson’s associated pathologies in Caenorhabditis elegans. J Funct Foods. 2017;31:32–43. [Google Scholar]
- Sun X, Chen W-D, Wang Y-D. DAF-16/FOXO transcription factor in aging and longevity. Front Pharmacol. 2017;8:548. doi: 10.3389/fphar.2017.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi S, Maurya P, Sammi SR, Gupta MM, Pandey R. 5-Desmethylnobiletin augments synaptic ACh levels and nicotinic ACh receptor activity: a potential candidate for alleviation of cholinergic dysfunction. Neurosci Lett. 2017;657:84–90. doi: 10.1016/j.neulet.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Müller F. Influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620–0. [DOI] [PubMed]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Yang X, Wang H, Li T, Chen L, Zheng B, Liu RH. Nobiletin delays aging and enhances stress resistance of Caenorhabditis elegans. Int J Mol Sci. 2020;21:341. doi: 10.3390/ijms21010341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Zhu X, Pan S, Fang Y, Jiang F, Phillips GO, Xu X. Antimicrobial activity of nobiletin and tangeretin against Pseudomonas. Food Chem. 2012;132:1883–1890. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 3.64 mb)