Abstract
Background
The impact of active cancer in COVID-19 patients is poorly defined; however, most studies showed a poorer outcome in cancer patients compared to the general population.
Methods
We analysed clinical data from 557 consecutive COVID-19 patients. Uni-multivariable analysis was performed to identify prognostic factors of COVID-19 survival; propensity score matching was used to estimate the impact of cancer.
Results
Of 557 consecutive COVID-19 patients, 46 had active cancer (8%). Comorbidities included diabetes (n = 137, 25%), hypertension (n = 284, 51%), coronary artery disease (n = 114, 20%) and dyslipidaemia (n = 122, 22%). Oncologic patients were older (mean age 71 vs 65, p = 0.012), more often smokers (20% vs 8%, p = 0.009), with higher neutrophil-to-lymphocyte ratio (13.3 vs 8.2, p = 0.046). Fatality rate was 50% (CI 95%: 34.9;65.1) in cancer patients and 20.2% (CI 95%: 16.8;23.9) in the non-oncologic population. Multivariable analysis showed active cancer (HRactive: 2.26, p = 0.001), age (HRage>65years: 1.08, p < 0.001), as well as lactate dehydrogenase (HRLDH>248mU/mL: 2.42, p = 0.007), PaO2/FiO2 (HRcontinuous: 1.00, p < 0.001), procalcitonin (HRPCT>0.5ng/mL: 2.21, p < 0.001), coronary artery disease (HRyes: 1.67, p = 0.010), cigarette smoking (HRyes: 1.65, p = 0.041) to be independent statistically significant predictors of outcome. Propensity score matching showed a 1.92× risk of death in active cancer patients compared to non-oncologic patients (p = 0.013), adjusted for ICU-related bias. We observed a median OS of 14 days for cancer patients vs 35 days for other patients.
Conclusion
A near-doubled death rate between cancer and non-cancer COVID-19 patients was reported. Active cancer has a negative impact on clinical outcome regardless of pre-existing clinical comorbidities.
Subject terms: Risk factors, Cancer therapy
Background
Since the beginning of the COVID-19 pandemic, cancer patients have been regarded as a vulnerable population.1–4 Early data reported a near two-fold risk of Sars-CoV-2 infection, a complicated course of infection and a higher fatality rate compared to non-oncologic patients.4–8 However, detailed data on the extent of the oncologic disease and anti-cancer therapies at Sars-CoV-2 diagnosis were often scant. Later analyses suggested a downsised risk of infection in cancer patients. We previously reported the experience of a referral Cancer Center in the epicentre of the Italian outbreak, with only 17 cases of Sars-CoV-2 being diagnosed among 1267 cancer patients on active medical treatment.9 In line with initial clinical suggestions, we registered a higher COVID-19 fatality in oncologic patients compared to the general population.10–12 In this uncertain scenario, major oncological societies released position papers recommending extreme caution in the management of cancer treatment, focusing on the undefined risk of a medical therapy impacting on the immune system.3,13–15 The worldwide spread of Sars-CoV-2 infection imposed a tough challenge for medical oncologists bearing the responsibility to treat cancer, an equally fatal disease.16–21 Consequently, efforts have been conducted to optimise cancer therapy during the pandemic and to better identify the features of poor outcome of the infection in cancer patients.22,23 Some published studies analysed demographic and clinical characteristics in this subgroup of patients detailing comorbidities, specific laboratory findings as well as radiological imaging at Sars-CoV-2 diagnosis.24,25
As a Cancer and COVID-19 referral centre, we also collected the aforementioned variables on the whole population of infected patients admitted in our Institution during the most intense period of the pandemic. We retrospectively analysed in a multivariate model, and confirmed by a propensity score, the weight of some of the most important aspects recognised as a risk factor for Sars-CoV-2 outcome, focusing on active cancer.12,26,27
Methods
Study design
We retrospectively reviewed the medical records of all consecutive adult patients admitted for COVID-19 at our Institution (a tertiary cancer centre with 662 beds, including 42 ICU beds) between February 27 and May 20, 2020. The diagnosis of Sars-CoV-2 infection was confirmed by a reverse-transcriptase polymerase chain reaction (RT-PCR) of nasopharyngeal swab or bronchoalveolar lavage (BAL). We collected data on demographics, smoking habits and comorbidities, including coronary artery disease (CAD), onco-haematologic disease, diabetes and hypertension. We collected also the clinical characteristics of Sars-CoV-2 at presentation, the ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2) laboratory findings including full blood count (FBC), inflammatory indexes (procalcitonin, PCT, CRP, ferritin, IL-6), lactate dehydrogenase (LDH) and radiological CT findings. We analysed SARS-CoV-2 active cancer patients focusing on the type of malignancy (solid tumour vs haematologic disease), the diagnosis (lung cancer, genitourinary-GU cancer, gastrointestinal-GI cancer, breast cancer and other)), the extent (localised vs metastatic) and the status of disease at the COVID-19 diagnosis, i.e. progressive disease (PD) vs non-PD (CR/PR/SD/NED). Active cancer was defined by the presence of localised or metastatic disease at the time of the viral infection, despite the received oncological treatment. Patients undergoing radical surgery or radical radio-chemotherapy within 4 weeks from COVID-19 diagnosis were also included in the analysis. Conversely, patients with a history of cancer or on adjuvant hormonal treatment were not considered in the cancer subgroup. Surrogate endpoints for COVID-19 survival included the length of hospitalisation, the ICU admission and the in-hospital fatality rate. The absence of prospective informed consent was waived by the Ethics Committee due to the emergency situation of the clinical scenario of the current pandemic.
Statistical analyses
Demographic and clinical characteristics were summarised as number and percentage or as median and range. Differences in distribution were estimated using the Chi-square or the Fisher exact test (when appropriate). Patients survival was calculated from the hospitalisation until death or discharge. Survival curves were generated using the Kaplan–Meier method. Median follow-up was estimated using the inverse Kaplan–Meier method. Differences between groups were evaluated using the log-rank test. The Cox proportional hazard regression model was used to calculate the hazard ratios (HRs) and their 95% confidence intervals (CI) in univariate and multivariate analysis. ICU was included in the model as a time-dependent variable starting from the first day of ICU admission. A propensity score matching was performed to estimate the effect of cancer by accounting for the covariates statistically significant in the multivariable model. For each cancer patient, four comparable patients were selected in the non-cancer population (1:4 ratio). All the reported p-values were two-sided. All analyses were carried out with the SAS software v. 9.4.
Results
Demographics and clinical features
We reported on 557 consecutive COVID-19 patients admitted at our Institution between February 27 and May 20, 2020, of whom 46 had active cancer (8%). Demographics, clinical and laboratory findings of COVID-19 patients are reported in Table 1. Most patients were men (n = 375, 67%), with a median age of 67 (range 27–96). Forty-eight patients (9%) were active smokers. With respect to comorbidities, 137 had diabetes (25%), 284 hypertension (51%), 114 CAD (20%) and 122 dyslipidaemia (22%). Comparing oncologic (n = 46) and non-oncologic patients (n = 511), the former were older (mean age 71 vs 65; p = 0.012), more often smokers (20% vs 8%; p = 0.009) and with higher neutrophil-to-lymphocyte ratio (NLR) (mean 13.3 vs 8.2; p = 0.046). The mean value of PaO2/FiO2 recorded in the emergency department was 283 in cancer patients vs 292 in non-cancer patients, which resulted not statistically significant (p = 0.558, Table 1).
Table 1.
Overall and by cancer demographics and clinic characteristics of 557 hospitalised patients with COVID-19.
| All patients | Non-cancer patients | Cancer patients | p-value | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| 557 | 100.0 | 511 | 91.7 | 46 | 8.3 | ||
| Gender | |||||||
| Male | 375 | 67.3 | 344 | 67.3 | 31 | 67.4 | 0.992 |
| Female | 182 | 32.7 | 167 | 32.7 | 15 | 32.6 | – |
| Age | |||||||
| (mean CI 95%) | 65 (64;68) | 65 (64;66) | 71 (67;74) | 0.012 | |||
| BMI | 27 | ||||||
| (mean 95% CI) | 27 (26.5;27.4) | – | 27.1 | 26.6–27.6 | 25.2 | 23.7–26.8 | 0.026 |
| <30 | 384 | 68.9 | 346 | 76.2 | 38 | 90.5 | 0.034 |
| ≥30 | 112 | 20.1 | 108 | 23.8 | 4 | 9.5 | |
| Missing | 61 | 11 | |||||
| Diabetes | |||||||
| No | 419 | 75.2 | 385 | 75.5 | 34 | 73.9 | 0.812 |
| Yes | 137 | 24.6 | 125 | 24.5 | 12 | 26.1 | – |
| Missing | 1 | 0.2 | – | – | – | – | – |
| Hypertension | |||||||
| No | 272 | 48.8 | 247 | 48.4 | 25 | 54.3 | 0.442 |
| Yes | 284 | 51.0 | 263 | 51.6 | 21 | 45.7 | – |
| Missing | 1 | 0.2 | – | – | – | – | – |
| Dyslipidemia | |||||||
| No | 434 | 77.9 | 401 | 78.6 | 33 | 71.7 | 0.28 |
| Yes | 122 | 21.9 | 109 | 21.4 | 13 | 28.3 | – |
| Missing | 1 | 0.2 | – | – | – | – | – |
| Smoking | |||||||
| No/former | 507 | 91 | 47.0 | 92.0 | 37 | 80.4 | 0.009 |
| Yes | 50 | 9.0 | 41 | 8.0 | 9 | 19.6 | – |
| CAD | |||||||
| No | 442 | 79.4 | 410 | 80.4 | 32 | 69.6 | 0.082 |
| Yes | 114 | 20.5 | 100 | 19.6 | 14 | 30.4 | – |
| Missing | 1 | 0.2 | – | – | – | – | – |
| Lymphocytes | |||||||
| <1000 | 282 | 50.6 | 251 | 49.1 | 31 | 67.4 | 0.018 |
| ≥1000 | 275 | 49.4 | 260 | 50.9 | 15 | 32.6 | – |
| LDH | |||||||
| <248 | 146 | 26.2 | 135 | 26.7 | 11 | 24.4 | 0.739 |
| ≥248 | 404 | 72.5 | 370 | 73.3 | 34 | 75.6 | – |
| Missing | 7 | 1.3 | – | – | – | – | – |
| IL-6 | |||||||
| No | 352 | 63.2 | 325 | 82.9 | 27 | 87.1 | 0.802 |
| Yes | 71 | 12.7 | 67 | 17.1 | 4 | 12.9 | – |
| Missing | 134 | 24.1 | – | – | – | – | – |
| PCT | |||||||
| <0.5 | 411 | 73.8 | 381 | 74.7 | 30 | 65.2 | 0.16 |
| ≥0.5 | 145 | 26.0 | 129 | 25.3 | 16 | 34.8 | – |
| Missing | 1 | 0.2 | – | – | – | – | – |
| CRP | |||||||
| <0.5 | 21 | 3.8 | 19 | 3.7 | 2 | 4.3 | 0.83 |
| ≥0.5 | 536 | 96.2 | 492 | 96.3 | 44 | 95.7 | – |
| Ferritin | |||||||
| <336.2 | 196 | 35.2 | 183 | 38.2 | 13 | 35.1 | 0.711 |
| ≥336.2 | 320 | 57.4 | 296 | 61.8 | 24 | 64.9 | – |
| Missing | 41 | 7.4 | – | – | – | – | – |
| NLR | |||||||
| (mean 95% CI) | 5.84 | 0.06;85 | 8.22 | 7.56;8.89 | 13.32 | 8.37;18.26 | 0.046 |
| PaO2/FiO2 | |||||||
| (mean 95% CI) | 304 (46;561) | 291.6 (283.3;300) | 283 (253.4, 312.5) | 0.558 | |||
| Ground glass opacities | |||||||
| No | 26 | 4.7 | 22 | 4.5 | 4 | 9.8 | 0.135 |
| Yes | 504 | 90.5 | 467 | 95.5 | 37 | 90.2 | |
| Missing | 27 | 4.8 | |||||
| Pulmonary consolidations | |||||||
| No | 383 | 68.8 | 355 | 72.6 | 28 | 68.3 | 0.554 |
| Yes | 147 | 26.4 | 134 | 27.4 | 13 | 31.7 | |
| Missing | 27 | 4.8 | |||||
| Pleural effusion | |||||||
| No | 462 | 82.9 | 434 | 88.8 | 28 | 68.3 | <0.001 |
| Yes | 68 | 12.3 | 55 | 11.2 | 13 | 31.7 | |
| Missing | 27 | 4.8 | |||||
| Pulmonary adenopathy | |||||||
| No | 375 | 67.3 | 345 | 70.6 | 30 | 73.2 | 0.723 |
| Yes | 155 | 27.9 | 144 | 29.4 | 11 | 26.8 | |
| Missing | 27 | 4.8 | |||||
CAD coronary artery disease, BMI body mass index, CI confidence interval, LDH lactate dehydrogenase, IL-6 interleukin-6, PCT procalcitonin, NLR neutrophil–lymphocyte ratio.
Statistically significant p < 0.05 values are in bold.
Survival analysis
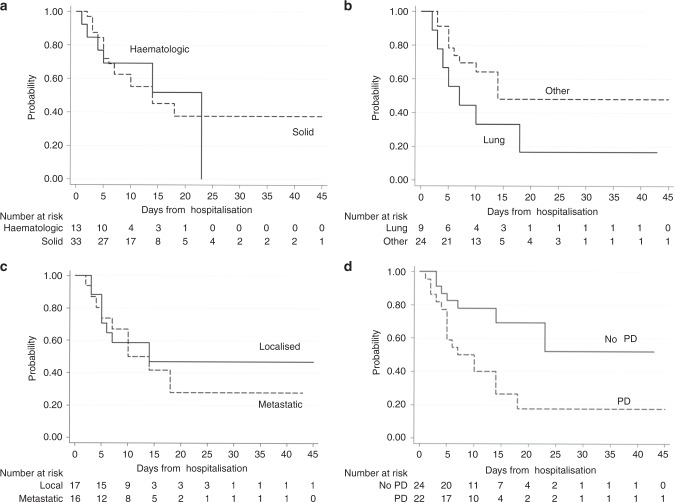
With a median follow-up of 12 days (range 0–76), 126 patients died (23%), of whom 23 were cancer patients. Considering the cancer patients cohort, the fatality rate was 50% (CI 95%: 34.9;65.1), whereas in the non-cancer subgroup was 20.2% (CI 95%: 16.8;23.9). Factors influencing the outcome in the univariable evaluation were age, hypertension, dyslipidaemia, diabetes, CAD, cancer, lymphocyte count, LDH level, PCT, smoking, NLR, PaO2/FiO2. Table 2 shows the survival-related hazard ratios (HR), 95% confidence interval (CI) and p-values. Multivariable Cox regression model (Table 3) confirmed the impact of active cancer (HR: 2.26, 95%, CI:1.39;3.66, p = 0.001, Fig. 1) adjusted for age (HRcontinuous: 1.08, p < 0.001), LDH (HRLDH>248: 2.42, p < 0.007), PaO2/FiO2 (HRcontinuous: 1.00, p < 0.001), PCT (HRPCT>0.5: 2.21, p < 0.001), CAD (HRyes: 1.67, p = 0.010) and cigarette smoking (HRyes: 1.65, p = 0.041) as independent statistically significant predictors of outcome. Propensity score matching performed considering multivariable statistically significant factors, demonstrated in the active cancer population a 1.92× risk of death compared to the non-cancer population, irrespectively of ICU admission (CI 95%: 1.15;3.21, p = 0.013) (Table 4) 1). Indeed, ICU admission was included as a time-dependent variable in the model (HRyes: 0.55, CI 95%: 0.25;1.20, p = 0.131) but did not influence the outcome. Hence, we registered a median OS of 14 days for cancer patients compared to 35 days for other patients (Fig. 1). Considering the cancer cohort, we did not observe any difference between solid and haematologic tumours (HR 1.04, CI 95%: 0.41;2.65, p = 0.931, Fig. 2a). Noteworthy, lung cancer patients showed a poor prognosis compared to other cancer diagnosis (Fig. 2b), albeit not statistically significant (HR: 1.93, CI 95%: 0.79;4.71, p = 0.148; 7 vs 14 median days of hospitalisation, p = 0.128). A full comparison in survival probability between tumour types is available online as Supplementary Material (Supplementary. Material 1). We did not report any differences in outcome between localised and metastatic disease (HR: 0.8; CI 95%: 0.31;2.08, p = 0.649, Fig. 2c) but, considering disease status at COVID-19 diagnosis, we reported a significantly worse COVID-19 outcome in patients with progressive disease (PD) compared to non-PD patients (HR: 2.931, CI 95% 1.2;7.14, p = 0.018, Fig. 2d). Extent of disease and delivered treatment are reported in Table 5 for each tumour type.
Table 2.
Univariable analysis in whole population: OS stratified by principal demographics and clinical characteristics
| Characteristics | HR | Lower 95% CI | Upper 95% CI | p-value |
|---|---|---|---|---|
| Gender | ||||
| Male vs female | 1.24 | 0.86 | 1.79 | 0.259 |
| Age | ||||
| Continuous values | 1.08 | 1.07 | 1.10 | <0.001 |
| BMI | ||||
| Continuous values | 0.98 | 0.94 | 1.02 | 0.312 |
| ≥30 | — | |||
| <30 | 0.66 | 0.40 | 1.03 | 0.113 |
| Diabetes | ||||
| No | — | |||
| Yes | 1.54 | 1.06 | 2.22 | 0.023 |
| Hypertension | ||||
| No | — | |||
| Yes | 1.68 | 1.15 | 2.45 | 0.008 |
| Dysplidaemia | ||||
| No | — | |||
| Yes | 1.63 | 1.12 | 2.37 | 0.011 |
| CAD | ||||
| No | — | |||
| Yes | 2.85 | 1.98 | 4.09 | <0.001 |
| Cancer | ||||
| No | — | |||
| Yes | 2.79 | 1.76 | 4.42 | <0.001 |
| Lymphocytes | ||||
| <1000 | — | |||
| ≥1000 | 0.53 | 0.36 | 0.77 | 0.001 |
| LDH | ||||
| <248 | — | |||
| ≥248 | 2.85 | 1.57 | 5.18 | 0.001 |
| IL-6 | ||||
| No | — | |||
| Yes | 0.71 | 0.36 | 1.38 | 0.306 |
| PCT | ||||
| <0.5 | — | |||
| ≥0.5 | 3.24 | 2.27 | 4.62 | <0.001 |
| CRP | ||||
| <0.5 | ||||
| ≥0.5 | 3.59 | 0.50 | 25.68 | 0.203 |
| Ferritin | ||||
| <336.2 | ||||
| ≥336.2 | 1.32 | 0.83 | 2.09 | 0.238 |
| Smoking | ||||
| No/former | ||||
| Yes | 3.17 | 2.03 | 4.95 | <0.001 |
| NLR | ||||
| 1.03 | 1.01 | 1.04 | <0.001 | |
| PaO2/FiO2 | ||||
| 0.99 | 0.99 | 1.00 | <0.001 | |
| Ground glass | ||||
| No | ||||
| Yes | 1.43 | 0.59 | 3.51 | 0.431 |
| Pulmonary consolidations | ||||
| No | — | |||
| Yes | 1.42 | 0.99 | 2.05 | 0.60 |
| Pleural effusion | ||||
| No | — | |||
| Yes | 1.10 | 0.75 | 1.60 | 0.625 |
| Pulmonary adenopathy | ||||
| No | — | |||
| Yes | 1.43 | 0.59 | 3.51 | 0.431 |
CAD coronary artery disease, BMI body mass index, CI confidence interval, LDH lactate dehydrogenase, IL-6 interleukin-6, PCT procalcitonin, NLR neutrophil–lymphocyte ratio.
Statistically significant p < 0.05 values are in bold.
Table 3.
Multivariable analysis in the whole hospitalised population.
| Variable | HR | Lower 95% CI | Upper 95% CI | p-value |
|---|---|---|---|---|
| Age (continuous values) | 1.08 | 1.06 | 1.1 | <0.001 |
| Cancer vs non-cancer | 2.26 | 1.39 | 3.657 | 0.001 |
| LDH (>248 vs <248 U/L) | 2.42 | 1.276 | 4.603 | <0.007 |
| PaO2/FiO2 | 0.99 | 0.994 | 0.998 | 0.001 |
| PCT (>0.5 vs <0.5 ng/mL) | 2.21 | 1.506 | 3.234 | <0.001 |
| CAD vs no CAD | 1.67 | 1.128 | 2.465 | 0.01 |
| Smoking vs no smoking | 1.65 | 1.02 | 2.679 | 0.041 |
CAD coronary artery disease, CI confidence interval, LDH lactate dehydrogenase, PCT procalcitonin.
Fig. 1. COVID-19 survival in cancer and non-cancer patients.
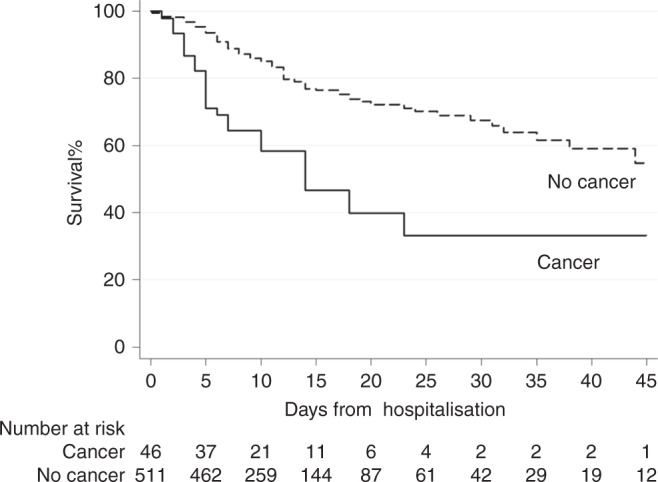
Cancer patients showed a poorer COVID-19 survival (HR: 2.26; CI 95%: 1.39;3.66, p = 0.001).
Table 4.
Propensity score matching.
| Non-cancer | Cancer | p-value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| All | 180 | 45 | |||
| Age (continuous values, mean CI 95%) | |||||
| 69.5 | 67.5;71.5 | 70.6 | 67.0;74.2 | 0.614 | |
| Diabetes | |||||
| No | 138 | 76.67 | 34 | 75.56 | 0.875 |
| Yes | 42 | 23.33 | 11 | 24.44 | |
| Hypertension | |||||
| No | 105 | 58.33 | 25 | 55.56 | 0.736 |
| Yes | 75 | 41.67 | 20 | 44.44 | |
| Dysplidemia | |||||
| No | 138 | 76.67 | 33 | 73.33 | 0.64 |
| Yes | 42 | 23.33 | 12 | 26.67 | |
| CAD | |||||
| No | 136 | 75.56 | 32 | 71.11 | 0.54 |
| Yes | 44 | 24.44 | 13 | 28.89 | |
| LDH (U/L) | |||||
| <248 | 40 | 22.22 | 11 | 24.44 | 0.75 |
| ≥248 | 140 | 77.78 | 34 | 75.56 | |
| PCT (ng/mL) | |||||
| <0.5 | 124 | 68.89 | 29 | 64.44 | 0.568 |
| ≥0.5 | 56 | 31.11 | 16 | 35.56 | |
| Smoking | |||||
| No ex | 149 | 82.78 | 36 | 80.00 | 0.663 |
| Yes | 31 | 17.22 | 9 | 20.00 | |
| PaO2/FiO2 | |||||
| (mean CI 95%) | 284.8 | 272;297.5 | 280.9 | 251;310.8 | 0.795 |
CAD coronary artery disease, CI confidence interval, LDH lactate dehydrogenase, PCT procalcitonin.
Fig. 2. COVID-19 survival stratified by subtype of cancer and status of disease.
a solid cancer vs haematologic cancer; b tumour type (lung vs other); c localised vs metastatic disease; d disease status at COVID-19 diagnosis (PD vs non-PD cancer patients).
Table 5.
Extent of disease, status of disease at COVID-19 diagnosis and treatment received according to tumour diagnosis.
| Diagnosis | Patients (n = 46) | Extent of disease | Status of disease at COVID-19 diagnosis | Treatment received | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Localised | Metastatic/Systemic | PD | non-PD | NED | Naive | Surgery | RT | CT | Ig | Target | Hormone | ||
| Solid tumour | 33 | 17 | 16 | 19 | 10 | 4 | 15 | 3 | 4 | 8 | 2 | 1 | 4 |
| Lung | 9 | 2 | 7 | 6 | 2 | 1 | 5 | 1 | 1 | 1 | 1 | 0 | 0 |
| GI | 10 | 8 | 2 | 7 | 1 | 2 | 6 | 2 | 1a | 2a | 0 | 0 | 0 |
| Breast | 3 | 1 | 2 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 2 |
| GU | 6 | 3 | 3 | 4 | 2 | 0 | 3 | 0 | 0 | 1b | 1b | 1 | 1 |
| Otherc | 5 | 3 | 2 | 2 | 3 | 0 | 1 | 0 | 2a | 3a | 0 | 0 | 1 |
| Haematologic | 13 | 0 | 13 | 3 | 8 | 2 | 6 | 0 | 1 | 5 | 2 | 0 | 0 |
| AML | 3 | 0 | 3 | 3 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 |
| MDS | 4 | 0 | 4 | 0 | 4 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 0 |
| LLC | 3 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| LMC | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1a,b | 1b | 0 | 0 |
| NHL | 2 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | 1a | 2a,b | 1b | 0 | 0 |
GI gastrointestinal, GU genitourinary, AML acute myeloid leukemia, MDS myelodysplastic syndrome, CLL chronic lymphocytic leukemia, CML chronic myeloid leukemia, NHL non-Hodgkin lymphoma.
aOne patient underwent chemo-radiation.
bOne patient underwent immuno-chemotherapy.
cTwo patients had head&neck cancer, one had glioblastoma, one had neuroendocrine tumour and one had unknown primary tumour.
Discussion
In our retrospective analysis, we have reported that both the epidemiology and clinical presentation of COVID-19 in active cancer patients in Italy are similar to the non-cancer population This notwithstanding, we observed how the natural course of the COVID-19, as well as the final outcome, are significantly worse in cancer patients, resulting in an almost double fatality rate (HR: 1.92, propensity score result). Working at an Institution extensively involved in the COVID-19 emergency, we had the opportunity to evaluate a large number of admitted patients, collecting detailed clinical, laboratory and radiological data, including comorbidities such as cancer and related treatment.9 In the current analysis, the demographics and clinical characteristics of cancer and non-cancer patients were similar including BMI.28 A male predominance in COVID-19, possibly explained by differences in innate and adaptive immunity, has been confirmed.29 At the time of COVID-19 diagnosis, the clinical presentation was similar among the two cohorts of patients. Unexpectedly, the respiratory impairment evaluated through PaO2/FiO2, as well as chest CT scan performed in the Emergency Department did not show any significant differences. As we had previously published,9 active cancer and relative treatments, including chemotherapy, immunotherapy and targeted therapies, did not result in an increased risk of Sars-CoV-2 infection.9 The lack of standardised criteria to define active cancer patients might have been responsible for the initial worries regarding the reported high incidence of cancer patients among Sars-CoV-2 infected individuals.30,31 A detailed analysis of published case-series showed most of them were likely patients with a history of cancer, rather than with active cancer.
Even if the risk for Sars-CoV-2 infection and the clinical presentation of COVID-19 are similar, it does result in a double mortality rate in cancer patients compared to non-cancer patients in a multivariable analysis (HRactive: 2.21). Unexpectedly, we did not notice any differences between solid and haematologic cancers. However, focusing on the histological diagnosis, we observed that only few patients were affected by aggressive blood diseases (e.g. AML or NHL) rather than chronic, indolent disease that would arguably affect the course of the infection. Several efforts have been made to decipher the negative influence of cancer on COVID-19 outcome. Our results confirm the higher mortality rate among cancer patients compared to non-oncological populations.6,10–12,32 In stark contrast with such reports, a matched cohort study from the Presbyterian Hospital (New York, USA) reported similar outcomes in cancer and non-cancer COVID-19 patients. Unlike previous studies, the authors included in the cancer subgroup, either patients who received active cancer treatment and patients on follow-up who received the last oncologic therapy up to 6 months before the admission for COVID-19. We adopted more stringent criteria including in the cancer cohort only those patients with localised or metastatic disease who received diagnosis or therapy within 4 weeks before the admission for COVID-19. Several factors could play a role in the fatal course of COVID-19 in patients with active cancer. First of all, cancer-related inflammation, as well as the associated prothrombotic status typically related to uncontrolled solid or haematologic cancer growth, could be responsible for the unfavourable prognosis in hospitalised COVID-19 patients.33–35 We suspected also a higher incidence of bacterial co-infection in the oncologic cohort, with a potential detrimental effect on outcome. Still, our study did not support this hypothesis as PCT values were comparable among the two groups. In line with our findings, a recent meta-analysis on 3834 patients showed a low proportion of COVID-19 patients having bacterial co-infection.36
Our study has some limitations. We acknowledge that ascribing the ultimate cause of death in cancer patients with COVID-19 is challenging. However, our results highlight that patients with newly diagnosed uncontrolled cancer, as well as progressive disease, are more likely to show a poor prognosis in case of COVID-19 infection, which may be related to an impaired immunological response. A further potential bias might be represented by the availability of intensive care in the ICU in a scenario of limited resources. Despite the low number of events, we proved by the propensity score analysis that admission to the ICU did not account for differences in outcome between the two cohorts of patients. Finally, the mono-institutional nature of our study prevented us from recruiting a large number of patients, thus limiting our analysis, especially in some specific histiotypes (e.g. aggressive blood disease). In conclusion, despite a comparable clinical presentation, we report a near two-fold increase in death rate between cancer and non-cancer COVID-19 patients admitted at a tertiary referral Italian hospital. Our data suggest uncontrolled cancer diagnosis to independently impact on clinical outcome regardless of other clinical characteristics including pre-existing comorbidities. To date, the understanding of the natural course of COVID-19 in active cancer patients is limited, and requires further cooperative efforts to be unfolded. Considering the vulnerable status of patients with active cancer in the current pandemic, state-of-the art cancer care should guarantee the continuity of treatment along with a direct engagement of multidisciplinary stakeholders to meet patients’ needs.37,38
Supplementary information
Acknowledgements
Not applicable.
Author contributions
A.F.B., M.C.—Drafting of manuscript, Data Interpretation, Study Concept; A.F.B., N.G., A.M., U.C., A.S.—Drafting of manuscript, Data Interpretation; M.C., A.S., M.A., V.L.Q.—Drafting of manuscript, Study Supervisors; A.D., A.F.B., A.M., M.C.—Drafting of manuscript, Data collection; L.G.—Drafting of manuscript, Statistical analysis.
Ethics approval and consent to participate
Ethics approval was provided by the local ethics committee of Humanitas Clinical and Research Center; the study was performed in accordance with the Declaration of Helsinki. Consent to participate was obtained from the patients involved in the study.
Consent to publish
Consent for publication have be obtained from the patients involved in the study.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Funding information
The authors received no specific funding for this work.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Alexia F. Bertuzzi, Michele Ciccarelli
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01396-9.
References
- 1.Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kutikov A, Weinberg DS, Edelman MJ, Horwitz EM, Uzzo RG, Fisher RI. A war on two fronts: cancer care in the time of COVID-19. Ann. Intern. Med. 2020;172:756–758. doi: 10.7326/M20-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia Y, Jin R, Zhao J, Li W, Shen H. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21:e180. doi: 10.1016/S1470-2045(20)30150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sidaway P. COVID-19 and cancer: what we know so far. Nat. Rev. Clin. Oncol. 2020;17:336. doi: 10.1038/s41571-020-0366-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee LY, Cazier J-B, Angelis V, Arnold R, Bisht V, Campton NA, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ofori-Asenso R, Ogundipe O, Agyeman AA, Chin KL, Mazidi M, Ademi Z, et al. Cancer is associated with severe disease in COVID-19 patients: a systematic review and meta-analysis. Ecancermedicalscience. 2020;14:1047. doi: 10.3332/ecancer.2020.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang K, Sheng Y, Huang C, Jin Y, Xiong N, Jiang K, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mei H, Dong X, Wang Y, Tang L, Hu Y. Managing patients with cancer during the COVID-19 pandemic: frontline experience from Wuhan. Lancet Oncol. 2020;21:634–636. doi: 10.1016/S1470-2045(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertuzzi, A. F., Marrari, A., Gennaro, N., Cariboni, U., Ciccarelli, M., Giordano, L. et al. Low incidence of SARS-CoV-2 in patients with solid tumours on active treatment: an observational study at a Tertiary Cancer Centre in Lombardy, Italy. Cancers (Basel). 12, 2352 (2020). [DOI] [PMC free article] [PubMed]
- 10.Gosain R, Abdou Y, Singh A, Rana N, Puzanov I, Ernstoff MS. C. O. V. I. D.-1. 9. and Cancer: a Comprehensive Review. Curr. Oncol. Rep. 2020;22:53. doi: 10.1007/s11912-020-00934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ElGohary, G. M., Hashmi, S., Styczynski, J., Kharfan-Dabaja, M. A., Alblooshi, R. M., de la Cámara, R. et al. The risk and prognosis of COVID-19 infection in cancer patients: a systematic review and meta-analysis. Hematol. Oncol. Stem Cell Ther. (2020). in press. [DOI] [PMC free article] [PubMed]
- 13.ASCO Statement on Novel Coronavirus (COVID-19). ASCO Annual Meeting. https://meetings.asco.org/am/asco-statement-novel-coronavirus-covid-19 (2020).
- 14.COVID-19 and Cancer. ESMO. https://www.esmo.org/covid-19-and-cancer (2020).
- 15.Burki TK. Cancer guidelines during the COVID-19 pandemic. Lancet Oncol. 2020;21:629–630. doi: 10.1016/S1470-2045(20)30217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giuliani, J. & Bonetti, A. Cancer prevales on COVID-19: To maintain high quality standard concerning diagnosis and oncological care even during a pandemic. J. Med. Virol.93, 118–119 (2020). [DOI] [PMC free article] [PubMed]
- 17.de Joode K, Dumoulin DW, Engelen V, Bloemendal HJ, Verheij M, van Laarhoven HWM, et al. Impact of the coronavirus disease 2019 pandemic on cancer treatment: the patients’ perspective. Eur. J. Cancer. 2020;136:132–139. doi: 10.1016/j.ejca.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Quteimat OM, Amer AM. The impact of the COVID-19 pandemic on cancer patients. Am. J. Clin. Oncol. 2020;43:452–455. doi: 10.1097/COC.0000000000000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciążyńska, M., Pabianek, M., Szczepaniak, K., Ułańska, M., Skibińska, M., Owczarek, W. et al. Quality of life of cancer patients during coronavirus disease (COVID-19) pandemic. Psychooncology 1–3 (2020). [DOI] [PMC free article] [PubMed]
- 20.Richards M, Anderson M, Carter P, Ebert BL, Mossialos E. The impact of the COVID-19 pandemic on cancer care. Nat. Cancer. 2020;1:565–567. doi: 10.1038/s43018-020-0074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omarini C, Maur M, Luppi G, Narni F, Luppi M, Dominici M, et al. Cancer treatment during the coronavirus disease 2019 pandemic: do not postpone, do it! Eur. J. Cancer. 2020;133:29–32. doi: 10.1016/j.ejca.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh WK. COVID-19 infection in cancer patients: early observations and unanswered questions. Ann. Oncol. 2020;31:838–839. doi: 10.1016/j.annonc.2020.03.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poortmans PM, Guarneri V, Cardoso M-J. Cancer and COVID-19: what do we really know? Lancet. 2020;395:1884–1885. doi: 10.1016/S0140-6736(20)31240-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allegra, A., Pioggia, G., Tonacci, A., Musolino, C. & Gangemi, S. Cancer and SARS-CoV-2 infection: diagnostic and therapeutic challenges. Cancers (Basel). 12, 1481(2020). [DOI] [PMC free article] [PubMed]
- 25.Venkatesulu, B. P., Chandrasekar, V. T., Girdhar, P., Advani, P., Sharma, A., Elumalai, T. et al. A systematic review and meta-analysis of cancer patients affected by a novel coronavirus. medRxiv (2020). [DOI] [PMC free article] [PubMed]
- 26.Li Q, Chen L, Li Q, He W, Yu J, Chen L, et al. Cancer increases risk of in-hospital death from COVID-19 in persons <65 years and those not in complete remission. Leukemia. 2020;34:2384–2391. doi: 10.1038/s41375-020-0986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jyotsana, N. & King, M. R. The impact of COVID-19 on cancer risk and treatment. Cell Mol. Bioeng. 13, 285–291 (2020). [DOI] [PMC free article] [PubMed]
- 28.Huang Y, Lu Y, Huang Y-M, Wang M, Ling W, Sui Y, et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metab. Clin. Exp. 2020;113:154378. doi: 10.1016/j.metabol.2020.154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi, T., Ellingson, M. K., Wong, P., Israelow, B., Lucas, C., Klein, J. et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature588, 315–320 (2020). [DOI] [PMC free article] [PubMed]
- 30.Curigliano G. Cancer patients and risk of mortality for COVID-19. Cancer Cell. 2020;38:161–163. doi: 10.1016/j.ccell.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinato, D. J., Zambelli, A., Aguilar-Company, J., Bower, M., Sng, C., Salazar, R. et al. Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Cancer Discov. 10, 1465–1474 (2020). [DOI] [PMC free article] [PubMed]
- 33.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singhania, N., Bansal, S., Nimmatoori, D. P., Ejaz, A. A., McCullough, P. A., Singhania, G. Current overview on hypercoagulability in COVID-19. Am. J. Cardiovasc. Drugs4, 1–11 (2020). [DOI] [PMC free article] [PubMed]
- 35.Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management. Thromb. Res. 2020;194:101–115. doi: 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J. Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertuzzi, A. F., Gennaro, N., Marrari, A. & Santoro, A. The blurred line between the art and science of medicine. Radiother. Oncol. 4, 61 (2020). [DOI] [PMC free article] [PubMed]
- 38.Rocque G, Miller-Sonnet E, Balch A, Stricker C, Seidman J, Stiles S, et al. Engaging multidisciplinary stakeholders to drive shared decision-making in oncology. J. Palliat. Care. 2019;34:29–31. doi: 10.1177/0825859718810723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.