Abstract
PURPOSE
This study aims to compare the volumetric change, degree of conversion (DOC), and cytotoxicity of 3D-printed restorations post-cured under three different conditions.
MATERIALS AND METHODS
3D-printed interim restorations were post-cured under three different conditions and systems: 5 min, 30 min, and 24 h. Three-unit and six-unit fixed dental prostheses (n = 30 for each case) were printed; ten specimens from each group were post-cured and then scanned to compare their volumetric changes. Root-mean-squared (RMS) values of the data were acquired by superimposing the scanned files with original files. Thirty disk-shaped specimens were printed to evaluate the DOC ratio. Fourier transform infrared spectroscopy was used to compare the DOCs of 10 specimens from each group. Human gingival fibroblasts were used to measure the cell viability of every specimen (n = 7). The data from this experiment were employed for one-way analysis of variance and Tukey's post-hoc comparisons.
RESULTS
Differences between the three-unit restorations were statistically insignificant, regardless of the post-curing conditions. However, for the six-unit restorations, a high RMS value was acquired when the post-curing duration was 30 min. The average DOC was approximately 56 – 62%; the difference between each group was statistically insignificant. All the groups exhibited cell viability greater than 70%, rendering them clinically acceptable.
CONCLUSION
The post-curing conditions influenced the volume when the length of the restoration was increased. However, this deviation was found to be clinically acceptable. Additionally, post-curing did not significantly influence the DOC and cytotoxicity of the restorations.
Keywords: 3D printing, Interim restoration, Post-curing
INTRODUCTION
Three-dimensional (3D) printing technology is widely used in clinical dentistry.1 This technology facilitates printing at any time by using the saved patient information. Additionally, simulated implant surgery on dental computer-aided design (CAD) software can be actualized using a 3D printed surgical guide.2 Interim restorations can be manufactured faster and are more aesthetic when created using 3D printing technology, as compared to conventional methods.3 Moreover, the development of biocompatible 3D printing materials for permanent use is expected to accelerate the application of 3D printing technology in dentistry.4
Various mechanisms are involved in 3D printing.1 The stereolithography apparatus (SLA), which uses photopolymerizing material, is widely adopted in clinical dentistry. For high efficiency, vat polymerization with digital light processing (DLP) or liquid crystal display is utilized in SLA methods.5,6 As multiple restorations can be printed simultaneously using this technique, the printing is economical.
The process of 3D printing begins with CAD or scanning.7 The acquired data are exported to a standard triangulated language (STL) file and then printed using the appropriate materials and conditions. The printed resin is rinsed with isopropyl alcohol; then, the support structure is removed. The remnant monomer of the resin is eliminated during the post-curing process. Previous studies have focused on the accuracy and speed of printing, as well as the mechanical properties of 3D-printed dental prostheses.8,9,10,11,12 However, apart from the printing process itself, saving time in the other stages is the key to achieving a more efficient workflow. Among the multiple printing stages, this study focuses on post-curing, which is the last stage of printing.
It is well known that the remnant monomer in a dental resin causes several issues.13,14,15,16 In particular, irritation of the oral mucosa and a decrease in the solidity of the monomer are significant concerns. Therefore, the remnant monomer must be removed through a process called post-curing. In general, dental clinicians consider long post-curing durations to be safe. However, innovative solutions and techniques can potentially reduce the post-curing duration. Thus far, there have been no evaluations of the currently used post-curing systems that simultaneously consider the volumetric changes and biological effect of the remnant monomer after post-curing.
Therefore, this study aims to evaluate the volumetric changes, degrees of conversion (DOCs), and cytotoxicity of 3D-printed interim restorations developed under various post-curing durations. The null hypothesis of this study is that there would be no significant differences in the volumes and biological traits of the interim restorations due to the different post-curing conditions.
MATERIALS AND METHODS
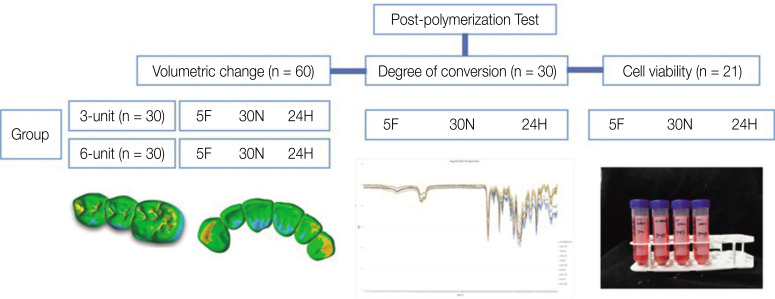
Fig. 1 depicts the experimental protocol of the study. Because this study aimed to evaluate the influence of post-curing alone on the 3D-printing workflow, a single type of 3D printer and material were used for the experiment. Moreover, three- and six-unit fixed dental prostheses for the posterior molar area and anterior teeth, respectively, were selected as the final designs for printing. After extracting the first molar on a standard dentiform, a three-unit fixed dental prosthesis was prepared. Then, the dentiform was scanned and designed using a standard CAD process. For the six-unit fixed dental prosthesis model, both the lateral incisors were removed, and both canines and central incisors were prepared. The following CAD process was undertaken, using the same method to prepare both the three- and six-unit fixed dental prostheses: the group with a post-curing duration of 30 min (group name: 30N) was considered as the control group for this study, as this is the most common post-curing duration.17,18,19 The group with a short post-curing time (5 min), wherein a higher intensity light source was employed (group name: 5F), and that with a post-curing duration of 24 h, wherein natural light was employed without a post-curing machine (group name: 24H), were considered the experimental groups. The printed specimens were cleaned with isopropyl alcohol for 20 min; subsequently, post-curing was performed under the three different conditions. The materials and equipment used in the experiment are summarized in Table 1, and the experimental groups are listed in Table 2.
Fig. 1. Experimental protocol.
Table 1. Summary of materials, as well as composition, manufacturers, and features of equipment used.
| Material | Brands | Manufacturer | Features |
|---|---|---|---|
| 3D Printing resin | Dio C&B | DIO Implant Co. | Methacrylates |
| 3D Printer | CMC Printer | CMC | DLP method |
| Post-polymerization system (5F) | Cure M | SONA GLOBAL Co. Ltd | Attached narrow-angle lens on the LED component |
| Post-polymerization system (30N) | LC-3DPrint Box | NextDent | Peaks at approximately 360 and 435 nm |
Table 2. Experimental groups.
| Group | Post-polymerization system | Time | Condition |
|---|---|---|---|
| 5F | Cure M | 5 min | Fast curing |
| 30N | LC-3DPrint Box | 30 min | Normal curing |
| 24H | Natural sunlight | 24 h | Very slow curing |
The following experiment was performed on the three-unit (n = 30) and six-unit fixed dental prostheses (n = 30) to examine the influence of post-curing on volumetric changes. A statistical power analysis was conducted to estimate the sample size required from each group. With an effect size of 0.22, an alpha of 0.05, and a power of 0.80, the calculations revealed that eight specimens per group would be needed to detect the postulated effect size. For this study, ten specimens from each group were post-cured under the abovementioned conditions. The post-cured specimens were scanned using a model scanner (DOF Freedom; DOF), and the STL files were acquired. The original and scanned STL files were superimposed using the best-fit algorithm to determine the influence of post-curing conditions on volumetric changes using a 3D data analysis program (Geomagic control X, 3D systems, Santa Ana, CA, USA).20 The volumetric changes were calculated using the root mean square (RMS) formula.21
Next, to measure the remnant monomer after post-curing under the various conditions, the DOCs were calculated. Disk-shaped specimens (with a diameter of 5 cm and thickness of 2 cm) were printed and post-cured using the same methods as employed for the fixed dental prosthesis specimens. A spectrometer (Fourier-transform infrared, FTIR, spectrometer, Perkin–Elmer Corporation, Norwalk, CT, USA) was used to examine ten specimens from each post-curing group. Then, the FTIR equation was used to calculate the DOCs by referencing the results obtained before post-curing.22
Human gingival fibroblasts (hGF, ATCC CRL-2014, American Type Culture Collection, Manassas, VA, USA) were used for the cell viability tests. 3D-printed specimens were designed to mimic three-unit fixed dental prostheses, and the specimens for all the three groups were prepared using the same process as in the previous experiment. An additional group of specimens that were only printed and rinsed without post-curing was included to further validate the research. The specimens were transferred into 50 cm3 tubes and incubated in 40 cm3 of Dulbecco's modified Eagle's medium (DMEM, Invitrogen, Carlsbad, CA, USA) for 24 h, which was supplemented with 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA, USA) and 1% antibiotics (100 U/cm3 of penicillin and 100 µg/cm3 of streptomycin, Invitrogen, Carlsbad, USA) at 37℃ in a humid atmosphere containing 5% CO2. Extracts were collected and thlen filtered using 0.20 µm filters (Minisart, Sartorius Stedim Biotech, Goettingen, Germany). The experimental groups were treated with their respective material extracts. In this test, the control group was set to media only. The hGF were seeded into 96-well culture plates at a density of 1 × 104 cells per well and incubated in the DMEM, containing 10% FBS and 1% antibiotics, for 24 h to promote adhesion. Then, the medium was replaced with 100 mm3 of each material extract and incubated for another 24 h. The hGF with the medium were used as controls. The cell viability was examined using 4-[3-(4-Idophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulphonate (WST-1 assay) (EZ-Cytox, Daeil Lab Service, Seoul, Korea), according to the manufacturer's instructions. Next, 10 mm3 of Ez-Cytox (tetrazolium salts) was added to the culture medium and incubated with the cells at 37℃ for 2 h. The absorbance of each well was measured at a wavelength of 450 nm using a microplate spectrophotometer (Multiskan GO Microplate Spectrophotometer, Thermo Scientific, Vantaa, Finland) and SkanIt Software 4.1 (Thermo Scientific Multiskan GO™, Waltham, MA, USA). All procedures of this experiment were based on those in previous studies.23 The schematics of all experiments are summarized in Fig. 1.
Statistical analyses were performed using statistical software (IBM SPSS Statistics, version 22.0, IBM Corp, Chicago, IL, USA). The data from the experiment were employed for a one-way analysis of variance, and Tukey's post-hoc test was used to determine any statistically significant differences. A P-value of < .05 was considered statistically significant.
RESULTS
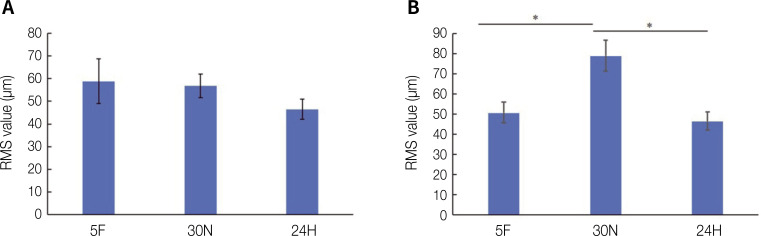
The comparison of the volumetric changes under the three different post-curing conditions is illustrated in Fig. 2. The RMS values of the three-unit fixed dental prostheses were 58.82 ± 9.81 µm for the 5F group, 56.86 ± 5.16 µm for the 30N group, and 46.47 ± 4.38 µm for the 24H group. These outcomes were not statistically significant. In contrast, the RMS values for the six-unit fixed dental prostheses were 46.58 ± 5.02, 79.00 ± 7.59, and 50.90 ± 4.60 µm for the 5F, 30N, and 24H groups, respectively. The specimens of the 30N group exhibited the highest RMS values. The results of Tukey's post-hoc test revealed statistically significant differences between the 5F and 30N groups and between the 30N and 24H groups. However, no significant difference was observed between the specimens of the 5F and 24H groups. A P-value of < .05 was considered statistically significant.
Fig. 2. Volumetric change corresponding to three different post-curing conditions by RMS value. (A) RMS values for three-unit fixed dental prosthesis, and (B) RMS values for six-unit fixed dental prosthesis. The columns connected by the black lines showed statistically significant differences (*P < .05).
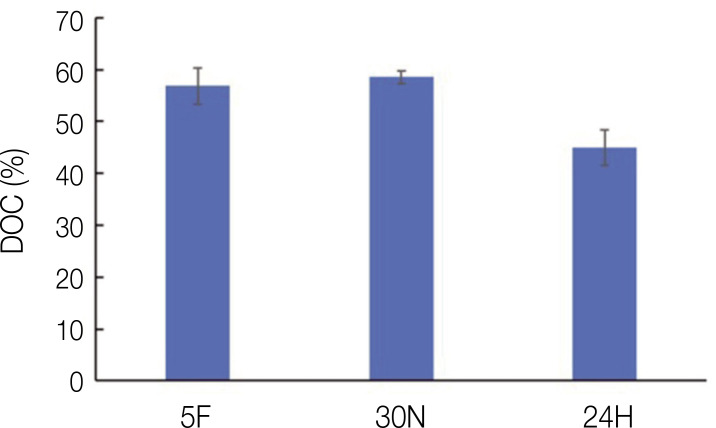
The DOC results are depicted in Fig. 3. In summary, the DOCs were 56% for the 5F group, 62% for the 30N group, and 56% for the 24H group. These results were not statistically significant.
Fig. 3. Degree of conversion corresponding to three different post-curing conditions. All values were not statistically significant.
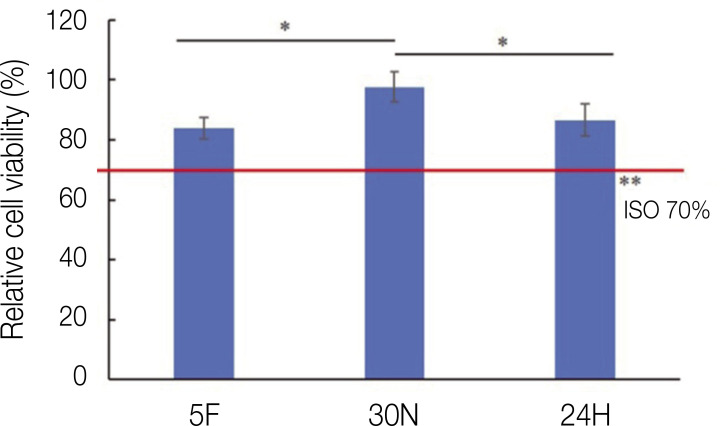
The cell viability results are depicted in Fig. 4. The specimens that did not undergo post-curing exhibited relative cell viabilities of 0.839 ± 0.05, 0.976 ± 0.05, and 0.866 ± 0.02 for the 5F, 30N, and 24H groups, respectively. The results show that cell viability was statistically lower after 30 min of post-curing using the standard method. However, each group surpassed the cut-off level (70%), which is certified by ISO 10993-5.
Fig. 4. Relative cell viability of each group. The columns connected by the black lines showed statistically significant differences (*P < .05). **ISO10993-5:2009 describes test methods to assess the in vitro cytotoxicity of medical devices. These methods are designed to determine the biological response of mammalian cells in vitro using appropriate biological parameters.
DISCUSSION
In this study, we compared the volumetric and biological changes of 3D printed interim restorations prepared following various post-curing procedures. Among the interim restorations, the volumetric changes for the six-unit fixed dental prosthesis specimens were statistically significant. In contrast, there was no statistically significant difference in the DOCs between groups. However, a significant difference was observed in cell viability. Therefore, the null hypothesis of this study was partially rejected.
The polymerization process begins with the radiation of a photopolymer under light at a wavelength at which the polymerizing initiators of the photopolymer are sensitive.24 The wavelengths for the polymerization of conventional composite resins and 3D-printing resins are known to be different. Dental products for 3D printing are polymerized in the presence of light with wavelengths of approximately 405 nm.25,26 Consequently, their polymerization itself may differ from conventional composite resins; therefore, additional experiments for post-curing under multiple conditions are required. 3D-printed specimens that exhibit a significant volumetric change after post-curing cannot be used in clinical practice. Hence, it is crucial to develop a means to minimize post-curing contraction.17,27 Gradual and sufficient post-curing is the key to minimizing contraction and the remnant monomer; however, high production efficiencies cannot be achieved if the post-curing duration is extremely long. Thus, determining the shortest post-curing duration that results in clinically acceptable shrinkage and remnant monomer is important. If the polymerization is performed in a short duration with minimal contraction and remnant monomer, not only can practitioners save time, but patients can also be treated more comfortably. To this end, this study focused on reducing the post-curing duration to maximize comfort in clinical practice.
Various ideas have been developed and adopted to cure resins rapidly. In this experiment, for the 5F post-curing group, a low-angle lens was attached to the ultraviolet LED lamp of the fast post-curing machine. Consequently, the machine could emit light at ~60 mW/cm2, which was twice its usual radiation intensity. Thus, the post-curing duration could theoretically be reduced from 30 min to 5 min, while maintaining adequate mechanical properties, as regulated by ISO 10477. Reduction in the post-curing duration to maximize comfort in clinical practice can only be achieved when the outcomes are accurate. However, because post-curing with intense light induces contraction28, the printed specimens that undergo post-curing using this method need to be clinically evaluated.
In this experiment, three- and six-unit fixed dental prostheses for the posterior teeth and anterior teeth, respectively, were selected. Because the amount of resin used is directly proportional to the amount of contraction, no problem should occur with general crown restorations if there are no flaws with these long fixed dental prosthesis units. The volumetric change results indicate that for the three- and six-unit fixed dental prosthesis interim specimens, the RMS values were 45 – 55 µm and 45 – 80 µm, respectively. The DLP 3D printer used in this experiment is known to print with deviations of up to 50 µm; a deviation of approximately 100 µm is considered to be clinically acceptable. Although the deviation of the six-unit fixed dental prosthesis that was post-cured for 30 min was greater than that of the other specimens, it was still within the clinically acceptable range.
The average DOC of the dental composite resin's C=C is 50 – 70%,20 which also applies to dental resins for 3D printing. The DOC is affected by the filler content and monomer constitution. Higher DOCs can be achieved by the refraction and scattering of light, which increases the depth of curing. An increase in the DOC implies that the polymers in the interim restorations are stable, resulting in enhanced mechanical properties. Previous studies have demonstrated the correlation of hardness and tensile strength with the DOC.8 Therefore, evaluating the DOCs of various specimens with diverse post-curing conditions can help in indirectly evaluating the mechanical properties of 3D printed interim restorations. In this experiment, even the post-curing group with the shortest duration (group 5F) exhibited a DOC of 56%, with no statistically significant differences between the groups. Furthermore, this DOC value was similar to the expected value provided by the manufacturer. Therefore, in terms of DOC, every post-curing technique and duration set for this experiment was found to be clinically acceptable.
Without appropriate post-curing, monomers may remain in the printed dental resin. Remnant monomers can be harmful in the oral environment and reduce the biocompatibility of the printed resin.15,16 hGF were used to evaluate the cell viability of printed interim restorations. Without proper post-curing, the cytotoxicity of the printed specimens is likely to be high. In this experiment, every group exhibited an acceptable cell viability, as certified by ISO10993. Additionally, a group of specimens without post-curing was evaluated to further validate our findings. However, even specimens without post-curing exhibited cytotoxicity levels that complied with the ISO standards. This may be attributed to the sufficient light exposure during the original printing process in all the groups. Therefore, the techniques used in the post-cured groups and the control group can be used in clinical practice.
However, this study has several limitations. For example, various post-curing durations were considered. A more controlled experiment could be realized if a single type of post-curing machine was used for the experiment, while varying the post-curing duration alone. Moreover, a scientifically controlled, shorter post-curing duration was not considered in this study. Nevertheless, as more data are accumulated, the 3D printing of dental prosthetics is expected to provide increased comfort to patients and exhibit higher production efficiency.
CONCLUSION
In this study, various experiments were conducted to assess the influence of volumetric change, DOC, and cytotoxicity on multiple groups of specimens. The results revealed some differences relative to the post-curing time; however, these differences were statistically insignificant.
In conclusion, 3D-printed interim restorations that underwent post-curing for a duration of 5 min were found to exhibit clinically acceptable volumetric changes. The post-curing duration was found to have an insignificant influence on the DOC and cell viability. Therefore, based on the results of the current study, a post-curing duration of 5 min for 3D-printed interim restorations is considered to be clinically acceptable and can thus be applied in clinical practice.
Footnotes
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (No. 2019R1F1A1060935, 2019R1A5A2027521, and 2021R1C1C1008165), a grant (BCRI20030 and BCRI21030) of Chonnam National University Hospital Biomedical Research Institute, and the Technology development Program (S2975345 and S2850798) funded by the Ministry of SMEs and Startups (MSS, Korea).
References
- 1.Dawood A, Marti Marti B, Sauret-Jackson V, Darwood A. 3D printing in dentistry. Br Dent J. 2015;219:521–529. doi: 10.1038/sj.bdj.2015.914. [DOI] [PubMed] [Google Scholar]
- 2.Abduo J, Lyons K, Bennamoun M. Trends in computer-aided manufacturing in prosthodontics: a review of the available streams. Int J Dent. 2014;2014:783948. doi: 10.1155/2014/783948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barazanchi A, Li KC, Al-Amleh B, Lyons K, Waddell JN. Additive technology: update on current materials and applications in dentistry. J Prosthodont. 2017;26:156–163. doi: 10.1111/jopr.12510. [DOI] [PubMed] [Google Scholar]
- 4.Quan H, Zhang T, Xu H, Luo S, Nie J, Zhu X. Photo-curing 3D printing technique and its challenges. Bioact Mater. 2020;5:110–115. doi: 10.1016/j.bioactmat.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seime L, Hardeberg JY. Characterisation of LCD and DLP projection displays. Color Imaging Conf Final Progr Proc. 2002:277–282. [Google Scholar]
- 6.van Noort R. The future of dental devices is digital. Dent Mater. 2012;28:3–12. doi: 10.1016/j.dental.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 7.McGurk M, Amis AA, Potamianos P, Goodger NM. Rapid prototyping techniques for anatomical modelling in medicine. Ann R Coll Surg Engl. 1997;79:169–174. [PMC free article] [PubMed] [Google Scholar]
- 8.Tahayeri A, Morgan M, Fugolin AP, Bompolaki D, Athirasala A, Pfeifer CS, Ferracane JL, Bertassoni LE. 3D printed versus conventionally cured provisional crown and bridge dental materials. Dent Mater. 2018;34:192–200. doi: 10.1016/j.dental.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taft RM, Kondor S, Grant GT. Accuracy of rapid prototype models for head and neck reconstruction. J Prosthet Dent. 2011;106:399–408. doi: 10.1016/S0022-3913(11)60154-6. [DOI] [PubMed] [Google Scholar]
- 10.Goodacre BJ, Goodacre CJ, Baba NZ, Kattadiyil MT. Comparison of denture base adaptation between CAD-CAM and conventional fabrication techniques. J Prosthet Dent. 2016;116:249–256. doi: 10.1016/j.prosdent.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Steinmassl O, Dumfahrt H, Grunert I, Steinmassl PA. CAD/CAM produces dentures with improved fit. Clin Oral Investig. 2018;22:2829–2835. doi: 10.1007/s00784-018-2369-2. [DOI] [PubMed] [Google Scholar]
- 12.Favero CS, English JD, Cozad BE, Wirthlin JO, Short MM, Kasper FK. Effect of print layer height and printer type on the accuracy of 3-dimensional printed orthodontic models. Am J Orthod Dentofacial Orthop. 2017;152:557–565. doi: 10.1016/j.ajodo.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Park SH, Lee CS. The difference in degree of conversion between light-cured and additional heat-cured composites. Oper Dent. 1996;21:213–217. [PubMed] [Google Scholar]
- 14.Susila AV, Balasubramanian V. Correlation of elution and sensitivity of cell lines to dental composites. Dent Mater. 2016;32:e63–e72. doi: 10.1016/j.dental.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Samanidou VF, Kerezoudi C, Tolika E, Palaghias G. A simple isocratic HPLC method for the simultaneous determination of the five most common residual monomers released from resin-based dental restorative materials. J Liq Chromatogr Relat Technol. 2015;38:740–749. [Google Scholar]
- 16.Bourbia M, Ma D, Cvitkovitch DG, Santerre JP, Finer Y. Cariogenic bacteria degrade dental resin composites and adhesives. J Dent Res. 2013;92:989–994. doi: 10.1177/0022034513504436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reymus M, Lümkemann N, Stawarczyk B. 3D-printed material for temporary restorations: impact of print layer thickness and post-curing method on degree of conversion. Int J Comput Dent. 2019;22:231–237. [PubMed] [Google Scholar]
- 18.Reymus M, Stawarczyk B. In vitro study on the influence of postpolymerization and aging on the Martens parameters of 3D-printed occlusal devices. J Prosthet Dent. 2020;19:S0022-3913(20)30077-9. doi: 10.1016/j.prosdent.2019.12.026. [DOI] [PubMed] [Google Scholar]
- 19.Kalberer N, Mehl A, Schimmel M, Müller F, Srinivasan M. CAD-CAM milled versus rapidly prototyped (3D-printed) complete dentures: an in vitro evaluation of trueness. J Prosthet Dent. 2019;121:637–643. doi: 10.1016/j.prosdent.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Kang SY, Park JH, Kim JH, Kim WC. Accuracy of provisional crowns made using stereolithography apparatus and subtractive technique. J Adv Prosthodont. 2018;10:354–360. doi: 10.4047/jap.2018.10.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaefer O, Watts DC, Sigusch BW, Kuepper H, Guentsch A. Marginal and internal fit of pressed lithium disilicate partial crowns in vitro: a three-dimensional analysis of accuracy and reproducibility. Dent Mater. 2012;28:320–326. doi: 10.1016/j.dental.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Leloup G, Holvoet PE, Bebelman S, Devaux J. Raman scattering determination of the depth of cure of light-activated composites: influence of different clinically relevant parameters. J Oral Rehabil. 2002;29:510–515. doi: 10.1046/j.1365-2842.2002.00889.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee BN, Hong JU, Kim SM, Jang JH, Chang HS, Hwang YC, Hwang IN, Oh WM. Anti-inflammatory and osteogenic effects of calcium silicate-based root canal sealers. J Endod. 2019;45:73–78. doi: 10.1016/j.joen.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Fuh J, Lu L, Tan C, Shen Z, Chew S. Processing and characterising photo-sensitive polymer in the rapid prototyping process. J Mater Process Technol. 1999;89:211–217. [Google Scholar]
- 25.Piedra-Cascón W, Sadeghpour M, Att W, Revilla-León M. A vat-polymerized 3-dimensionally printed dual-material occlusal device: a dental technique. J Prosthet Dent. 2020:S0022-3913(20)30438-8. doi: 10.1016/j.prosdent.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Lin CH, Lin YM, Lai YL, Lee SY. Mechanical properties, accuracy, and cytotoxicity of UV-polymerized 3D printing resins composed of Bis-EMA, UDMA, and TEGDMA. J Prosthet Dent. 2020;123:349–354. doi: 10.1016/j.prosdent.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Reymus M, Fabritius R, Keßler A, Hickel R, Edelhoff D, Stawarczyk B. Fracture load of 3D-printed fixed dental prostheses compared with milled and conventionally fabricated ones: the impact of resin material, build direction, post-curing, and artificial aging-an in vitro study. Clin Oral Investig. 2020;24:701–710. doi: 10.1007/s00784-019-02952-7. [DOI] [PubMed] [Google Scholar]
- 28.Par M, Marovic D, Attin T, Tarle Z, Tauböck TT. The effect of rapid high-intensity light-curing on micromechanical properties of bulk-fill and conventional resin composites. Sci Rep. 2020;10:10560. doi: 10.1038/s41598-020-67641-y. [DOI] [PMC free article] [PubMed] [Google Scholar]