Abstract
The Mekong and Chao Phraya rivers harbor a species-rich freshwater mussel assemblage containing a large radiation of the Pseudodontini species. Members of the genera Bineurus Simpson 1900 and Thaiconcha Bolotov et al., 2020 primarily inhabit small and medium-sized tributaries of these rivers. Here, we present an integrative taxonomic review of these genus-level clades. We show that Bineurus contains four species: B. mouhotii (Lea, 1863), B. exilis (Morelet, 1866) stat. rev., B. anodontinum (Rochebrune, 1882) stat. rev., and B. loeiensis sp. nov. In its turn, Thaiconcha comprises three species: T. callifera (Martens, 1860), T. munelliptica sp. nov., and T. thaiensis sp. nov. Two species, Pseudodon ovalis Morlet, 1889 and P. thomsoni Morlet, 1884, are considered here as questionable taxa. These findings further highlight that Southeast Asia represents a significant evolutionary hotspot of freshwater mussels, which requires further international collaborative research and conservation efforts.
Subject terms: Zoology, Taxonomy
Introduction
The Mekong is the largest freshwater basin in Southeast Asia harboring a species-rich fauna of the Unionidae1–3. Several recent phylogenetic studies have substantially revised the systematics of various freshwater mussel taxa from the Mekong basin, especially among representatives of the tribes Indochinellini (e.g. Scabies Haas, 19114, 5, Harmandia Rochebrune, 18824, and Unionetta Haas, 19554), Rectidentini (Ensidens Frierson, 19116, 7, and Hyriopsis Conrad, 18533, 7, 8), and Contradentini (Contradens Haas, 19117, 9–11). Less taxonomic focus has been paid to the most species-rich tribe in Southeast Asia, the Pseudodontini. It was shown that the genus- and species-level diversity of this clade needs greater systematic attention, as it was largely underestimated12.
The genus Bineurus Simpson 1900 represents a divergent clade within the Pseudodontini, with at least two valid species: B. mouhotii (Lea, 1863) and B. exilis (Morelet, 1866)12. Originally, it was described as a subgenus within Pseudodon Gould, 1844 with three species: Pseudodon mouhotii, P. exilis, and P. avae (Theobald, 1873)13, 14. Later, Prashad15 transferred Pseudodon avae to the genus Indopseudodon Prashad, 1922. Afterwards Haas16 synonymized Pseudodon exilis with P. mouhotii and placed only two species in the section Bineurus, i.e. P. mouhotii and P. hageni (Strubell, 1897). Bineurus has remained to be a valid subgenus/section until a comprehensive revision of Brandt1 who recognized the single genus Pseudodon (without subgenera and sections) containing four species, i.e. P. mouhoti, P. inoscularis (Gould, 1844), P. cambodjensis (Petit, 1865), and P. vondembuschianus (Lea, 1840) (the last three taxa with several subspecies). Recently, Bolotov et al.17 resurrected Bineurus as a valid genus based on the results of an integrative taxonomic analysis. However, the species-level taxonomy of Bineurus taxa remains poorly understood, and yet to be revised using DNA sequence data. Furthermore, the genus Thaiconcha Bolotov et al., 2020 was introduced to separate the Pseudodon callifera/P. ellipticum group from the Bineurus clade12. Pseudodon callifera (Martens, 1860) was assigned as the type species for the genus, whereas P. ellipticum Conrad, 1865 and P. thomsoni Morlet, 1884 were synonymized with the first species12. Finally, the nominal taxon Pseudodon ovalis Morlet, 1889 was also placed in Thaiconcha but that solution was based solely on conchological features12.
The present study aims to (1) revise the genera Bineurus and Thaiconcha using integrative approach combining DNA-based, morphological, and biogeographic evidence, and (2) describe three freshwater mussel species new to science discovered in the Mekong and Chao Phraya basins.
Results
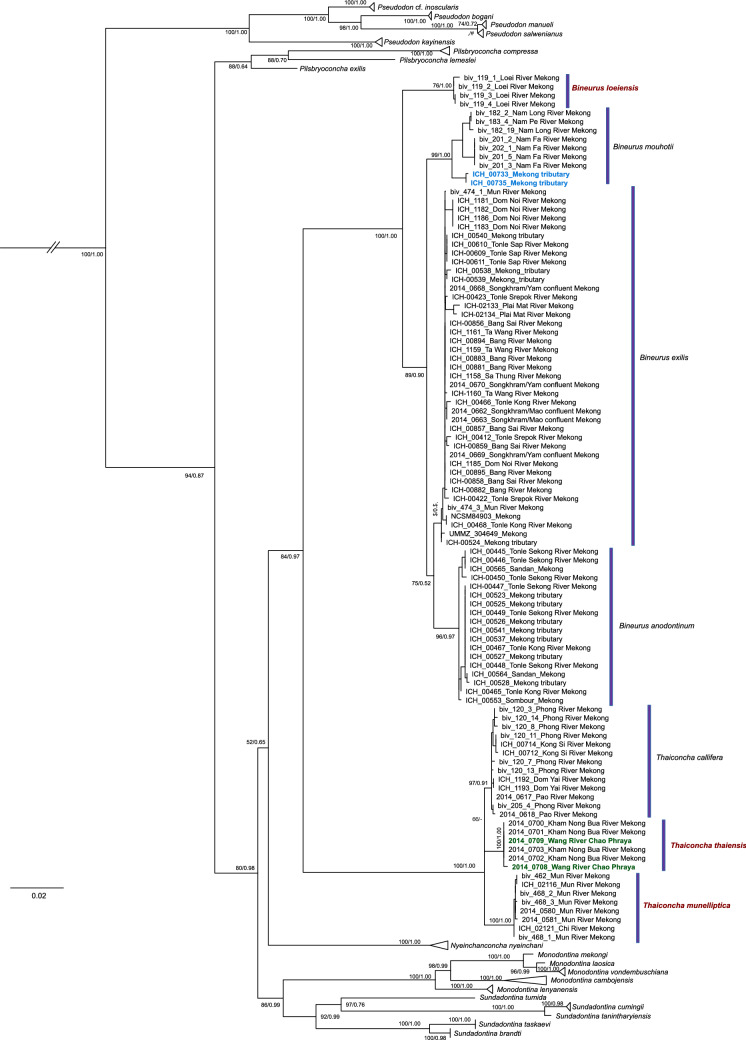
Our multi-locus phylogeny based on three molecular markers (COI + 16S rRNA + 28S rRNA) included 143 individuals from the Pseudodontini (Fig. 1, Supplementary Table 1 and Fig. 1). The tree topology was mainly identical for both the Bayesian and the maximum-likelihood phylogenetic analyses (Fig. 1 and Supplementary Fig. 1). Bineurus and Thaiconcha were both recovered as strongly supported clades (BS/BPP = 100/1.00). We identified four species-level Bineurus subclades corresponding to B. mouhotii, B. exilis, B. anodontinum, and B. loeiensis sp. nov. The Thaiconcha clade contained three species-level subclades, including T. callifera and two undescribed lineages, i.e. T. munelliptica sp. nov. and T. thaiensis sp. nov. All the novel subclades represented well-supported species-level groups with diagnostic nucleotide substitutions (Table 1). The level of genetic divergence (uncorrected COI p-distance) among these species varies from 2.4 to 5.8% (Table 1).
Figure 1.
Maximum likelihood phylogeny of the complete data set of mitochondrial and nuclear sequences (five partitions: three codons of COI + 16S rRNA + 28S rRNA). Outgroup is not shown. Black numbers near nodes are ML Bootstrap Support values (BS)/Bayesian Posterior Probabilities (BPP). The new species names are red; the divergent lineage of Bineurus mouhotii is blue; the Chao Phraya’s lineage of Thaiconcha thaiensis is green.
Table 1.
Molecular diagnoses of new Bineurus and Thaiconcha species from Mekong and Chao Phraya basins.
| Species | Mean COI p-distance from the nearest neighbor, % | The nearest neighbor of new species | Fixed nucleotide differences based on the sequence alignment of congeners | ||
|---|---|---|---|---|---|
| COI | 16S rRNA | 28S rRNA | |||
| B. loeiensis sp. nov | 5.8 | B. exillis | 41C, 56 A, 95 A, 122 G, 221 C, 275 C, 303 C, 329 A, 398 C, 413 G, 419 C, 486 T, 557 G, 584 C, 590 C, 608 A, 629 T, 644 G | 11 G, 15 T, 234 G, 255 T, 390 G, 432 T | 653 G |
| T. munelliptica sp. nov | 3.1 | T. callifera | 20 G, 41 C, 161 A, 197 A, 293 A, 401 C, 476 G, 558 C, 602 C, 641 C | 132 G, 258 G, 259 G, 318 G | n/a |
| T. thaiensis sp. nov | 2.4 | T. callifera | 116 C, 134 G, 158 C, 206 C, 264 C, 374 T, 407 C, 617 T | n/a | n/a |
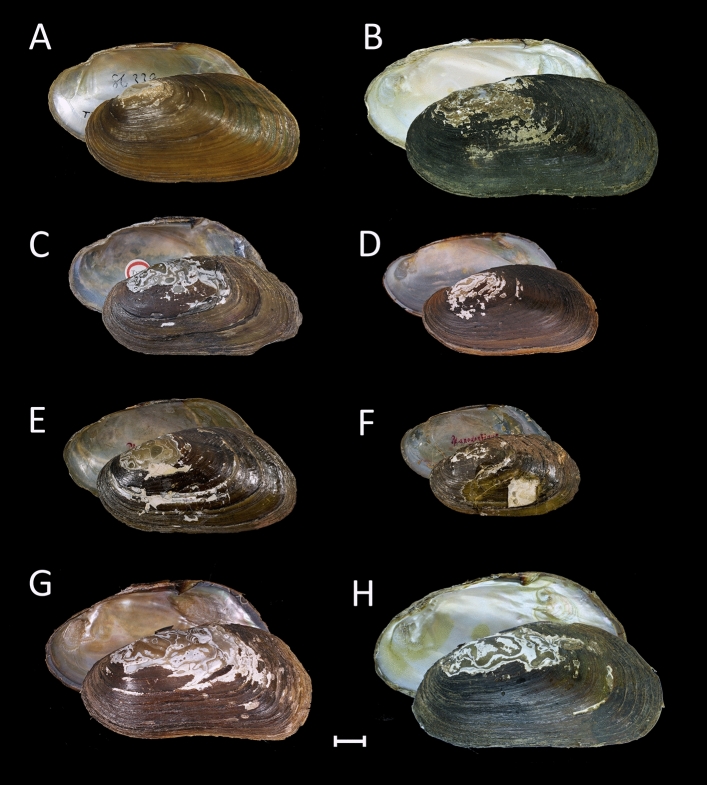
Past studies on the genus Bineurus have demonstrated that species within this clade are difficult to distinguish morphologically1, 17. All four Bineurus species are characterized by having rhomboidal or kidney-like shell outlines, rather thin and compressed shells, and tubercle-like pseudocardinal teeth (Fig. 2). Prospective topotypes of Monocondylaea exilis Morelet 1866 from a tributary of the Tonle Sap River in Cambodia were generally similar to the lectotype of this nominal taxon, according to the shell shape, hinge plate, and underdeveloped muscle scars, although several topotypes share wrinkles on the posterior margin. The nominal taxon Pseudodon pierrei Rochebrune, 1882 synonymized with Bineurus exilis also shared the same features. Prospective topotypes of Pseudodon anodontinum Rochebrune, 1882 found in the Lower Mekong upstream of Sambour and genetically similar specimens from other localities of this river were rather variable in the shell shape but most of these samples had well-developed posterior muscle attachment scars even in young individuals. The latter feature was also observed in a syntype MNHN-IM-2000-1638 of Pseudodon anodontinum Rochebrune, 1882. Bineurus mouhotii is somewhat similar to B. anodontinum by conchological traits but its geographic distribution is primarily restricted to the Upper Mekong. All generic features are also shared by an undescribed lineage of Bineurus (B. loeiensis sp. nov.), and it is characterized by having a smooth periostracum without wrinkles, and higher posterior margin.
Figure 2.
Shells of Bineurus species from the Mekong basin. (A) B. mouhotii (Lea, 1863) [holotype USNM 86339]; (B) B. mouhotii [RMBH biv182_19, Nam Long River, Nam Ou basin, Mekong, Laos]; (C) B. exilis (Morelet, 1866) stat. rev. [lectotype NHMUK 1893.2.4.1981]; (D) B. exilis stat. rev. [topotype UF 507440 (ICH-00610), tributary of Tonle Sap River, Mekong River basin, Cambodia]; (E) Pseudodon pierrei Rochebrune, 1882 syn. nov. [syntype MNHN-IM-2000-1760] (= B. exilis); (F) B. anodontinum (Rochebrune, 1882) stat. rev. [syntype MNHN-IM-2000-1638]; (G) B. anodontinum stat. rev. [topotype UF 507419 (ICH-00553), Mekong River basin, Sambour, Cambodia]; (H) B. loeiensis sp. nov. [holotype RMBH biv119_1, Loei River, Thailand]. Scale bar = 1 cm. Photos: Ilya V. Vikhrev [A]; Ekaterina S. Konopleva [B, H]; Kevin Webb (NHMUK Photographic Unit) [C]; Manuel Caballer (ANR-11-INBS-0004; 2018 MNHN Project “RECOLNAT”) [E, F]; and John M. Pfeiffer [D, G].
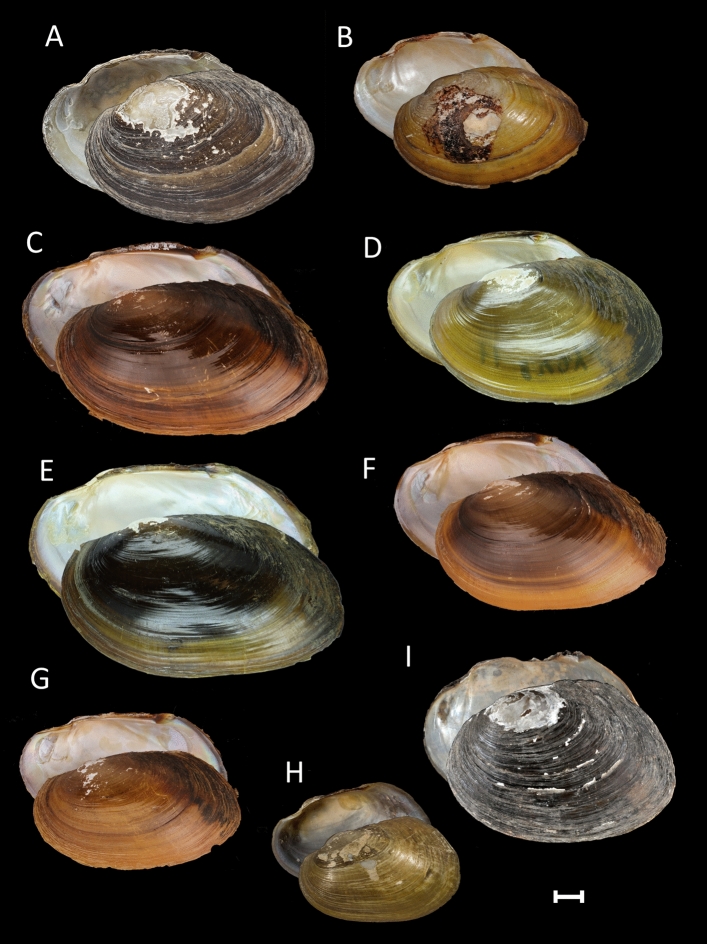
The Thaiconcha species share elliptical shells, small umbo, more or less developed pseudocardinal teeth and muscle scars, and elongated posterior end (Fig. 3). Among Thaiconcha callifera specimens, there were two primary conchological forms. The first form contained individuals with more circular shell and low, triangular posterior wing, thus being similar to the syntype of this nominal taxon (Fig. 3D). The second form occurred more commonly, and mainly had an elongated, elliptical shell (Fig. 3C). An undescribed lineage of Thaiconcha from the Mun basin (i.e. T. munelliptica sp. nov.) (Figs. 3E,F, 4) differs from other taxa by having a larger size and greater inflation. Young specimens usually have winged posterior margin but this feature is typically lost with age. The shell of Thaiconcha thaiensis sp. nov. is broadly rounded in the posterior margin, and has a less prominent posterior wing.
Figure 3.
Shells of Thaiconcha species from the Mekong basin and questionable taxa. (A) T. callifera (Martens, 1860) [syntype NHMUK 1859-8-1-20]; (B) Pseudodon ellipticum Conrad, 1865 [syntype ANSP 41763] (= T. callifera); (C) T. callifera [UF 507741 (ICH-00712), Kong Si River, Mekong River basin, Thailand]; (D) T. callifera [RMBH biv120_7, Phong River, Mekong River basin, Thailand]; (E) T. munelliptica sp. nov. [holotype RMBH biv468_2, Mun River upstream of Tha Tum village, Mekong River basin, Thailand]; (F) T. munelliptica sp. nov. [UF 507607 (2014-0580), Mun River, Thailand]; (G) T. thaiensis sp. nov. [holotype UF 567706 (2014-0700), Kham Nong Bua River, Mekong River basin, Thailand]. (H) Pseudodon thomsoni Morlet, 1884 [syntype MNHN-IM-2000-1800]; (I) Pseudodon ovalis Morlet, 1889 [syntype MNHN-IM-2000-35801]. Scale bar = 1 cm. Photos: Kevin Webb (NHMUK Photographic Unit) [A]; ANSP database [B]; John M. Pfeiffer [C, F, G]; Manuel Caballer (ANR-11-INBS-0004; 2018 MNHN Project “RECOLNAT”) [H]; Philippe Maestrati (MNHN) [I]; and Ekaterina S. Konopleva [D, E].
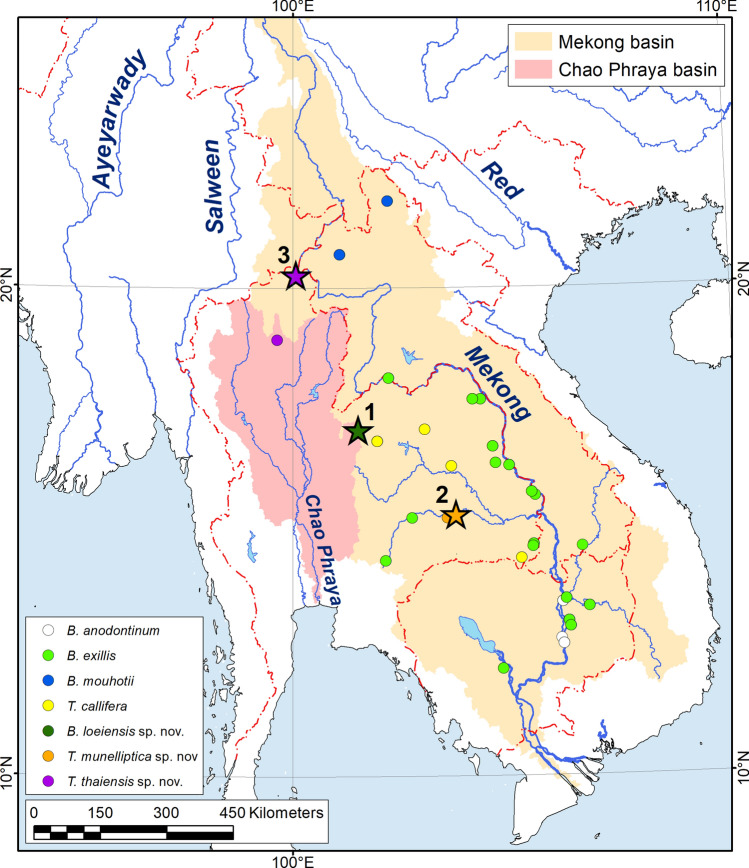
Figure 4.
Distribution ranges of Bineurus and Thaiconcha from Mekong and Chao Phraya basins. The type localities of new species are marked by stars: 1—Bineurus loeiensis sp. nov., Loei River, Thailand; 2—Thaiconcha munelliptica sp. nov., Mun River, Thailand; 3—Thaiconcha thaiensis sp. nov., Kham Nong Bua River, Thailand. The corresponding river basins are highlighted in color. The map was developed using ESRI ArcGIS 10 software (www.esri.com/arcgis). The topographic base of the map was compiled with Natural Earth Free Vector and Raster Map Data (www.naturalearthdata.com), GSHHG version 2.3.7 (http://www.soest.hawaii.edu/pwessel/gshhg)18, and the HydroSHEDS database (http://www.hydrosheds.org)19, 20. (Map: Mikhail Yu. Gofarov).
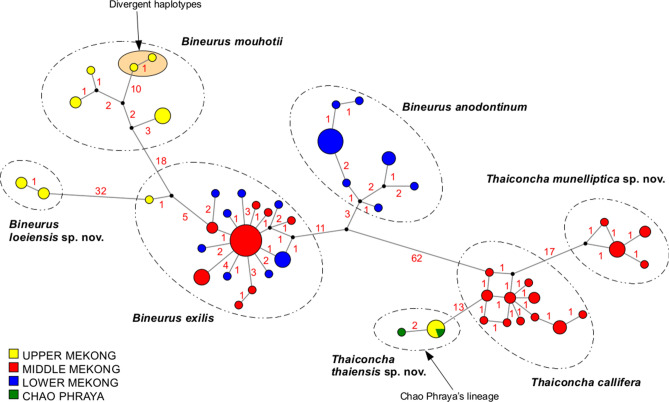
The range of Bineurus includes the Mekong basin in Laos, Thailand, Cambodia, and southern Vietnam. Bineurus exilis is the most widespread species, and is distributed throughout the Middle and Lower Mekong (Fig. 4). Bineurus anodontinum inhabits small tributaries of the Lower Mekong in Cambodia. Bineurus mouhotii is distributed primarily in the Upper Mekong basin in Laos. However, there is a Bineurus mouhotii population (catalog No. UF 507756) around Nong Khai in Thailand (18.1511° N, 102.1790° E), although it represents a rather divergent intraspecific lineage (Fig. 5). An undescribed Bineurus lineage (B. loeiensis sp. nov.) was discovered from the Loei River, a tributary of the Upper Mekong (Fig. 4). We assume that this new species represents an endemic lineage to this watershed.
Figure 5.
Median joining network of the COI sequences of Bineurus and Thaiconcha species (N = 102). The red numbers near branches indicate the numbers of nucleotide substitutions between haplotypes. Size of circles corresponds to the number of available sequences for each haplotype (smallest circle = one sequence). The Mekong was divided into sections based on Halls and Kshatriya21, 22.
The Thaiconcha taxa mainly inhabit the Middle Mekong basin, although one species (T. thaiensis sp. nov.) also occurs in at least one northern tributary of the Chao Phraya (Fig. 4). The T. thaiensis sp. nov. specimens (catalog No. UF 507663) from the Chao Phraya population were genetically identical, or very similar (2 nucleotide difference), to specimens from the Kong Si River of the Upper Mekong (Fig. 5).
Additional morphological research using the type specimens of Pseudodon thomsoni Morlet, 1884 (syntype Cat. no. MNHN-IM-2000-1800) and P. ovalis Morlet, 1889 (syntype Cat. no. MNHN-IM-2000-35801) have demonstrated that these species more likely do not belong to the genus Thaiconcha. Both species share a rather specific shell shape that seems to be higher posteriorly than that of Thaiconcha taxa. The type specimen of Pseudodon ovalis differs from Thaiconcha by having a compressed circular shell with weakly developed wing. A syntype of Pseudodon thomsoni is obovate, moderately inflated, truncated posteriorly, and has a distinct ridge on the posterior slope. These nominal taxa seem to be representatives of Monodontina Conrad, 1853. However, due to the lack of molecular data we transfer these taxa to the questionable group (see Taxonomic account below).
Finally, a taxonomic key was designed for more accurate identification of the genera, belonging to the tribe Pseudodontini.
Key to the Pseudodontini genera based on conchological features
| 1 | Shell rhomboidal, compressed, very thin, no teeth… | Pilsbryoconcha |
| – | Shell of other shape, pseudocardinal teeth present… | 2 |
| 2 | Shell elongate-rhomboid or kidney-shaped, usually with concave ventral margin, compressed, with a knob-like pseudocardinal tooth in each valve… | Bineurus |
| – | Shell elliptical, ovate or rounded, ventral margin not concave (convex or straight)… | 3 |
| 3 | Shell elliptical, elongated posteriorly… | Thaiconcha |
| – | Shell ovate or rounded… | 4 |
| 4 | Pseudocardinal teeth weakly developed, shell winged and truncated posteriorly… | Monodontina |
| – | Pseudocardinal teeth strongly developed, knob-like… | 5 |
| 5 | Shell without prominent wing, rather thick, usually with dark brown or black periostracum… | Sundadontina |
| – | Shell winged or somewhat winged… | 6 |
| 6 | Shell somewhat winged, dorsal and posterior margins usually covered by fine wrinkles… | Pseudodon |
| – | Shell winged, rather thin, usually with light brown periostracum with green radial lines posteriorly; posterior area with weakly developed corrugate plication… | Nyeinchanconcha |
Taxonomic account
Family Unionidae Rafinesque, 1820.
Subfamily Gonideinae Ortmann, 1916.
Tribe Pseudodontini Frierson, 1927.
Type genus: Pseudodon Gould, 1844 (by original designation).
Genus Bineurus Simpson, 1900
Type species: Monocondyloea mouhotii Lea, 1863 (by original designation).
Diagnosis: Bineurus can be distinguished from its sister genera by an elongated rhomboid/kidney-shaped, inequilateral, compressed, and rather thin shell, usually with straight or concave ventral margin. Hinge plate narrow, with a small, tubercle-like pseudocardinal tooth in each valve. Muscle scars are usually more or less developed.
Distribution: Mekong River basin.
Comments: This genus contains four species (Table 2), one of which is new to science and described here.
Table 2.
Taxonomic review of freshwater mussel taxa in the genera Bineurus and Thaiconcha from Mekong and Chao Phraya basins.
| Genus | Species | Type and type locality | Distribution |
|---|---|---|---|
| Bineurus Simpson, 1900 |
B. mouhotii (Lea, 1863) [= Monocondyloea mouhotii Lea, 1863] |
Holotype USNM 86339: Laos Mts., Cambodia, Siam [mountain river/stream belonging to the Mekong River basin between Kenethao (17.6908° N, 101.3775° E) and Luang Prabang (19.8949° N, 102.1334° E), Laos] | Mekong basin in Northern Laos (Nam Ou) and Northern Thailand |
| B. anodontinum (Rochebrune, 1882) [= Pseudodon anodontinum Rochebrune, 1882] | Syntype MNHN-IM-2000-1638: Sombor-Sombor, Mekong, Cochinchine [Mekong River at Sambour, approx. 12.7726° N, 105.9629° E, Cambodia] | Lower Mekong in Cambodia | |
| B. exilis (Morelet, 1866) [= Monocondylaea exilis Morelet, 1866; aPseudodon pierrei Rochebrune, 1882] |
Lectotype NHMUK 1893.2.4.1981: in torrentibus montanis Cambodia (Label data: Lac Tonli-Sap, Cambodia) [Lake Tonle Sap, Cambodia] |
Mekong basin in Thailand, Cambodia and southern Vietnam | |
| Bineurus loeiensis sp. nov | Holotype RMBH biv 119_1: Loei River, 17.0982° N, 101.4814° E, Mekong Basin, Thailand | Mekong basin, Thailand | |
| Thaiconcha Bolotov et al., 2020 | T. callifera (Martens, 1860) [= Anodonta callifera Martens, 1860; Pseudodon ellipticum Conrad, 1865] | Syntype NHMUK 1859-8-1-20: Siam [Thailand] | Mekong basin in Cambodia and Thailand |
| T. munelliptica sp. nov | Holotype RMBH biv 468_2: Mun River, upstream of Tha Tum village, 15.3575° N, 103.6637° E, Thailand | Mun and Chi Rivers in Thailand | |
| T. thaiensis sp. nov | Holotype UF 567706 (2014-0700), Kham Nong Bua River, trib. of Mekong River, at Rt. 1016 bridge 0.3 mi west of Rt. 1290, 20.2681° N, 100.0721° E, Chiang Rai, Thailand | Mekong and Chao Phraya basins in Thailand |
aThese nominal taxa were placed to the corresponding genera or to synonymy on the basis of conchological features alone, and they are in need of future molecular study.
Bineurus mouhotii (Lea, 1863)
= Monocondyloea mouhotii Lea (1863): 19023.
= Monocondylaea mouhotiana Lea (1866): 6524.
= Pseudodon (Bineurus) mouhoti (Lea, 1863): 19025.
= Pseudodon mouhoti (Lea, 1863): 265–2661.
Figure 2A,B.
Type and type locality: Holotype USNM 86339 (examined by us; Fig. 2A); Laos Mts., Cambodia, Siam.
Material examined: LAOS: tributary of Nam Fa River near Vieng Phou Kha, 20.6820° N, 101.0794° E, Mekong River basin, 24.v.2016, 6 specimens [RMBH biv 201 and RMBH biv 202, including RMBH biv 201_2, RMBH biv 201_3, RMBH biv 201_5, and RMBH biv 202_1 sequenced], Spitsyn leg.; Nam Long River, 21.7700° N, 102.1863° E, Nam Ou River basin, Mekong drainage, 4–12.v.2012, 2 specimens [RMBH biv 182, including specimens RMBH biv 182_2, biv 182_19 sequenced], Bolotov and Vikhrev leg. (Fig. 2B); Nam Pe River, 21.5905° N, 102.0829° E, Nam Ou basin, Mekong drainage, 4–12.v.2012, 1 specimen [RMBH biv 183_4, sequenced], Bolotov and Vikhrev leg. THAILAND: tributary of Mekong River at Rt. 211 bridge approximately 3 km south of Ban Muang, 18.1511° N, 102.1790° E, Mekong River basin, Nong Khai, 24.i.2016, UF 507756, 3 specimens [ICH-00735, ICH-00733 sequenced], Pfeiffer and Page leg.
Distribution: Mekong River basin in western and northern Laos (including Nam Ou River) and northern Thailand.
Comments: Isaac Lea briefly described two new freshwater mussel species using a shell sample labelled as “Laos Mountains, Cambodia, Siam. Monsieur Mouhot, per H. Cuming, Esq.”, i.e. Monocondyloea mouhotii and Unio laosensis Lea, 1863 (= Gibbosula laosensis; Margaritiferidae)23. An expanded re-description of these taxa was published three years later23. Henri Mouhot, an adventurous French naturalist, collected this sample somewhere during his travel throughout Siam, Cambodia and Laos in the 1858–1861. Lea24 noted that Mouhot’s collection contained only five or six freshwater mussel species with the two new taxa. The collector died near Luang Prabang (Laos) in 1861, two years before the description of the taxa. Mouhot’s field diary and correspondence were published in a two-volume work entitled “Travels in the central parts of Indo-China (Siam), Cambodia, and Laos, during the years 1858, 1859, and 1860”26, 27. It is likely that the vague type locality of the two taxa was taken by Lea (1863)17 from the general route of Mouhot’s travel. In the field notes, Mouhot26, 27 did not mention any samples of freshwater shells, although several samples of land snails and seashells were noted. However, Samuel Stevens, a well-known British naturalist, in a letter to Mouhot’s brother (p. 294)27 wrote that Mouhot collected freshwater shells only from the Laos Mountains.
Bineurus exilis (Morelet, 1866) stat. rev
= Monocondylaea exilis Morelet (1866): 6328 [lectotype NHMUK 1893.2.4.1981; in torrentibus montanis Cambodia (Label: lac Tonli-Sap, Cambodia)] [CAMBODIA: Lake Tonle Sap; examined by us; Fig. 2C].
= Pseudodon mouhoti (Lea, 1863): 265–2661.
= Pseudodon pierrei Rochebrune (1882) syn. nov.: 4129 [syntype MNHN-IM-2000-1760: Shigloni Breithon, Cochinchine; examined by us; Fig. 2E].
Figure 2C–E.
Topotypes examined: CAMBODIA: a tributary of Tonle Sap River, 12.1889° N, 104.6610° E, Mekong River basin, 2-7.i.2016, UF 507440, 24 specimens [ICH-00609, ICH-00610, and ICH-00611 sequenced], Pfeiffer and Page leg (Fig. 2D).
Other material examined: CAMBODIA: Tonle Srepok River, 13.4434° N, 106.6033° E, Mekong River basin, Khoum Kbal Romeas, 2-7.i.2016, UF 507381, 3 specimens [ICH-00412, ICH-00422, ICH-00423 sequenced], Pfeiffer and Page leg.; Tonle Kong River, 13.6094° N, 106.0924° E, Krong Stung Treng, 2-7.i.2016, UF 567737, 2 specimens [ICH-00466, ICH-00468 sequenced], Pfeiffer and Page leg.; unknown tributary of Mekong just north of Phumi Prêk Preah, 13.1512° N, 106.1452° E, Mekong River basin, Phumi Prêk Preah, 2-7.i.2016, UF 559380, 1 specimen [ICH-00524 sequenced], Pfeiffer and Page leg.; unknown tributary of Mekong just north of Phumi Prêk Preah, 13.04° N, 106.1808°E, Mekong River basin, Phumi Prêk Preah, 2-7.i.2016, UF 507413, 40 specimens [ICH-00538, ICH-00539, ICH-00540 sequenced], Pfeiffer and Page leg. THAILAND: Songkhram River at confluence with Mao River, 17.7020° N, 104.2558° E, Mekong River basin, Nakhon Phanom, 08.i.2015, UF 507639, 2 specimens [2014-0662, 2014-0663 sequenced], Pfeiffer and Page leg.; Songkhram River at confluence with Yam River, 17.7091° N, 104.0767° E, Mekong River basin, Sakon Nakhon, 08.i.2015, UF 507644, 3 specimens [2014-0668, 2014-0669, 2014-0670 sequenced], Pfeiffer and Page leg.; Bang Sai River at Rt. 2292 bridge, 16.7368° N, 104.5195° E, Mekong River basin, Mukdahan, 29.i.2016, UF 507816, 70 specimens [ICH-00856, ICH-00857, ICH-00858, ICH-00859 sequenced], Pfeiffer and Page leg.; Bang River, 16.3464° N, 104.8832° E, Mekong River basin, Mukdahan, 30.i.2016, UF 507828, 18 specimens [ICH-00881, ICH-00882, ICH-00883 sequenced], Pfeiffer and Page leg.; Bang River, 16.3959° N, 104.5715° E, Mekong River basin, Mukdahan, 31.i.2016, UF 507835, 10 specimens [ICH-00894, ICH-00895 sequenced], Pfeiffer and Page leg.; Sa Thung River, 15.7307° N, 105.4511° E, Mekong River basin, Ubon Ratchathani, 01.ii.2016, UF 507845, 3 specimens [ICH-01158 sequenced], Pfeiffer and Page leg.; Ta Wang River, 15.7929° N, 105.3761° E, Mekong River basin, Ubon Ratchathani, 01.ii.2016, UF 507846, 51 specimens [ICH-01159, ICH-01160, ICH-01161 sequenced], Pfeiffer and Page leg.; Dom Noi River, 14.7286° N, 105.3986° E, Mekong River basin, Ubon Ratchathani, 02.ii.2016, UF 507854, 20 specimens [ICH-01181, ICH-01182, ICH-01183, ICH-01185, ICH-01186 sequenced], Pfeiffer and Page leg.; Plai Mat River, 15.2866° N, 102.6853° E, Mekong River basin, Nakhon Ratchasima, 16-28.i.2017, UF 507476, 2 specimens [ICH-02133, ICH-02134 sequenced], Pfeiffer and Page leg.; a tributary of Mekong, 18.1511° N, 102.1790° E, Mekong River basin, Nakhon Ratchasima, 24.i.2016, UF 541633, 1 specimen [ICH-00734 sequenced], Pfeiffer and Page leg.; upstream of upper reservoir, 14.4138° N, 102.0821° E, Mun River, Mekong River basin, 12.iii.2018, 7 specimens [RMBH biv 474, including RMBH biv 474_1 and RMBH biv 474_3 sequenced], Bolotov and Vikhrev leg.
Distribution: Mekong River basin in Thailand, Cambodia, and southern Vietnam.
Comments: The nominal taxon Pseudodon pierrei is considered here to be a junior synonym of Bineurus exilis based on conchological similarity of the type specimen (Fig. 2E) and geographic proximity of the type locality.
Bineurus anodontinum (Rochebrune, 1882) stat. rev
= Pseudodon anodontinum Rochebrune (1882): 4129.
= Pseudodon (Bineurus) mouhoti (Lea, 1863): 265–2661.
= Pseudodon mouhoti (Lea, 1863): 265–2661.
Figure 2F,G.
Type and type locality: Syntype MNHN-IM-2000-1638 (examined by us; Fig. 2F); Sombor-Sombor, Mekong, Cochinchine [CAMBODIA: Mekong River at Sambour, approx. 12.7726° N, 105.9629° E].
Topotypes examined: CAMBODIA: an anabranch of Mekong River about 1.4 miles upstream from Sambour, 12.7946° N, 105.9726° E (Fig. 2G), 2-7.i.2016, UF 507419, 3 specimens [ICH-00553 sequenced], Pfeiffer and Page leg.
Other material examined: CAMBODIA: Tonle Sekong River, 13.5450° N, 106.0135° E, Mekong River basin, Krong Stung Treng, 2-7.i.2016, UF 507391, 9 specimens [ICH-00445, ICH-00446, ICH-00447, ICH-00448, ICH-00449, ICH-00450 sequenced], Pfeiffer and Page leg.; Tonle Kong River just upstream of island, 13.6094° N, 106.0924° E, Mekong River basin, Krong Stung Treng, 2-7.i.2016, UF 507896, 4 specimens [ICH-00465, ICH-00467 sequenced], Pfeiffer and Page leg.; unknown tributary of Mekong just north of Phumi Prêk Preah, 13.1512° N, 106.1452° E, Mekong River basin, Phumi Prêk Preah, 2-7.i.2016, UF 507408, 28 specimens [ICH-00523, ICH-00525, ICH-00526, ICH-00527, ICH-00528 sequenced], Pfeiffer and Page leg.; downstream of Sandan, 12.6869° N, 106.0170° E, Mekong River, Sandan, 2-7.i.2016, UF 507424, 5 specimens [ICH-00564, ICH-00565 sequenced], Pfeiffer and Page leg.; unknown tributary of Mekong just north of Phumi Prêk Preah, 13.0401° N, 106.1808° E, Mekong River basin, Phumi Prêk Preah, 2-7.i.2016, UF 559262, 2 specimens [ICH-00537, ICH-00541 sequenced], Pfeiffer and Page leg.
Distribution: Lower Mekong in Cambodia.
Comments: Our samples were linked to this nominal taxon based on conchological similarity to the type specimen and DNA sequences of topotypes.
Bineurus loeiensis sp. nov
LSID: http://zoobank.org/urn:lsid:zoobank.org:act:095BDBBE-CE3B-4AE7-A011-06A2DD0CEAC8
Type and type locality: Holotype RMBH biv119_1; THAILAND: Loei River, 17.0982° N, 101.4814° E, Mekong basin, 08.iv.2014, Bolotov and Vikhrev leg.
Paratypes: THAILAND: type locality, same collecting date and collectors, 10 specimens [RMBH biv119_2, RMBH biv119_3, RMBH biv119_4, RMBH biv119_5, RMBH biv119_6, RMBH biv119_7, RMBH biv119_8, RMBH biv119_9, RMBH biv119_10, and RMBH biv119_11].
Etymology: The name of this species is derived from the Loei River in Thailand, its type locality.
Diagnosis: The new species is conchologically and genetically similar to Bineurus exillis but differs from it by a more developed pseudocardinal tooth and the lack of prominent wrinkles posteriorly. It can also be distinguished from other Bineurus taxa by fixed nucleotide substitutions in the COI, 16S rRNA, and 28S rRNA gene fragments (Table 1).
Description: Specimens medium-sized: length 36.8–77.1 mm; height 19.5–38.7 mm; width 9.3–21.3 mm. Shell kidney-shaped, inequilateral, moderately thick, rather compressed, rounded anteriorly and truncated posteriorly; dorsal margin slightly curved, ventral margin straight or slightly curved. The umbones not elevated; strongly eroded. Periostracum dark-brown, smooth; nacre whitish with yellow flecks. Pseudocardinal teeth tubercular-like on each valve. Anterior muscle attachment scars rather developed, somewhat drop-like; posterior ones less prominent, rounded.
Habitat and ecology: The species was found only in the Loei River, northern Thailand. It inhabits river sections with silt-sandy substrate, rocks, and boulders.
Distribution: Loei River, northeast Thailand.
Comments: Bolotov et al.17 listed this species as Bineurus aff. mouhotii (Lea, 1863) sp.2.
Genus Thaiconcha Bolotov et al., 2020
Type species: Anodonta callifera Martens, 1860 (by original designation).
Diagnosis: Shell large, elliptical, moderately thick, and inflated. Pseudocardinal teeth rather well developed, muscle attachment scars deep.
Distribution: Mekong and Chao Phraya basins in Thailand and Cambodia.
Comments: This genus contains three species (Table 2), two of which are new to science and described here.
Thaiconcha callifera (Martens, 1860)
= Anodonta callifera Martens (1860): 1530.
= Pseudodon ellipticum Conrad (1865): 352 [syntype ANSP 41763: Cambodia; examined by us; Fig. 3B]31.
= Pseudodon inoscularis callifer (Martens, 1860): 2671.
= Pseudodon vendembuschianus ellipticus Conrad, 1860: 2701.
Figure 3A–D.
Type and type locality: Syntype NHMUK 1859–8-1–20 (examined by us; Fig. 3A); Siam [THAILAND].
Material examined: THAILAND: Phong River, 16.8616° N, 101.9105° E, Mekong River basin, 09.iv.2014, 17 specimens [RMBH biv 120 and biv 205, including RMBH biv 120_3, biv 120_4, biv 120_7, biv 120_8, biv 120_11, biv 120_12, biv 120_13, biv 120_14, biv 120_15, and biv 205_4 sequenced], Bolotov and Vikhrev leg. (Fig. 3D); Kong Si River at bridge ESE of Ban Kumphawapi, 17.0969° N, 102.9885° E, Mekong River basin, Udon Thani, 21.i.2016, UF 507741, 5 specimens [ICH-00712, ICH-00714 sequenced], Pfeiffer and Page leg. (Fig. 3C); Dom Yai River at Rt. 2248 bridge approx. 15 km ESE of Nam Yuen, 14.4460° N, 105.1211° E, Mekong River basin, Ubon Ratchathani, 02.ii.2016, UF 507860, 5 specimens [ICH-01192, ICH-01193 sequenced], Pfeiffer and Page leg.; Pao River, tributary of Chi River at Rt. 214 bridge, 16.3402° N, 103.5758° E, Mun River drainage, Mekong River basin, Kalasin, 06.i.2015, UF 507621, 8 specimens [2014-0617, 2014-0618 sequenced], Pfeiffer and Page leg.
Distribution: Mekong basin in Cambodia and Thailand.
Thaiconcha munelliptica sp. nov
LSID: http://zoobank.org/urn:lsid:zoobank.org:act:4591D422-38FB-4FED-A080-B6285E0BC4BC
Type and type locality: Holotype RMBH biv468_1; THAILAND: pool site with clay bottom, Mun River upstream of Tha Tum village, 15.3575° N, 103.6637° E, Mekong basin, Surin Province, 7.iii.2018, Bolotov and Vikhrev leg.
Paratypes: THAILAND: type locality, same collecting date and collectors as for the holotype, 5 specimens [sequenced specimens RMBH biv468_2 and RMBH biv468_3; RMBH biv468_4, RMBH biv468_5, and RMBH biv468_6]; Mun River at Tha Tum village, 15.3297° N, 103.6821° E, Mekong basin, Surin Province, 7.iii.2018, 1 specimen [sequenced RMBH biv462], local villager leg.; Mun River, 15.3304° N, 103.6818° E, Mekong basin, Surin Province, 06.i. 2015, UF 507607, 2 specimens [sequenced 2014-0580, 2014-0581], Pfeiffer and Page leg.; Mun River, 15.2927° N, 103.5074° E, 2.8 km north of Ban Taklang in Na Nong Phai, Surin Province, 16-28.i.2017, UF 507466, 2 specimens [sequenced ICH-02116], Pfeiffer and Page leg.; Chi River, 15.2838° N, 103.4695° E, 3.4 km WNW of Ban Taklang in Tha Muang, Mekong basin, Buri Ram Province, 16-28.i.2017, UF 507470, 1 specimen [sequenced ICH-02121], Pfeiffer and Page leg.
Etymology: The name of this species is a combination of words “Mun” (i.e. Mun River, after its type locality) and “elliptica” (previously known nominal taxon in this genus).
Diagnosis: This new taxon differs from its sister species Thaiconcha callifera by having a massive, more oblong, and inflated shell, and less prominent pseudocardinal teeth. It can also be distinguished from the sister taxon by fixed nucleotide substitutions in the COI and 16S rRNA gene fragments (Table 1).
Description: Specimens medium or large; shell measurements: length 51.8–98.3 mm, height 30.6–56.9 mm, width 15.6–35.1 mm. Shell elongate-ovate, inequilateral, moderately thick, somewhat inflated; anterior and posterior margins rounded, dorsal and ventral sides curved. Younger shells thinner with developed posterior wing; the periostracum olive-greenish with clear radial striola stretching from the umbones. Older specimens with dark-brown periostracum and lighter ventral margin. The nacre bluish, shining. The umbones slightly elevated, eroded. The pseudocardinal teeth tubercular in each valve. Adductor muscle scars somewhat drop-like, contiguous with pedal retractor scars. The posterior muscle scars shallow.
Habitat and ecology: Slow flowing sections of medium-sized rivers with clay or other soft substrate.
Distribution: Mun and Chi rivers in northeast Thailand.
Thaiconcha thaiensis sp. nov
LSID: http://zoobank.org/urn:lsid:zoobank.org:act:6067A20D-E8A1-4D6E-921B-00BFC0CB1B0F
Type and type locality: Holotype UF 567706; THAILAND: Kham Nong Bua River, trib. of Mekong River, at Rt. 1016 bridge 0.3 mi west of Rt. 1290, 20.2681° N, 100.0721° E, Chiang Rai, 13.i.2015, Pfeiffer and Page leg.
Paratypes: THAILAND: type locality, same collecting date and collectors as holotype, UF 507660, 16 specimens [sequenced 2014-0701, 2014-0702, 2014-0703].
Etymology: The name of this species is derived from the country of Thailand, in which its type locality is situated.
Diagnosis: This taxon is conchologically similar to Thaiconcha califera and T. munelliptica but it may be distinguished from these taxa by its more yellow periostracum (especially in young individuals), more broadly rounded posterior margin, and less prominent posterior wing. It can also be distinguished from the sister taxon by fixed nucleotide substitutions in the COI gene fragment (Table 1).
Description: Specimens small to large: length 34.0–93.8 mm; height 20.5–53.9 mm; width 9.1–28.9 mm. Shell elongate-ovate, inequilateral, moderately thick, somewhat compressed; anterior and posterior margins rounded, dorsal and ventral margins curved. Smaller specimens thinner shelled and more lightly colored, and broadly rounded posteriorly. Larger specimens thicker shelled, darker, and more pointed posteriorly. Radial striola occasionally present, faint. Nacre faintly purple to blue, often with brown stains, strongly reflective. Umbones slightly elevated, commonly eroded (even in small individuals). Lateral teeth absent, large ligamental fosset. Pseudocardinal tooth, one in each valve, mostly smooth, some faint ridges dorsally. Adductor muscles faint, deepening with age, contiguous. Pedal elevator scars distinct, numerous, in straight line.
Habitat and ecology: Small to medium-sized rivers with slow to moderate flow; often found in deeper parts of the stream in stable mud and sand.
Distribution: Mekong and Chao Phraya river basins.
Questionable Taxa
?Pseudodon thomsoni Morlet, 1884
= Pseudodon thomsoni Morlet (1884): 40132.
= Pseudodon (Bineurus) thomsoni Graf & Cummings (2007): 31133.
= Thaiconcha callifera Bolotov et al. (2020) [partim]: 10, 1411.
Type and type locality: Syntype MNHN-IM-2000-1800 (examined by us; Fig. 3H); Cambodia.
Distribution: Mekong basin in Cambodia.
Comments: Haas15 omitted this nominal taxon. There is no available molecular data for it.
?Pseudodon ovalis Morlet, 1889
= Pseudodon ovalis Morlet (1889): 166, 19734.
= Pseudodon (Monodontina) ellipticus Haas (1969) 13416.
= Pseudodon vondembuschianus ellipticus Brandt (1974): 2701.
= Thaiconcha ovalis Bolotov et al. (2020): 1012.
Type and type locality: Syntype MNHN-IM-2000-35801 (examined by us; Fig. 3I); Riviere de Srakeo (Siam) [THAILAND: Bang Pakong River].
Distribution: Bang Pakong basin in Thailand.
Comments: No DNA sequences are available for this taxon.
Discussion
Taxonomic issues
Study of the morphology, phylogeny and distribution of the Pseudodontini from Mekong and Chao Phraya revealed four distant species-level taxa in the genus Bineurus and three such taxa in the genus Thaiconcha. Species in both genera are difficult to distinguish based on conchological traits but are genetically distinct and often times, species are allopathically distributed, which aids in their identification. However, at least one species, Bineurus exilis, does co-occurs with its congeners: B. mouhotii in the northern part of its distribution (Nong Khai Province, Thailand) and B. anodontium in the southern part of distribution, (Kratie Province, Cambodia). These species are morphologically indistinguishable but are divergent phylogenetically. Despite the morphological similarity, their sympatry and high level of genetic divergence suggest that these lineages represent biological species and are intrinsically reproductively isolated. The presence of morphologically similar but phylogenetically distant species among unionids has been demonstrated for a number of genera, e.g. Pleurobema35, Hyriopsis36, Sinanodonta37–40, Fusconaia41, and Etheria42.
The representatives of the genus Thaiconcha are rather variable morphologically and mainly differed by shell shape, especially within T. callifera. Such shape plasticity could be driven by environmental conditions and hydrology which may influence the conchological characteristics43–45. Bineurus and Thaiconcha species were mainly collected from small and medium sized rivers, as well as at the confluence of small tributaries of the Mekong proper, usually with soft (clay, sandy, or somewhat silty) substrate. Less specimens were found in the mainstreams of the Mekong and Chao Phraya.
Our analyses demonstrated a well-resolved phylogeny with high and moderate supports for all available taxa under discussion. Three species new to science, i.e. Bineurus loeiensis sp. nov., Thaiconcha munelliptica sp. nov. and T. thaiensis sp. nov. were recognized and described under the framework of this study. The minimum level of genetic divergence was 2.4% (Table 1), what is generally appropriate for the species delimitation41, 46. The lineage of Bineurus mouhotii from a Mekong’s tributary around Nong Khai (catalog No. UF 507756) is rather divergent phylogenetically (based on the COI gene p-distance = 2.2%), and it may belong to a separate taxon (Fig. 5). However, the morphological similarity and the lack of sequences for 16S rRNA and 28S rRNA gene fragments preclude any final solution on the taxonomic status of this lineage (Supplementary Table 1).
Based on conchological analyses of the type specimens, Pseudodon ovalis and P. thomsoni have been transferred to the group of questionable taxa. Features such as winged and circular shell, small umbo, and weakly developed pseudocardinal teeth are not specific for Thaiconcha members. However, the type of Pseudodon thomsoni represents a young individual that complicates morphological studies, because small specimens of Thaiconcha spp. can often be winged. The taxonomic status of both species is yet to be confirmed based on DNA sequence data.
Distributional and biogeographic notes
The genera Bineurus and Thaiconcha are primarily restricted to the Mekong basin but T. thainesis sp. nov. has also been found in the Upper Chao Phraya basin. This disjunct population suggests a historical stream capture event between the Mekong and Upper Chao Phraya drainages. The hypothesis of past connections between upper parts of these large river basins was supported by a wide array of papers concerning mussels7 and fishes47, 48, as well as summarized in several biogeographic works49, 50. Genetically related snakehead populations of Channa striata (Bloch, 1793) were observed in the Mekong (Chiang Rai) and Chao Phraya (Chiang Mai, Lumphun, Sukhothai and others in the northern and central parts of Thailand)47. The cyprinid fish Garra theunensis Kottelat, 1998, known from the Mekong basin, was found in the Upper Nan River belonging to the Chao Phraya drainage48. Among freshwater mussels, Lens contradens (Lea, 1838) represents an opposite example to Thaiconcha thaiensis as it primarily ranged throughout the Chao Phraya, with a few geographically disjunctive populations in the Mekong (Ing, Kok, and Loei)7.
The rearrangements of Southeast Asian river systems such as separations, connections, and stream captures repeatedly occurred in the past mainly caused by tectonic processes and sea level fluctuations51–53. The Wang River represents a part of the Northern Chao Phraya drainage, raising from the Phi Pan Nam Mountain Range and flowing through Chiang Rai and Lumpang to the Tak Province, where it joins the Ping River54. The tributaries starting from the northern slopes of this range belong to the Mekong basin, while tributaries of the western slopes fell to the Salween River55. The Phi Pan Nam Mountains serve as a geographic barrier separating these large river basins. Hence, a disjunctive range of Thaiconcha thainesis sp. nov. may reflect past rearrangements of the northern paleo-Chao Phraya and the paleo-Mekong. A small genetic divergence between the COI haplotypes of Thaiconcha thaiensis sp. nov. from the Wang River and a tributary of the Upper Mekong indicated that these drainage rearrangements could have been triggered by relatively recent geological events (e.g. Pliocene to Pleistocene)56. It was found that Lens contradens also shares shallow molecular divergence between its populations from the Mekong and Chao Phraya7.
In any case, a more extensive sampling is required for these remote water bodies to reconstruct the history of past drainage rearrangements and to exclude a possibility of recent human-mediated introduction events for given taxa.
Methods
Data sampling
The specimens of Bineurus and Thaiconcha species were collected from different water bodies in Thailand, Laos, and Cambodia (Supplementary Table 1). Among them, prospective topotypes of Bineurus exilis from a tributary of the Tonle Sap River in Cambodia and of B. anodontinum from the Lower Mekong basin (upstream of Sambour) were collected. We studied the type specimens of nominal taxa under discussion and shell lots of other species in the following museum collections: Natural History Museum, London, Great Britain (BMNH); National Museum of Natural History, Smithsonian Institution, Washington D.C., USA (NMNH); Muséum national d’histoire naturelle, Paris, France (MNHN); Florida Museum of Natural History, Gainesville, USA (UF); and Russian Museum of Biodiversity Hotspots, Federal Center for Integrated Arctic Research of the Ural Branch of the Russian Academy of Sciences, Arkhangelsk, Russia (RMBH). Images of the type specimens were obtained from Academy of Natural Sciences of Drexel University, Philadelphia, USA (ANSP), BMNH, and MNHN.
DNA sequence data and phylogenetic analyses
Molecular analysis was based on three molecular markers, i.e. the COI, 16S rRNA, and 28S rRNA gene fragments. It was carried out using PCR primers and laboratory protocols as described in our earlier papers10, 17, 57. The newly generated sequences were checked using a sequence alignment editor (BioEdit v. 7.2.558) and aligned through MUSCLE algorithm in MEGA759. The alignments of three gene fragments were joined to the combined dataset using an online fasta sequence toolbox FaBox v. 1.4160.
Phylogenetic analyses were based on a five-partition dataset (3 codons of COI + 16S rRNA + 28S rRNA) of 147 sequences. Four representatives of the subfamily Gonideinae were used as outgroup, i.e. Gonidea angulata (Lea, 1838), Potomida littoralis (Cuvier, 1798), Leguminaia wheatleyi (Lea, 1862), and Lamprotula leaii (Gray in Griffith & Pidgeon, 1833) (Supplementary Table 1). The COI sequence of B. exilis (ICH-00734) was deleted from the dataset due to having some doubtful regions. The maximum likelihood (ML) analysis was performed through IQ-TREE (W-IQ-TREE) server61 using an automatic identification of most appropriate evolutionary models62 and an ultrafast bootstrap (UFBoot) algorithm with 5000 replicates63. Models of sequence evolution for each partition calculated through Model Finder64 based on Bayesian Information Criterion (BIC) were following: 1st codon COI − F81 + I; 2nd codon COI − TPM2u + G + I; 3rd codon COI − TN + G + I; 28S − HKY + G + I; TIM2e + G. Bayesian inference analysis (BI) was carried out in MrBayes v. 3.2.765 with four runs, each with three heated (temperature = 0.1) and one cold Markov chain, during 25 million generations and sampling every 1000th generation. The first 15% of trees were discarded as burn-in. The calculation was performed at San Diego Supercomputer Center through the CIPRES Science Gateway66. A trace analysis tool (Tracer v. 1.7) was used to check a convergence of the MCMC chains to a stationary distribution67. The effective sample size (ESS) for all the parameters was recorded as > 3000. To study the phylogeography of Bineurus and Thaiconcha species, a median joining network was constructed through Network v. 4.6.1.3 software21 based on 102 COI haplotypes (Supplementary Table 1). The Mekong basin was divided into three sections (upper, middle, and lower) based on fish migration scheme developed by Halls and Kshatriya22, 68. MEGA759 was used to calculate uncorrected p-distances between species and to check the fixed nucleotide differences.
Nomenclatural acts
The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature (ICZN), and hence the new names contained herein are available under that Code from the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank (http://zoobank.org), the online registration system for the ICZN. The LSID for this publication is: http://zoobank.org/urn:lsid:zoobank.org:pub:EE6080B0-3444-46D8-B24B-4B5D6B97EF4C. The electronic edition of this paper was published in a journal with an ISSN, and has been archived and is available from PubMed Central.
Supplementary Information
Acknowledgements
This work was partly supported by the Ministry of Science and Higher Education of the Russian Federation (projects No. AAAA-A18-118012390161-9 and No. 0793-2020-005), by grants of the Russian Foundation for Basic Research (grant No. 18-44-292001_r_mk) and the Russian Science Foundation (grant No. 21-17-00126, including analyses of widespread bivalve taxa of Southeast Asia in the Mekong basin). We would like to thank all museum curators, especially Dr. Virginie Heros from Muséum national d’histoire naturelle, Paris, France; Dr. Jonathan Ablett and Dr. Tom S. White from Natural History Museum, London, Great Britain for their generous assistance in studies of the type series of discussed taxa. We are also very grateful to Dr. Matthias Glaubrecht and one anonymous reviewer for their kind and valuable comments and suggestions.
Author contributions
E.S.K., I.N.B., and J.M.P. developed the concept of the study. J.M.P., E.S.K., I.N.B., I.V.V., S.T., and K.T. collected samples. A.A.T., J.M.P., E.S.K. and A.V.K. designed and processed molecular analyses. E.S.K. performed morphological research and phylogenetic modeling. M.Y.G. created the map. E.S.K., I.N.B. and J.M.P. wrote the paper, with input from I.V.V., A.V.K., and M.Y.G. All authors discussed the final version of the manuscript.
Data availability
Freshwater mussel sampling for this study was approved under permission of the Department of Fisheries, National Inland Fisheries Institute, Bangkok, Thailand. Our samples from Thailand were taken under the export permission No. 11501110316100766 dated on 15 March 2018 issued by the Suvarnabhumi Airport Fish Inspection Office. The type series of the new species are available in the UF—Florida Museum of Natural History, Gainesville, USA, and RMBH—Russian Museum of Biodiversity Hotspots, Federal Center for Integrated Arctic Research, Russian Academy of Sciences, Arkhangelsk, Russia. The sequences generated in this study are available from GenBank. The accession number and collecting locality for each specimen are presented in Supplementary Table 1. Shell measurements and reference DNA sequences for the type series of new freshwater mussel species from Mekong basin are given in Table 3.
Table 3.
Shell measurements and reference DNA sequences for the type series of new Bineurus and Thaiconcha species from Mekong basin.
| Species | Status of specimen | Specimen voucher | Shell length, mm | Shell height, mm | Shell width, mm | NCBI’s GenBank acc. nos | ||
|---|---|---|---|---|---|---|---|---|
| COI | 16S rRNA | 28S rRNA | ||||||
| B. loeiensis sp. nov | Holotype | biv119_1 | 76.6 | 38.7 | 20.8 | KX865879 | KX865650 | KX865750 |
| B. loeiensis sp. nov | Paratype | biv119_2 | 60.2 | 31.4 | 17.1 | KX865880 | KX865651 | KX865751 |
| B. loeiensis sp. nov | Paratype | biv119_3 | 60.1 | 29.9 | 17.2 | KX865881 | KX865652 | KX865752 |
| B. loeiensis sp. nov | Paratype | biv119_4 | 36.8 | 19.5 | 9.3 | KX865882 | KX865653 | KX865753 |
| B. loeiensis sp. nov | Paratype | biv119_5 | 73.2 | 35.7 | 20.1 | n/a | n/a | n/a |
| B. loeiensis sp. nov | Paratype | biv119_6 | 77.1 | 39.8 | 21.3 | n/a | n/a | n/a |
| B. loeiensis sp. nov | Paratype | biv119_7 | 54.6 | 29.0 | 15.6 | n/a | n/a | n/a |
| B. loeiensis sp. nov | Paratype | biv119_8 | 56.8 | 30.2 | 15.6 | n/a | n/a | n/a |
| B. loeiensis sp. nov | Paratype | biv119_9 | 68.5 | 36.0 | 18.7 | n/a | n/a | n/a |
| B. loeiensis sp. nov | Paratype | biv119_10 | 66.6 | 33.8 | 18.6 | n/a | n/a | n/a |
| B. loeiensis sp. nov | Paratype | biv119_11 | 71.8 | 38.0 | 18.3 | n/a | n/a | n/a |
| T. munelliptica sp. nov | Holotype | biv468_1 | 98.3 | 54.7 | 32.7 | MN275064 | MN307253 | MN307194 |
| T. munelliptica sp. nov | Paratype | biv468_2 | 95.1 | 53.8 | 33.5 | MN275065 | MN307254 | MN307195 |
| T. munelliptica sp. nov | Paratype | biv468_3 | 68.0 | 41.2 | 21.9 | MN275066 | MN307255 | MN307196 |
| T. munelliptica sp. nov | Paratype | biv462 | 62.7 | 39.4 | 19.8 | MN275063 | MN307252 | MN307193 |
| T. munelliptica sp. nov | Paratype | biv468_4 | 71.9 | 43.3 | 23.6 | n/a | n/a | n/a |
| T. munelliptica sp. nov | Paratype | biv468_5 | 57.8 | 34.7 | 17.6 | n/a | n/a | n/a |
| T. munelliptica sp. nov | Paratype | biv468_6 | 51.8 | 30.6 | 15.6 | n/a | n/a | n/a |
| T. munelliptica sp. nov | Paratype | UF 507607 (2014-0580) | 75.9 | 45.6 | 24.6 | MW603621 | n/a | n/a |
| T. munelliptica sp. nov | Paratype | UF 507607 (2014-0581) | 80.9 | 43.7 | 26.0 | MW603622 | n/a | n/a |
| T. munelliptica sp. nov | Paratype | UF 507466 (ICH-02116) | 77.7 | 47.3 | 24.9 | MW603691 | n/a | n/a |
| T. munelliptica sp. nov | Paratype | UF 507470 (ICH-02121) | 94.4 | 56.9 | 35.1 | MW603692 | n/a | n/a |
| T. thaiensis sp. nov | Holotype | UF 567706 (2014-0700) | 69.7 | 38.8 | 21.3 | MW603630 | n/a | MW647150 |
| T. thaiensis sp. nov | Paratype | UF 507660 (2014-0701) | 42.1 | 25.2 | 11.8 | MW603631 | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 (2014-0702) | 78.6 | 46.3 | 27.0 | MW603632 | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 (2014-0703) | 93.8 | 53.9 | 28.9 | MW603633 | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 74.2 | 40.9 | 24.0 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 71.1 | 40.4 | 23.6 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 67.6 | 41.4 | 22.1 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 70.6 | 42.8 | 23.1 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 73.1 | 42.1 | 24.3 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 73.6 | 42.3 | 25.6 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 50.1 | 29.2 | 14.7 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 44.2 | 25.9 | 12.8 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 44.1 | 25.7 | 12.7 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 41.4 | 24.5 | 11.0 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 39.3 | 24.0 | 10.8 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 49.0 | 28.2 | 14.2 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 34.0 | 20.5 | 9.1 | n/a | n/a | n/a |
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-87633-w.
References
- 1.Brandt RAM. The non-marine aquatic mollusca of Thailand. Archiv für Mollusckenkunde. 1974;105:1–423. [Google Scholar]
- 2.Bogan AE. Global diversity of freshwater mussels (Mollusca, Bivalvia) in freshwater. Hydrobiologia. 2008;595:139–147. doi: 10.1007/s10750-007-9011-7. [DOI] [Google Scholar]
- 3.Zieritz A, et al. Diversity, biogeography and conservation of freshwater mussels (Bivalvia: Unionida) in East and Southeast Asia. Hydrobiologia. 2018;810:29–44. doi: 10.1007/s10750-017-3104-8. [DOI] [Google Scholar]
- 4.Pfeiffer JM, Graf DL, Cummings KS, Page LM. Molecular phylogeny and taxonomic revision of two enigmatic freshwater mussel genera (Bivalvia: Unionidae incertae sedis: Harmandia and Unionetta) reveals a diverse clade of Southeast Asian Parreysiinae. J. Molluscan Stud. 2018;84:404–416. doi: 10.1093/mollus/eyy028. [DOI] [Google Scholar]
- 5.Jeratthitikul E, Sucharit C, Prasankok P. Molecular phylogeny of the Indochinese freshwater mussel genus Scabies Haas, 1911 (Bivalvia: Unionidae) Trop. Nat. Hist. 2019;19:21–36. [Google Scholar]
- 6.Muanta S, Jeratthitikul E, Panha S, Prasankok P. Phylogeography of the freshwater bivalve genus Ensidens (Unionidae) in Thailand. J. Molluscan Stud. 2019;85:224–231. doi: 10.1093/mollus/eyz013. [DOI] [Google Scholar]
- 7.Pfeiffer, J.M., Graf, D.L., Cummings, K.S. & Page, L.M. Taxonomic revision of a radiation of Southeast Asian freshwater mussels (Unionidae: Gonideinae: Contradentini+Rectidentini). Invertebr. Syst. (2020) (accepted).
- 8.Zieritz A, et al. Towards the conservation of Borneo’s freshwater mussels: Rediscovery of the endemic Ctenodesma borneensis and first record of the non-native Sinanodonta lauta. Biodivers. Conserv. 2020;29:2235–2253. doi: 10.1007/s10531-020-01971-1. [DOI] [Google Scholar]
- 9.Jeratthitikul E, et al. Integrative taxonomy reveals phenotypic plasticity in the freshwater mussel Contradens contradens (Bivalvia: Unionidae) in Thailand, with a description of a new species. Syst. Biodivers. 2019;17:134–147. doi: 10.1080/14772000.2018.1554607. [DOI] [Google Scholar]
- 10.Konopleva ES, et al. A new genus and two new species of freshwater mussels (Unionidae) from western Indochina. Sci. Rep. 2019;9:1–14. doi: 10.1038/s41598-019-39365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konopleva ES, et al. A new Contradens from Laos (Bivalvia: Unionidae: Contradentini) Ecol. Montenegrina. 2019;24:25–31. doi: 10.37828/em.2019.24.5. [DOI] [Google Scholar]
- 12.Bolotov IN, et al. New freshwater mussel taxa discoveries clarify biogeographic division of Southeast Asia. Sci. Rep. 2020;10:6616. doi: 10.1038/s41598-020-63612-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simpson CT. Synopsis of the naiades, or pearly freshwater mussels. Proc. U.S. Natl. Mus. 1900;22:501–1044. doi: 10.5479/si.00963801.22-1205.501. [DOI] [Google Scholar]
- 14.Simpson, C. T. A Descriptive Catalogue of the Naiades, or Pearly Fresh-Water Mussels (Bryant Walker, 1914).
- 15.Prashad B. A revision of the Burmese Unionidae. Rec. Indian Mus. 1922;24:91–111. [Google Scholar]
- 16.Haas F. Superfamilia Unionacea. Das Tierreich. 1969;88:1–663. [Google Scholar]
- 17.Bolotov IN, et al. New taxa of freshwater mussels (Unionidae) from a species-rich but overlooked evolutionary hotspot in Southeast Asia. Sci. Rep. 2017;7:1–18. doi: 10.1038/s41598-017-11957-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wessel P, Smith WHFA. Global self-consistent, hierarchical, high-resolution shoreline database. J. Geophys. Res. 1996;101:8741–8743. doi: 10.1029/96JB00104. [DOI] [Google Scholar]
- 19.Lehner B, Grill G. Global river hydrography and network routing: Baseline data and new approaches to study the world’s large river systems. Hydrol. Process. 2013;27(15):2171–2186. doi: 10.1002/hyp.9740. [DOI] [Google Scholar]
- 20.Lehner B, Verdin K, Jarvis A. New global hydrography derived from spaceborne elevation data. Eos Trans. AGU. 2008;89(10):93–94. doi: 10.1029/2008EO100001. [DOI] [Google Scholar]
- 21.Bandelt HJ, Forster P, Röhl A. Median-joining networks for inferring intra-specific phylogenies. Mol. Biol. Evol. 1999;16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- 22.Halls, A.S. & Kshatriya, M. Modelling the cumulative barrier and passage effects of mainstream hydropower dams on migratory fish populations in the lower Mekong basin. MRC technical Paper25. Mekong River Commission, Vientiane, Lao PDR (2009).
- 23.Lea I. Description of a new species of Unio and a Monocondylaea. Proc. Acad. Natl. Sci. Phila. 1863;15:190. [Google Scholar]
- 24.Lea I. Description of five new species of the genus Unio. Proc. Acad. Nat. Sci. Phila. 1866;18:133. [Google Scholar]
- 25.Suvatti, C. Fauna of Thailand (Department of Fisheries, 1950).
- 26.Mouhot, A. H. Travels in the Central Parts of Indo-China (Siam), Cambodia, and Laos, During the Years 1858, 1859, and 1860 Vol. 1 (Murray, 1864).
- 27.Mouhot, A. H. Travels in the Central Parts of Indo-China (Siam), Cambodia, and Laos, During the Years 1858, 1859, and 1860 Vol. 2 (Murray, 1864).
- 28.Morelet A. Description d’espèces appartenant à la faune malacologique de l’Indo-Chine. J. Conchyliol. 1866;14:62–64. [Google Scholar]
- 29.Rochebrune A-T. Documents sur la faune malacologique de la Cochinchine et du Cambodge. Bulletin de la Société philomathique de Paris. 1882;6:35–74. [Google Scholar]
- 30.Martens E. On the Mollusca of Siam (Communicated by Dr. A. Günther, Foreign Member) Proc. Zool. Soc. Lond. 1860;1860:6–18. [Google Scholar]
- 31.Conrad TA. Description of a new species of Pseudodon. Am. J. Conchol. 1865;1:352. [Google Scholar]
- 32.Morlet L. Description d’espèce nouvelles de coquilles, recueillies par M. Pavie, au Cambodge. J. Conchyliol. 1884;32:386–403. [Google Scholar]
- 33.Graf DL, Cummings KS. Review of the systematics and global diversity of freshwater mussel species (Bivalvia: Unionoida) J. Molluscan Stud. 2007;73:291–314. doi: 10.1093/mollus/eym029. [DOI] [Google Scholar]
- 34.Morlet L. Catalogue des Coquilles recueillies, par M. Pavie, dans le Cambodge et le Royaume de Siam, et description d’espèce nouvelles. J. Conchyliol. 1889;37:121–200. [Google Scholar]
- 35.Gangloff, M.M., Williams, J.D. & Feminella, J.W. A new species of freshwater mussel (Bivalvia: Unionidae), Pleurobema athearni, from the Coosa River Drainage of Alabama, USA. Zootaxa1118, 43–56. https://doi.org/10.11646/zootaxa.1118.1.2 (2006).
- 36.Zieritz A, et al. Factors driving changes in freshwater mussel (Bivalvia, Unionida) diversity and distribution in Peninsular Malaysia. Sci. Total Environ. 2016;571:1069–1078. doi: 10.1016/j.scitotenv.2016.07.098. [DOI] [PubMed] [Google Scholar]
- 37.Bolotov IN, et al. Spreading of the Chinese pond mussel, Sinanodonta woodiana, across Wallacea: One or more lineages invade tropical islands and Europe. Biochem. Syst. Ecol. 2016;67:58–64. doi: 10.1016/j.bse.2016.05.018. [DOI] [Google Scholar]
- 38.Bespalaya YV, et al. DNA barcoding reveals invasion of two cryptic Sinanodonta mussel species (Bivalvia: Unionidae) into the largest Siberian river. Limnologica. 2018;69:94–102. doi: 10.1016/j.limno.2017.11.009. [DOI] [Google Scholar]
- 39.Kondakov, A. V. et al. DNA analysis of a non-native lineage of Sinanodonta woodiana species complex (Bivalvia: Unionidae) from Middle Asia supports the Chinese origin of the European invaders. Zootaxa4462, 511–522. https://doi.org/10.11646/zootaxa.4462.4.4 (2018) . [DOI] [PubMed]
- 40.Kondakov AV, et al. The Asian pond mussels rapidly colonize Russia: Successful invasions of two cryptic species to the Volga and Ob rivers. BioInvasions Rec. 2020;9:504–518. doi: 10.3391/bir.2020.9.3.07. [DOI] [Google Scholar]
- 41.Pieri AM, et al. Molecular and morphometric analyses reveal cryptic diversity with freshwater mussels (Bivalvia: Unionidae) of the western Gulf coastal drainages of the USA. Biol. J. Lin. Soc. 2018;124:261–277. doi: 10.1093/biolinnean/bly046. [DOI] [Google Scholar]
- 42.Elderkin CL, Clewing C, Ndeo OW, Albrecht C. Molecular phylogeny and DNA barcoding confirm cryptic species in the African freshwater oyster Etheria elliptica Lamarck, 1807 (Bivalvia: Etheriidae) Biol. J. Lin. Soc. 2016;118:369–381. doi: 10.1111/bij.12734. [DOI] [Google Scholar]
- 43.Zieritz A, Hoffman JI, Amos W, Aldridge DC. Phenotypic plasticity and genetic isolation-by-distance in the freshwater mussel Unio pictorum (Mollusca: Unionoida) Evol. Ecol. 2010;24:923–938. doi: 10.1007/s10682-009-9350-0. [DOI] [Google Scholar]
- 44.Zieritz A, Aldridge DC. Sexual, habitat-constrained and parasite-induced dimorphism in the shell of a freshwater mussel (Anadonta anatina, Unionidae) J. Morphol. 2011;272:1365–1375. doi: 10.1002/jmor.10990. [DOI] [PubMed] [Google Scholar]
- 45.Bolotov IN, et al. Climate warming as a possible trigger of keystone mussel population decline in oligotrophic rivers at the continental scale. Sci. Rep. 2018;8:1–9. doi: 10.1038/s41598-017-18873-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith CH, Johnson NA, Inoue K, Doyle RD, Randklev CR. Integrative taxonomy reveals a new species of freshwater mussel, Potamilus streckersoni sp. Nov. (Bivalvia: Unionidae): implications for conservation and management. Syst. Biodivers. 2019;17:331–348. doi: 10.1080/14772000.2019.1607615. [DOI] [Google Scholar]
- 47.Hara M, Sekino M, Na-Nakorn U. Genetic differentiation of natural populations of the snake-head fish, Channa striatus in Thailand. Fish. Sci. 1998;64:882–885. doi: 10.2331/fishsci.64.882. [DOI] [Google Scholar]
- 48.Lothongkham A, Ratmuangkhwang S. First record of the Cyprinid Fish, Garra theunensis Kottelat, 1998 (Cypriniformes: Cyprinidae) from the Upper Nan River Basin of the Chao Phraya River System, Northern Thailand. Thailand Nat. Hist. Mus. J. 2018;12:19–27. [Google Scholar]
- 49.Hutchinson, C. S. Geological Evolution of South-East Asia (Clarendon Press, 1989).
- 50.Rainboth, W.J., Vidthayanon, C. & Mai, D.Y. Fishes of the Greater Mekong Ecosystem with Species List and Photographic Atlas (Michigan, 2012).
- 51.Gregory JW. The evolution of the river systems of south-eastern Asia. Scott. Geogr. Mag. 1925;41:129–141. [Google Scholar]
- 52.Woodruff DS. Neogene marine transgressions, palaeogeography and biogeographic transitions on the Thai-Malay Peninsula. J. Biogeogr. 2003;30:551–567. doi: 10.1046/j.1365-2699.2003.00846.x. [DOI] [Google Scholar]
- 53.Köhler, F., Panha, S. & Glaubrecht, M. Speciation and radiation in a river: Assessing the morphological and genetic differentiation in a species flock of viviparous gastropods (Cerithioidea: Pachychilidae) In Evolution in Action. Case Studies in Adaptive Radiation and the Origin of Biodiversity (ed. Glaubrecht, M.) 513–550 (Springer, 2010).
- 54.Petsut N, Kulabtong S. Field survey of freshwater fishes in Upper Wang River, North Thailand. Biodivers. J. 2015;6:513–516. [Google Scholar]
- 55.Glen, F. B. Geologic Reconnaissance of the Mineral Deposits of Thailand (Government Printing Office, 1951).
- 56.Bishop P. Drainage rearrangement by river capture, beheading and diversion. Prog. Phys. Geogr. 1995;19:449–473. doi: 10.1177/030913339501900402. [DOI] [Google Scholar]
- 57.Bolotov IN, et al. Ancient river inference explains exceptional Oriental freshwater mussel radiations. Sci. Rep. 2017;7:1–14. doi: 10.1038/s41598-017-02312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hall TA. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- 59.Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Villesen P. FaBox: An online toolbox for fasta sequences. Mol. Ecol. Notes. 2007;7:965–968. doi: 10.1111/j.1471-8286.2007.01821.x. [DOI] [Google Scholar]
- 61.Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chernomor O, von Haeseler A, Minh BQ. Terrace aware data structure for phylogenomic inference from supermatrices. Syst. Biol. 2016;65:997–1008. doi: 10.1093/sysbio/syw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018;35:518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ronquist F, et al. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller, M., Pfeiffer, W. & Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Gateway Computing Environments Workshop (GCE). 1–8 (IEEE, 2010).
- 67.Rambaut A, et al. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018;67:901–904. doi: 10.1093/sysbio/syy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allen, D.J., Smith, K.G., & Darwall, W.R.T. (Compilers) The Status and Distribution of Freshwater Biodiversity in Indo-Burma (IUCN, 2012).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Freshwater mussel sampling for this study was approved under permission of the Department of Fisheries, National Inland Fisheries Institute, Bangkok, Thailand. Our samples from Thailand were taken under the export permission No. 11501110316100766 dated on 15 March 2018 issued by the Suvarnabhumi Airport Fish Inspection Office. The type series of the new species are available in the UF—Florida Museum of Natural History, Gainesville, USA, and RMBH—Russian Museum of Biodiversity Hotspots, Federal Center for Integrated Arctic Research, Russian Academy of Sciences, Arkhangelsk, Russia. The sequences generated in this study are available from GenBank. The accession number and collecting locality for each specimen are presented in Supplementary Table 1. Shell measurements and reference DNA sequences for the type series of new freshwater mussel species from Mekong basin are given in Table 3.
Table 3.
Shell measurements and reference DNA sequences for the type series of new Bineurus and Thaiconcha species from Mekong basin.
| Species | Status of specimen | Specimen voucher | Shell length, mm | Shell height, mm | Shell width, mm | NCBI’s GenBank acc. nos | ||
|---|---|---|---|---|---|---|---|---|
| COI | 16S rRNA | 28S rRNA | ||||||
| B. loeiensis sp. nov | Holotype | biv119_1 | 76.6 | 38.7 | 20.8 | KX865879 | KX865650 | KX865750 |
| B. loeiensis sp. nov | Paratype | biv119_2 | 60.2 | 31.4 | 17.1 | KX865880 | KX865651 | KX865751 |
| B. loeiensis sp. nov | Paratype | biv119_3 | 60.1 | 29.9 | 17.2 | KX865881 | KX865652 | KX865752 |
| B. loeiensis sp. nov | Paratype | biv119_4 | 36.8 | 19.5 | 9.3 | KX865882 | KX865653 | KX865753 |
| B. loeiensis sp. nov | Paratype | biv119_5 | 73.2 | 35.7 | 20.1 | n/a | n/a | n/a |
| B. loeiensis sp. nov | Paratype | biv119_6 | 77.1 | 39.8 | 21.3 | n/a | n/a | n/a |
| B. loeiensis sp. nov | Paratype | biv119_7 | 54.6 | 29.0 | 15.6 | n/a | n/a | n/a |
| B. loeiensis sp. nov | Paratype | biv119_8 | 56.8 | 30.2 | 15.6 | n/a | n/a | n/a |
| B. loeiensis sp. nov | Paratype | biv119_9 | 68.5 | 36.0 | 18.7 | n/a | n/a | n/a |
| B. loeiensis sp. nov | Paratype | biv119_10 | 66.6 | 33.8 | 18.6 | n/a | n/a | n/a |
| B. loeiensis sp. nov | Paratype | biv119_11 | 71.8 | 38.0 | 18.3 | n/a | n/a | n/a |
| T. munelliptica sp. nov | Holotype | biv468_1 | 98.3 | 54.7 | 32.7 | MN275064 | MN307253 | MN307194 |
| T. munelliptica sp. nov | Paratype | biv468_2 | 95.1 | 53.8 | 33.5 | MN275065 | MN307254 | MN307195 |
| T. munelliptica sp. nov | Paratype | biv468_3 | 68.0 | 41.2 | 21.9 | MN275066 | MN307255 | MN307196 |
| T. munelliptica sp. nov | Paratype | biv462 | 62.7 | 39.4 | 19.8 | MN275063 | MN307252 | MN307193 |
| T. munelliptica sp. nov | Paratype | biv468_4 | 71.9 | 43.3 | 23.6 | n/a | n/a | n/a |
| T. munelliptica sp. nov | Paratype | biv468_5 | 57.8 | 34.7 | 17.6 | n/a | n/a | n/a |
| T. munelliptica sp. nov | Paratype | biv468_6 | 51.8 | 30.6 | 15.6 | n/a | n/a | n/a |
| T. munelliptica sp. nov | Paratype | UF 507607 (2014-0580) | 75.9 | 45.6 | 24.6 | MW603621 | n/a | n/a |
| T. munelliptica sp. nov | Paratype | UF 507607 (2014-0581) | 80.9 | 43.7 | 26.0 | MW603622 | n/a | n/a |
| T. munelliptica sp. nov | Paratype | UF 507466 (ICH-02116) | 77.7 | 47.3 | 24.9 | MW603691 | n/a | n/a |
| T. munelliptica sp. nov | Paratype | UF 507470 (ICH-02121) | 94.4 | 56.9 | 35.1 | MW603692 | n/a | n/a |
| T. thaiensis sp. nov | Holotype | UF 567706 (2014-0700) | 69.7 | 38.8 | 21.3 | MW603630 | n/a | MW647150 |
| T. thaiensis sp. nov | Paratype | UF 507660 (2014-0701) | 42.1 | 25.2 | 11.8 | MW603631 | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 (2014-0702) | 78.6 | 46.3 | 27.0 | MW603632 | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 (2014-0703) | 93.8 | 53.9 | 28.9 | MW603633 | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 74.2 | 40.9 | 24.0 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 71.1 | 40.4 | 23.6 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 67.6 | 41.4 | 22.1 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 70.6 | 42.8 | 23.1 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 73.1 | 42.1 | 24.3 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 73.6 | 42.3 | 25.6 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 50.1 | 29.2 | 14.7 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 44.2 | 25.9 | 12.8 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 44.1 | 25.7 | 12.7 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 41.4 | 24.5 | 11.0 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 39.3 | 24.0 | 10.8 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 49.0 | 28.2 | 14.2 | n/a | n/a | n/a |
| T. thaiensis sp. nov | Paratype | UF 507660 | 34.0 | 20.5 | 9.1 | n/a | n/a | n/a |